Experimental Investigation of Thermal and Pressure Performance in Computer Cooling Systems Using Different Types of Nanofluids
Abstract
:1. Introduction
2. Nanofluid Preparation and Characterization
3. Thermophysical Properties
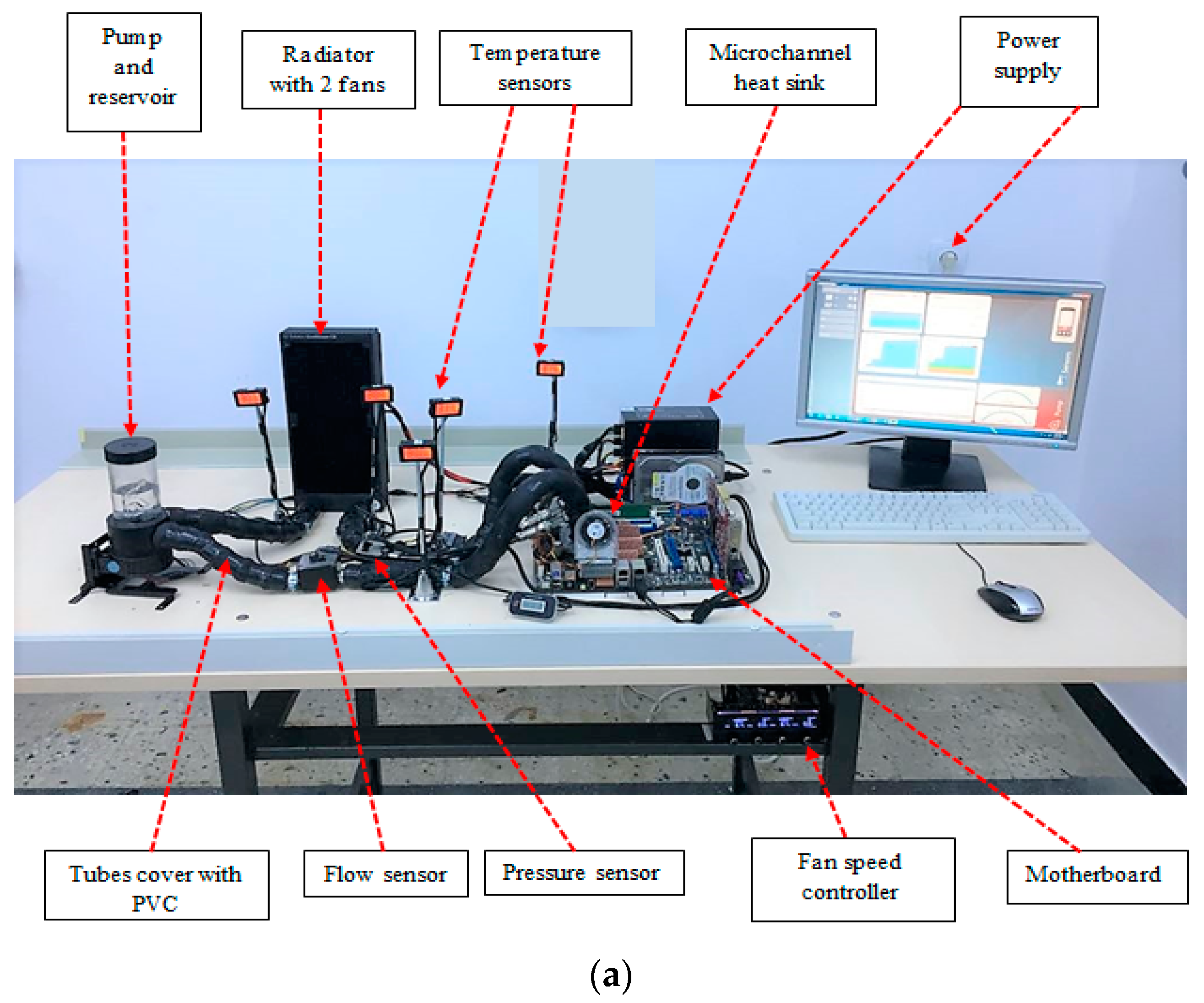
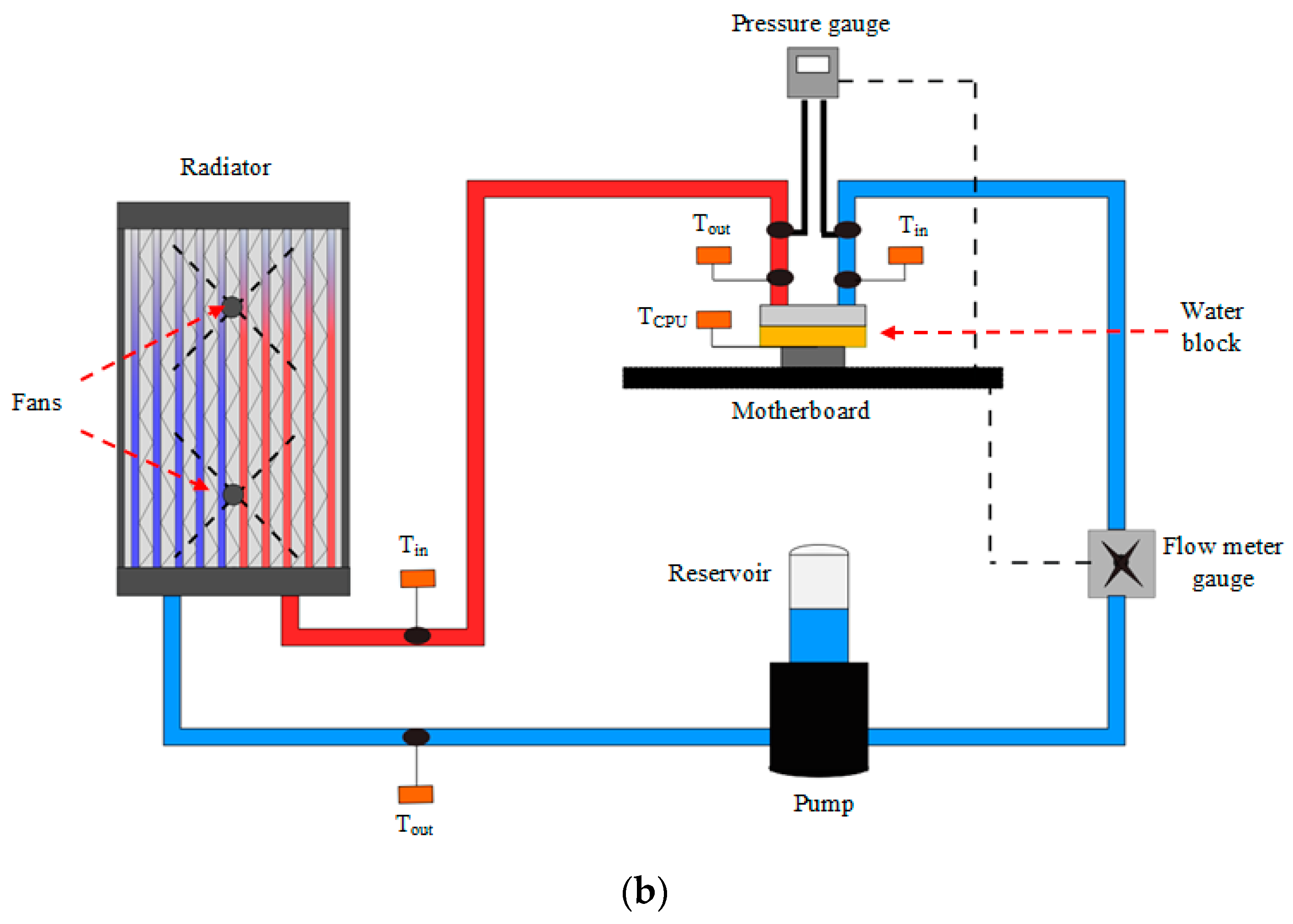
4. Experimental Setup
5. Data Processing
6. Results
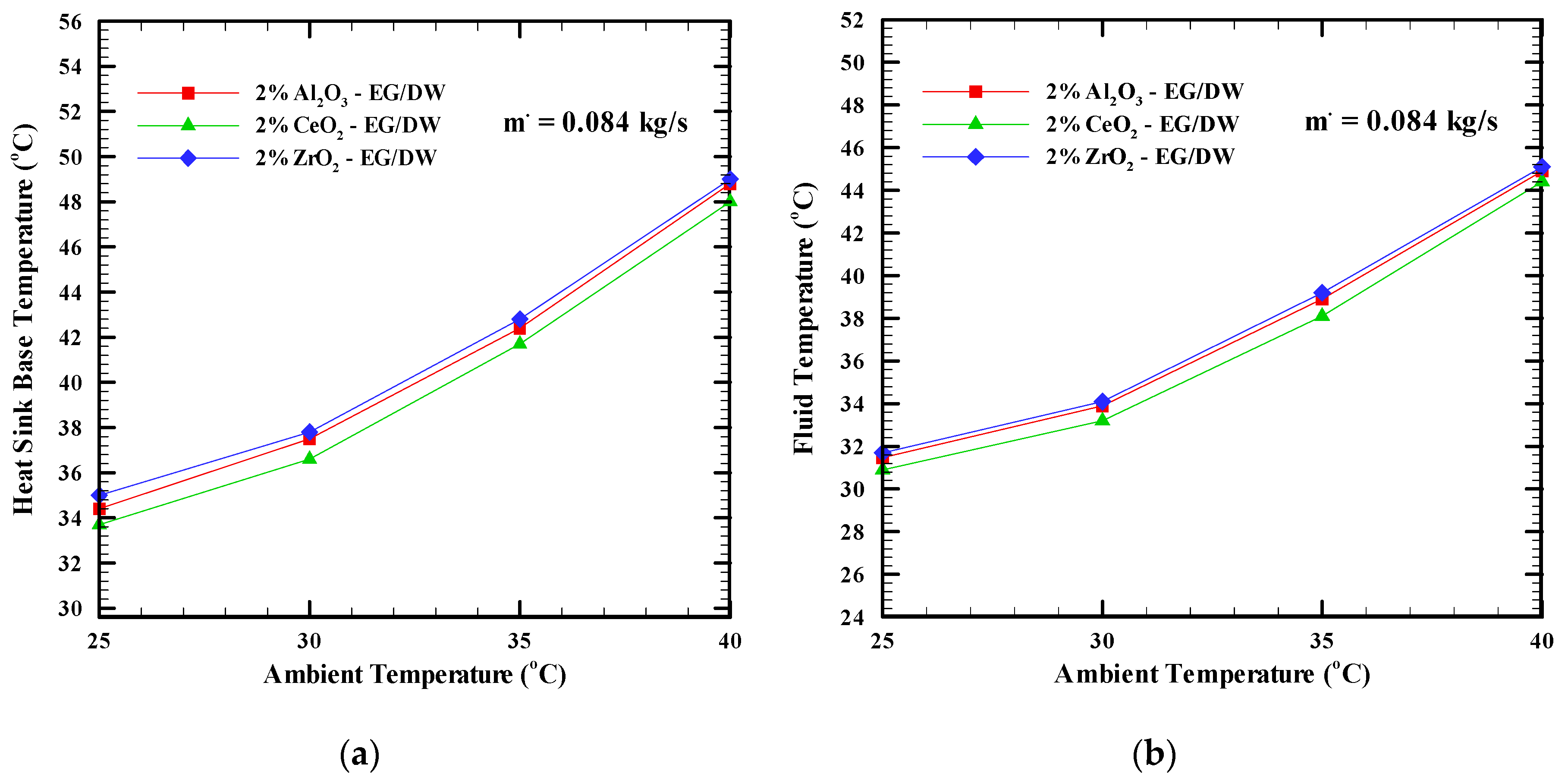
6.1. Heat Sink Base Temperature
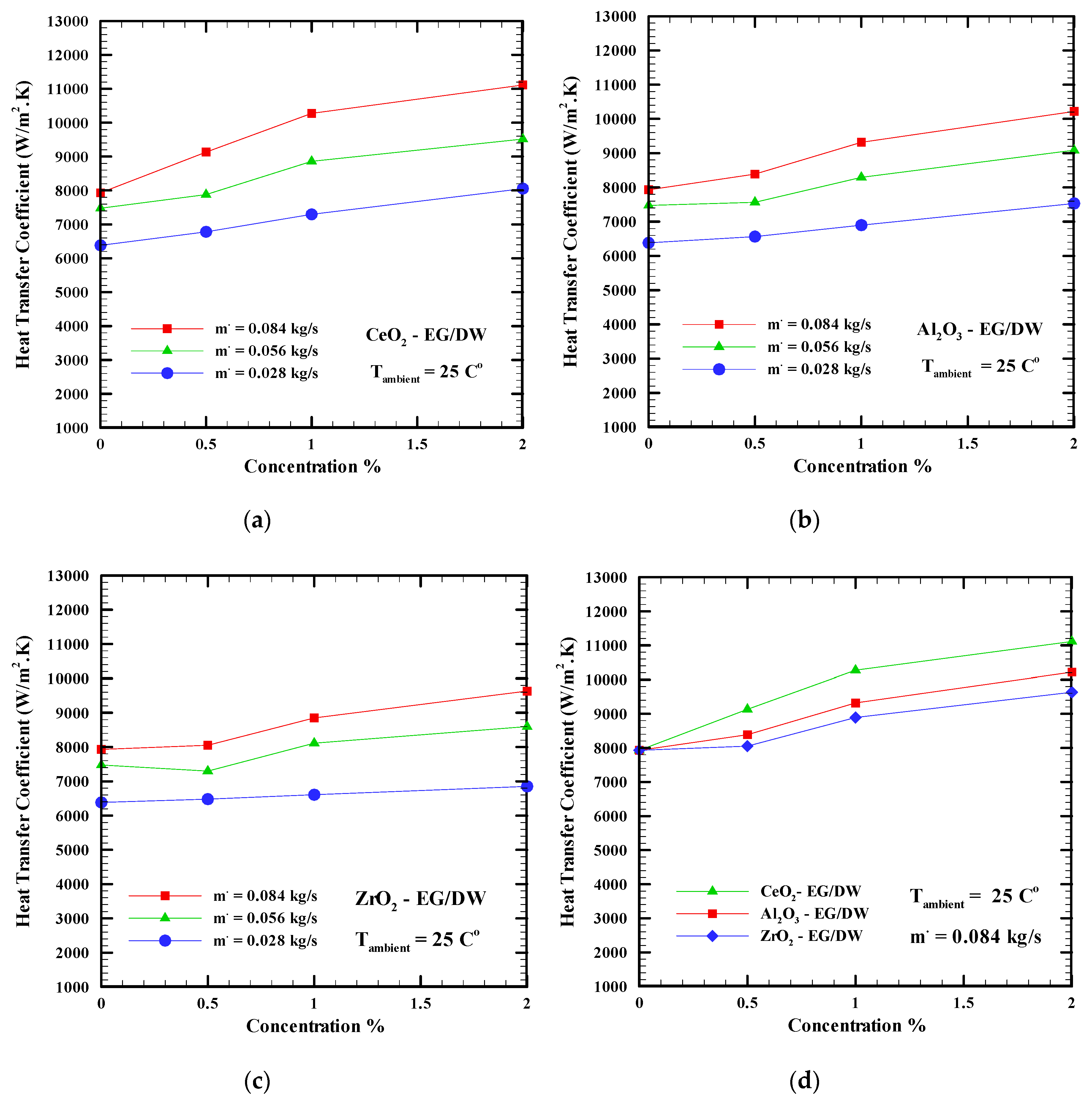
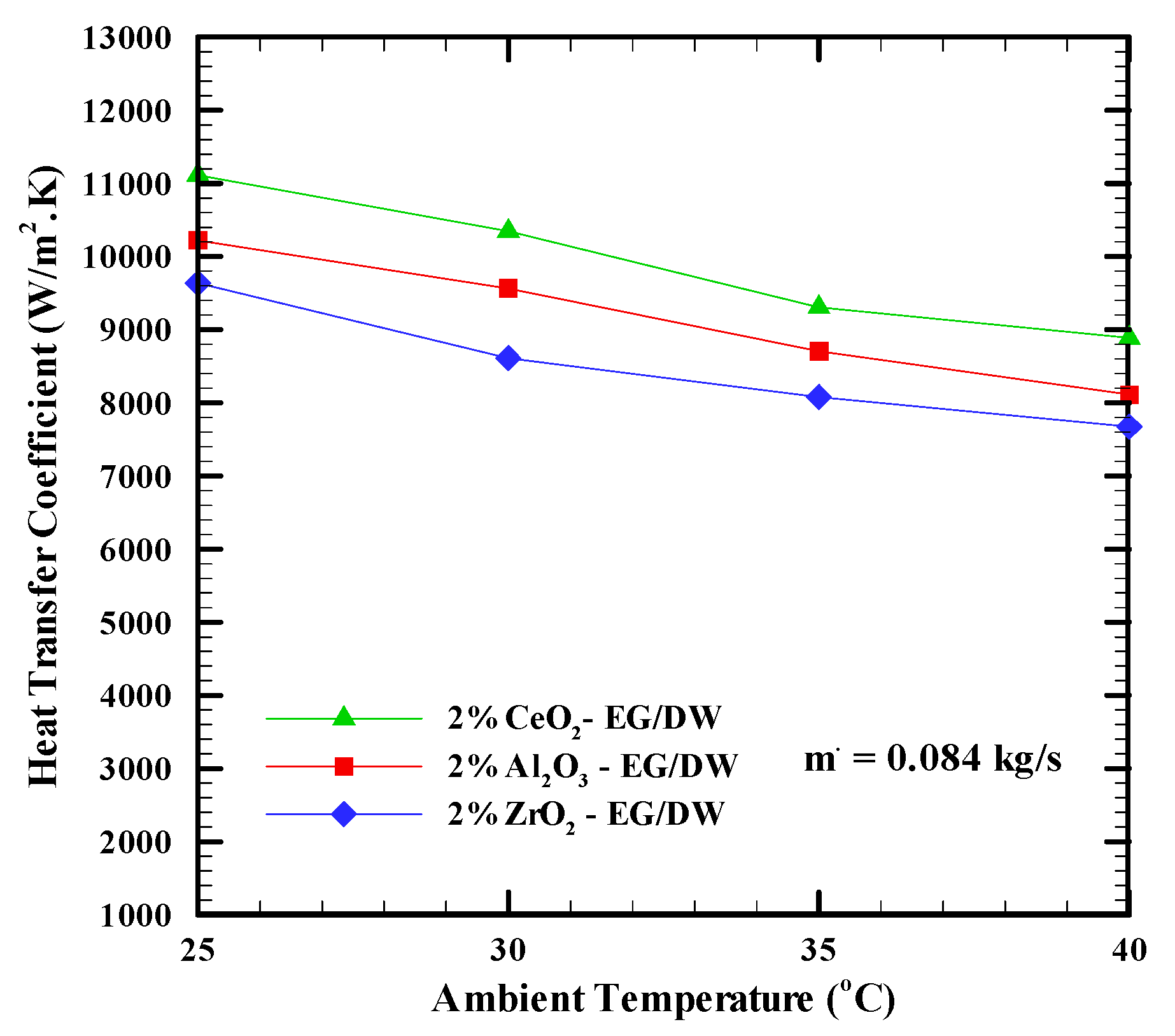
6.2. Heat Transfer Coefficients
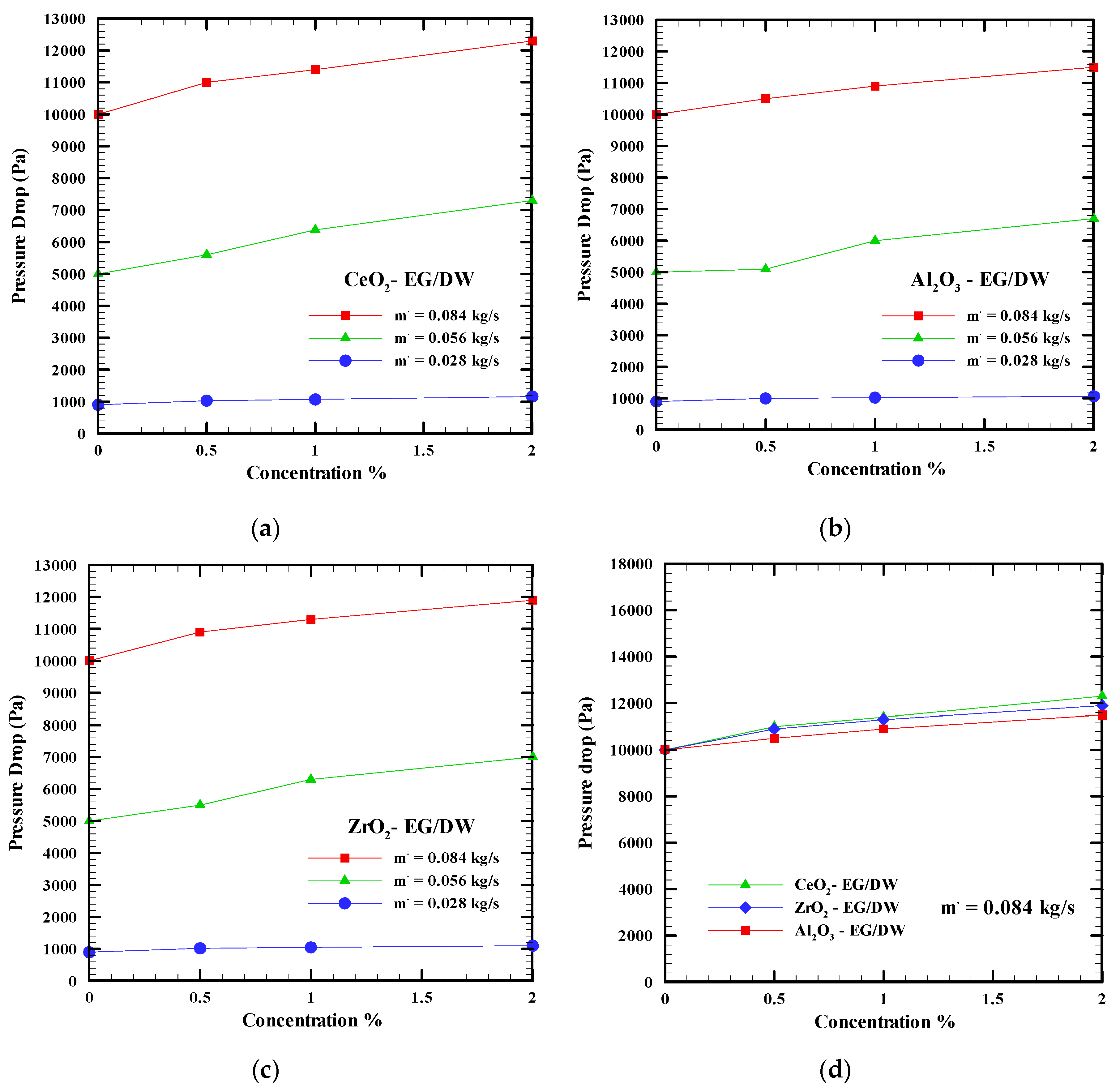
6.3. Pressure Drop
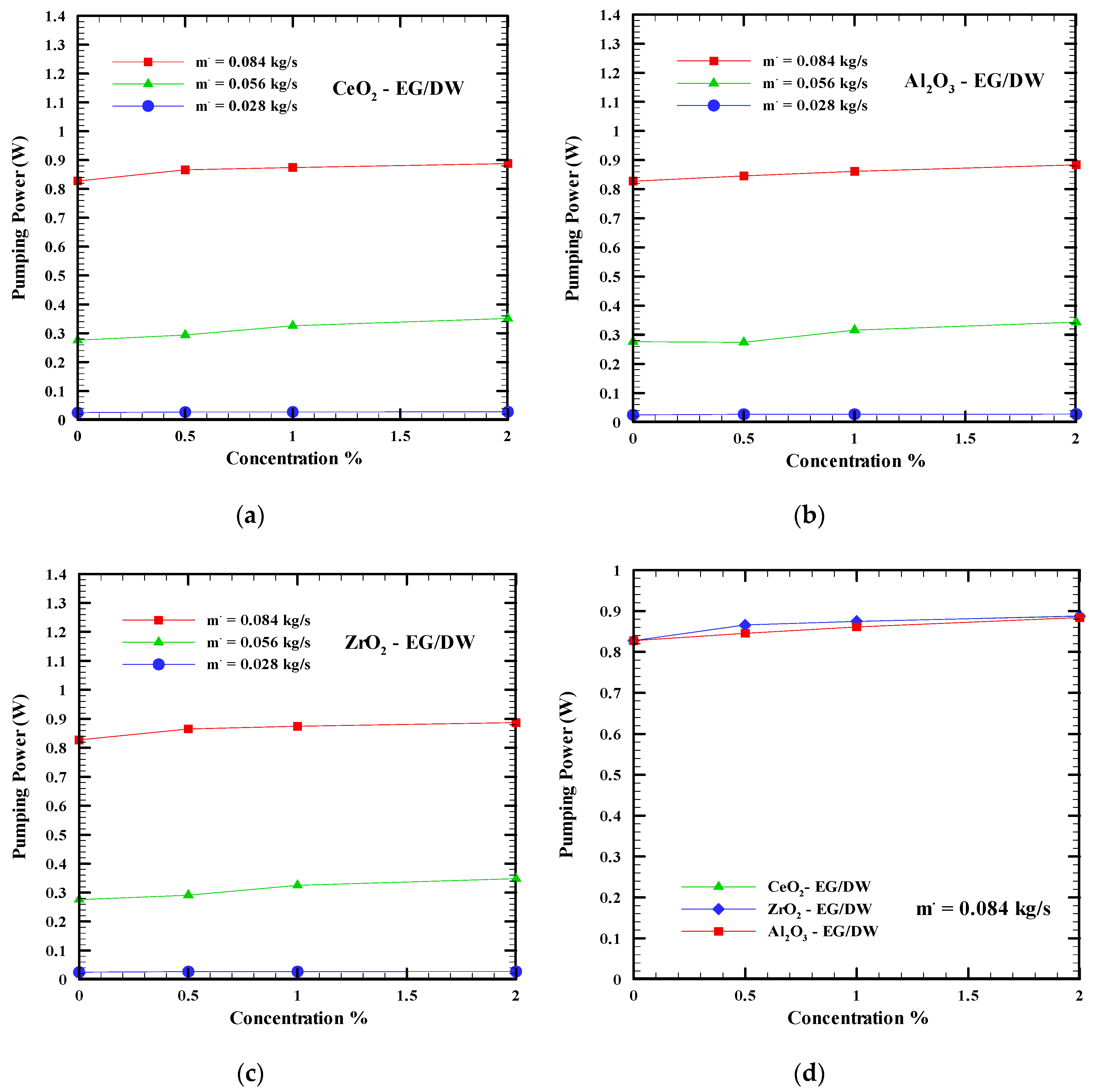
6.4. Pumping Power
7. Conclusions
- Increasing the concentration of the nanoparticles from 0.5% to 2% and increasing the mass flow rate causes a decrease in the base temperature of the heat sink more than the base fluid. The CeO2 nanofluid at a 2% concentration reduces the temperature with an 8.3% since the CeO2 nanofluid has the highest thermal conductivity and viscosity. At the same concentration, the Al2O3 and ZrO2 nanofluids show 6.1% and 4.2% temperature decrease, respectively, compared with the base fluid EG/DW (20:80). Moreover, increasing the ambient temperature from 25 °C to 40 °C led to an increase of the heat sink base temperature.
- Adding nanoparticles to the base fluid and increasing the mass flow rate leads to an increase in the heat transfer coefficient much more than the base fluid. The CeO2 nanofluid shows the highest heat transfer coefficient with a 29% enhancement, while the Al2O3 nanofluid shows a 22% enhancement. The ZrO2 showed a 17% enhancement. Furthermore, increasing the ambient temperature from 25 °C to 40 °C led to a decrease in the heat transfer coefficient.
- The pressure drops and pumping power increase when the mass flow rate and concentration of the nanofluid increases. The Al2O3-EG/DW shows the lowest value followed by ZrO2-EG/DW and CeO2-EG/DW. However, a slight increase of pumping power and pressure drop can be balanced by considering the high improvement of the nanofluid in computer cooling performance compared to the base fluid.
- The novelty of the study consists in providing information about the comparative behavior of CeO2, Al2O3 and ZrO2 nanoparticles suspended in EG/DW (20:80) when used as cooling fluids in computer cooling systems. Graphs are provided with the variation of the convective heat transfer coefficient, heat sink base temperature, pressure drop and pumping power, against the nanoparticles concentration in nanofluids and the mass flow rate of the cooling fluid.
- From an academic point of view, the work establishes a procedure for the preparation and characterization of nanofluids. An experimental setup was created to investigate the heat transfer performances of a computer cooling system with nanofluids as the cooling fluid. Of academic interest is the data processing developed in the work.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tuckerman, D.B.; Pease, R.F. High performance heat sinking for VLSI. IEEE Electron Device Lett. 1981, 2, 126–129. [Google Scholar] [CrossRef]
- Lee, J.; Mudawar, I. Assessment of the effectiveness of nanofluids for single-phase and two-phase heat transfer in micro-channels. Int. J. Heat Mass Transf. 2007, 50, 452–463. [Google Scholar] [CrossRef]
- Khonsue, O. Experimental on the liquid cooling system with thermoelectric for personal computer. Heat Mass Transf. 2012, 48, 1767–1771. [Google Scholar] [CrossRef] [Green Version]
- Tran, N.; Zhang, C.; Dang, T.; Teng, J. Numerical and experimental studies on pressure drop and performance index of an aluminum microchannel heat sink. In Proceedings of the 2012 International Symposium on Computer, Consumer and Control, Taichung, Taiwan, 4–6 June 2012; pp. 252–257. [Google Scholar]
- Ma, D.D.; Xia, G.D.; Wang, J.; Yang, Y.C.; Jia, Y.T.; Zong, L.X. An experimental study on hydrothermal performance of microchannel heat sinks with 4-ports and offset zigzag channels. Energy Convers. Manag. 2017, 152, 157–165. [Google Scholar] [CrossRef]
- Choi, S.U.; Eastman, J.A. Enhancing thermal conductivity of fluids with nanoparticles. In ASME International Mechanical Engineering Congress & Exposition; American Society of Mechanical Engineers: Fairfield, CT, USA, 1995. [Google Scholar]
- Raja, M.; Vijayana, R.; Dineshkuma, P.; Venkatesan, M. Review on nanofluids characterization, heat transfer characteristics and applications. Renew. Sustain. Energy Rev. 2016, 64, 163–173. [Google Scholar] [CrossRef]
- Lomascolo, M.; Colangelo, G.; Milanese, M.; Risi, A. Review of heat transfer in nanofluids: Conductive, convective and radiative experimental results. Renew. Sustain. Energy Rev. 2015, 43, 1182–1198. [Google Scholar] [CrossRef]
- Nandhakumar, R.; Senthilkumar, D. An experimental investigation on thermal performance of nanofluids in a minichannel heat sink. J. Adv. Chem. 2016, 12, 5152–5162. [Google Scholar]
- Jeong, J.; Li, C.; Kwon, Y.; Lee, J.; Kim, S.H.; Yun, R. Particle shape effect on the viscosity and thermal conductivity of ZnO nanofluids. Int. J. Refrig. 2013, 36, 2233–2241. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.U.; Li, S.; Eastman, J. Measuring thermal conductivity of fluids containing oxide nanoparticles. J. Heat Transf. 1999, 121, 280–289. [Google Scholar] [CrossRef]
- Alawi, O.A.; Sidik, N.A.C.; Xian, H.W.; Kean, T.H.; Kazi, S.N. Thermal conductivity and viscosity models of metallic oxides nanofluids. Int. J. Heat Mass Transf. 2018, 116, 1314–1325. [Google Scholar] [CrossRef]
- Namburu, P.K.; Das, D.K.; Tanguturi, K.M.; Vajjha, R.S. Numerical study of turbulent flow and heat transfer characteristics of nanofluids considering variable properties. Int. J. Therm. Sci. 2009, 48, 290–302. [Google Scholar] [CrossRef]
- Eastman, J.A.; Choi, S.U.S.; Li, S.; Yu, W.; Thompson, L.J. Anomalously increased Effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl. Phys. Lett. 2001, 78, 718–720. [Google Scholar] [CrossRef]
- Esfe, M.H.; Wongwises, S.; Naderi, A.; Asadi, A.; Safaei, M.R.; Rostamian, H.; Dahari, M.; Karimipour, A. Thermal conductivity of Cu/TiO2–Water/EG hybrid nanofluid: Experimental data and modeling using artificial neural network and correlation. Int. Commun. Heat Mass Transf. 2015, 66, 100–104. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.H.; Nabil, M.F.; Mamat, R. Experimental investigation of nanoparticle mixture ratios on TiO2–SiO2 nanofluids heat transfer performance under turbulent flow. Int. Commun. Heat Mass Transf. 2018, 118, 617–627. [Google Scholar] [CrossRef]
- Siddiqui, F.R.; Tso, C.Y.; Chan, K.C.; Fu, S.C.; Chao, C.Y.H. On trade-off for dispersion stability and thermal transport of Cu-Al2O3 hybrid nanofluid for various mixing ratios. Int. Commun. Heat Mass Transf. 2019, 132, 1200–1216. [Google Scholar] [CrossRef]
- Chopkar, M.; Kumar, S.; Bhandari, D.; Das, P.K.; Manna, I. Development and characterization of Al2Cu and Ag2Al nanoparticle dispersed water and ethylene glycol based nanofluid. Mater. Sci. Eng. B 2007, 139, 141–148. [Google Scholar] [CrossRef]
- Baby, T.T.; Ramaprabhu, S. Synthesis and nanofluid application of silver nanoparticles decorated grapheme. J. Mater. Chem. 2011, 21, 9702–9709. [Google Scholar] [CrossRef]
- Chen, L.F.; Cheng, M.; Yang, D.J.; Yang, L. Enhanced thermal conductivity of nanofluid by synergistic effect of multi-walled carbon nanotubes and Fe2O3 nanoparticles. Appl. Mech. Mater. 2014, 548, 118–123. [Google Scholar]
- Yiamsawasd, T.; Dalkilic, A.S.; Wongwisesa, S. Measurement of the thermal conductivity of Titania and alumina nanofluids. Thermochim. Acta 2012, 545, 48–56. [Google Scholar] [CrossRef]
- Kumaresan, V.; Velraj, R. Experimental investigation of the thermo-physical properties of water–ethylene glycol mixture based CNT nanofluids. Thermochim. Acta 2012, 545, 180–186. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Li, Y.; Chen, L.; Wang, Q. Experimental investigation on the heat transfer properties of Al2O3 nanofluids using the mixture of ethylene glycol and water as base fluid. Powder Technol. 2012, 230, 14–19. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.H.; Mamat, R.; Sharma, K.V. Experimental investigation on heat transfer performance of TiO2 nanofluids in water–ethylene glycol mixture. Int. Commun. Heat Mass Transf. 2016, 73, 16–24. [Google Scholar] [CrossRef]
- Namburu, P.K.; Kulkarni, D.P.; Misra, D.; Das, D.K. Viscosity of copper oxide nanoparticles dispersed in ethylene glycol and water mixture. Exp. Therm. Fluid Sci. 2007, 32, 67–71. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K. Experimental determination of thermal conductivity of three nanofluids and development of new correlations. Int. Commun. Heat Mass Transf. 2009, 52, 4675–4682. [Google Scholar] [CrossRef]
- Reddy, M.C.S.; Rao, V.V. Experimental studies on thermal conductivity of blends of ethylene glycol-water-based TiO2 nanofluids. Int. Commun. Heat Mass Transf. 2013, 46, 31–36. [Google Scholar] [CrossRef]
- Li, X.; Zou, C. Thermo-physical properties of water and ethylene glycol mixture based SiC nanofluids: An experimental investigation. Int. Commun. Heat Mass Transf. 2016, 101, 412–417. [Google Scholar] [CrossRef]
- Sundar, L.S.; Ramana, E.V.; Singh, M.K.; Sousa, A.C.M. Thermal conductivity and viscosity of stabilized ethylene glycol and water mixture Al2O3 nanofluids for heat transfer applications: An expermintal study. Int. Commun. Heat Mass Transf. 2014, 56, 86–95. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Sousa, A.C.M. Thermal conductivity of ethylene glycol and water mixture based Fe3O4 nanofluid. Int. Commun. Heat Mass Transf. 2013, 49, 17–24. [Google Scholar] [CrossRef]
- Ahmed, H.E.; Mohammed, H.A.; Yusoff, M.Z. An overview on heat transfer augmentation using vortex generators and nanofluids: Approaches and applications. Renew. Sustain. Energy Rev. 2012, 16, 5951–5993. [Google Scholar] [CrossRef]
- Xuan, Y.; Roetzel, W. Concepts for heat transfer correlation of nanofluids. Int. Commun. Heat Mass Transf. 2000, 43, 3701–3707. [Google Scholar] [CrossRef]
- Lazarus, G.; Raja, B.; Mohan, L.D.; Wongwises, S. Enhancement of heat transfer using nanofluids—An overview. Renew. Sustain. Energy Rev. 2010, 14, 629–641. [Google Scholar]
- Keblinski, P.; Eastman, J.A.; Cahill, D.G. Nanofluids for thermal transport. Mater. Today 2005, 8, 36–44. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Roy, G.; Galanis, N.; Suiro, S. Heat Transfer Enhancement by using Al2O3-Water Nanofluid in a Liquid Cooling System for Microprocessors. In Proceedings of the 4th WSEAS International Conference on Heat Transfer, Thermal Engineering and Environment, Venice, Italy, 20–22 November 2006; pp. 103–108. [Google Scholar]
- Chein, R.; Chuang, J. Experimental microchannel heat sink performance studies using nanofluids. Int. J. Therm. Sci. 2007, 46, 57–66. [Google Scholar] [CrossRef]
- Ho, C.J.; Wei, L.C.; Li, Z.W. An experimental investigation of forced convective cooling performance of a microchannel heat sink with Al2O3/water nanofluid. Appl. Therm. Eng. 2010, 30, 96–103. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Ghosh, P.; Sarkar, J. Performance comparison of the plate heat exchanger using different nanofluids. Exp. Therm. Fluid Sci. 2013, 49, 141–151. [Google Scholar] [CrossRef]
- Haghighi, E.B.; Anwar, Z.; Lumbreras, I.; Mirmohammadi, S.A.; Behi, M.; Khodabandeh, R.; Palm, B. Screening single phase laminar convective heat transfer of nanofluids in a micro-tube. J. Phys. Conf. Ser. 2012, 395, 012–036. [Google Scholar] [CrossRef]
- Rimbault, B.; Nguyen, C.T.; Galanis, N. Experimental investigation of CuO-water nanofluid flow and heat transfer inside a microchannel heat sink. Int. J. Therm. Sci. 2014, 84, 275–292. [Google Scholar] [CrossRef]
- Nazari, M.; Karami, M.; Ashouri, M. Comparing the thermal performance water, Ethylene Glycol, Alumina and CNT Nanofluids in CPU Cooling: Experimental study. Exp. Therm. Fluid Sci. 2014, 57, 371–377. [Google Scholar] [CrossRef]
- Sivakumar, A.; Alagumurthi, N.; Senthilvelan, T. Effect of serpentine grooves on heat transfer characteristics of microchannel heat sink with different nanofluids. Heat Transf. Asian Res. 2015, 46, 201–217. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, R. Design and Experimental Analysis of Rectangular Wavy Micro Channel Heat sink. Int. J. Innov. Res. Adv. Eng. 2016, 3, 23492763. [Google Scholar]
- Arslan, A.; Halelfadl, S.; Mare, T.; Estelle, P.; Doner, N.; Mohd-Ghazali, N. Experimental investigation of a microchannel heat sink performance using aqueous carbon nanotubes based nanofluid. In Proceedings of the 5th International Conference on Applied Energy, Beijing, China, 8–11 October 2016. [Google Scholar]
- Thansekhar, M.R.; Anbumeenakshi, C. Experimental investigation of thermal performance of microchannel heat sink with nanofluids Al2O3/Water and SiO2/Water. Exp. Tech. 2017, 41, 399–406. [Google Scholar] [CrossRef]
- Manay, E.; Sahin, B. Heat transfer and pressure drop of nanofluids in a microchannel heat sink. Heat Transf. Eng. 2017, 38, 510–522. [Google Scholar] [CrossRef]
- Esfe, M.H.; Afrand, M.; Karimipour, A.; Yanb, W.M.; Sina, N. An experimental study on thermal conductivity of MgO nanoparticles suspended in a binary mixture of water and ethylene glycol. Int. Commun. Heat Mass Transf. 2015, 67, 173–175. [Google Scholar] [CrossRef]
- Usri, N.A.; Azmi, W.H.; Mamat, R.; Hamid, K.A.; Najafi, G. Thermal conductivity enhancement of Al2O3 nanofluid in ethylene glycol and water mixture. Energy Procedia 2015, 79, 397–402. [Google Scholar] [CrossRef]
- DOW. Engineering and Operating Guide for DOWTHERM SR-1 and DOWTHERM 4000 Inhibited Ethylene Glycol-based Heat Transfer Fluids. North Amerca-USA and Canada. 2008, pp. 1–44. Available online: https://www.dow.com/en-us (accessed on 15 April 2019).
- Alfaryjat, A.A.; Mohammed, H.A.; Adam, N.M.; Stanciu, D.; Dobrovicescua, A. Numerical investigation of heat transfer enhancement using various nanofluids in hexagonal microchannel heat sink. Therm. Sci. Eng. Prog. 2018, 5, 252–262. [Google Scholar] [CrossRef]
- Available online: www.asus.com (accessed on 15 April 2019).
- Intel. Intel® Celeron® Dual-Core Processor, Thermal and Mechanical Design Guidelines; Intel: Santa Clara, CA, USA, 2017. [Google Scholar]
- Rafati, M.; Hamidi, A.A.; Niaser, M.S. Application of nanofluids in computer cooling systems (heat transfer performance of nanofluids). Appl. Therm. Eng. 2012, 45, 9–14. [Google Scholar] [CrossRef]
- Wu, X.; Wu, H.; Cheng, P. Pressure drop and heat transfer of Al2O3-H2O nanofluids through silicon microchannels. J. Micromech. Microeng. 2009, 19, 105020. [Google Scholar] [CrossRef]
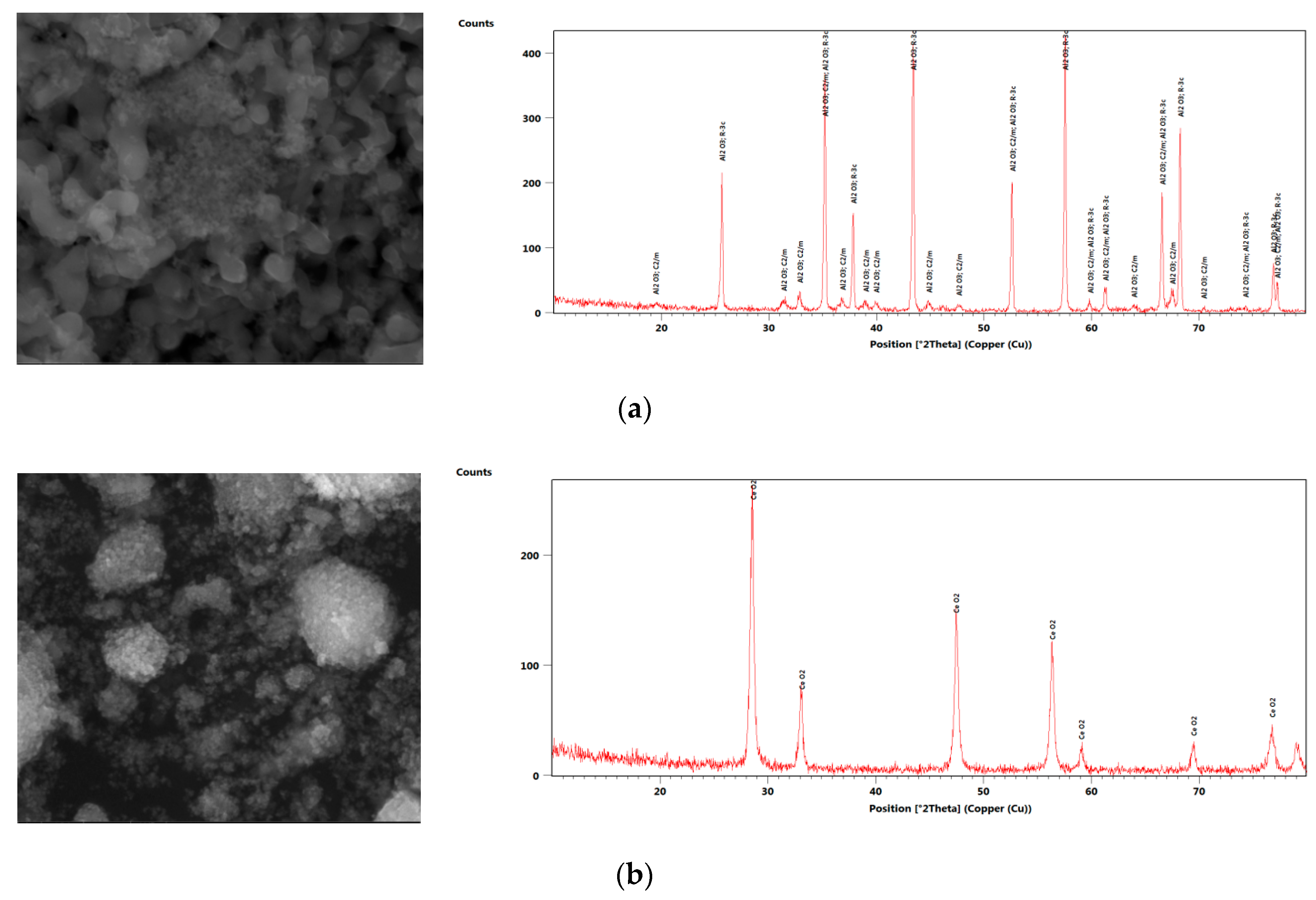
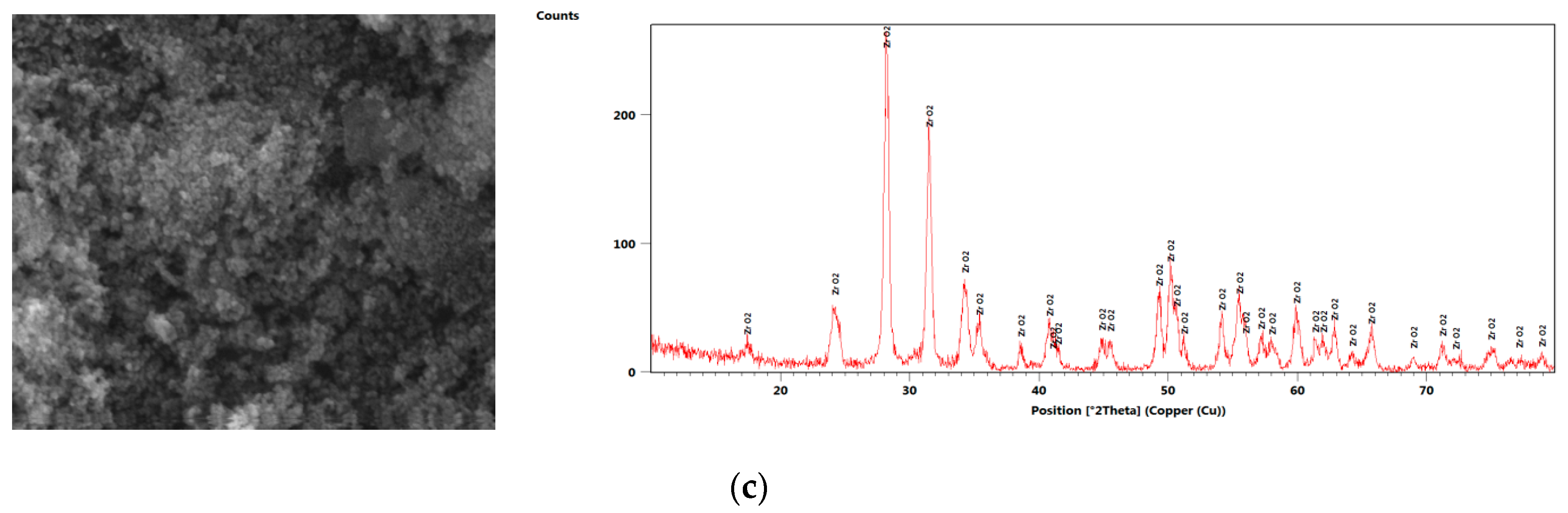
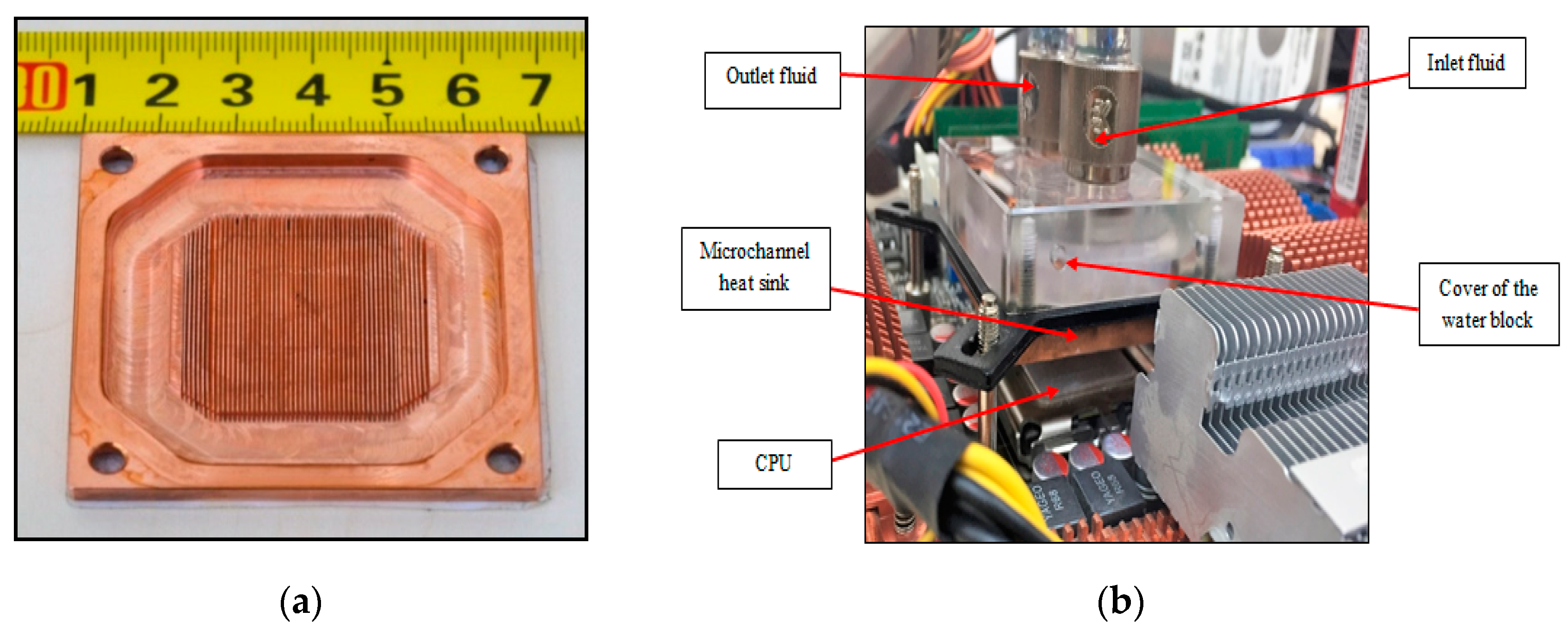
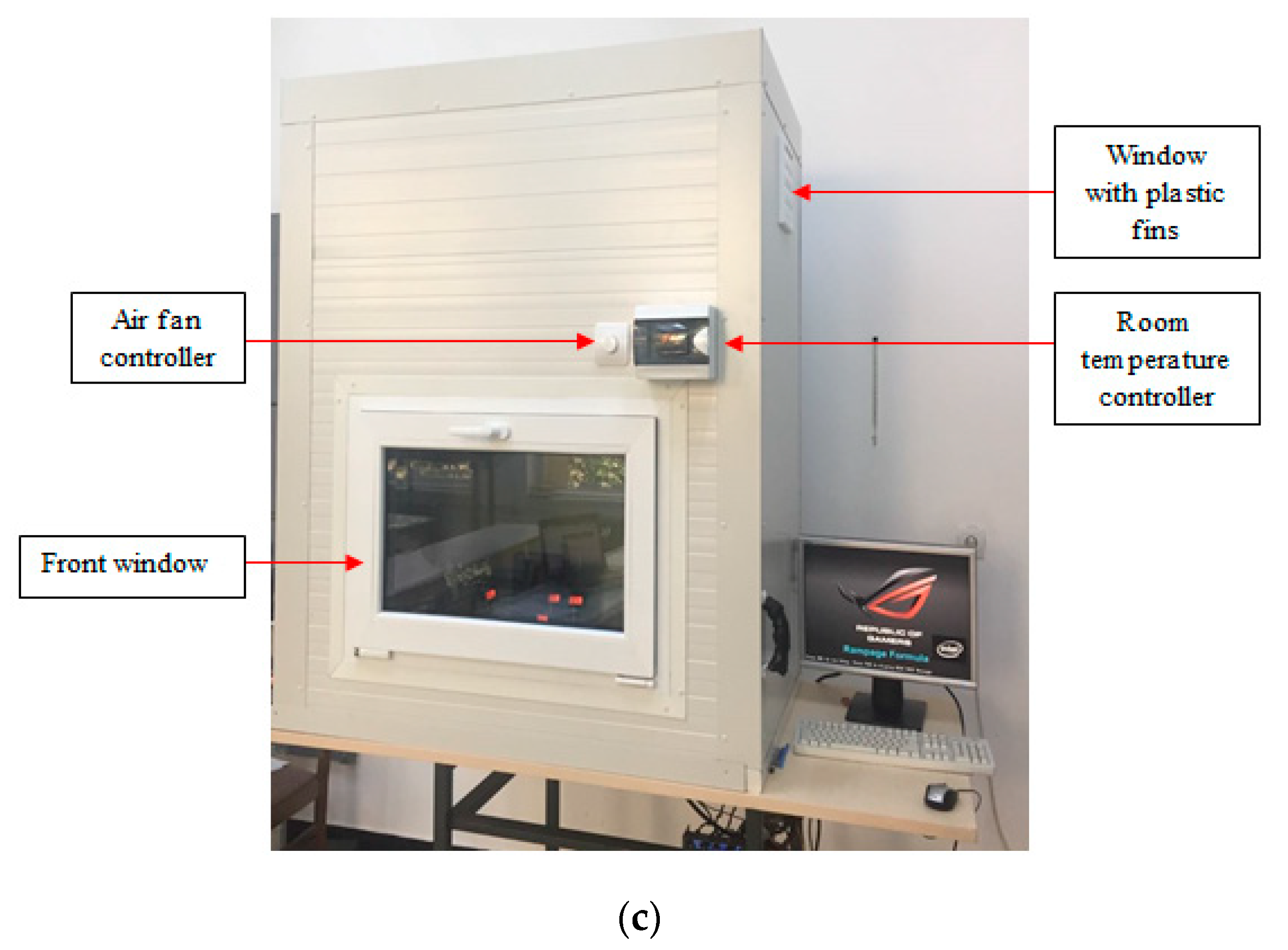

| Author | N.P Type | N.P Size (nm) | Base Fluid | V.F % | MCHS Enhancement |
|---|---|---|---|---|---|
| Nguyen et al. [35] | Al2O3 | 47 | Water | 0.69 to 4.5 |
|
| Chein and Chuang [36] | CuO | 20 to 80 | Water | 0.2 to 0.4 |
|
| Ho et al. [37] | Al2O3 | 33 | Water | 0 to 2 |
|
| Korpyś et al. [38] | CuO | 30 to 50 | Water | 0.0086 to 0.0225 |
|
| Nitiapiruk et al. [39] | TiO2 | --- | Water | 0.5, 1 2 |
|
| Rimbault et al. [40] | CuO | 29 | Water | 0.24, 1.03, 4.5 |
|
| Nazari et al. [41] | Al2O3 CNT | 40 | Water EG | 0.1, 0.25, 0.5 |
|
| Sivakumar et al. [42] | Al2O3 CuO | 15 | Water EG | 0.01 to 0.3 |
|
| Singh and Kumar [43] | Al2O3 | --- | Water | 1 to 3 |
|
| Arslan et al. [44] | CNT | --- | Water | 0.01 |
|
| Thansekhar and Anbumeenakshi [45] | Al2O3 SiO2 | 43 | Water | 0.1, 0.25 |
|
| Manay and Sahin [46] | TiO2 | 25 | Water | 0.25, 0.5, 1, 1.5, 2 |
|
| Nanoparticle | Purity | Diameter | Density | Shape | Used Nanoparticles Concentrations (%) |
|---|---|---|---|---|---|
| CeO2 | 99.9% | <50 nm | 7.22 g/cm3 | spherical | 0.5, 1, 2 |
| Al2O3 | 99.9% | <50 nm | 3.97 g/cm3 | Nearly spherical | 0.5, 1, 2 |
| ZrO2 | 99.9% | <50 nm | 5.6 g/cm3 | Nearly spherical | 0.5, 1, 2 |
| Working Fluid | Concentration % | K (W/m·K) | Cp (J/(kg·K)) | µ (Ns/m2) | |
|---|---|---|---|---|---|
| Base fluid | 0 | 0.498 | 3828 | 1029 | 0.00147 |
| CeO2 EG/DW | 0.5 | 0.527 | 3713 | 1057 | 0.00159 |
| 1 | 0.535 | 3605 | 1089 | 0.00173 | |
| 2 | 0.547 | 3406 | 1152 | 0.00188 | |
| Al2O3 EG/DW | 0.5 | 0.525 | 3771 | 1040 | 0.00153 |
| 1 | 0.532 | 3717 | 1058 | 0.00166 | |
| 2 | 0.545 | 3612 | 1086 | 0.00178 | |
| ZrO2 EG/DW | 0.5 | 0.503 | 3737 | 1047 | 0.00158 |
| 1 | 0.509 | 3650 | 1076 | 0.00171 | |
| 2 | 0.518 | 3487 | 1119 | 0.00187 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaryjat, A.; Miron, L.; Pop, H.; Apostol, V.; Stefanescu, M.-F.; Dobrovicescu, A. Experimental Investigation of Thermal and Pressure Performance in Computer Cooling Systems Using Different Types of Nanofluids. Nanomaterials 2019, 9, 1231. https://doi.org/10.3390/nano9091231
Alfaryjat A, Miron L, Pop H, Apostol V, Stefanescu M-F, Dobrovicescu A. Experimental Investigation of Thermal and Pressure Performance in Computer Cooling Systems Using Different Types of Nanofluids. Nanomaterials. 2019; 9(9):1231. https://doi.org/10.3390/nano9091231
Chicago/Turabian StyleAlfaryjat, Altayyeb, Lucian Miron, Horatiu Pop, Valentin Apostol, Mariana-Florentina Stefanescu, and Alexandru Dobrovicescu. 2019. "Experimental Investigation of Thermal and Pressure Performance in Computer Cooling Systems Using Different Types of Nanofluids" Nanomaterials 9, no. 9: 1231. https://doi.org/10.3390/nano9091231






