Facile Synthesis of Pd Nanocubes with Assistant of Iodide and Investigation of Their Electrocatalytic Performances Towards Formic Acid Oxidation
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Synthesis of Pd Nanocubes
2.3. Removal of Stabilizer and Capping Agent from Pd Nanoparticles As-Synthesized
2.4. Electrochemical Measurements
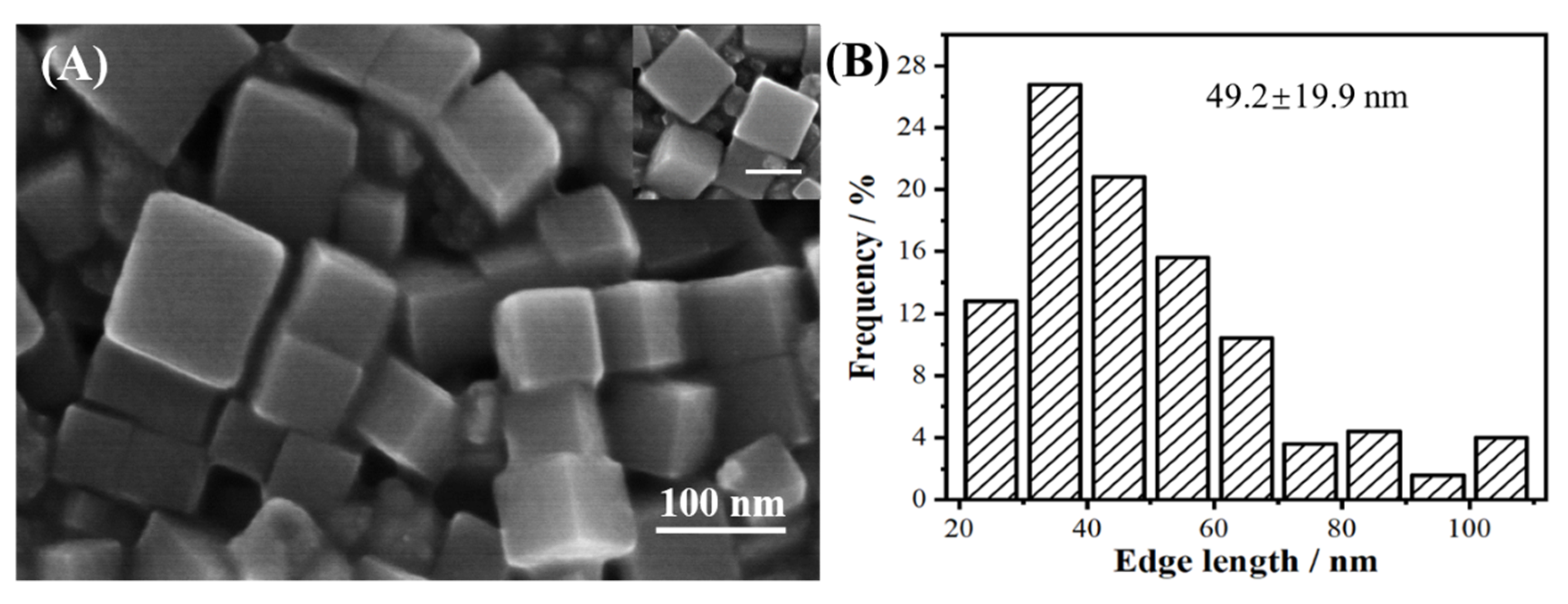
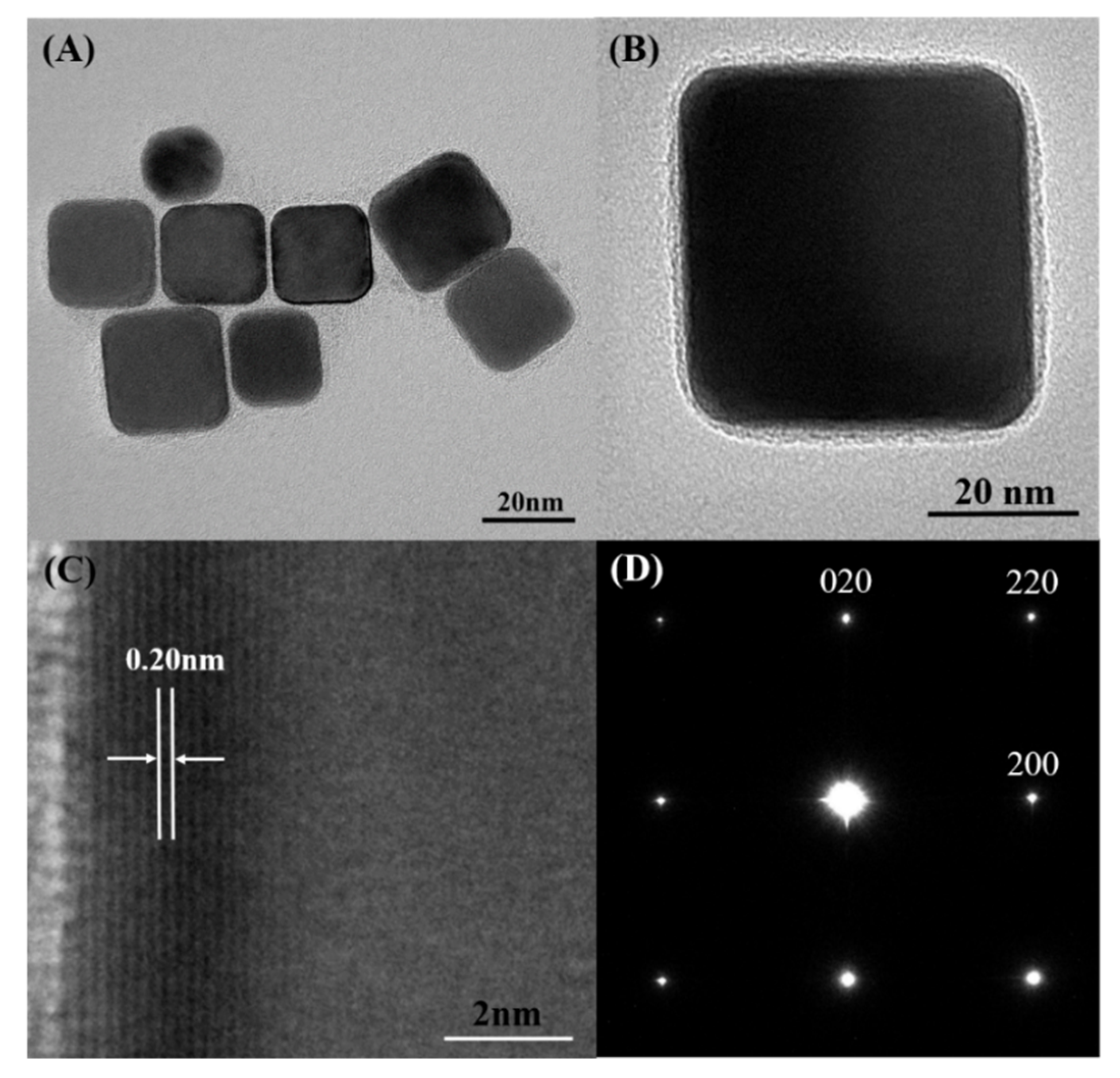
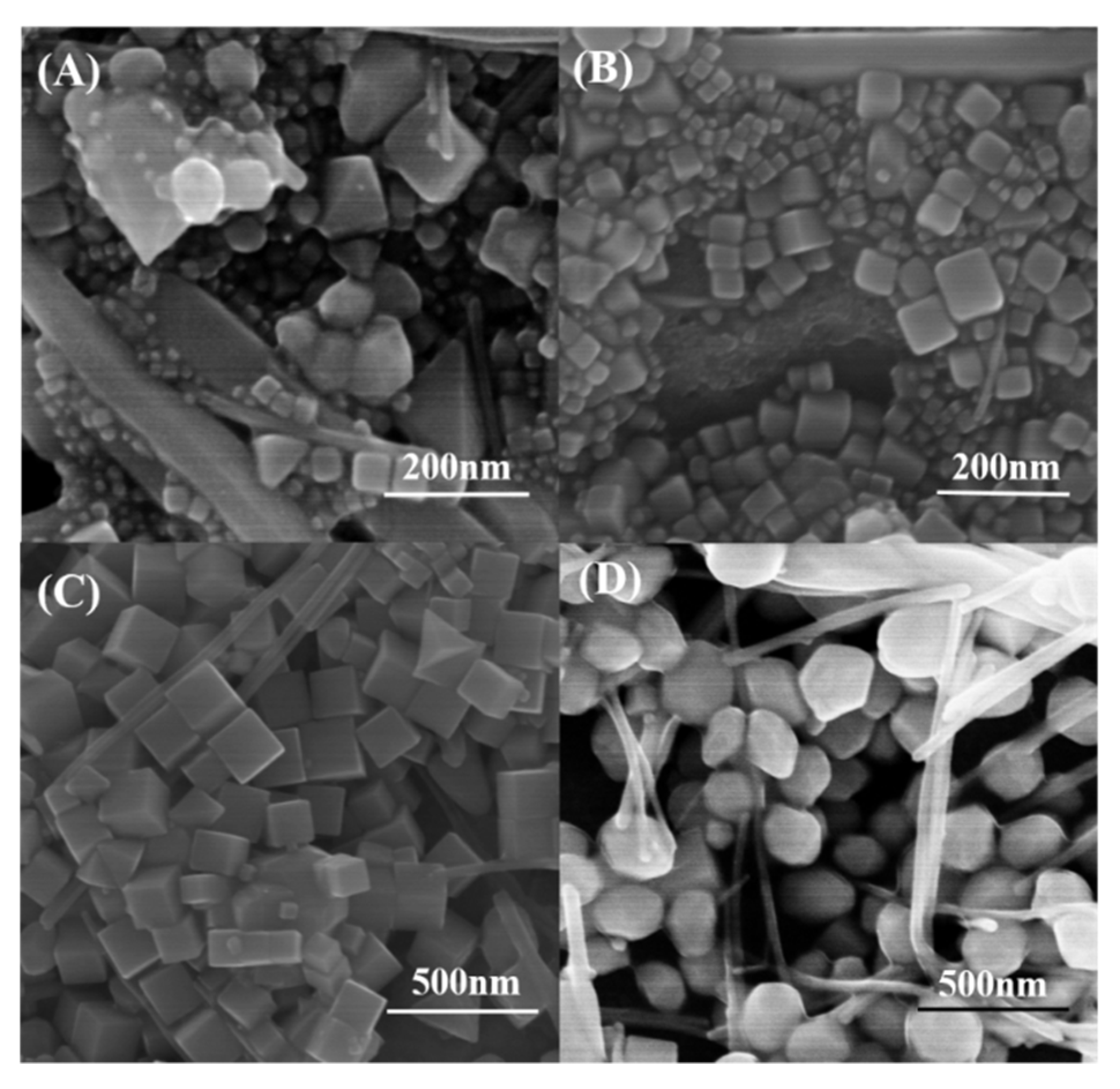
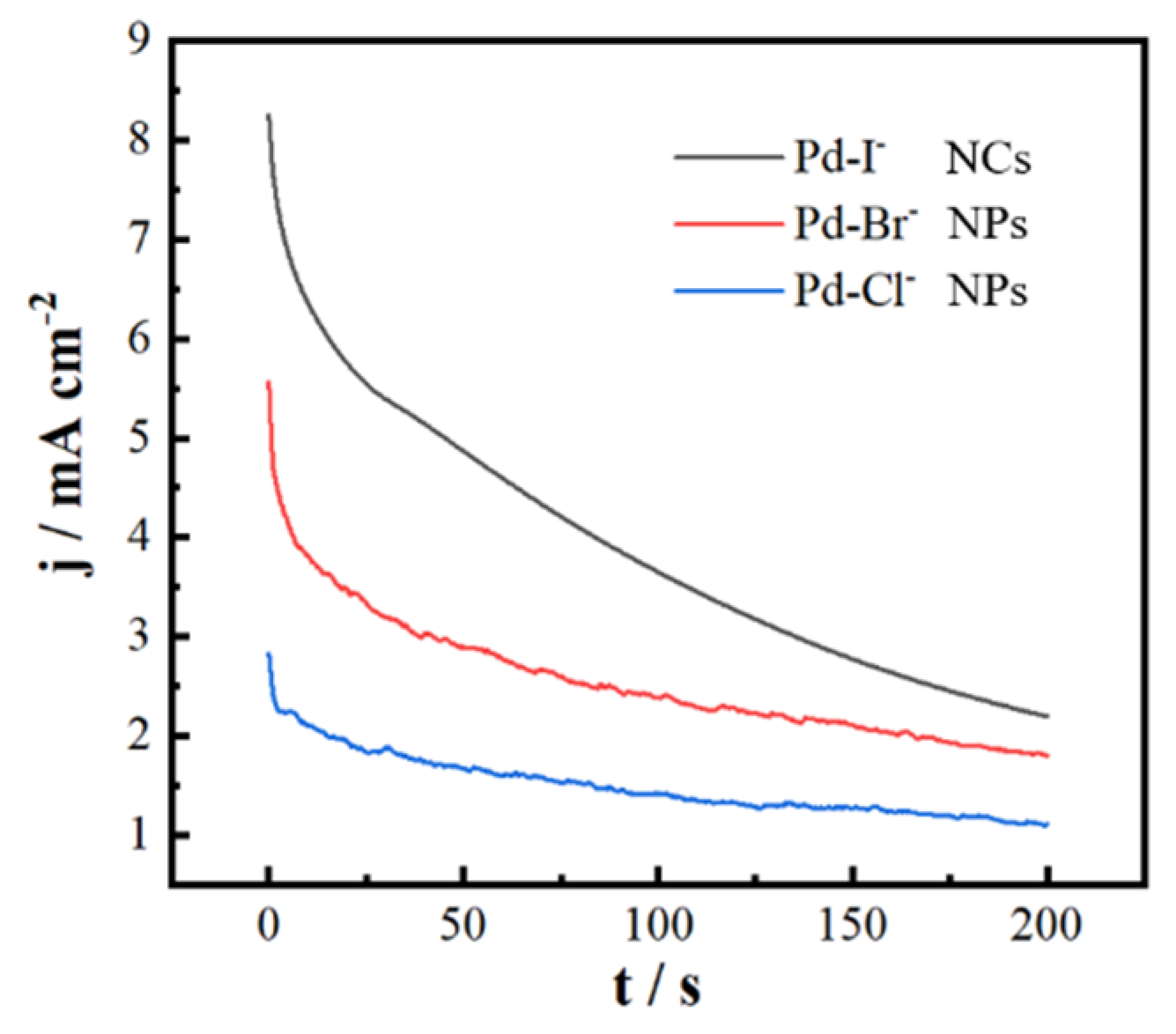
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Y.H.; Hung, H.H.; Huang, M.H. Seed-Mediated Synthesis of Palladium Nanorods and Branched Nanocrystals and Their Use as Recyclable Suzuki Coupling Reaction Catalysts. J. Am. Chem. Soc. 2009, 131, 9114–9121. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Li, Y.J.; Chen, Y.; Zhou, E.B.; Xu, Y.X.; Zhou, H.L.; Duan, X.F.; Huang, Y. Palladium-Based Nanostructures with Highly Porous Features and Perpendicular Pore Channels as Enhanced Organic Catalysts. Angew. Chem. 2013, 52, 2520–2524. [Google Scholar] [CrossRef] [PubMed]
- Antolini, E. Palladium in fuel cell catalysis. Energy Environ. Sci. 2009, 2, 915–931. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Tian, N.; Li, J.T.; Broadwell, I.; Sun, S.G. Nanomaterials of high surface energy with exceptional properties in catalysis and energy storage. Chem. Soc. Rev. 2011, 40, 4167–4185. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.X.; Zhang, L.; Xu, G.B. Shape-Controlled Synthesis of Single-Crystalline Palladium Nanocrystals. ACS Nano 2010, 4, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.S.; Zhang, H.; Xie, Z.X.; Xia, Y.N. Palladium nanocrystals enclosed by {100} and {111} facets in controlled proportions and their catalytic activities for formic acid oxidation. Energy Environ. Sci. 2012, 5, 6352–6357. [Google Scholar] [CrossRef]
- Jin, M.S.; Zhang, H.; Xie, Z.X.; Xia, Y.N. Palladium Concave Nanocubes with High-Index Facets and Their Enhanced Catalytic Properties. Angew. Chem. 2011, 50, 7850–7854. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Zheng, N.F. One-Pot, High-Yield Synthesis of 5-Fold Twinned Pd Nanowires and Nanorods. J. Am. Chem. Soc. 2009, 131, 4602–4603. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Tang, S.H.; Mu, X.L.; Dai, Y.; Chen, G.X.; Zhou, Z.Y.; Ruan, F.X.; Yang, Z.L.; Zheng, N.F. Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat. Nanotechnol. 2011, 6, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.B.; Gao, G.H.; Pan, Z.Y.; Wang, T.J.; Meng, X.Q.; Cai, L.T. Large-Scale Synthesis of Palladium Concave Nanocubes with High-Index Facets for Sustainable Enhanced Catalytic Performance. Sci. Rep.-UK 2015, 5, 8515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Wang, H.; Re, Y.S.; Cai, W.B. Palladium nanocrystals bound by {110} or {100} facets: from one pot synthesis to electrochemistry. Chem. Commun. 2012, 48, 8362–8364. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Xiong, Y.J.; Xia, Y.N. A water-based synthesis of octahedral, decahedral, and icosahedral Pd nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 9279–9282. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.X.; Li, Z.Y.; Shi, L.H.; Liu, X.Q.; Li, H.J.; Han, S.; Chen, J.; Xu, G.B. Seed-Mediated Growth of Nearly Monodisperse Palladium Nanocubes with Controllable Sizes. Cryst. Growth Des. 2008, 8, 4440–4444. [Google Scholar] [CrossRef]
- Figueroa-Cosme, L.; Gilroy, K.D.; Yang, T.H.; Vara, M.; Park, J.H.; Bao, S.X.; da Silva, A.G.M.; Xia, Y.N. Synthesis of Palladium Nanoscale Octahedra through a One-Pot, Dual-Reductant Route and Kinetic Analysis. Chem. Eur. J. 2018, 24, 6133–6139. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.J.; Cai, H.G.; Yin, Y.D.; Xia, Y.N. Synthesis and characterization of fivefold twinned nanorods and right bipyramids of palladium. Chem. Phys. Lett. 2007, 440, 273–278. [Google Scholar] [CrossRef]
- Huang, X.Q.; Tang, S.H.; Zhang, H.H.; Zhou, Z.Y.; Zheng, N.F. Controlled Formation of Concave Tetrahedral/Trigonal Bipyramidal Palladium Nanocrystals. J. Am. Chem. Soc. 2009, 131, 13916–13917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, M.S.; Zhu, E.B.; Zheng, Y.B.; Huang, Y.; Huang, X.Q. Seedless Growth of Palladium Nanocrystals with Tunable Structures: From Tetrahedra to Nanosheets. Nano Lett. 2015, 15, 7519–7525. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Zhou, Z.Y.; Yu, N.F.; Wang, L.Y.; Sun, S.G. Direct Electrodeposition of Tetrahexahedral Pd Nanocrystals with High-Index Facets and High Catalytic Activity for Ethanol Electrooxidation. J. Am. Chem. Soc. 2010, 132, 7580–7581. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Xu, C.D.; Huang, L.; Zhou, Z.Y.; Chen, S.P.; Sun, S.G. Electrochemically Shape-Controlled Synthesis of Pd Concave-Disdyakis Triacontahedra in Deep Eutectic Solvent. J. Phys. Chem. C 2016, 120, 15569–15577. [Google Scholar] [CrossRef]
- Huang, R.; Wen, Y.H.; Shao, G.F.; Zhu, Z.Z.; Sun, S.G. Thermal Stability and Shape Evolution of Tetrahexahedral Au-Pd Core-Shell Nanoparticles with High-Index Facets. J. Phys. Chem. C 2013, 117, 6896–6903. [Google Scholar] [CrossRef]
- Huang, R.; Wen, Y.H.; Zhu, Z.Z.; Sun, S.G. Pt-Pd Bimetallic Catalysts: Structural and Thermal Stabilities of Core-Shell and Alloyed Nanoparticles. J. Phys. Chem. C 2012, 116, 8664–8671. [Google Scholar] [CrossRef]
- Huang, X.Q.; Zhang, H.H.; Guo, C.Y.; Zhou, Z.Y.; Zheng, N.F. Simplifying the Creation of Hollow Metallic Nanostructures: One-Pot Synthesis of Hollow Palladium/Platinum Single-Crystalline Nanocubes. Angew. Chem. Int. Ed. 2009, 48, 4808–4812. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.; Ha, S.; Zakzeski, J.; Masel, R.I. Unusually active palladium-based catalysts for the electrooxidation of formic acid. J. Power Sources 2006, 157, 78–84. [Google Scholar] [CrossRef]
- Wang, R.F.; Liao, S.J.; Ji, S. High performance Pd-based catalysts for oxidation of formic acid. J. Power Sources 2008, 180, 205–208. [Google Scholar] [CrossRef]
- Chen, X.M.; Wu, G.H.; Chen, J.M.; Chen, X.; Xie, Z.X.; Wang, X.R. Synthesis of “Clean” and Well-Dispersive Pd Nanoparticles with Excellent Electrocatalytic Property on Graphene Oxide. J. Am. Chem. Soc. 2011, 133, 3693–3695. [Google Scholar] [CrossRef] [PubMed]
- Nalajala, N.; Saleha, W.F.G.; Ladewig, B.P.; Neergat, M. Sodium borohydride treatment: A simple and effective process for the removal of stabilizer and capping agents from shape-controlled palladium nanoparticles. Chem. Commun. 2014, 50, 9365–9368. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Kobayashi, H.; Camargo, P.H.C.; Allard, L.F.; Liu, J.Y.; Xia, Y.N. New Insights into the Growth Mechanism and Surface Structure of Palladium Nanocrystals. Nano Res. 2010, 3, 180–188. [Google Scholar] [CrossRef]
- Yin, A.X.; Min, X.Q.; Zhang, Y.W.; Yan, C.H. Shape-Selective Synthesis and Facet-Dependent Enhanced Electrocatalytic Activity and Durability of Monodisperse Sub-10 nm Pt-Pd Tetrahedrons and Cubes. J. Am. Chem. Soc. 2011, 133, 3816–3819. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Li, Y.J.; Li, Y.J.; Zhou, H.L.; Duan, X.F.; Huang, Y. Synthesis of PtPd Bimetal Nanocrystals with Controllable Shape, Composition, and Their Tunable Catalytic Properties. Nano Lett. 2012, 12, 4265–4270. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.J.; Cai, H.G.; Wiley, B.J.; Wang, J.G.; Kim, M.J.; Xia, Y.N. Synthesis and mechanistic study of palladium nanobars and nanorods. J. Am. Chem. Soc. 2007, 129, 3665–3675. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.S.; Liu, H.Y.; Zhang, H.; Xie, Z.X.; Liu, J.Y.; Xia, Y.N. Synthesis of Pd Nanocrystals Enclosed by {100} Facets and with Sizes <10 nm for Application in CO Oxidation. Nano Res. 2011, 4, 83–91. [Google Scholar]
- Xiong, Y.J.; Washio, I.; Chen, J.Y.; Cai, H.G.; Li, Z.Y.; Xia, Y.N. Poly(vinyl pyrrolidone): A dual functional reductant and stabilizer for the facile synthesis of noble metal nanoplates in aqueous solutions. Langmuir 2006, 22, 8563–8570. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.P.; Niu, W.X.; Luque, R.; Xu, G.B. Solvothermal synthesis of metal nanocrystals and their applications. Nano Today 2015, 10, 240–267. [Google Scholar] [CrossRef]
- Rice, C.; Ha, R.I.; Masel, R.I.; Waszczuk, P.; Wieckowski, A.; Barnard, T. Direct formic acid fuel cells. J. Power Sources 2002, 111, 83–89. [Google Scholar] [CrossRef]
- Deng, Y.J.; Tian, N.; Zhou, Z.Y.; Huang, R.; Liu, Z.L.; Xiao, J.; Sun, S.G. Alloy tetrahexahedral Pd-Pt catalysts: enhancing significantly the catalytic activity by synergy effect of high-index facets and electronic structure. Chem. Sci. 2012, 3, 1157–1161. [Google Scholar] [CrossRef]
- Hoshi, N.; Kida, K.; Nakamura, M.; Nakada, M.; Osada, K. Structural effects of electrochemical oxidation of formic acid on single crystal electrodes of palladium. J. Phys. Chem. B 2006, 110, 12480–12484. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Li, Z.; Wang, K.; Zhou, L.; Zhao, X.; Jiang, W.; Li, Q.; Deng, Y. Facile Synthesis of Pd Nanocubes with Assistant of Iodide and Investigation of Their Electrocatalytic Performances Towards Formic Acid Oxidation. Nanomaterials 2019, 9, 375. https://doi.org/10.3390/nano9030375
Liu X, Li Z, Wang K, Zhou L, Zhao X, Jiang W, Li Q, Deng Y. Facile Synthesis of Pd Nanocubes with Assistant of Iodide and Investigation of Their Electrocatalytic Performances Towards Formic Acid Oxidation. Nanomaterials. 2019; 9(3):375. https://doi.org/10.3390/nano9030375
Chicago/Turabian StyleLiu, Xuan, Zichao Li, Kuankuan Wang, Luming Zhou, Xihui Zhao, Wenhai Jiang, Qun Li, and Yujia Deng. 2019. "Facile Synthesis of Pd Nanocubes with Assistant of Iodide and Investigation of Their Electrocatalytic Performances Towards Formic Acid Oxidation" Nanomaterials 9, no. 3: 375. https://doi.org/10.3390/nano9030375
APA StyleLiu, X., Li, Z., Wang, K., Zhou, L., Zhao, X., Jiang, W., Li, Q., & Deng, Y. (2019). Facile Synthesis of Pd Nanocubes with Assistant of Iodide and Investigation of Their Electrocatalytic Performances Towards Formic Acid Oxidation. Nanomaterials, 9(3), 375. https://doi.org/10.3390/nano9030375




