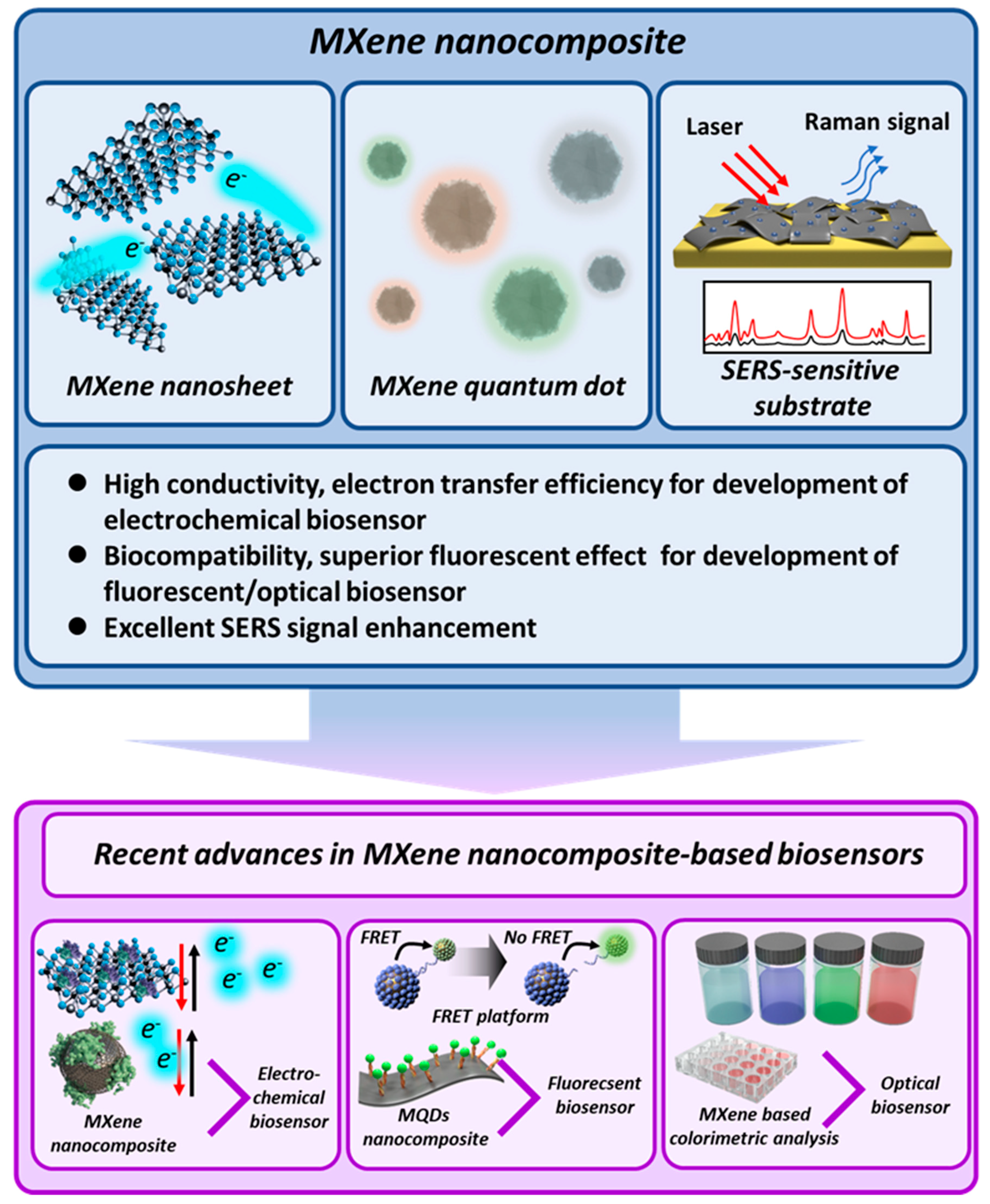
Recent Advances in MXene Nanocomposite-Based Biosensors
Abstract
1. Introduction
2. Definition and Characteristics of MXenes
2.1. Definition of MXenes
2.2. Synthesis Method and Characteristics
2.2.1. HF Etching Method-Based MXenes and Characteristics
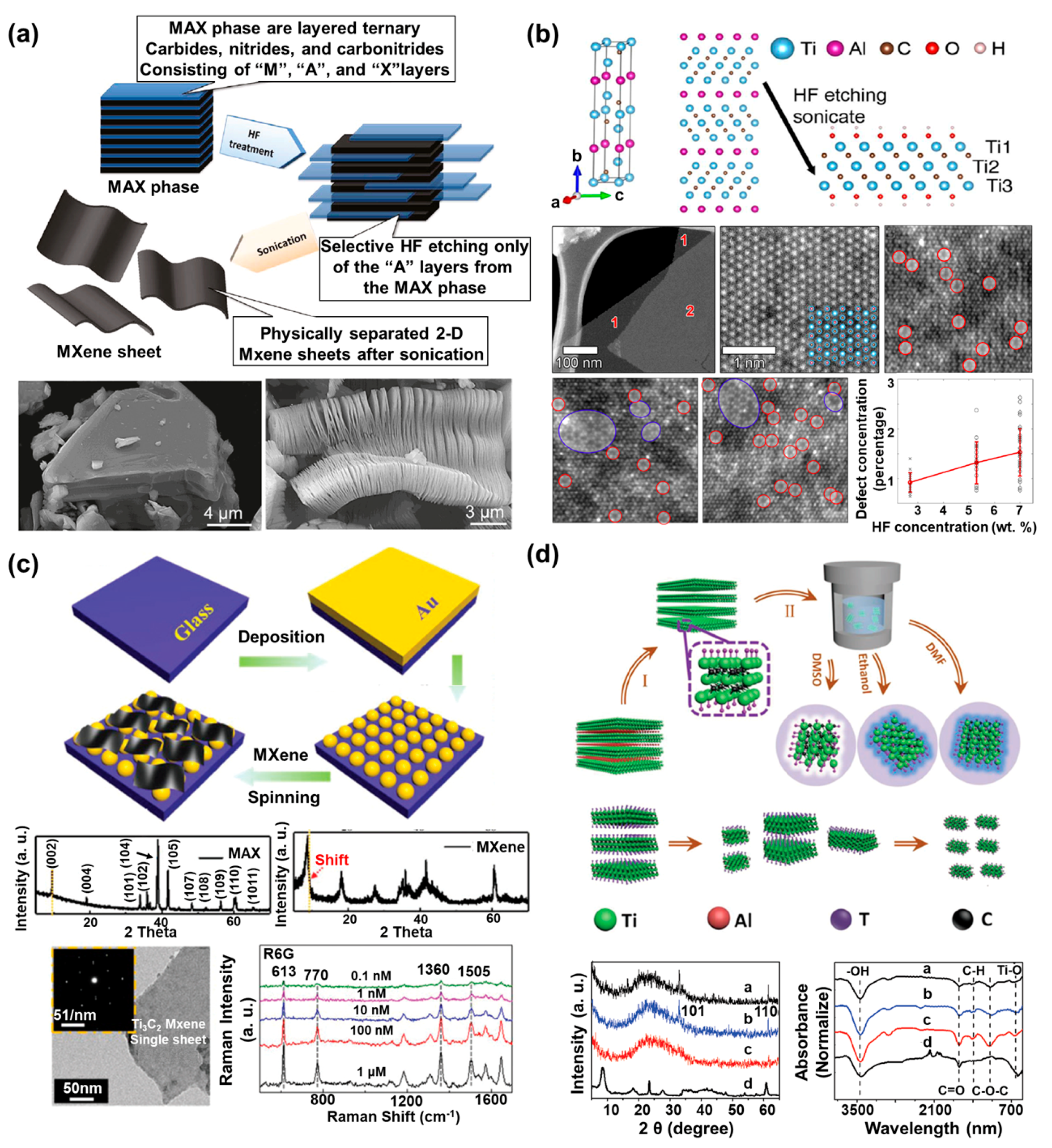
2.2.2. In situ HF Etching Method-Based MXenes and Characteristics
2.2.3. Synthesis of MQDs and Characteristics
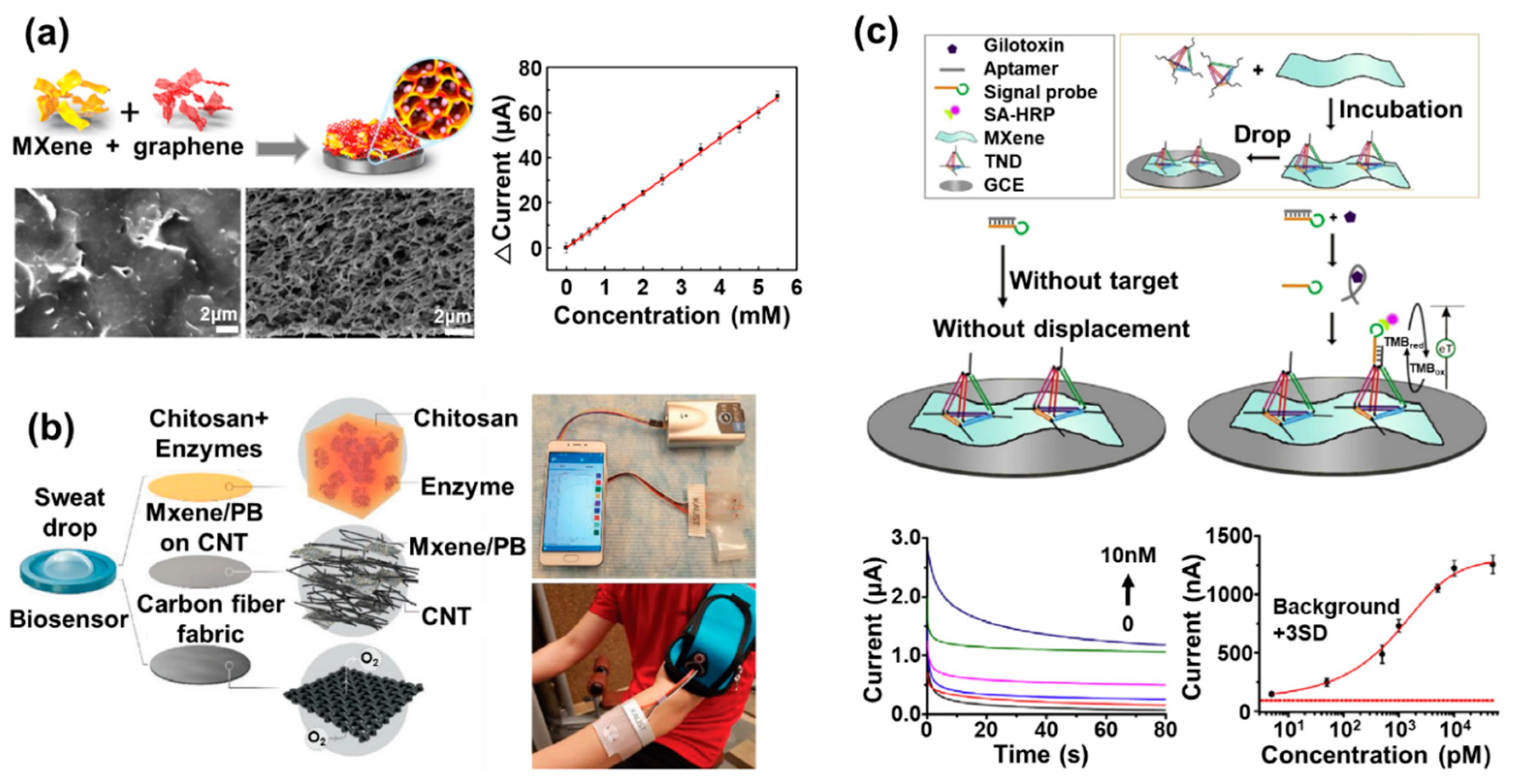
3. MXene-Based Electrochemical Biosensors
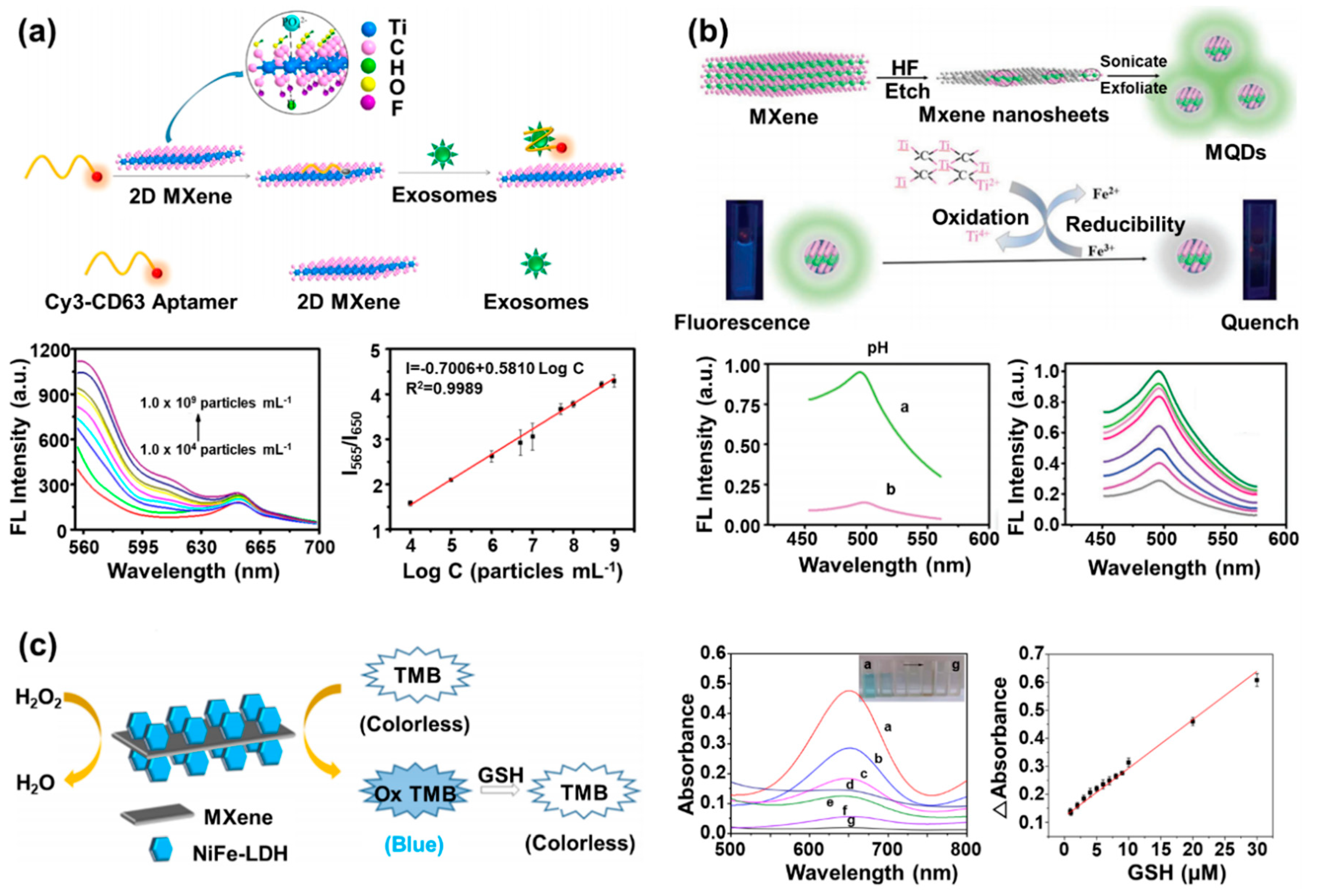
4. MXene-Based Fluorescent/Optical Biosensors
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kirsch, J.; Siltanen, C.; Zhou, Q.; Revzin, A.; Simonian, A. Biosensor technology: Recent advances in threat agent detection and medicine. Chem. Soc. Rev. 2013, 42, 8733–8768. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Ahn, J.; Choi, Y.S.; Jeong, J.-M.; Lee, S.J.; Lee, J.J.; Choi, B.G.; Lee, K.G. Flexible nanopillar-based immunoelectrochemical biosensor for noninvasive detection of Amyloid beta. Nano Converg. 2020, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Roointan, A.; Mir, T.A.; Wani, S.I.; Mati-ur-Rehman; Hussain, K.K.; Ahmed, B.; Abrahim, S.; Savardashtaki, A.; Gandomani, G.; Gandomani, M.; et al. Early detection of lung cancer biomarkers through biosensor technology: A review. J. Pharm. Biomed. Anal. 2019, 164, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Morales-Narváez, E.; Dincer, C. The impact of biosensing in a pandemic outbreak: COVID-19. Biosens. Bioelectron. 2020, 163, 112274. [Google Scholar] [CrossRef]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.C.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C.W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020, 14, 3822–3835. [Google Scholar] [CrossRef]
- Nunna, B.B.; Mandal, D.; Lee, J.U.; Singh, H.; Zhuang, S.; Misra, D.; Bhuyian, M.N.U.; Lee, E.S. Detection of cancer antigens (CA-125) using gold nano particles on interdigitated electrode-based microfuidic biosensor. Nano Converg. 2019, 6, 3. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, D.; Zeng, C.; Miao, Z.; Dai, L. Biocompatible graphene oxide-based glucose biosensors. Langmuir 2010, 26, 6158–6160. [Google Scholar] [CrossRef]
- Mohammadniaei, M.; Yoon, J.; Lee, T.; Choi, J.W. Spectroelectrochemical detection of microRNA-155 based on functional RNA immobilization onto ITO/GNP nanopattern. J. Biotechnol. 2018, 274, 40–46. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H.; Amini, B. A fluorescence Nano-biosensors immobilization on Iron (MNPs) and gold (AuNPs) nanoparticles for detection of Shigella spp. Mater. Sci. Eng. C Biomim. Mater. Sens. 2019, 105, 110113. [Google Scholar] [CrossRef]
- Hussein, M.A.; El-Said, W.A.; Abu-Zied, B.M.; Choi, J.W. Nanosheet composed of gold nanoparticle/graphene/epoxy resin based on ultrasonic fabrication for flexible dopamine biosensor using surface-enhanced Raman spectroscopy. Nano Converg. 2020, 7, 15. [Google Scholar] [CrossRef]
- Wee, Y.; Park, S.; Kwon, Y.H.; Ju, Y.; Yeon, K.M.; Kim, J. Tyrosinase-immobilized CNT based biosensor for highly-sensitive detection of phenolic compounds. Biosens. Bioelectron. 2019, 132, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, A.; Xing, Y.; Duan, H.; Xu, W.; Zhou, Q.; Wu, H.; Chen, C.; Chen, B. Three-dimensional graphene biointerface with extremely high sensitivity to single cancer cell monitoring. Biosens. Bioelectron. 2018, 105, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, H.M.; Ye, P. 2D nanomaterials: Beyond graphene and transition metal dichalcogenides. Chem. Soc. Rev. 2018, 47, 6009–6012. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, T.; Bapurao, G.B.; Jo, J.; Oh, B.K.; Choi, J.-W. Electrochemical H2O2 biosensor composed of myoglobin on MoS2 nanoparticle-graphene oxide hybrid structure. Biosens. Bioelectron. 2017, 93, 14–20. [Google Scholar] [CrossRef]
- Dou, X.M.; Zhao, L.; Li, X.Q.; Qin, L.X.; Han, S.; Kang, S.Z. Ag nanoparticles decorated mesh-like MoS2 hierarchical nanostructure fabricated on Ti foil: A highly sensitive SERS substrate for detection of trace malachite green in flowing water. Appl. Surf. Sci. 2020, 509, 145331. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.Q.; Qiu, Q.; Wen, Q.R.; Zhang, Q.; Yang, W.J.; Yuwen, L.H.; Weng, L.X.; Wang, L.H. Efficient biofunctionalization of MoS2 nanosheets with peptides as intracellular fluorescent biosensor for sensitive detection of caspase-3 activity. J. Colloids Interface Sci. 2019, 543, 96–105. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Gadhari, N.S.; Li, X.; Rao, Z.; Navale, S.T.; Shen, Y.; Patil, V.R.; Huang, Y. Recent advances in MXene–based electrochemical sensors and biosensors. TrAC Trends Anal. Chem. 2019, 120, 115643. [Google Scholar] [CrossRef]
- Sinha, A.; Dhanjai; Zhao, H.M.; Huang, Y.J.; Lu, X.B.; Chen, J.P.; Jain, R. MXene: An emerging material for sensing and biosensing. TrAC Trends Anal. Chem. 2018, 105, 424–435. [Google Scholar] [CrossRef]
- Chen, J.L.; Tong, P.; Huang, L.T.; Yu, Z.H.; Tang, D.P. Ti3C2 MXene nanosheet-based capacitance immunoassay with tyramine-enzyme repeats to detect prostate-specific antigen on interdigitated micro-comb electrode. Electrochim. Acta 2019, 319, 375–381. [Google Scholar] [CrossRef]
- Chen, X.; Sun, X.; Xu, W.; Pan, G.; Zhou, D.; Zhu, J.; Wang, H.; Bai, X.; Dong, B.; Song, H. Ratiometric photoluminescence sensing based on Ti3C2 MXene quantum dots as an intracellular pH sensor. Nanoscale 2018, 10, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Sarycheva, A.; Makaryan, T.; Maleski, K.; Satheeshkumar, E.; Melikyan, A.; Minassian, H.; Yoshimura, M.; Gogotsi, Y. Two-Dimensional Titanium Carbide (MXene) as Surface-Enhanced Raman Scattering Substrate. J. Phys. Chem. C 2017, 121, 19983–19988. [Google Scholar] [CrossRef]
- Meng, R.; Huang, J.; Feng, Y.; Zu, L.; Peng, C.; Zheng, L.; Zheng, L.; Chen, Z.; Liu, G.; Chen, B.; et al. Black Phosphorus Quantum Dot/Ti3C2 MXene Nanosheet Composites for Efficient Electrochemical Lithium/Sodium-Ion Storage. Adv. Energy Mater. 2018, 8, 1801514. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, L.; Xie, X.-Y.; Li, X.; Ma, Y.; Liu, Q.; Fang, W.-H.; Shi, X.; Cui, G.; Sun, X. Ti3C2Tx (T = F, OH) MXene nanosheets: Conductive 2D catalysts for ambient electrohydrogenation of N2 to NH3. J. Mater. Chem. A 2018, 6, 24031–24035. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Shi, J. Insights into 2D MXenes for Versatile Biomedical Applications: Current Advances and Challenges Ahead. Adv. Sci. 2018, 5, 1800518. [Google Scholar] [CrossRef]
- Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124. [Google Scholar] [CrossRef]
- Dai, C.; Lin, H.; Xu, G.; Liu, Z.; Wu, R.; Chen, Y. Biocompatible 2D Titanium Carbide (MXenes) Composite Nanosheets for pH-Responsive MRI-Guided Tumor Hyperthermia. Chem. Mater. 2017, 29, 8637–8652. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, X.; Zhang, R.; Zhang, Y.; Wu, L.; Lu, W.; Li, J.; Li, Y.; Zhang, H. MXene-Enabled Electrochemical Microfluidic Biosensor: Applications toward Multicomponent Continuous Monitoring in Whole Blood. Adv. Funct. Mater. 2019, 29, 1807326. [Google Scholar] [CrossRef]
- Guan, Q.; Ma, J.; Yang, W.; Zhang, R.; Zhang, X.; Dong, X.; Fan, Y.; Cai, L.; Cao, Y.; Zhang, Y.; et al. Highly fluorescent Ti3C2 MXene quantum dots for macrophage labeling and Cu2+ ion sensing. Nanoscale 2019, 11, 14123–14133. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, P.; Xue, T.; Ge, Y.; Ai, S.; Sheng, Y.; Wu, R.; Xu, L.; Tang, K.; Wen, Y. A novel graphene-like titanium carbide MXene/Au–Ag nanoshuttles bifunctional nanosensor for electrochemical and SERS intelligent analysis of ultra-trace carbendazim coupled with machine learning. Ceram. Int. 2021, 47, 173–184. [Google Scholar] [CrossRef]
- Jun, B.M.; Kim, S.; Heo, J.; Park, C.M.; Her, N.; Jang, M.; Huang, Y.; Han, J.; Yoon, Y. Review of MXenes as new nanomaterials for energy storage/delivery and selected environmental applications. Nano Res. 2019, 12, 471–487. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Jin, H.; Kim, B.; Baek, D.S.; Joo, S.H.; Lee, K. MXene: An emerging two-dimensional material for future energy conversion and storage applications. J. Mater. Chem. A 2017, 5, 24564–24579. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2T × MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Sajid, M. MXenes: Are they emerging materials for Analytical Chemistry applications?—A review. Anal. Chim. Acta 2020, in press. [Google Scholar] [CrossRef]
- Srivastava, P.; Mishra, A.; Mizuseki, H.; Lee, K.R.; Singh, A.K. Mechanistic Insight into the Chemical Exfoliation and Functionalization of Ti3C2 MXene. ACS Appl. Mater. Interfaces 2016, 8, 24256–24264. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Zhao, J.B.; Wen, J.; Bai, L.N.; Xiao, J.P.; Zheng, R.D.; Shan, X.Y.; Li, L.; Gao, H.; Zhang, X.T. One-step synthesis of few-layer niobium carbide MXene as a promising anode material for high-rate lithium ion batteries. Dalton Trans. 2019, 48, 14433–14439. [Google Scholar] [CrossRef] [PubMed]
- Sang, X.; Xie, Y.; Lin, M.-W.; Alhabeb, M.; Van Aken, K.L.; Gogotsi, Y.; Kent, P.R.; Xiao, K.; Unocic, R.R. Atomic Defects in Monolayer Titanium Carbide (Ti3C2Tx) MXene. ACS Nano 2016, 10, 9193–9200. [Google Scholar] [CrossRef]
- Feng, A.; Yu, Y.; Wang, Y.; Jiang, F.; Yu, Y.; Mi, L.; Song, L. Two-dimensional MXene Ti3C2 produced by exfoliation of Ti3AlC2. Mater. Des. 2017, 114, 161–166. [Google Scholar] [CrossRef]
- Xu, G.; Niu, Y.; Yang, X.; Jin, Z.; Wang, Y.; Xu, Y.; Niu, H. Preparation of Ti3C2Tx MXene-Derived Quantum Dots with White/Blue-Emitting Photoluminescence and Electrochemiluminescence. Adv. Opt. Mater. 2018, 6, 1800951. [Google Scholar] [CrossRef]
- Khazaei, M.; Arai, M.; Sasaki, T.; Chung, C.Y.; Venkataramanan, N.S.; Estili, M.; Sakka, Y.; Kawazoe, Y. Novel electronic and magnetic properties of two-dimensional transition metal carbides and nitrides. Adv. Funct. Mater. 2013, 23, 2185–2192. [Google Scholar] [CrossRef]
- Zhu, J.; Ha, E.N.; Zhao, G.L.; Zhou, Y.; Huang, D.S.; Yue, G.Z.; Hu, L.S.; Sun, N.; Wang, Y.; Lee, L.Y.S.; et al. Recent advance in MXenes: A promising 2D material for catalysis, sensor and chemical adsorption. Coord. Chem. Rev. 2017, 352, 306–327. [Google Scholar] [CrossRef]
- Soomro, R.A.; Jawaid, S.; Zhu, Q.Z.; Abbas, Z.; Xu, B. A mini-review on MXenes as versatile substrate for advanced sensors. Chin. Chem. Lett. 2020, 31, 922–930. [Google Scholar] [CrossRef]
- Yu, M.; Liu, S.; Su, D.; Jiang, S.; Zhang, G.; Qin, Y.; Li, M.Y. Controllable MXene nano-sheet/Au nanostructure architectures for the ultra-sensitive molecule Raman detection. Nanoscale 2019, 11, 22230–22236. [Google Scholar] [CrossRef] [PubMed]
- Lipatov, A.; Alhabeb, M.; Lukatskaya, M.R.; Boson, A.; Gogotsi, Y.; Sinitskii, A. Effect of Synthesis on Quality, Electronic Properties and Environmental Stability of Individual Monolayer Ti3C2 MXene Flakes. Adv. Electron. Mater. 2016, 2, 1600255. [Google Scholar] [CrossRef]
- Xie, H.; Li, P.; Shao, J.; Huang, H.; Chen, Y.; Jiang, Z.; Chu, P.K.; Yu, X.-F. Electrostatic Self-Assembly of Ti3C2Tx MXene and Gold Nanorods as an Efficient Surface-Enhanced Raman Scattering Platform for Reliable and High-Sensitivity Determination of Organic Pollutants. ACS Sens. 2019, 4, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.H.; Niu, Y.S.; Liu, M.L.; Zhang, K.X.; Xu, G.F.; Wang, Y.; Wang, X.W.; Xu, Y.H.; Li, J.H. One-step hydrothermal synthesis of fluorescent MXene-like titanium Chock for carbonitride quantum dots. Inorg. Chem. Commun. 2019, 105, 151–157. [Google Scholar] [CrossRef]
- Feng, X.M.; Liu, Y.Y.; Kong, Q.C.; Ye, J.S.; Chen, X.H.; Hu, J.Q.; Chen, Z.W. Direct electrochemistry of myoglobin immobilized on chitosan-wrapped rod-constructed ZnO microspheres and its application to hydrogen peroxide biosensing. J. Solid State Electrochem. 2010, 14, 923–930. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Bulko, T.V.; Archakov, A.I. Electrochemical reduction of cytochrome P450 as an approach to the construction of biosensors and bioreactors. J. Inorg. Biochem. 2005, 99, 1051–1063. [Google Scholar] [CrossRef]
- Gu, H.; Xing, Y.; Xiong, P.; Tang, H.; Li, C.; Chen, S.; Zeng, R.; Han, K.; Shi, G. Three-Dimensional Porous Ti3C2Tx MXene–Graphene Hybrid Films for Glucose Biosensing. ACS Appl. Nano Mater. 2019, 2, 6537–6545. [Google Scholar] [CrossRef]
- Lei, Y.; Zhao, W.; Zhang, Y.; Jiang, Q.; He, J.H.; Baeumner, A.J.; Wolfbeis, O.S.; Wang, Z.L.; Salama, K.N.; Alshareef, H.N. A MXene-Based Wearable Biosensor System for High-Performance In Vitro Perspiration Analysis. Small 2019, 15, 1901190. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, H.; Huang, Y.; Xiong, M.; Wang, F.; Li, C. A label-free electrochemical biosensor for highly sensitive detection of gliotoxin based on DNA nanostructure/MXene nanocomplexes. Biosens. Bioelectron. 2019, 142, 111531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, F.; Zhang, H.; Zhang, Y.; Liu, M.; Liu, Y. Universal Ti3C2 MXenes Based Self-Standard Ratiometric Fluorescence Resonance Energy Transfer Platform for Highly Sensitive Detection of Exosomes. Anal. Chem. 2018, 90, 12737–12744. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Liu, M.; Liu, Y. Selective detection of Fe3+ ions based on fluorescence MXene quantum dots via a mechanism integrating electron transfer and inner filter effect. Nanoscale 2020, 12, 1826–1832. [Google Scholar] [CrossRef]
- Li, H.; Wen, Y.; Zhu, X.; Wang, J.; Zhang, L.; Sun, B. Novel Heterostructure of a MXene@NiFe-LDH Nanohybrid with Superior Peroxidase-Like Activity for Sensitive Colorimetric Detection of Glutathione. ACS Sustain. Chem. Eng. 2019, 8, 520–526. [Google Scholar] [CrossRef]
- Wu, L.X.; Lu, X.B.; Dhanjai; Wu, Z.S.; Dong, Y.F.; Wang, X.H.; Zheng, S.H.; Chen, J.P. 2D transition metal carbide MXene as a robust biosensing platform for enzyme immobilization and ultrasensitive detection of phenol. Biosens. Bioelectron. 2018, 107, 69–75. [Google Scholar] [CrossRef]
- Koyappayil, A.; Chavan, S.G.; Mohammadniaei, M.; Go, A.; Hwang, S.Y.; Lee, M.-H. β-Hydroxybutyrate dehydrogenase decorated MXene nanosheets for the amperometric determination of β-hydroxybutyrate. Microchim. Acta 2020, 187, 277. [Google Scholar] [CrossRef]
- Ma, X.; Tu, X.L.; Gao, F.; Xie, Y.; Huang, X.G.; Fernandez, C.; Qu, F.L.; Liu, G.B.; Lu, L.M.; Yu, Y.F. Hierarchical porous MXene/amino carbon nanotubes-based molecular imprinting sensor for highly sensitive and selective sensing of fisetin. Sens. Actuators B Chem. 2020, 309, 127815. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.Y.; Yao, M.Y.; Dong, J.; Zhang, Q.H.; Zhang, L.L.; Zhao, X. Facile fabrication of flexible rGO/MXene hybrid fiber-like electrode with high volumetric capacitance. J. Power Source 2020, 448, 227398. [Google Scholar] [CrossRef]
- Rakhi, R.; Nayak, P.; Xia, C.; Alshareef, H.N. Novel amperometric glucose biosensor based on MXene nanocomposite. Sci. Rep. 2016, 6, 36422. [Google Scholar] [CrossRef] [PubMed]
- Mohammadniaei, M.; Koyappayil, A.; Sun, Y.; Min, J.; Lee, M.H. Gold nanoparticle/MXene for multiple and sensitive detection of oncomiRs based on synergetic signal amplification. Biosens. Bioelectron. 2020, 159, 112208. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, M.; Xia, J.; Zhang, F.; Wang, Z. An electrochemical biosensor based on AuNPs/Ti3C2 MXene three-dimensional nanocomposite for microRNA-155 detection by exonuclease III-aided cascade target recycling. J. Electroanal. Chem. 2020, 878, 114669. [Google Scholar] [CrossRef]
- Kheyrabadi, L.K.; Koyappayil, A.; Kim, T.; Cheon, Y.-P.; Lee, M.-H. A MoS2@Ti3C2Tx MXene hybrid-based electrochemical aptasensor (MEA) for sensitive and rapid detection of Thyroxine. Bioelectrochemistry 2021, 137, 107674. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Y.; Jiao, S.; Zhu, S.; Liu, X. A novel label-free strategy for the ultrasensitive miRNA-182 detection based on MoS2/Ti3C2 nanohybrids. Biosens. Bioelectron. 2019, 137, 45–51. [Google Scholar] [CrossRef]
- Li, M.H.; Fang, L.; Zhou, H.; Wu, F.; Lu, Y.; Luo, H.J.; Zhang, Y.X.; Hu, B.S. Three-dimensional porous MXene/NiCo-LDH composite for high performance non-enzymatic glucose sensor. Appl. Surf. Sci. 2019, 495, 143554. [Google Scholar] [CrossRef]
- Dang, Y.; Guan, X.; Zhou, Y.; Hao, C.; Zhang, Y.; Chen, S.; Ma, Y.; Bai, Y.; Gong, Y.; Gao, Y. Biocompatible PB/Ti3C2 hybrid nanocomposites for the non-enzymatic electrochemical detection of H2O2 released from living cells. Sens. Actuators B Chem. 2020, 319, 128259. [Google Scholar] [CrossRef]
- Feng, Y.F.; Zhou, F.R.; Deng, Q.H.; Peng, C. Solvothermal synthesis of in situ nitrogen-doped Ti3C2 MXene fluorescent quantum dots for selective Cu2+ detection. Ceram. Int. 2020, 46, 8320–8327. [Google Scholar] [CrossRef]
- Zhu, X.H.; Pang, X.; Zhang, Y.Y.; Yao, S.Z. Titanium carbide MXenes combined with red-emitting carbon dots as a unique turn-on fluorescent nanosensor for label-free determination of glucose. J. Mater. Chem. B 2019, 7, 7729–7735. [Google Scholar] [CrossRef]
- Desai, M.L.; Basu, H.; Singhal, R.K.; Saha, S.; Kailasa, S.K. Ultra-small two dimensional MXene nanosheets for selective and sensitive fluorescence detection of Ag+ and Mn2+ ions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 565, 70–77. [Google Scholar] [CrossRef]
- Xu, Q.; Ding, L.; Wen, Y.; Yang, W.; Zhou, H.; Chen, X.; Street, J.; Zhou, A.; Ong, W.-J.; Li, N. High photoluminescence quantum yield of 18.7% by using nitrogen-doped Ti3C2 MXene quantum dots. J. Mater. Chem. C 2018, 6, 6360–6369. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, J.H.; Chueng, S.D.; Pongkulapa, T.; Yang, L.; Cho, H.Y.; Choi, J.W.; Lee, K.B. Nondestructive Characterization of Stem Cell Neurogenesis by a Magneto-Plasmonic Nanomaterial-Based Exosomal miRNA Detection. ACS Nano 2019, 13, 8793–8803. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, Y.; Lu, D.; Guo, Y.; Guo, S. Ultrathin Ti3C2 nanosheets based “off-on” fluorescent nanoprobe for rapid and sensitive detection of HPV infection. Sens. Actuator B Chem. 2019, 286, 222–229. [Google Scholar] [CrossRef]
- Li, S.H.; Li, Y.C.; Cao, J.; Zhu, J.; Fan, L.Z.; Li, X.H. Sulfur-Doped Graphene Quantum Dots as a Novel Fluorescent Probe for Highly Selective and Sensitive Detection of Fe3+. Anal. Chem. 2014, 86, 10201–10207. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhou, J.; He, Y.; Cai, Z.; Ge, Y.; Zhou, J.; Song, G. ε-Poly-L-lysine-protected Ti3C2 MXene quantum dots with high quantum yield for fluorometric determination of cytochrome c and trypsin. Microchim. Acta 2019, 186, 770. [Google Scholar] [CrossRef]
- Ma, L.; Liu, F.Y.; Lei, Z.; Wang, Z.X. A novel upconversion@polydopamine core@shell nanoparticle based aptameric biosensor for biosensing and imaging of cytochrome c inside living cells. Biosens. Bioelectron. 2017, 87, 638–645. [Google Scholar] [CrossRef]
- Ertürk, G.; Hedström, M.; Mattiasson, B. A sensitive and real-time assay of trypsin by using molecular imprinting-based capacitive biosensor. Biosens. Bioelectron. 2016, 86, 557–565. [Google Scholar] [CrossRef]
- Liu, M.; He, Y.; Zhou, J.; Ge, Y.; Zhou, J.; Song, G. A “naked-eye” colorimetric and ratiometric fluorescence probe for uric acid based on Ti3C2 MXene quantum dots. Anal. Chim. Acta 2020, 1103, 134–142. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Liu, M.; Liu, Y. 2D titanium carbide MXenes as emerging optical biosensing platforms. Biosens. Bioelectron. 2021, 171, 112730. [Google Scholar] [CrossRef]
- Shao, B.; Xiao, Z. Recent achievements in exosomal biomarkers detection by nanomaterials-based optical biosensors—A review. Anal. Chim. Acta 2020, 1114, 74–84. [Google Scholar] [CrossRef]
- Li, Y.; Kang, Z.; Kong, L.; Shi, H.; Zhang, Y.; Cui, M.; Yang, D.P. MXene-Ti3C2/CuS nanocomposites: Enhanced peroxidase-like activity and sensitive colorimetric cholesterol detection. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 110000. [Google Scholar] [CrossRef] [PubMed]
| MAX Phases | Etching Solution and Condition | MXenes | Characteristics | Reference |
|---|---|---|---|---|
| Ti3AlC2 powder | 50% HF, room temperature, 2 h | Ti3C2 (nanosheet) | Excellent electrical conductivity and hydrophilic | [37] |
| Nb2AlC powder | 40% HF, 60 °C, 90 h | Nb2CTx (nanosheet) | Excellent electrical conductivity | [38] |
| Ti3AlC2 powder | LiF in 6 M HCl, 35 °C, 24 h | Ti3C2Tx (nanosheet) | Defect structure and excellent electrical conductivity | [39] |
| Ti3AlC2 powder | 1 M bifluoride (NaHF2, KHF2, NH4HF2), 60 °C, 8 h | Ti3C2 (nanosheet) | Large interplanar spacing | [40] |
| Ti3AlC2 powder | 49% HF, toom temperature, 24 h and hydrothermal reaction with DMF, ethanol, DMSO | Ti3C2Tx (quantum dot) | Excellent optical properties and biocompatibility | [41] |
| Biosensors Based on the MXene Nanocomposite | |||||||
|---|---|---|---|---|---|---|---|
| Type | Composition | Sensing Probe | Target | Utilized Technique | Sensitivity | Stability | Reference |
| MXene-based electrochemical biosensors | Ti3C2Tx MXene/graphene/GOx/glassy carbon | GOx | Glucose | Cyclic voltammetry | Detection sensitivity: 12.10 μA/mM | Negligible current decrease over 300 scanning cycles | [51] |
| MXene/CNT/Prussian blue/enzymes/carbon fiber | GOx and lactate oxidase | Glucose and lactate in the sweat | Chrono amperometry | Detection limit: 0.33 µM, detection sensitivity: 35.3 µA/mMcm2 for glucose, detection limit: 0.67 µM, detection sensitivity: 11.4 µA/mMcm2 for lactate | Retention of its detection sensitivity over 15 days | [52] | |
| Ti3C2 MXene/TDN/HRP/gliotoxin aptamer/signal probe/glassy carbon | Gliotoxin aptamer/signal probe | Glio toxin | Amperometry | Limit of detection: 1.63 pg/mL, 5 Pm | Retention of its current response over 7 days | [53] | |
| MXene-based fluorescent/optical biosensors | MXene nanosheets/Cy3-labeled CD63 aptamer | Cy3-labeled CD63 aptamer | Exo some | Fluorescence | Detection limit: 1.4 × 103 exosomes/mL | Stable signal in pH6.4–8.4 range and retention of signal over 10 days | [54] |
| MQDs/Fe3+ | MQDs | Fe3+ | Fluorescence | Limit of detection: 310 nM | Stable signal in pH 6.4–8.4 range and RSD for 10 and 250 μM of Fe3+ was 1.1% and 1.2% | [55] | |
| MXene/NiFe-LDH/ TMB/H2O2 | TMB | GSH | Colorimetry | Detection limit: 84 nM | - | [56] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.; Shin, M.; Lim, J.; Lee, J.-Y.; Choi, J.-W. Recent Advances in MXene Nanocomposite-Based Biosensors. Biosensors 2020, 10, 185. https://doi.org/10.3390/bios10110185
Yoon J, Shin M, Lim J, Lee J-Y, Choi J-W. Recent Advances in MXene Nanocomposite-Based Biosensors. Biosensors. 2020; 10(11):185. https://doi.org/10.3390/bios10110185
Chicago/Turabian StyleYoon, Jinho, Minkyu Shin, Joungpyo Lim, Ji-Young Lee, and Jeong-Woo Choi. 2020. "Recent Advances in MXene Nanocomposite-Based Biosensors" Biosensors 10, no. 11: 185. https://doi.org/10.3390/bios10110185
APA StyleYoon, J., Shin, M., Lim, J., Lee, J.-Y., & Choi, J.-W. (2020). Recent Advances in MXene Nanocomposite-Based Biosensors. Biosensors, 10(11), 185. https://doi.org/10.3390/bios10110185






