Short-Time Hydrothermal Synthesis of CuBi2O4 Nanocolumn Arrays for Efficient Visible-Light Photocatalysis
Abstract
:1. Introduction
2. Materials and Methods
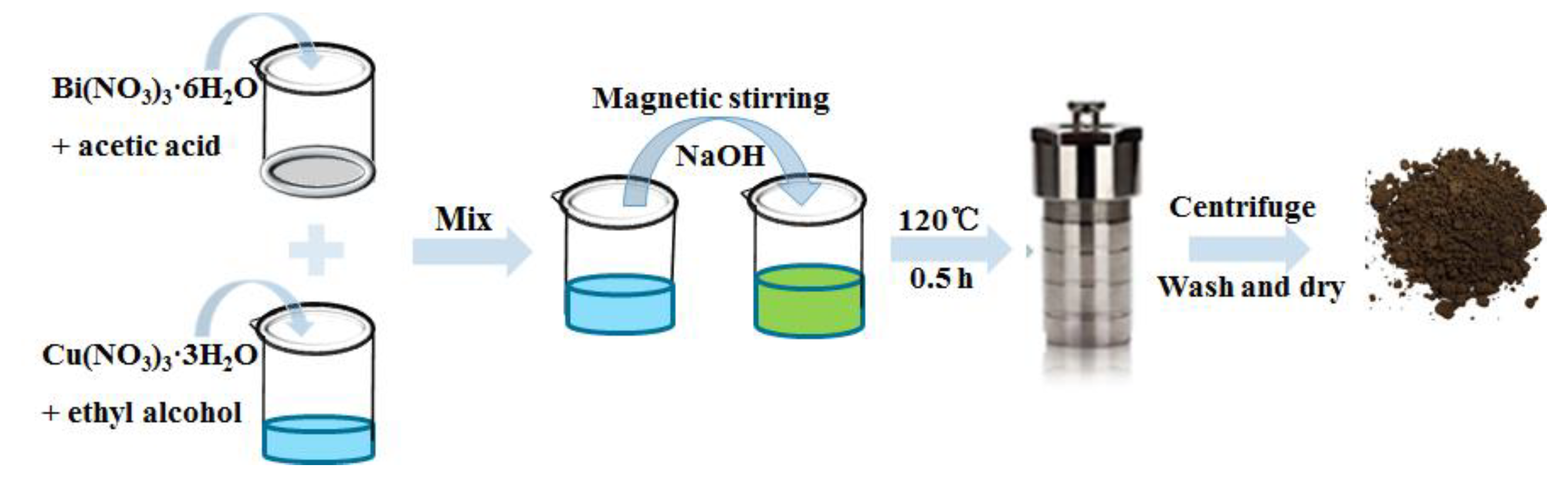
2.1. Material Preparation
2.2. Characterization
2.3. Photocatalytic Activity Measurement
3. Results and Discussion
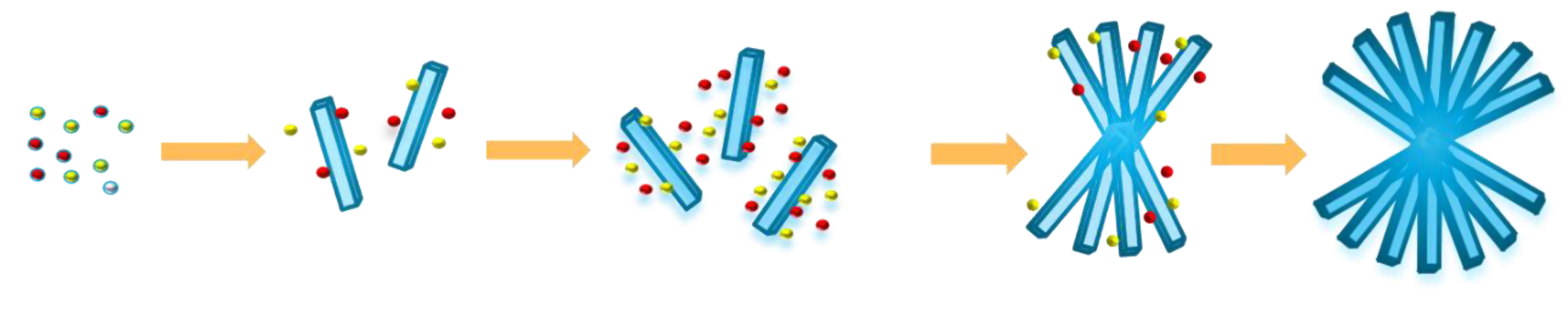
3.1. Synthetic Procedures of CuBi2O4 Nanocolumn Arrays
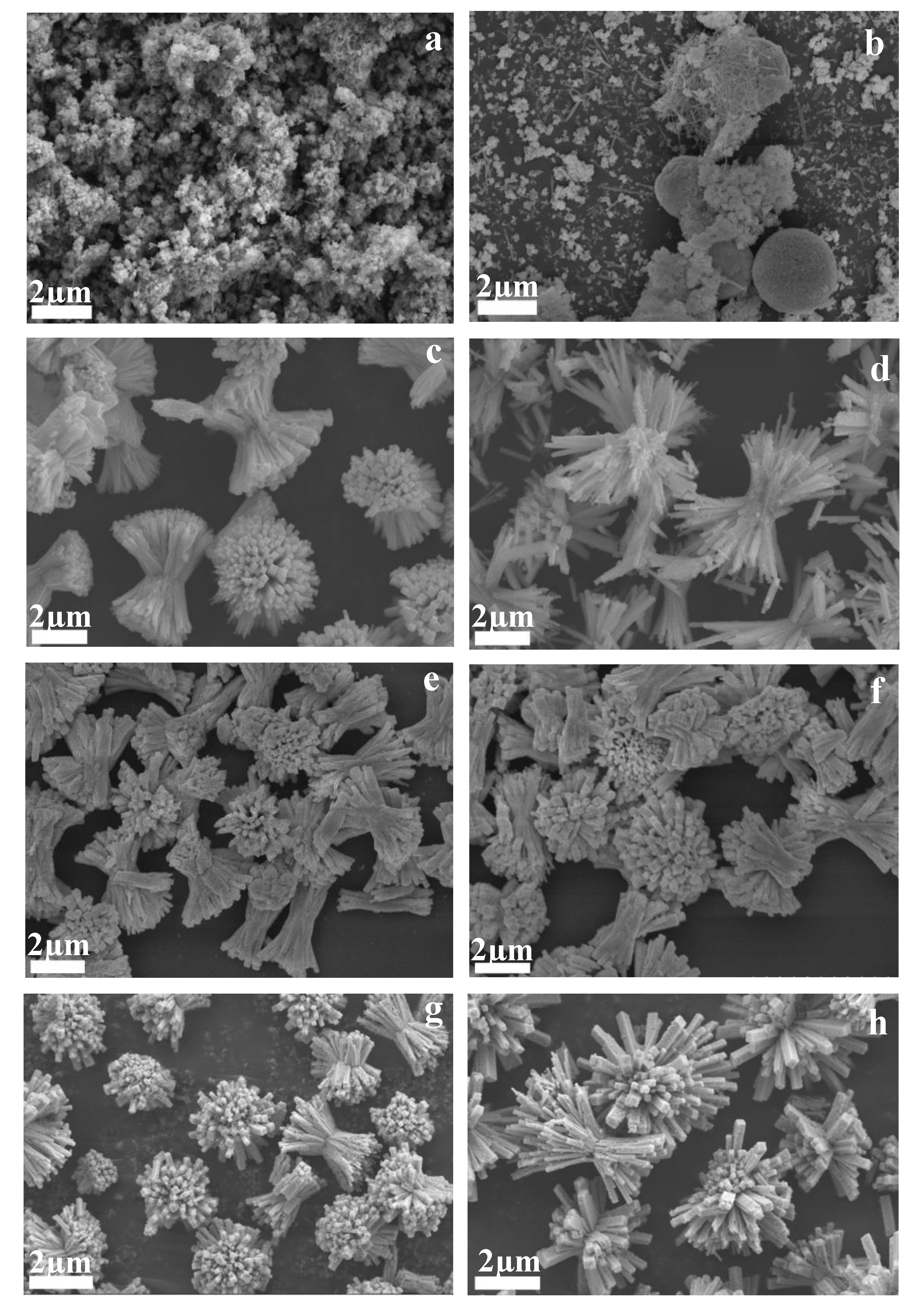
3.2. Characterization of CuBi2O4 Nanocolumn Arrays
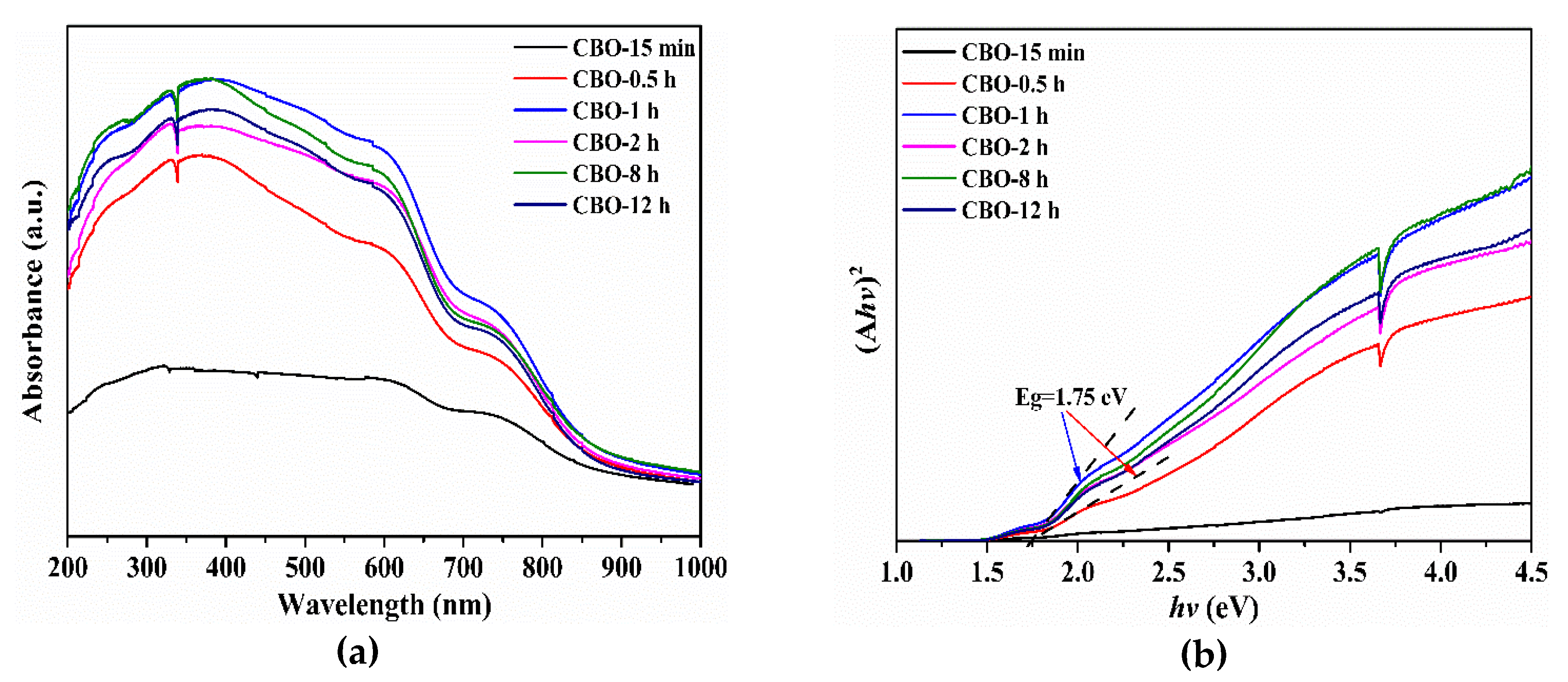
3.3. Optical Absorption Properties
3.4. Photocatalytic Activity of CuBi2O4
3.5. Stability of the CuBi2O4 Nanocolumn Arrays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Wang, H.; Woldu, A.R.; Zhang, X.; He, T. Optimization of charge behavior in nanoporous CuBi2O4 photocathode for photoelectrochemical reduction of CO2. Cataly. Today 2019, 335, 388–394. [Google Scholar] [CrossRef]
- Wang, F.; Yang, H.; Zhang, Y. Enhanced photocatalytic performance of CuBi2O4 particles decorated with Ag nanowires. Mater. Sci. Semicon. Proc. 2018, 73, 58–66. [Google Scholar] [CrossRef]
- Kang, D.; Hill, J.C.; Park, Y.; Choi, K.S. Photoelectrochemical Properties and Photostabilities of High Surface Area CuBi2O4 and Ag-Doped CuBi2O4 Photocathodes. Chem. Mater. 2016, 2812, 4331–4340. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Wang, H.; Huang, H.; Liu, Y.; Kang, Z. Fabrication of a CuBi2O4/g-C3N4 p–n heterojunction with enhanced visible light photocatalytic efficiency toward tetracycline degradation. Inorg. Chem. Front. 2017, 410, 1714–1720. [Google Scholar] [CrossRef]
- Abdulkarem, A.M.; Li, J.; Aref, A.A.; Ren, L.; Elssfah, E.M.; Wang, H.; Ge, Y.; Yu, Y. CuBi2O4 single crystal nanorods prepared by hydrothermal method: Growth mechanism and optical properties. Mater. Res. Bull. 2011, 469, 1443–1450. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Li, J.; Yang, G.; Bai, T.; Wang, j. Effects of synthetic conditions on the morphology and catalytic properties of hierarchical CuBi2O4 nanoflowers grown by low-temperature solution process. J. Alloys Compd. 2013, 580, 172–175. [Google Scholar] [CrossRef]
- Li, M.Y.; Tang, Y.B.; Shi, W.L.; Chen, F.Y.; Shi, Y.; Gu, H.C. Design of visible-light-response core–shell Fe2O3/CuBi2O4 heterojunctions with enhanced photocatalytic activity towards the degradation of tetracycline: Z-scheme photocatalytic mechanism insight. Inorg. Chem. Front. 2018, 512, 3148–3154. [Google Scholar] [CrossRef]
- Chen, X.; Dai, Y.; Guo, J. Hydrothermal synthesis of well-distributed spherical CuBi2O4 with enhanced photocatalytic activity under visible light irradiation. Mater. Lett. 2015, 161, 251–254. [Google Scholar] [CrossRef]
- Nishikawa, M.; Hiura, S.; Mitani, Y.; Nosaka, Y. Enhanced photocatalytic activity of BiVO4 by co-grafting of metal ions and combining with CuBi2O4. J. Photoch. Photobiol. A 2013, 262, 52–56. [Google Scholar] [CrossRef]
- Elaziouti, A.; Laouedj, N.; Bekka, A.; Vannier, R.N. Preparation and characterization of p–n heterojunction CuBi2O4/CeO2 and its photocatalytic activities under UVA light irradiation. J. King Saud Univ. Sci. 2015, 272, 120–135. [Google Scholar] [CrossRef]
- Nishikawa, M.; Yuto, S.; Hasegawa, T.; Shiroishi, W.; Honghao, H.; Nakabayashi, Y.; Nosaka, Y.; Saito, N. Compositing effects of CuBi2O4 on visible-light responsive photocatalysts. Mater. Sci. Semicon. Proc. 2017, 57, 12–17. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y. Preparation, characterization and visible photocatalytic activity of CuBi2O4 photocatalyst by a novel sol–gel method. J. Mater. Sci. Mater. Electron. 2015, 266, 4308–4312. [Google Scholar] [CrossRef]
- Najafian, H.; Manteghi, F.; Beshkar, F.; Salavati-Niasari, M. Fabrication of nanocomposite photocatalyst CuBi2O4/Bi3ClO4 for removal of acid brown 14 as water pollutant under visible light irradiation. J. Hazard. Mater. 2019, 361, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Yang, H.; Wang, W.P.; Zhang, H.M.; Li, R.S.; Wang, X.X.; Yu, R.C. A promising supercapacitor electrode material of CuBi2O4 hierarchical microspheres synthesized via a coprecipitation route. J. Alloys Compd. 2016, 684, 707–713. [Google Scholar] [CrossRef]
- Henmi, C. Kusachiite, CuBi2O4, a new mineral from Fuka, Okayama Prefecture, Japan. Mineral. Mag. 1995, 59, 545–548. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Karthikeyan, K.; Kalpana, D.; Lee, Y.S.; Selvan, R.K. Surfactant-free hydrothermal synthesis of hierarchically structured spherical CuBi2O4 as negative electrodes for Li-ion hybrid capacitors. J. Colloid Interf. Sci. 2016, 469, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.D.; Lua, S.K.; Dong, Z.; Lim, T.T. A novel three-dimensional spherical CuBi2O4 consisting of nanocolumn arrays with persulfate and peroxymonosulfate activation functionalities for 1H-benzotriazole removal. Nanoscale 2015, 717, 8149–8158. [Google Scholar] [CrossRef]
- Oh, W.D.; Dong, Z.; Lim, T.T. Hierarchically-structured Co–CuBi2O4 and Cu–CuBi2O4 for sulfanilamide removal via peroxymonosulfate activation. Cataly. Today 2017, 280, 2–7. [Google Scholar] [CrossRef]
- Muthukrishnaraj, A.; Vadivel, S.; Joni, I.M.; Balasubramanian, N. Development of reduced graphene oxide/CuBi2O4 hybrid for enhanced photocatalytic behavior under visible light irradiation. Ceram. Inter. 2015, 415, 6164–6168. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Zhu, R.; Li, N.; Chen, J.; Li, S.; Xia, W.; Xu, S.; Wang, H.; Chen, X. Ag3PO4 Deposited on CuBi2O4 to Construct Z-Scheme Photocatalyst with Excellent Visible-Light Catalytic Performance Toward the Degradation of Diclofenac Sodium. Nanomaterials 2019, 9, 959. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Wang, H.; Han, M.; Guan, W.; Huang, H.; Liu, Y.; Kang, Z. Study on highly enhanced photocatalytic tetracycline degradation of type AgI/CuBi2O4 and Z-scheme AgBr/CuBi2O4 heterojunction photocatalysts. J. Hazard. Mater. 2018, 349, 111–118. [Google Scholar] [CrossRef]
- Wang, F.; Chemseddine, A.; Abdi, F.F.; van de Krol, R.; Berglund, S.P. Spray pyrolysis of CuBi2O4 photocathodes: Improved solution chemistry for highly homogeneous thin films. J. Mater. Chem. A 2017, 525, 12838–12847. [Google Scholar] [CrossRef]
- Wang, F.; Septina, W.; Chemseddine, A.; Abdi, F.F.; Friedrich, D.; Bogdanoff, P.; van de Krol, R.; Tilley, S.D.; Berglund, S.P. Gradient Self-Doped CuBi2O4 with Highly Improved Charge Separation Efficiency. J. Am. Chem. Soc. 2017, 13942, 15094–15103. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, D.H.; Hong, S.H. CuBi2O4 Prepared by the Polymerized Complex Method for Gas-Sensing Applications. ACS Appl. Mater. Inter. 2018, 1017, 14901–14913. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chen, Y.; Chen, B.; Ma, J. Preparation, characterization and photocatalytic activity of CuBi2O4/NaTaO3 coupled photocatalysts. J. Alloys Compd. 2013, 559, 116–122. [Google Scholar] [CrossRef]
- He, Y.; Fishman, Z.S.; Yang, K.R.; Ortiz, B.; Liu, C.; Goldsamt, J.; Batista, V.S.; Pfefferle, L.D. Hydrophobic CuO Nanosheets Functionalized with Organic Adsorbates. J. Am. Chem. Soc. 2018, 1405, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, T.; Yang, G.; Lu, C.; Dong, F.; Li, Y.; Guan, W. The precursor-guided hydrothermal synthesis of CuBi2O4/WO3 heterostructure with enhanced photoactivity under simulated solar light irradiation and mechanism insight. J. Hazard. Mater. 2019, 120956. [Google Scholar] [CrossRef] [PubMed]
- Berglund, S.P.; Abdi, F.F.; Bogdanoff, P.; Chemseddine, A.; Friedrich, D.; van de Krol, R. Comprehensive Evaluation of CuBi2O4 as a Photocathode Material for Photoelectrochemical Water Splitting. Chem. Mater. 2016, 2812, 4231–4242. [Google Scholar] [CrossRef]
- Shi, W.; Guo, F.; Yuan, S. In situ synthesis of Z-scheme Ag3PO4/CuBi2O4 photocatalysts and enhanced photocatalytic performance for the degradation of tetracycline under visible light irradiation. Appl. Cataly. B Environ. 2017, 209, 720–728. [Google Scholar] [CrossRef]
- Yuan, X.; Shen, D.; Zhang, Q.; Zou, H.; Liu, Z.; Peng, F. Z-scheme Bi2WO6/CuBi2O4 heterojunction mediated by interfacial electric field for efficient visible-light photocatalytic degradation of tetracycline. Chem. Eng. J. 2019, 369, 292–301. [Google Scholar] [CrossRef]
- Wang, L.; Yang, G.; Wang, D.; Lu, C.; Guan, W.; Li, Y.; Deng, J.; Crittenden, J. Fabrication of the flower-flake-like CuBi2O4/Bi2WO6 heterostructure as efficient visible-light driven photocatalysts: Performance, kinetics and mechanism insight. Appl. Surf. Sci. 2019, 495, 143521. [Google Scholar] [CrossRef]
- Zhang, F.J.; Zhu, S.F.; Xie, F.Z.; Zhang, J.; Meng, Z.D. Plate-on-plate structured Bi2MoO6/Bi2WO6 heterojunction with high-efficiently gradient charge transfer for decolorization of MB. Sep. Purif. Technol. 2013, 113, 1–8. [Google Scholar] [CrossRef]
- Guo, F.; Li, M.; Ren, H.; Huang, X.; Hou, W.; Wang, C.; Shi, W.; Lu, C. Fabrication of p-n CuBi2O4/MoS2 heterojunction with nanosheets-on-microrods structure for enhanced photocatalytic activity towards tetracycline degradation. Appl. Surf. Sci. 2019, 491, 88–94. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cai, F.; Guo, P.; Lei, Y.; Xi, Q.; Wang, F. Short-Time Hydrothermal Synthesis of CuBi2O4 Nanocolumn Arrays for Efficient Visible-Light Photocatalysis. Nanomaterials 2019, 9, 1257. https://doi.org/10.3390/nano9091257
Wang Y, Cai F, Guo P, Lei Y, Xi Q, Wang F. Short-Time Hydrothermal Synthesis of CuBi2O4 Nanocolumn Arrays for Efficient Visible-Light Photocatalysis. Nanomaterials. 2019; 9(9):1257. https://doi.org/10.3390/nano9091257
Chicago/Turabian StyleWang, Yi, Fang Cai, Pengran Guo, Yongqian Lei, Qiaoyue Xi, and Fuxian Wang. 2019. "Short-Time Hydrothermal Synthesis of CuBi2O4 Nanocolumn Arrays for Efficient Visible-Light Photocatalysis" Nanomaterials 9, no. 9: 1257. https://doi.org/10.3390/nano9091257
APA StyleWang, Y., Cai, F., Guo, P., Lei, Y., Xi, Q., & Wang, F. (2019). Short-Time Hydrothermal Synthesis of CuBi2O4 Nanocolumn Arrays for Efficient Visible-Light Photocatalysis. Nanomaterials, 9(9), 1257. https://doi.org/10.3390/nano9091257




