Fast Deoxynivalenol Determination in Cereals Using a White Light Reflectance Spectroscopy Immunosensor
Abstract
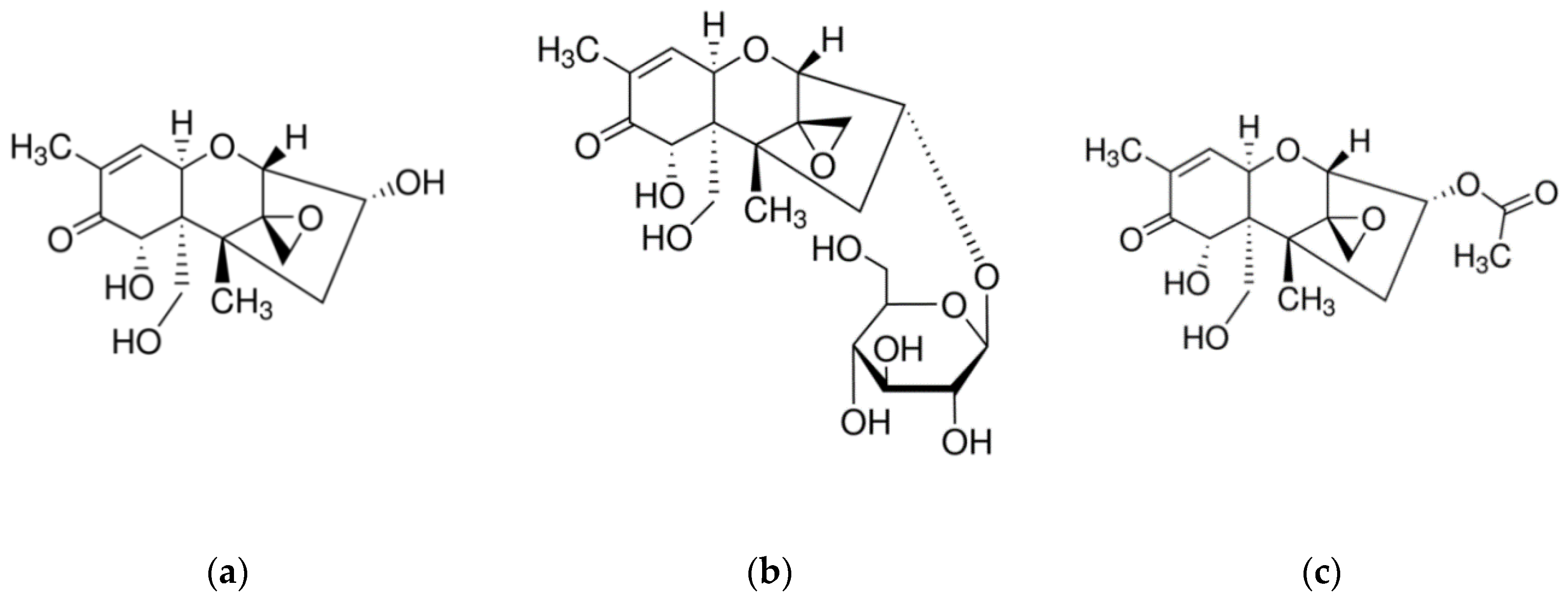
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Preparation of Calibrators and Samples
2.4. Chip Bio Functionalization and Assay Protocol
3. Results
3.1. Assay Optimization
3.2. Analytical Characteristics of DON Immunosensor
3.3. Evaluation of Assay Specificity
3.4. Chip Stability and Regeneration Potential
3.5. Optimization of Grain Extraction Protocol
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Process Nutr. 2019, 1, 6. [Google Scholar] [CrossRef]
- Rotter, B.A. Toxicology of deoxinivalenol (vomitoxin). J. Toxicol.Environ. Health 1996, 48, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdisc. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Numanoglu, E.; Gokmen, V.; Uygun, U.; Koksel, H. Thermal degradation of deoxynivalenol during maize bread baking. Food Add. Contamin. A 2012, 29, 423–430. [Google Scholar] [CrossRef]
- Nagl, V.; Schatzmayr, G. Deoxynivalenol and its masked forms in food and feed. Curr. Opin.Food Sci. 2015, 5, 43–49. [Google Scholar] [CrossRef]
- Bryla, M.; Waskiewicz, A.; Ksieniewicz-Wozniak, E.; Szymczyk, K.; Jedrzejczak, R. Modified fusarium mycotoxins in cereals and their products—Metabolism, occurrence, and toxicity: An updated review. Molecules 2018, 23, 963. [Google Scholar] [CrossRef]
- Maresca, M. From the gut to the brain: Journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar] [CrossRef]
- Wan, D.; Huang, L.; Pan, Y.; Wu, Q.; Chen, D.; Tao, Y.; Wang, X.; Liu, Z.; Li, J.; Wang, L.; et al. Metabolism, distribution, and excretion of deoxynivalenol with combined techniques of radiotracing, high-performance liquid chromatography ion trap Time-of-Flight mass spectrometry, and online radiometric detection. J. Agric. Food Chem. 2014, 62, 288–296. [Google Scholar] [CrossRef]
- Schwartz-Zimmermann, H.E.; Hametner, C.; Nagl, V.; Slavik, V.; Moll, W.-D.; Franz Berthiller, F. Deoxynivalenol (DON) sulfonates as major DON metabolites in rats: From identification to biomarker method development, validation and application. Anal. Bioanal. Chem. 2014, 406, 7911–7924. [Google Scholar] [CrossRef]
- Yan, P.; Liu, Z.; Liu, S.; Yao, L.; Liu, Y.; Wu, Y.; Gong, Z. Natural occurrence of deoxynivalenol and its acetylated derivatives in Chinese maize and wheat collected in 2017. Toxins 2020, 12, 200. [Google Scholar] [CrossRef]
- Schmeitz, C.; Warth, B.; Fruhmann, P.; Michlmayr, H.; Malachová, A.; Berthiller, F.; Schuhmacher, R.; Krska, R.; Adam, G. The metabolic fate of deoxynivalenol and its acetylated derivatives in a wheat suspension culture: Identification and detection of DON-15-O-glucoside, 15-acetyl-DON-3-O-glucoside and 15-acetyl-DON-3-sulfate. Toxins 2015, 7, 3112–3126. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: Differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 49, 5–24.
- Guidance for Industry and FDA: Advisory Levels for Deoxynivalenol (DON) in Finished Wheat Products for Human Consumption and Grains and Grain By-Products used for Animal Feed. July 2010. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-and-fda-advisory-levels-deoxynivalenol-don-finished-wheat-products-human (accessed on 10 August 2020).
- Anfossi, L.; Giovannoli, C.; Baggiani, C. Mycotoxin detection. Curr. Opin. Biotechnol. 2016, 37, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in analysis and detection of major mycotoxins in foods. Foods 2020, 9, 518. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Cramer, B.; Dall’Asta, C.; Iha, M.H.; Lattanzio, V.M.T.; Maragos, C.; Solfrizzo, M.; Stranska, M.; Stroka, J.; Sumarah, M. Developments in mycotoxin analysis: An update for 2018-19. World Mycotox. J. 2020, 13, 3–24. [Google Scholar] [CrossRef]
- Spanjer, M.C.; Rensen, P.M.; Scholten, J.M. LC–MS/MS multi-method for mycotoxins after single extraction, with validation data for peanut, pistachio, wheat, maize, cornflakes, raisins and figs. Food Add. Contam. A 2008, 25, 472–489. [Google Scholar] [CrossRef]
- De Santis, B.; Debegnach, F.; Gregori, E.; Russo, S.; Marchegiani, F.; Moracci, G.; Brera, C. Development of a LC-MS/MS method for the multi-mycotoxin determination in composite cereal-based samples. Toxins 2017, 9, 169. [Google Scholar] [CrossRef]
- Gonçalves, C.; Mischke, C.; Stroka, J. Determination of deoxynivalenol and its major conjugates in cereals using an organic solvent-free extraction and IAC clean-up coupled in-line with HPLC-PCD-FLD. Food Add. Contam. A 2020, 37, 1765–1776. [Google Scholar] [CrossRef]
- Nolan, P.; Auer, S.; Spehar, A.; Elliot, C.T.; Katrina Campbell, K. Current trends in rapid tests for mycotoxins. Food Add. Contam. A 2019, 36, 800–814. [Google Scholar] [CrossRef]
- Song, S.; Li, N.; Zhao, Z.; Njumbe Ediage, E.; Wu, S.; Sun, C.; De Saeger, S.; Wu, A. Multiplex lateral flow immunoassay for mycotoxin determination. Anal. Chem. 2014, 86, 4995–5001. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, H.; Li, C.; Zhang, S.; Shen, J.; Wang, Z. Gold nanoparticles-based lateral flow immunoassay with silver staining for simultaneous detection of fumonisin B1 and deoxynivalenol. Food Control 2015, 54, 347–352. [Google Scholar] [CrossRef]
- Foubert, A.; Beloglazova, N.V.; Gordienko, A.V.; Tessier, M.D.; Drijvers, E.; Hens, Z.; De Saeger, S. Development of a rainbow lateral flow immunoassay for the simultaneous detection of four mycotoxins. J. Agric. Food Chem. 2017, 65, 7121–7130. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zhang, Q.; Wang, D.; Li, P.; Tang, X.; Zhang, W. Determination of deoxynivalenol by ELISA and immunochromatographic strip assay based on monoclonal antibodies. Toxin Rev. 2019. [Google Scholar] [CrossRef]
- Li, R.; Meng, C.; Wen, Y.; Fu, W.; He, P. Fluorometric lateral flow immunoassay for simultaneous determination of three mycotoxins (aflatoxin B1, zearalenone and deoxynivalenol) using quantum dot microbeads. Microchim. Acta 2019, 186, 748. [Google Scholar] [CrossRef]
- Huang, X.; Huang, T.; Li, X.; Huang, Z. Flower-like gold nanoparticles-based immunochromatographic test strip for rapid simultaneous detection of fumonisin B1 and deoxynivalenol in Chinese traditional medicine. J. Pharm. Biomed. Anal. 2020, 177, 112895. [Google Scholar] [CrossRef]
- Maragos, C.M.; McCormick, S.P. Monoclonal antibodies for the mycotoxins deoxynivalenol and 3-acetyl-deoxynivalenol. Food Agric. Immunol. 2000, 12, 181–192. [Google Scholar] [CrossRef]
- Maragos, C.M.; Jolley, M.E.; Nasir, M.S. Fluorescence polarization as a tool for the determination of deoxynivalenol in wheat. Food Add.Contam. 2002, 19, 400–407. [Google Scholar] [CrossRef]
- Lippolis, V.; Pascale, M.; Visconti, A. Optimization of a fluorescence polarization immunoassay for rapid quantification of deoxynivalenol in durum wheat-beat products. J. Food Protect. 2006, 69, 2712–2719. [Google Scholar] [CrossRef]
- Kuzdraliński, A.; Solarska, E.; Muszyńska, M. Deoxynivalenol and zearalenone occurrence in beers analysed by an enzyme-linked immunosorbent assay method. Food Control 2013, 29, 22–24. [Google Scholar] [CrossRef]
- Valenzano, S.; Lippolis, V.; Pascale, M.; De Marco, A.; Maragos, C.M.; Suman, M.; Visconti, A. Determination of deoxynivalenol in wheat Bran and whole-wheat flour by fluorescence polarization immunoassay. Food Anal. Method 2014, 7, 806–813. [Google Scholar] [CrossRef]
- Yu, S.; Yu, F.; Li, Y.; Liu, L.; Zhang, H.; Qu, L.; Wu, Y. Magnetic nanoparticles replacing microplate as immobile phase could greatly improve the sensitivity of chemiluminescence enzymatic immunoassay for deoxynivalenol. Food Control 2016, 60, 500–504. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Lu, Y.; Ma, D.-Y.; Qi, M.G.; Wang, S. A competitive direct enzyme-linked immunosorbent assay for the rapid detection of deoxynivalenol: Development and application in agricultural products and feedstuff. Food Agric. Immunol. 2017, 28, 516–527. [Google Scholar] [CrossRef]
- Sanders, M.; McPartlin, D.; Moran, K.; Guo, Y.; Eeckhout, M.; O’Kennedy, R.; De Saeger, S.; Maragos, C. Comparison of enzyme-linked immunosorbent assay, surface plasmon resonance and biolayer interferometry for screening of deoxynivalenol in wheat and wheat dust. Toxins 2016, 8, 103. [Google Scholar] [CrossRef]
- Kadota, T.; Takezawa, Y.; Hirano, S.; Tajima, O.; Maragos, C.M.; Nakajima, T.; Tanaka, T.; Kamata, Y.; Sugita-Konishi, Y. Rapid detection of nivalenol and deoxynivalenol in wheat using surface Plasmon resonance immunoassay. Anal. Chim. Acta 2010, 673, 173–178. [Google Scholar] [CrossRef]
- Meneely, J.; Fodey, T.; Armstrong, L.; Sulyok, M.; Krska, R.; Elliott, C. Rapid surface plasmon resonance immunoassay for the determination of deoxynivalenol in wheat, wheat products, and maize-based baby food. J. Agric. Food Chem. 2010, 58, 8936–8941. [Google Scholar] [CrossRef]
- Joshi, S.; Segarra-Fas, A.; Peters, J.; Zuilhof, H.; van Beek, T.A.; Nielen, M.W.F. Multiplex surface plasmon resonance biosensing and its transferability towards imaging nanoplasmonics for detection of mycotoxins in barley. Analyst 2016, 141, 1307–1318. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Maragos, C.M. Gold nanoparticle-enhanced multiplexed imaging surface Plasmon resonance (iSPR) detection of Fusarium mycotoxins in wheat. Biosens. Bioelectron. 2018, 101, 245–252. [Google Scholar] [CrossRef]
- Maragos, C.M. Detection of deoxynivalenol using biolayer interferometry. Mycotox. Res. 2011, 27, 157–165. [Google Scholar] [CrossRef]
- Maragos, C.M. Signal amplification using colloidal gold in a biolayer interferometry-based immunosensor for the mycotoxin deoxynivalenol. Food Add. Contam. A 2012, 29, 1108–1117. [Google Scholar] [CrossRef]
- Pagkali, V.; Petrou, P.S.; Makarona, E.; Peters, J.; Haasnoot, W.; Jobst, G.; Moser, I.; Gajos, K.; Budkowski, A.; Economou, A.; et al. Simultaneous determination of aflatoxin B1, fumonisin B1 and deoxynivalenol in beer samples with a label-free monolithically integrated optoelectronic biosensor. J. Hazard Mater. 2018, 359, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Koukouvinos, G.; Petrou, P.S.; Misiakos, K.; Drygiannakis, D.; Raptis, I.; Goustouridis, D.; Kakabakos, S.E. A label-free flow-through immunosensor for determination of total- and free-PSA in human serum samples based on white-light reflectance spectroscopy. Sens. Actuators B 2015, 209, 1041–1048. [Google Scholar] [CrossRef]
- Koukouvinos, G.; Petrou, P.; Goustouridis, D.; Misiakos, K.; Kakabakos, S.; Raptis, I. Development and bioanalytical applications of a white light reflectance spectroscopy label-free sensing platform. Biosensors 2017, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Koukouvinos, G.; Τsialla, Z.; Petrou, P.S.; Misiakos, K.; Goustouridis, D.; Moreno, A.U.; Fernandez-Alba, A.R.; Raptis, I.; Kakabakos, S.E. Fast simultaneous detection of three pesticides by a white light reflectance spectroscopy sensing platform. Sens. Actuators B 2017, 238, 1214–1223. [Google Scholar] [CrossRef]
- Anastasiadis, V.; Koukouvinos, G.; Petrou, P.S.; Economou, A.; Dekker, J.; Harjanne, M.; Heimala, P.; Goustouridis, D.; Raptis, I.; Kakabakos, S.E. Multiplexed mycotoxins determination employing white light reflectance spectroscopy and silicon chips with silicon oxide areas of different thickness. Biosens. Bioelectron. 2020, 153, 112035. [Google Scholar] [CrossRef]
- Varga, E.; Malachova, A.; Schwartz, H.; Krska, R.; Berthiller, F. Survey of deoxynivalenol and its conjugates deoxynivalenol-3-glucoside and 3-acetyldeoxynivalenol in 374 beer samples. Food Add. Contam. A 2013, 30, 137–146. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Varga, E.; Maragos, C.; Baumgartner, S.; Adam, G.; Berthiller, F. Cross-reactivity of commercial and non-commercial deoxynivalenol-antibodies to emerging trichothecenes and common deoxynivalenol-derivatives. World Mycotox. J. 2019, 12, 45–53. [Google Scholar] [CrossRef]






| Extraction Method | %R | ||
|---|---|---|---|
| 20 ng/mL (1000 μg/kg) | 50 ng/mL (2500 μg/kg) | 200 ng/mL (10,000 μg/kg) | |
| H2O | 98.0 ± 4.0 | 97.0 ± 3.0 | 105 ± 4.0 |
| MeOH/H2O 80:20 | 55.0 ± 4.5 | 57.0 ± 5.0 | 53.5 ± 4.0 |
| MeOH/H2O 60:40 | 66.0 ± 3.0 | 64.0 ± 3.5 | 67.0 ± 4.0 |
| MeOH/H2O 40:60 | 81.0 ± 4.0 | 78.0 ± 5.0 | 82.0 ± 3.0 |
| MeOH/H2O 20:80 | 96.0 ± 2.0 | 94.0 ± 3.0 | 98.0 ± 3.0 |
| AcN/H2O 80:20 | 92.0 ± 4.0 | 93.0 ± 2.5 | 95.0 ± 3.0 |
| AcN/H2O 60:40 | 82.0 ± 3.0 | 85.0 ± 4.0 | 84.0 ± 3.5 |
| AcN/H2O 40:60 | 85.0 ± 4.0 | 87.0 ± 2.5 | 83.0 ± 4.0 |
| AcN/H2O 20:80 | 92.0 ± 2.0 | 89.0 ± 4.0 | 88.0 ± 3.0 |
| H2O/OKM1 | 97.0 ± 3.5 | 98.0 ± 4.0 | 102 ± 5.0 |
| H2O/AKM1 | 93.0 ± 3.0 | 96.0 ± 5.0 | 92.0 ± 3.0 |
| H2O/ΕΝΚ1 | 100 ± 4.0 | 92.0 ± 3.0 | 95.0 ± 4.0 |
| Method | LOD (μg/kg) | Sample Type | Assay Duration (min) | EU Maximum Levels (μg/kg) | Ref. Number |
|---|---|---|---|---|---|
| WLRS | 62.5 | maize wheat | 12(17 *) | 1750 | this work |
| SPR | 50 | wheat | 5(7 *) | 1750 | 36 |
| SPR | 57 | wheat | 4(6 *) | 1750 | 37 |
| 9 | wheat-based breakfast cereal | 500 | |||
| 6 | maize-based baby food | 200 | |||
| iSPR | 64 | barley | 4(15 *) | 1250 | 38 |
| iSPR | 15 | wheat | <9(17.5 *) | 1750 | 39 |
| BLI | 128 | wheat | 5(6.5 *) | 1750 | 35 |
| 737 | wheat dust | - | |||
| BLI | 100 | wheat flour | 4(7 *) | 750 | 40 |
| BLI | 90 | wheat flour | 4(6 *) | 750 | 41 |
| MZI | 20 | beer | 12(17 *) | - | 42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasiadis, V.; Raptis, I.; Economou, A.; Kakabakos, S.E.; Petrou, P.S. Fast Deoxynivalenol Determination in Cereals Using a White Light Reflectance Spectroscopy Immunosensor. Biosensors 2020, 10, 154. https://doi.org/10.3390/bios10110154
Anastasiadis V, Raptis I, Economou A, Kakabakos SE, Petrou PS. Fast Deoxynivalenol Determination in Cereals Using a White Light Reflectance Spectroscopy Immunosensor. Biosensors. 2020; 10(11):154. https://doi.org/10.3390/bios10110154
Chicago/Turabian StyleAnastasiadis, Vasileios, Ioannis Raptis, Anastasios Economou, Sotirios E. Kakabakos, and Panagiota S. Petrou. 2020. "Fast Deoxynivalenol Determination in Cereals Using a White Light Reflectance Spectroscopy Immunosensor" Biosensors 10, no. 11: 154. https://doi.org/10.3390/bios10110154
APA StyleAnastasiadis, V., Raptis, I., Economou, A., Kakabakos, S. E., & Petrou, P. S. (2020). Fast Deoxynivalenol Determination in Cereals Using a White Light Reflectance Spectroscopy Immunosensor. Biosensors, 10(11), 154. https://doi.org/10.3390/bios10110154









