Nanofluidic Immobilization and Growth Detection of Escherichia coli in a Chip for Antibiotic Susceptibility Testing
Abstract
:1. Introduction
2. Materials and Methods
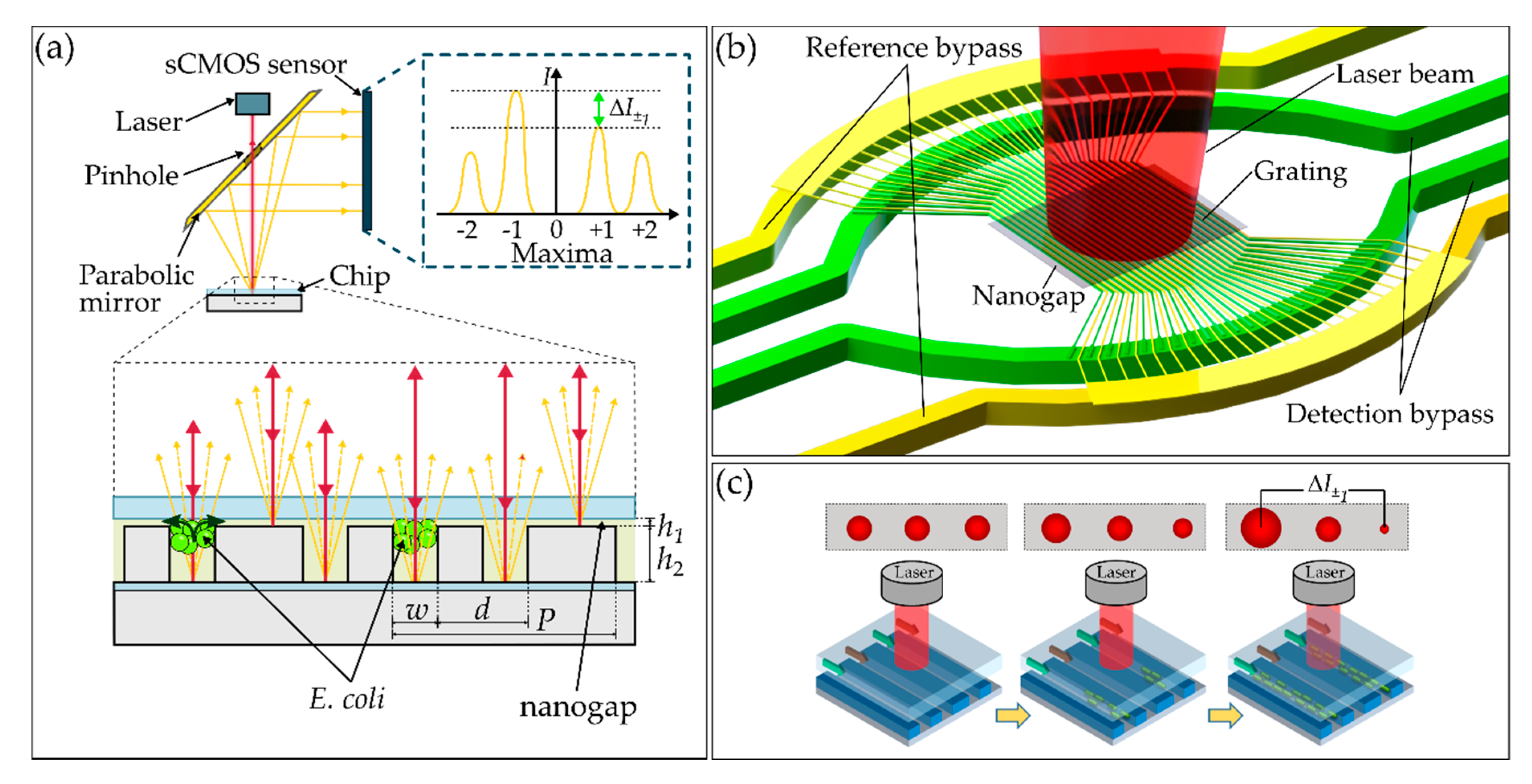
2.1. Nano-Grating Chip Concept
2.2. Optofluidic Measurement Setup
2.3. Chip Fabrication
2.4. Bacterial Strain and Growth Medium
2.5. Determination of Growth Rates
3. Results and Discussion
3.1. Device Characterization
3.2. Bacterial Capture Experiments
3.3. Growth Experiments with Fluorescence
3.4. Growth Detection with Diffraction Signal Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Avesar, J.; Rosenfeld, D.; Truman-Rosentsvit, M.; Ben-Arye, T.; Geffen, Y.; Bercovici, M.; Levenberg, S. Rapid phenotypic antimicrobial susceptibility testing using nanoliter arrays. Proc. Natl. Acad. Sci. USA 2017, 114, E5787–E5795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerremans, J.J.; Verboom, P.; Stijnen, T.; Hakkaart-van Roijen, L.; Goessens, W.; Verbrugh, H.A.; Vos, M.C. Rapid identification and antimicrobial susceptibility testing reduce antibiotic use and accelerate pathogen-directed antibiotic use. J. Antimicrob. Chemother. 2008, 61, 428–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Belkum, A.; Durand, G.; Peyret, M.; Chatellier, S.; Zambardi, G.; Schrenzel, J.; Shortridge, D.; Engelhardt, A.; Dunne, W.M. Rapid clinical bacteriology and its future impact. Ann. Lab. Med. 2013, 33, 14–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laxminarayan, R.; Chaudhury, R.R. Antibiotic Resistance in India: Drivers and Opportunities for Action. PLoS Med. 2016, 13, e1001974. [Google Scholar] [CrossRef] [Green Version]
- Hsia, Y.; Lee, B.R.; Versporten, A.; Yang, Y.; Bielicki, J.; Jackson, C.; Newland, J.; Goossens, H.; Magrini, N.; Sharland, M. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): An analysis of paediatric survey data from 56 countries. Lancet Glob. Health 2019, 7, e861–e871. [Google Scholar] [CrossRef] [Green Version]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef]
- Khan, Z.A.; Siddiqui, M.F.; Park, S. Progress in antibiotic susceptibility tests: A comparative review with special emphasis on microfluidic methods. Biotechnol. Lett. 2019, 41, 221–230. [Google Scholar] [CrossRef]
- Mach, K.E.; Mohan, R.; Baron, E.J.; Shih, M.-C.; Gau, V.; Wong, P.K.; Liao, J.C. A biosensor platform for rapid antimicrobial susceptibility testing directly from clinical samples. J. Urol. 2011, 185, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Baltekin, Ö.; Boucharin, A.; Tano, E.; Andersson, D.I.; Elf, J. Antibiotic susceptibility testing in less than 30 min using direct single-cell imaging. Proc. Natl. Acad. Sci. USA 2017, 114, 9170–9175. [Google Scholar] [CrossRef] [Green Version]
- Evangelista, A.T.; Karlowsky, J.A. Automated and Manual Systems for Antimicrobial Susceptibility Testing of Bacteria. In Manual of Commercial Methods in Clinical Microbiology; Truant, A.L., Tang, Y.-W., Waites, K.B., Bébéar, C., Rennie, R.P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 414–432. ISBN 9781119021872. [Google Scholar]
- Murray, C.; Adeyiga, O.; Owsley, K.; Di Carlo, D. Research highlights: Microfluidic analysis of antimicrobial susceptibility. Lab Chip 2015, 15, 1226–1229. [Google Scholar] [CrossRef]
- Besant, J.D.; Sargent, E.H.; Kelley, S.O. Rapid electrochemical phenotypic profiling of antibiotic-resistant bacteria. Lab Chip 2015, 15, 2799–2807. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jung, Y.-G.; Kim, J.; Kim, S.; Jung, Y.; Na, H.; Kwon, S. Rapid antibiotic susceptibility testing by tracking single cell growth in a microfluidic agarose channel system. Lab Chip 2013, 13, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; An, Y.; Hjort, K.; Hjort, K.; Sandegren, L.; Wu, Z. Time lapse investigation of antibiotic susceptibility using a microfluidic linear gradient 3D culture device. Lab Chip 2014, 14, 3409. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Yoo, J.; Lee, M.; Kim, E.-G.; Lee, J.S.; Lee, S.; Joo, S.; Song, S.H.; Kim, E.-C.; Lee, J.C.; et al. A rapid antimicrobial susceptibility test based on single-cell morphological analysis. Sci. Transl. Med. 2014, 6, 267ra174. [Google Scholar] [CrossRef] [PubMed]
- Baltekin, O.; Boucharin, A.; Tano, E.; Andersson, D.I.; Elf, J. Fast Antibiotic Susceptibility Testing based on single cell growth rate measurements. BioRxiv 2016. [Google Scholar] [CrossRef]
- Yang, Y.; Gupta, K.; Ekinci, K.L. All-electrical monitoring of bacterial antibiotic susceptibility in a microfluidic device. Proc. Natl. Acad. Sci. USA 2020, 117, 10639–10644. [Google Scholar] [CrossRef] [PubMed]
- Sadanandan, S.K.; Baltekin, O.; Magnusson, K.E.G.; Boucharin, A.; Ranefall, P.; Jalden, J.; Elf, J.; Wahlby, C. Segmentation and Track-Analysis in Time-Lapse Imaging of Bacteria. IEEE J. Sel. Top. Signal Process. 2016, 10, 174–184. [Google Scholar] [CrossRef]
- Van Leest, T.; Caro, J. Cavity-enhanced optical trapping of bacteria using a silicon photonic crystal. Lab Chip 2013, 13, 4358–4365. [Google Scholar] [CrossRef] [Green Version]
- Boedicker, J.Q.; Li, L.; Kline, T.R.; Ismagilov, R.F. Detecting bacteria and determining their susceptibility to antibiotics by stochastic confinement in nanoliter droplets using plug-based microfluidics. Lab Chip 2008, 8, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Churski, K.; Kaminski, T.S.; Jakiela, S.; Kamysz, W.; Baranska-Rybak, W.; Weibel, D.B.; Garstecki, P. Rapid screening of antibiotic toxicity in an automated microdroplet system. Lab Chip 2012, 12, 1629–1637. [Google Scholar] [CrossRef]
- Liu, X.; Painter, R.E.; Enesa, K.; Holmes, D.; Whyte, G.; Garlisi, C.G.; Monsma, F.J.; Rehak, M.; Craig, F.F.; Smith, C.A. High-throughput screening of antibiotic-resistant bacteria in picodroplets. Lab Chip 2016, 16, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Páez-Avilés, C.; Juanola-Feliu, E.; Punter-Villagrasa, J.; Del Moral Zamora, B.; Homs-Corbera, A.; Colomer-Farrarons, J.; Miribel-Català, P.L.; Samitier, J. Combined Dielectrophoresis and Impedance Systems for Bacteria Analysis in Microfluidic On-Chip Platforms. Sensors 2016, 16, 1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostova, E.; Ben Adiba, C.; Dietler, G.; Sekatskii, S.K. Kinetics of Antibody Binding to Membranes of Living Bacteria Measured by a Photonic Crystal-Based Biosensor. Biosensors 2016, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Purr, F.; Eckardt, M.-F.; Kieserling, J.; Gronwald, P.-L.; Burg, T.P.; Dietzel, A. Robust Smartphone Assisted Biosensing Based on Asymmetric Nanofluidic Grating Interferometry. Sensors 2019, 19, 2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purr, F.; Bassu, M.; Lowe, R.D.; Thürmann, B.; Dietzel, A.; Burg, T.P. Asymmetric nanofluidic grating detector for differential refractive index measurement and biosensing. Lab Chip 2017. [Google Scholar] [CrossRef] [Green Version]
- Purr, F.; Lowe, R.D.; Stehr, M.; Singh, M.; Burg, T.P.; Dietzel, A. Biosensing based on optimized asymmetric optofluidic nanochannel gratings. Micro Nano Eng. 2020, 8, 100056. [Google Scholar] [CrossRef]
- Bates, R.; Thamban, P.L.; Goeckner, M.; Overzet, L.; Bates, R.L.; Stephan Thamban, P.L.; Goeckner, M.J.; Overzet, L.J. Silicon etch using SF6/C4F8/Ar gas mixtures // Silicon etch using SF 6 /C 4 F 8 /Ar gas mixtures. Am. Vac. Soc. 2014, 32, 41302. [Google Scholar] [CrossRef]
- Busche, J.F.; Möller, S.; Stehr, M.; Dietzel, A. Cross-Flow Filtration of Escherichia coli at a Nanofluidic Gap for Fast Immobilization and Antibiotic Susceptibility Testing. Micromachines 2019, 10, 691. [Google Scholar] [CrossRef] [Green Version]
- Lange, H.; Taillandier, P.; Riba, J.-P. Effect of high shear stress on microbial viability. J. Chem. Technol. Biotechnol. 2001, 76, 501–505. [Google Scholar] [CrossRef]
- Männik, J.; Driessen, R.; Galajda, P.; Keymer, J.E.; Dekker, C. Bacterial growth and motility in sub-micron constrictions. Proc. Natl. Acad. Sci. USA 2009, 106, 14861–14866. [Google Scholar] [CrossRef] [Green Version]
- Tandogan, N. Effects of Applied Pressure on Bacterial Transport through Confined Spaces. In Proceedings of the 40th Annual Northeast Bioengineering Conference (NEBEC), Boston, MA, USA, 25–27 April 2014; IEEE: Piscataway, NJ, USA, 2014. ISBN 9781479937288. [Google Scholar]
- Gibson, B.; Wilson, D.J.; Feil, E.; Eyre-Walker, A. The distribution of bacterial doubling times in the wild. Proc. Biol. Sci. 2018, 285. [Google Scholar] [CrossRef] [PubMed]






| Pressure Applied (mbar) at the Inlets and Outlets | |||||||
|---|---|---|---|---|---|---|---|
| D1,I | D2,O | D3,O | D4,O | R5,I | R6,O | R7,O | R8,O |
| 380 | 350 | 350 | 350 | 200 | 180 | 180 | 180 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busche, J.F.; Möller, S.; Klein, A.-K.; Stehr, M.; Purr, F.; Bassu, M.; Burg, T.P.; Dietzel, A. Nanofluidic Immobilization and Growth Detection of Escherichia coli in a Chip for Antibiotic Susceptibility Testing. Biosensors 2020, 10, 135. https://doi.org/10.3390/bios10100135
Busche JF, Möller S, Klein A-K, Stehr M, Purr F, Bassu M, Burg TP, Dietzel A. Nanofluidic Immobilization and Growth Detection of Escherichia coli in a Chip for Antibiotic Susceptibility Testing. Biosensors. 2020; 10(10):135. https://doi.org/10.3390/bios10100135
Chicago/Turabian StyleBusche, Jan F., Svenja Möller, Ann-Kathrin Klein, Matthias Stehr, Foelke Purr, Margherita Bassu, Thomas P. Burg, and Andreas Dietzel. 2020. "Nanofluidic Immobilization and Growth Detection of Escherichia coli in a Chip for Antibiotic Susceptibility Testing" Biosensors 10, no. 10: 135. https://doi.org/10.3390/bios10100135
APA StyleBusche, J. F., Möller, S., Klein, A.-K., Stehr, M., Purr, F., Bassu, M., Burg, T. P., & Dietzel, A. (2020). Nanofluidic Immobilization and Growth Detection of Escherichia coli in a Chip for Antibiotic Susceptibility Testing. Biosensors, 10(10), 135. https://doi.org/10.3390/bios10100135






