3.1. TiO2 Nanocrystals (TNCs)—Dots, Rods, and Plates
Diethylene glycol (DEG) stabilized titania nanodots (TNDs) were acquired commercially as yellowish granular material, which was dispersible in water. For the spin-coating fabrication of TND films (see below) it was necessary to transfer the particles into chloroform. To this end the original DEG ligands were, at least to some extent, exchanged by oleylamine (OLAM). The ligand exchange was confirmed by an elemental analysis (
Supplementary Materials, Tables S1 and S2), revealing an N/C ratio of ~1/21. This finding suggests a ~1.6 fold molar excess of OLAM over residual DEG. Using thermogravimetric analysis (TGA) a total organic mass fraction of ~40% was measured (
Supplementary Materials, Figure S3). Assuming a 0.2 nm
2 molecular footprint of OLAM [
31] and a spherical particle shape with a diameter of 5 nm, the total amount of OLAM corresponded roughly to a monolayer coverage of the TNDs’ surface (
Supplementary Materials, Figure S4). However, a precise determination of the particle size via transmission electron microscopy (TEM) was difficult because the TNDs aggregated on the TEM substrates and occasionally observed isolated nanocrystals showed only faint imaging contrast. The crystalline domains, as seen in the TEM image in
Figure 1A, indicated particle diameters roughly in the range from 3 to 7 nm, which is in agreement with the specifications provided by the manufacturer (7 ± 3 nm). Powder XRD measurements were consistent with the anatase polymorph (
Supplementary Materials, Figure S5). The crystalline domain sizes were determined using the widths of the (101) and (200) diffraction peaks according to the Scherrer equation [
32]. In agreement with the TEM analysis this method provided crystalline domain sizes of 5–6 nm (
Table S4).
Titania nanorods (TNRs) were synthesized following the seeded growth approach of Gordon et al. [
29]. As the procedure requires the addition of OLAC as oxygen source and an excess of OLAM as shape-directing agent, this method provided TNRs, which were stabilized by a mixture of OLAM and OLAC. Elemental analysis revealed an N/C ratio of ~1/32, suggesting an OLAM/OLAC molar ratio of ~1.3/1.0 (
Supplementary Materials, Table S3). As determined by TGA, the total organic mass fraction was ~25%. Assuming a footprint of 0.2 nm
2 for both, OLAM [
30] and OLAC [
33], suggests that the amount of ligands within the final TNR stock solution corresponded to roughly half a monolayer coverage of the nanorods’ surface (
Supplementary Materials, Figure S4). TEM characterization revealed an average rod length of 29.3 ± 3.7 nm with an average diameter of 3.4 ± 0.4 nm. A representative TEM image is shown in
Figure 1B. Powder XRD measurements revealed that brookite was the dominant crystalline phase, while a small amount of anatase phase was still present, in agreement with literature [
28,
29] (
Supplementary Materials, Figure S5).
Titania nanoplates (TNPs) were synthesized using the same approach as for the TNR synthesis, with minor modifications [
29]. Here, titanium(IV)tetrafluoride was used as a precursor. Since fluoride ions bind strongly to the {001} facets of the anatase polymorph the crystal growth in these directions is inhibited. Thus, resulting particles have a plate-shaped morphology with a quadratic base area. TEM analysis revealed an average edge length of 33.6 ± 4.2 nm and a plate thickness of ~6 nm. A representative TEM image is shown in
Figure 1C. Powder XRD measurements confirmed the anatase phase (
Supplementary Materials, Figure S5). As in the case of the TNR synthesis, OLAC was used as oxygen source and a huge excess of OLAM was added to the reaction mixture as an additional shape-directing agent. Accordingly, the synthesis provided OLAM/OLAC-stabilized TNPs. Elemental analysis revealed an N/C ratio of 1/25, suggesting an OLAM/OLAC molar ratio of ~2.7/1.0 (
Supplementary Materials, Table S3). As determined by TGA, the total organic mass fraction was ~25%, suggesting that the amount of OLAM/OLAC ligands within the final TNP stock solution corresponded to roughly a monolayer coverage of the nanoplates’ surface (
Supplementary Materials, Figure S4).
3.2. Layer-by-Layer (LbL) Preparation and Characterization of Substrate Supported TNC Films
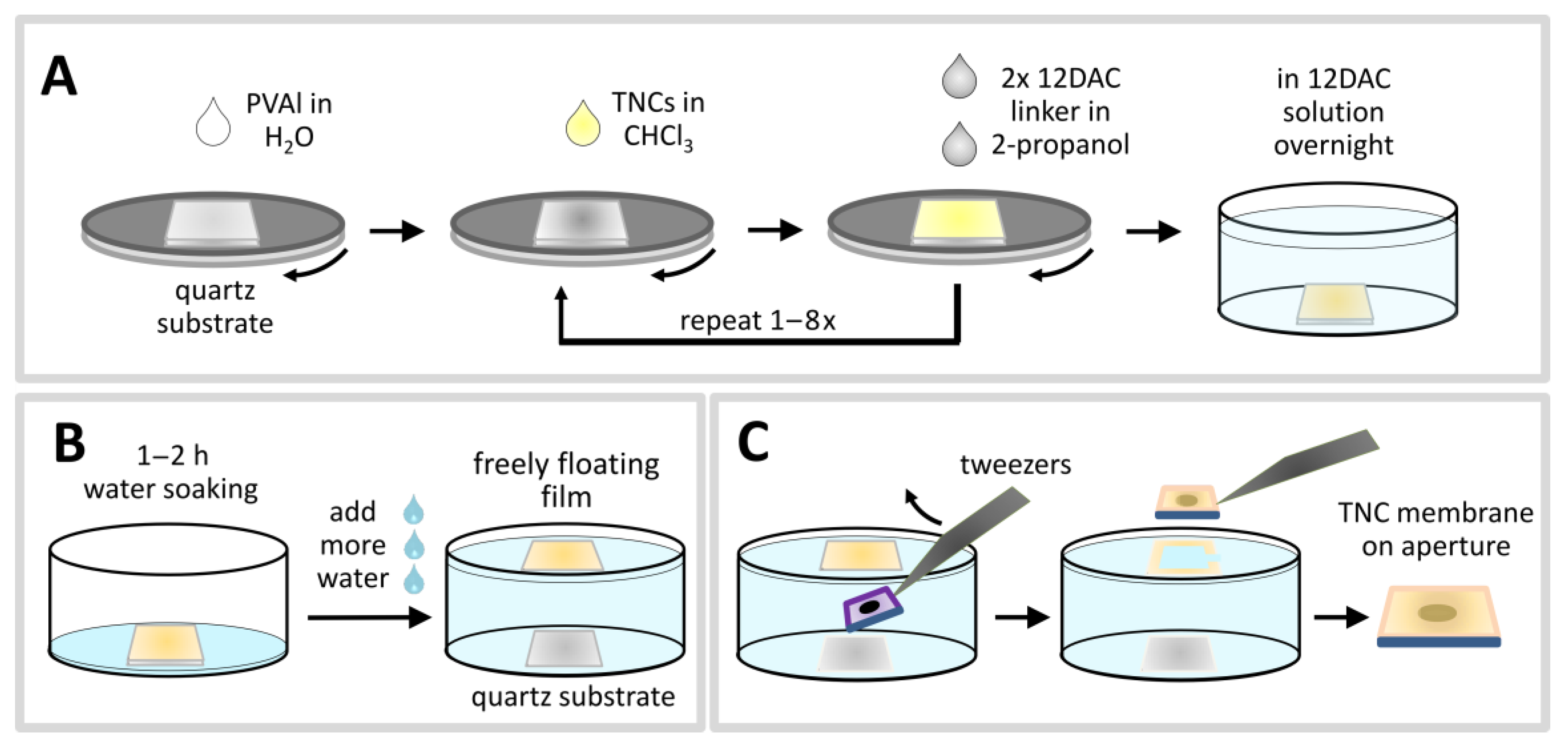
For the layer-by-layer (LbL) preparation of TNC films freshly cleaned quartz substrates were first spin-coated with polyvinyl alcohol (PVAl). The water-soluble polymer formed a smooth homogeneous layer with a thickness of 100 to 200 nm. This film served as sacrificial layer to enable the preparation of freestanding TNC membranes, as detailed below (
Scheme 1). 1,12-dodecanedioic acid (12DAC) and the TNCs were then deposited alternatingly via spin-coating onto the PVAl layer.
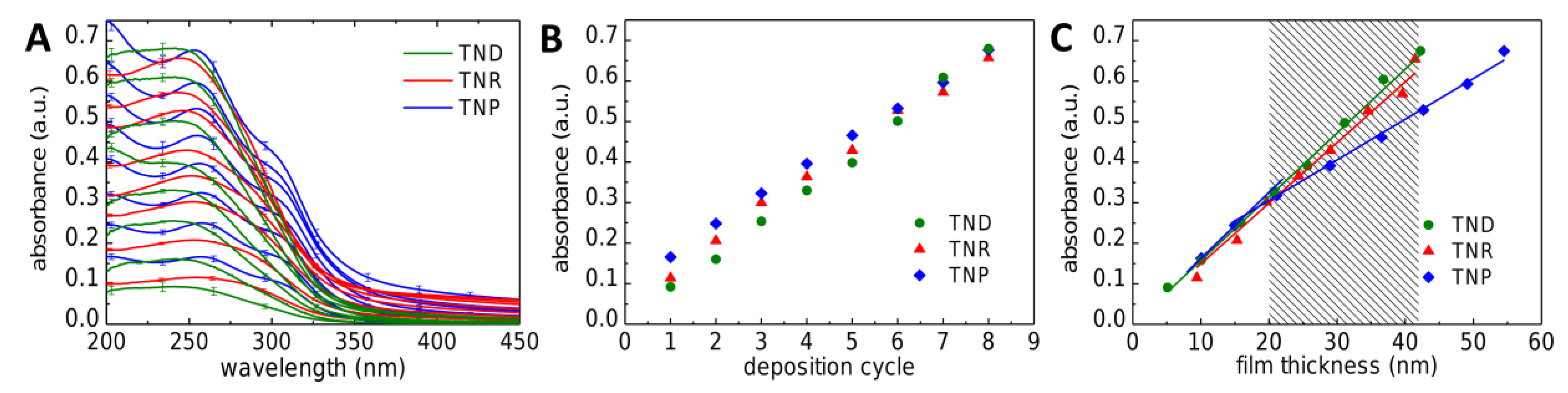
Scheme 1A depicts the whole spin-coating process. Representative absorbance spectra of TND, TNR, and TNP films recorded after each completed deposition cycle are shown in
Figure 2A. The TND and TNR films showed a broad absorbance band below ~350 nm, with a maximum developing at ~250 nm for the thicker TNR films. Similar absorbance spectra were reported for thin titania films prepared by a sol–gel process [
34]. The TNP films featured a more structured absorbance spectrum with a maximum at ~255 nm and a pronounced shoulder at ~305 nm, similar to thin TiO
2 films prepared via chemical vapor deposition (CVD) [
35]. Plotting the absorbance at 250 nm against the number of deposition cycles (
Figure 2B) confirmed the well-controlled increase in film thickness with each deposition cycle. The actual thickness of the TNC films was determined by AFM after transferring them onto silicon substrates (see below).
Figure 2C shows the absorbance (at 250 nm) plotted vs. film thickness. Obeying Lambert’s law the absorbance of the TND and TNR films increased linearly with increasing thickness, revealing that the optical density did not change with the number of deposition cycles. The linear fits to the data returned decadic attenuation coefficients of 1.58 × 10
5 cm
−1 and 1.50 × 10
5 cm
−1 for the TND and TNR films, respectively. In case of the TNP film, the absorbance increased more steeply with the first two deposition cycles (decadic attenuation coefficient: 1.63 × 10
5 cm
−1) and then continued to increase linearly with a significantly smaller slope (1.00 × 10
5 cm
−1). This finding suggests that the volume fraction of titania was significantly lower within the upper layers than within the first two layers deposited. Furthermore, comparing the attenuation coefficients to those of the other two TNC films suggests that the volume fraction of titania within the upper layers of the TNP film was significantly lower than that of the TND or TNR films (provided that the differently shaped titania nanocrystals had similar volume-normalized extinction coefficients). This finding suggests that during the first two deposition cycles the nanoplates aligned with the substrate’s surface and, therefore, formed a quite densely packed arrangement. With increasing thickness, however, the film surface became rougher and TNPs were deposited in a more randomly packed arrangement with a more porous morphology.
For comparison, Mills et al. [
35] studied the optical properties of TiO
2 thin films deposited via CVD onto quartz substrates. They measured a decadic attenuation coefficient of ~2.20 × 10
5 cm
−1 (at 250 nm). Assuming similar volume-normalized extinction coefficients for the differently shaped nanocrystals, this comparison suggests that all three TNC films had a lower volume fraction of titania than CVD deposited films, most likely due to the presence of organic material (ligands and the 12DAC linker) and a more porous structure (see structural characterization below).
For successfully performing the LbL deposition of TNC films it was mandatory to use orthogonal solvents. The TNCs were dissolved in chloroform whereas 12DAC was dissolved in 2-propanol before spin-coating. These solvents do not dissolve the sacrificial PVAl layer. Furthermore, 2-propanol does not dissolve the hydrophobic OLAM/OLAC stabilized TNCs and, thus, the TNCs are not washed off the substrate when applying the 12DAC solution. After the final spin-coating cycle, the coated substrates were immersed overnight into the 12DAC solution to enhance cross-linking of TNCs. Finally, excess 12DAC was removed by washing the films with 2-propanol. When treating the films with chloroform the obtained coatings did not re-dissolve or degrade, suggesting that the particles were successfully cross-linked. Since carboxylic acids are known to bind strongly to TiO2 surfaces, it is expected that OLAM ligands at the TNC surfaces are replaced, at least to some extent, by 12DAC, leading to a cross-linked TNC network.
In order to further characterize the TNC films they were transferred onto suitable substrates. To this end, the TNC coated quartz substrates were placed into a glass container, which was slowly filled with water to dissolve the sacrificial layer of PVAl underneath the TNC film (
Scheme 1B). This way, the TNC films detached from their original substrates and remained freely floating on the water surface. The films were then skimmed manually from the water surface using suitable substrates.
The thickness and surface roughness of the TNC films were measured by AFM (
Supplementary Materials, Figures S6 and S7). For this purpose, the films were transferred onto silicon substrates. Measured film thicknesses are displayed in
Figure 2C and correlated with the UV-absorbance at 250 nm. Typical arithmetic average surface roughnesses (
Ra) of TNC films with thicknesses of 20 to 25 nm were 4.17 nm, 1.58 nm, and 4.56 nm for the TND, TNR, and TNP films, respectively.
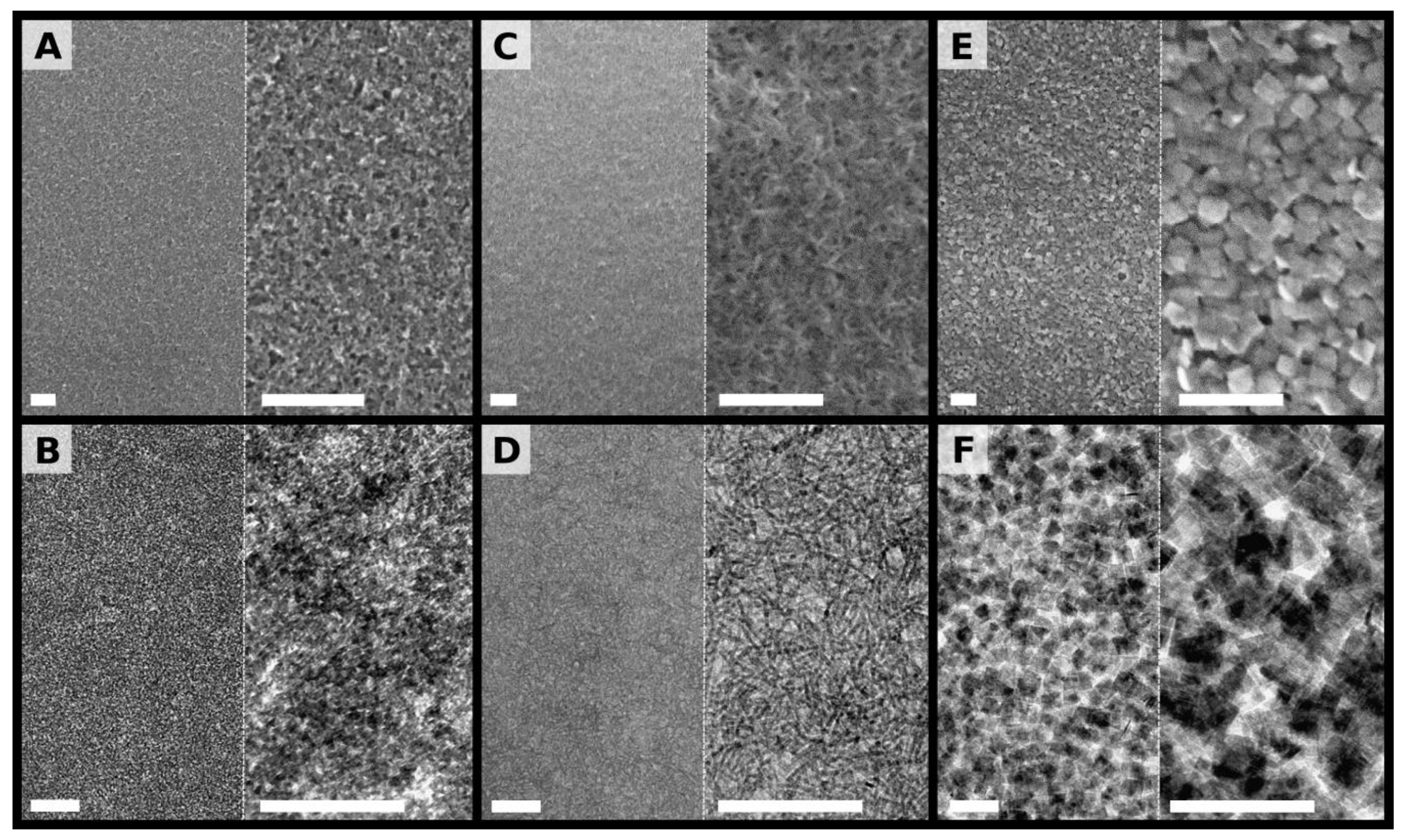
Figure 3 shows SEM and TEM images of the three TNC films transferred onto silicon substrates and carbon coated copper grids, respectively. At low magnification the TND films show a quite homogeneous coverage of the substrates (
Figure 3A, left). At higher magnification, however, a disordered microporous structure is observed (
Figure 3A, right). The TEM images shown in
Figure 3B confirm the formation of a granular film, which forms a quite uniform layer on the µm-scale, but appears disordered and rather inhomogeneous on the ~100 nm scale. This finding is consistent with relatively high surface roughness determined by AFM.
The low magnification SEM image of the TNR film (
Figure 3C, left) reveals a uniform and homogeneous coverage of the substrate, similar as the TND film. In contrast, however, a fibrous porous structure is clearly seen, especially at higher magnification (
Figure 3C, right). This fibrous structure is also observed in the TEM images shown in
Figure 3D. The TEM images indicate that the TNRs do not have any preferred orientation within the xy-plane of the film. Further, there is no indication of extended side-by-side alignment and bundling.
Due to the larger size of the TNPs, respective films have a more coarse-grained structure, as seen in the SEM images in
Figure 3E. Here, single particles can easily be recognized. On the µm-scale the film forms a uniform layer covering the substrate. At higher magnification (
Figure 3E, right panel) a highly disordered, porous structure with randomly orientated TNPs is observed. This finding is in agreement with the relatively high surface roughness determined by AFM (see above). The TEM images shown in
Figure 3F confirm the random orientation of the TNPs. There is no obvious indication for alignment or stacking of TNPs.
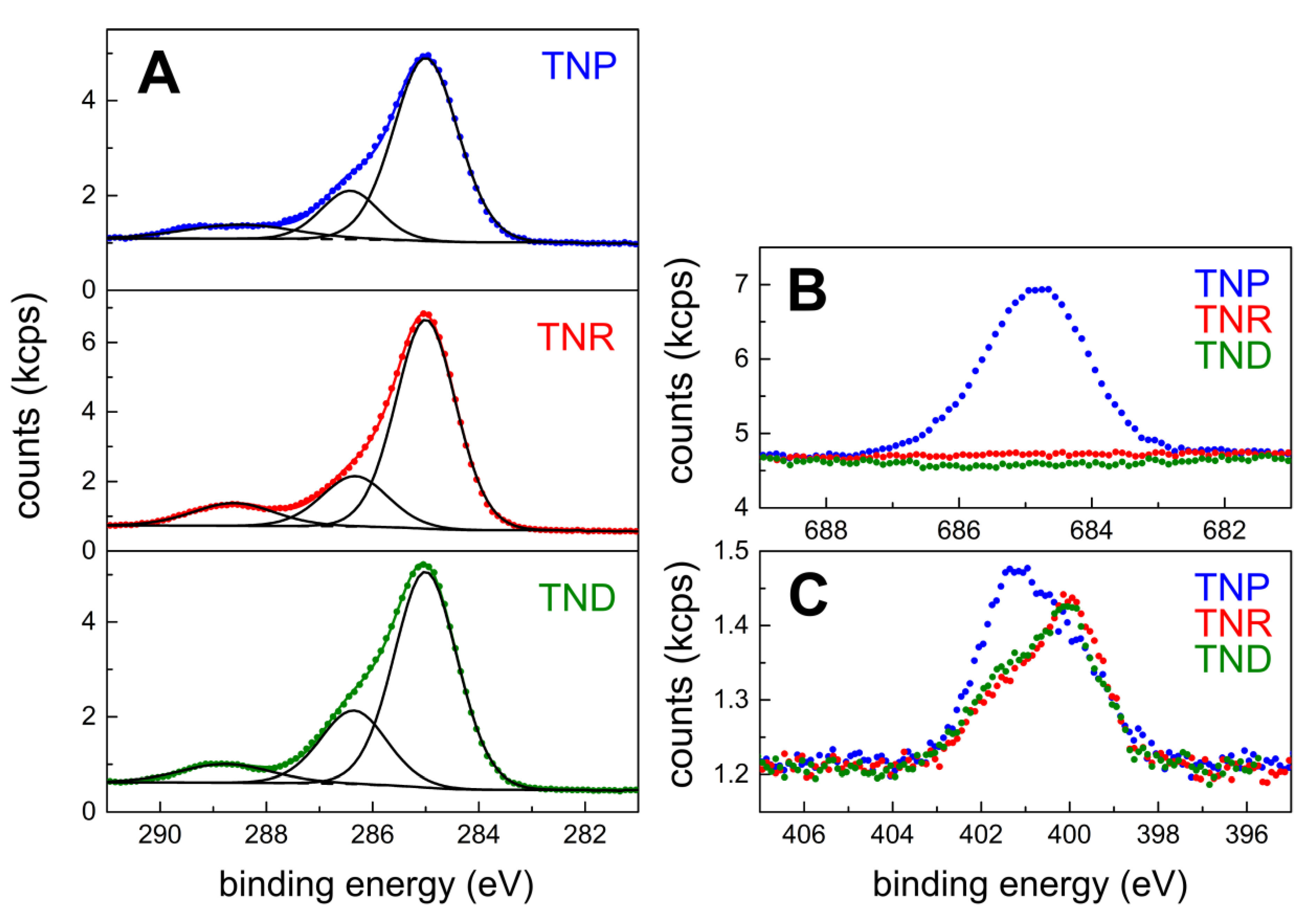
The chemical composition of the TNC films was analyzed by X-ray photoelectron spectroscopy (XPS). The signals of the C 1s core level confirmed the presence of aliphatic hydrocarbon and carboxy species. The latter are attributed to the carboxylic acid groups of residual OLAC (in the case of TNR and TNP films) and added 12DAC cross-linker, see
Figure 4A. A comparison of the XPS data with the findings of the elemental analyses of the TNC samples used for film preparation (see above) reveals that the amount of carboxylic carbon relative to total carbon increased significantly (
Supplementary Materials). Thus, these data confirm the deposition of the 12DAC cross-linker during film formation. The assignment of the carboxylic group in the C 1s region at 288.6 eV and 286.3 eV correspond to the O 1s level signals at 532.4 eV and 531.0 eV (
Supplementary Materials, Figure S8C).
As expected, the TNP sample contained significant amounts of fluorine from the TiF
4 precursor, see
Figure 4B. Fluoride is known to bind to anatase {001} facets [
29]. In the TNR and TND films fluorine could not be detected. In the case of the TNR sample, only a very faint Cl 2p signal (noise level) from the TiCl
4 precursor could be observed (
Supplementary Materials, Figure S8D).
As seen in
Figure 4C, the N 1s signal revealed the presence of at least two nitrogen species. In a previous study [
36], the signals at 399.9 eV and 401.5 eV were assigned to free and H-bonded amines, respectively. Thus, these signals may indicate the presence of residual OLAM. However, the assignment of the binding energies of N-containing species on titania surfaces is a matter of debate. For example, a signal at 399.6 eV has also been assigned to O–Ti–N linkages [
37] Furthermore, as noted previously, contamination of titania surfaces by N-containing species is unavoidable [
36]. Thus, a more specific analysis of the measured N 1s signal is difficult and beyond the scope of our current study.
3.3. Freestanding TNC Membranes and AFM-Bulge Tests
Freestanding TNC membranes were prepared by skimming the films from the water surface using silicon substrates with circular apertures (
Scheme 1C). The diameters of the apertures ranged from 32–111 µm. In initial experiments it was observed that most of the transferred TNC films were dragged into the orifice, stretched, and cracked while drying under ambient air. This failure was attributed to capillary action. Thus, in order to reduce the capillary force the hydrophilic character of the silicon substrates was decreased by treating them with triethoxy(isobutyl)silane. With this modification the majority (~75%) of the TNC films formed stable, freestanding membranes spanning the substrates’ aperture.
AFM-bulge tests were performed using the same equipment as in our previous work [
26]. In a first preliminary set of experiments, full-scan images (100 × 100 µm
2, 512 × 512 pixel
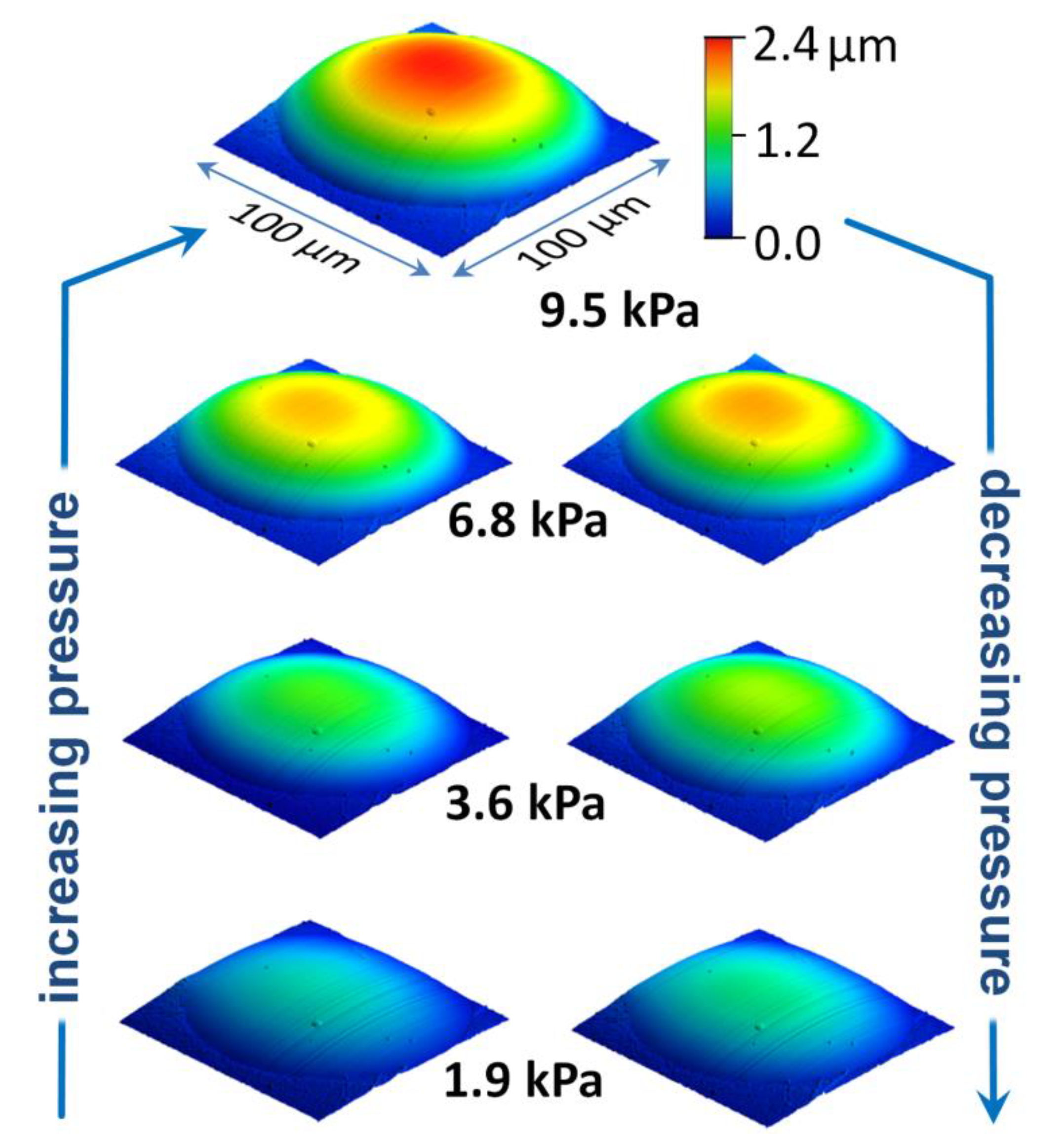
2) of the bulged TNC membranes were acquired whilst applying constant pressure differences ranging from 1 to 10 kPa. At sufficiently high overpressure a regular dome with a circular base area was formed. These images enabled a qualitative assessment of the membranes’ bulging behavior. For example, the series of images in
Figure 5 shows the reversible deflection of a TNR membrane under various pressure loads. Similar results were obtained for the TND and TNP membranes. These measurements clearly demonstrate that LbL prepared TNC films are well suited for probing their micromechanical properties via AFM-bulge tests: First, their deflection under pressure load can be controlled and measured accurately with the AFM setup. Second, under pressure load the membranes formed fairly regular bulges that can be analyzed using the spherical cap model with approximations of a thin-walled pressure vessel (see below). Third, during the AFM measurements the membranes remained fixed to the substrate along the aperture’s circumference without indications of detachment.
To characterize the membranes’ elastic properties quantitatively a full-scan AFM image of the bulge was acquired. This image was used to localize the apex of the bulge. The pressure dependent deflection of the membrane was then measured by acquiring line scans traversing the apex of the bulge. In these experiments the pressure
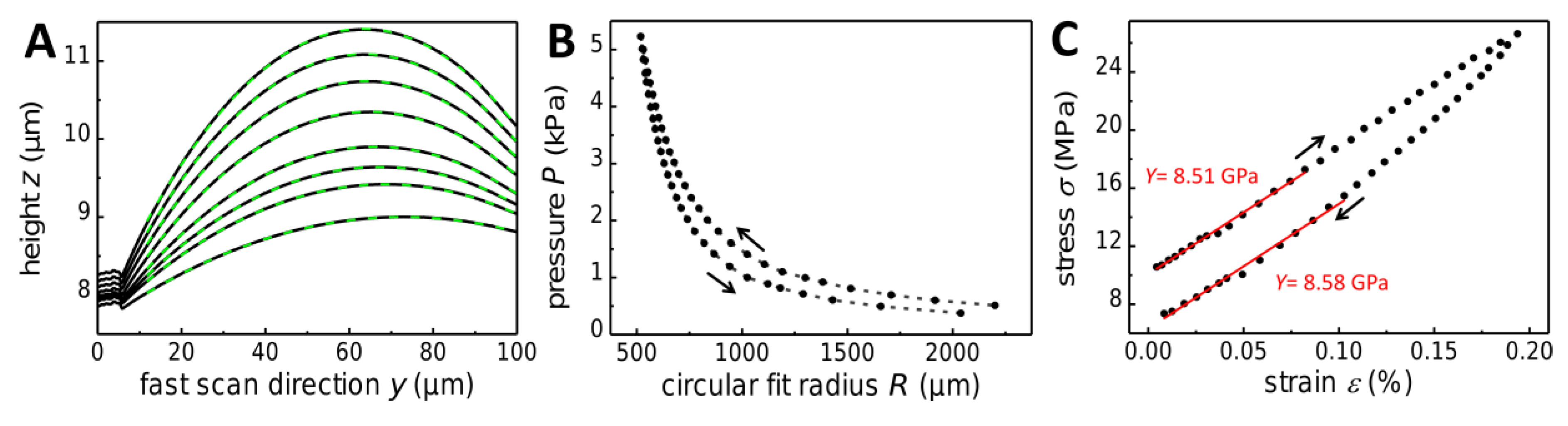
P was varied stepwise, typically within the range of ~0.1 to ~6 kPa. A representative set of line scans used to extract the membranes’ biaxial modulus is shown in
Figure 6A. According to the model of the thin-walled pressure vessel the biaxial stress
σ of the bulged membrane is given by Equation (1):
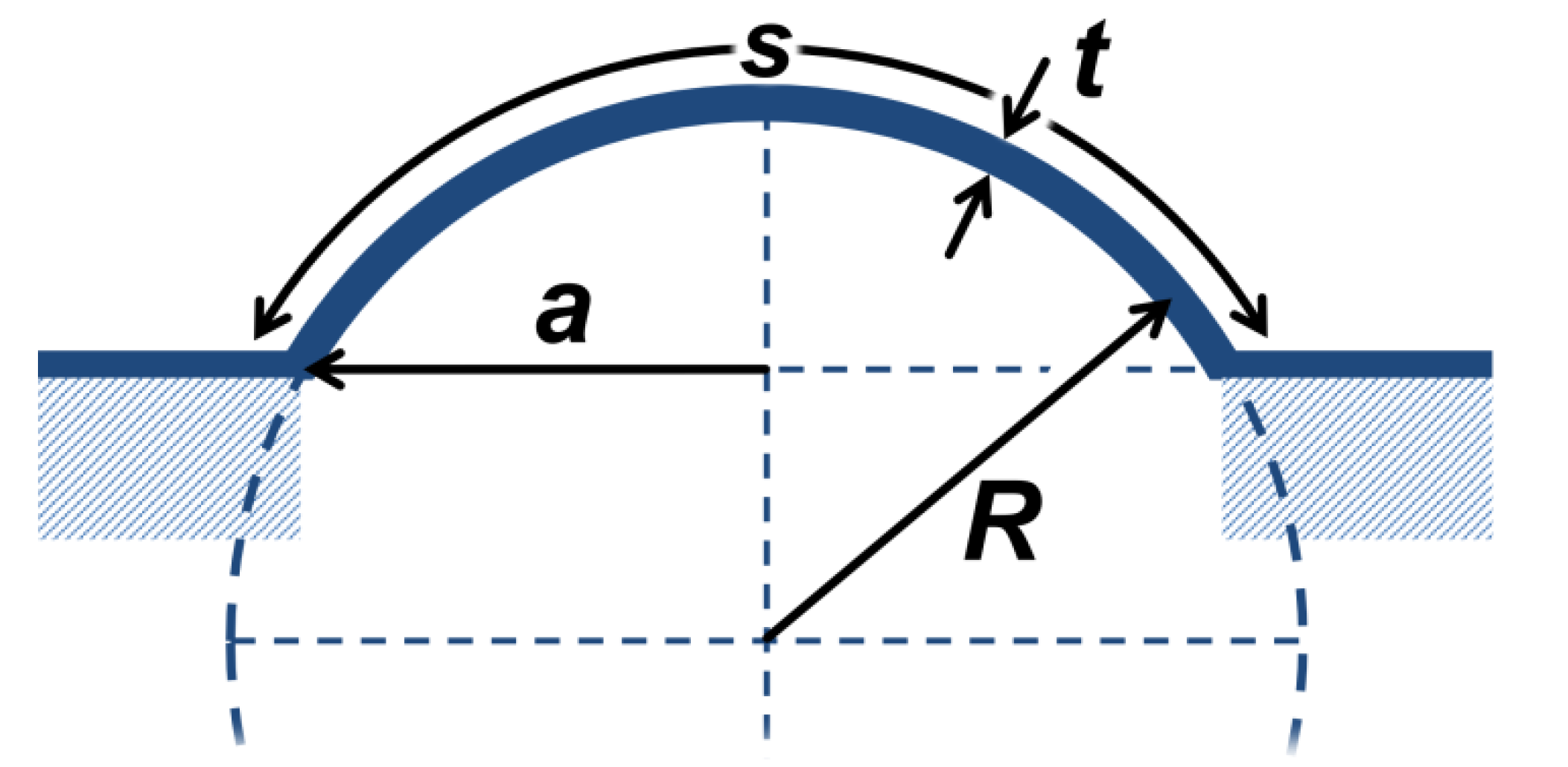
Here,
R is the radius of the pressure vessel, and
t is the thickness of the membrane, as illustrated in
Scheme 2. This model is applicable as long as
R >>
t. The thickness
t of the membranes was determined by UV/vis spectroscopy, using the calibration curves in
Figure 2C. The bulge radius
R was determined by fitting circles to the measured arc profiles, as indicated in
Figure 6A (dashed green lines). This method, which was referred to as “circular-fit method” in a previous study [
26], is fairly robust against substrate tilt.
Figure 6B shows the change of the bulge radius
R with increasing and decreasing overpressure
P.
The strain
ε of the bulged membranes was determined using Equation (2):
Here,
s is the arc length of the bulge, which is confined by the aperture’s diameter 2
a (
Scheme 2). Using the bulge radius
R and the aperture radius
a the arc length
s can be computed, according to Equation (3):
According to Equation (4) the biaxial modulus
Y and the pre-stress
can be extracted as the slope and the ordinate intercept of linear fits to the stress–strain data:
Figure 6C shows the stress–strain correlation diagram of a TNR membrane. The observed deviation of ascending data (loading) from descending data (unloading), which is also seen in the
P vs.
R plot (
Figure 6B), is due to viscoelastic deformation. As a result, the residual stress of the membrane (i.e., the stress observed at the end of the experiment at zero strain) was somewhat lower than the initial pre-stress
(which is determined by extrapolating the initial loading curve to the ordinate intercept). This observation is attributed to pure viscous creep and/or retarded elastic deformation, as suggested previously for membranes of alkanedithiol cross-linked gold nanoparticles [
26]. Within the lower strain range, both, loading and unloading curves were linear with very similar slopes. Therefore, in the following, only the slopes of linear fits to the stress–strain loading curves were considered to determine the biaxial moduli of the membranes.
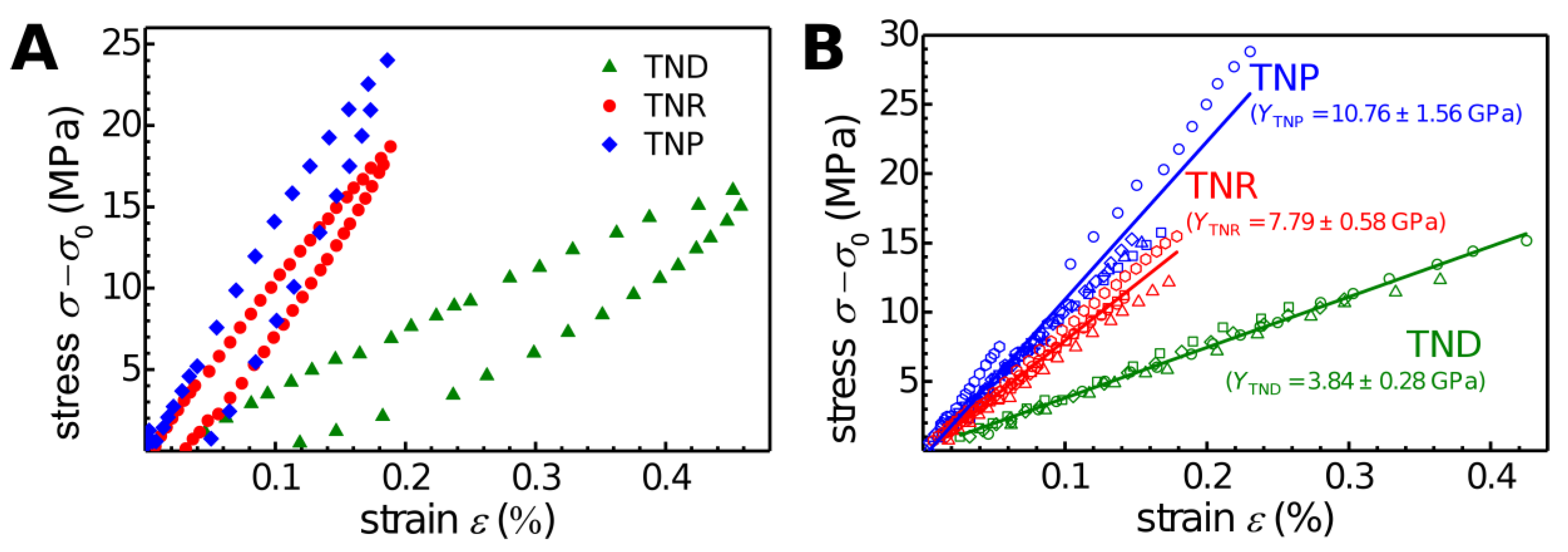
In
Figure 7A representative loading and unloading stress–strain data were plotted for the three types of TNC membranes studied. All three materials showed qualitatively similar stress–strain curves. However, the different slopes of these data clearly suggest a decrease of the membranes’ biaxial modulus in the order TNP > TNR >> TND.
In order to confirm this trend, several membranes of the different TNC materials were prepared and characterized. The stress–strain loading data of all 18 membranes studied are provided in the
Supplementary Materials (Figures S9–S11), together with values of their biaxial modulus
Y and their pre-stress
(
Supplementary Materials, Table S6). Most membranes studied were taut and had positive pre-stress values, scattering between 0.9 to 21 MPa without a clear difference between the three types of membrane materials. Only one TNP membrane was slack and had a negative pre-stress of −8.2 MPa. The scattering of the
values is most likely due to the manual procedure used to transfer the membranes onto the AFM substrates.
Figure 7B shows the (
σ–
) vs.
ε data of all TNC membranes studied. These data clearly confirm that the biaxial modulus decreased in the order TNP > TNR >> TND. Taking into account that for these bulge tests membranes with different thickness, diameter, and pre-stress were used (
Supplementary Materials, Table S6), the similarity of extracted biaxial moduli referring to the same type of material was quite remarkable and underlined the robustness of the AFM-bulge test method. The average biaxial modulus
Y of each membrane material was determined by averaging the slopes of the linear fits to the data for each individual membrane of respective material. In addition, the biaxial modulus was converted into Young’s modulus
E, according to Equation (5):
Here,
ν is the Poisson ratio, which was assumed to equal 0.33 [
10,
17]. The average values of
Y and
E are presented in
Table 1 for all three membrane materials.
The mechanical properties of composites from ligand stabilized inorganic nanoparticles depend on various interdependent parameters, including the particles’ size, shape, their spatial arrangement, the size and molecular structure of the ligands and added cross-linkers, the efficiency of ligand–ligand interactions and cross-linking, the chemical binding of the ligands, and cross-linkers to the nanoparticles’ surfaces, which may involve different crystal facets and different crystallite phases, and, in general, the overall volume fraction of stiff (inorganic) to soft (organic) matter, and the materials’ microstructure (e.g., porosity). Here, an increasing stiffness of the TNC films was observed when increasing the TNCs’ size and dimensionality from dots (0D) to rods (1D) to plates (2D). As suggested by the optical attenuation coefficients, the titania volume fractions of the TND and TNR films were similar, nevertheless the elastic modulus of the TNR films was approximately twice as high as that of the TND films. According to TEM and SEM characterization (
Figure 3), the higher stiffness of the TNR membranes is attributed to the haystack-like network of stiff TNRs with numerous inter-rod contacts cross-linked by 12DAC. The higher surface roughness of the TND films, as detected by AFM (see above), suggests also that their stiffness was reduced by the more discontinuous film morphology. When further increasing the size and dimensionality of the TNCs by using TNPs for film assembly the stiffness of the composite increased only moderately. SEM and TEM images revealed that the nanoplates were randomly orientated within the TNP films (
Figure 3E,F). Accordingly, a coarse-grained porous structure was formed and cross-linking of TNPs via the comparably small 12DAC molecules (~1 nm in length) can occur only at points of close inter-plate contacts. Therefore, the density of cross-linked inter-particle contacts was probably much lower than in the case of TND or TNR films. Thus, due to the larger size of the TNPs (~100× and ~25× larger volume than that of TNDs and TNRs, respectively) these films showed an overall increase in stiffness. However, the particle size effect was most likely compromised by less efficient cross-linking and a discontinuous film morphology. Further, cross-linking of the TNPs may have been compromised additionally by fluoride ions inserted into the {001} surfaces as well as residual OLAC/OLAM ligands, which may have hindered the efficient binding of 12DAC to the nanoparticle surface.
The elastic moduli of our disordered TNC membranes are, in general, similar to those reported for membranes of highly ordered nanocrystals with interdigitating ligand shells, as well as for membranes of gold nanocrystals covalently cross-linked by
α,
ω-alkanedithiols [
15,
17,
26] He et al. [
17] studied freestanding membranes of Fe/Fe
2O
3, Au, and CoO nanocrystals with core diameters ranging from 5 to 14 nm. Due to efficient ligand interdigitation remarkably stiff materials were formed with average elastic moduli ranging from 1 to 14 GPa, as determined by AFM force-indentation measurements. Similar elastic properties were reported by Shevchenko and co-workers [
10] and Pileni and coworkers [
11,
12,
13,
14,
15] who studied the micromechanical properties of supercrystals from densely packed nanocrystals by nanoindentation. These studies showed that the interplay of the nanocrystals’ packing density, the degree of ordering, and the efficiency of ligand interdigitation is crucial for setting the overall material’s mechanical properties.
According to previous findings, it should be possible to increase the stiffness of TNC nanocomposites by increasing the degree of cross-linking. Liaqat et al. prepared multilayered titania nanoparticle/DOPA-polymer composites [
38]. These films, which were prepared in a layer-by-layer spin-coating procedure, consisted of several bilayers, each formed by one ~46 nm thick TND layer (TND diameter: ~8 nm) and one ~4 nm thick polymer layer. After each spin-coating cycle the layers were heated to 120 °C. Since the polymer infiltrated into the nanoparticle layers a strongly cross-linked hybrid network was formed, with strong binding of the polymer’s catechol groups to the nanoparticles’ surfaces. As a result, the composite had a high elastic modulus of 17.5 ± 2.5 GPa, as determined by nanoindentation measurements.
Finally, it is interesting to compare the elastic properties of our TNC membranes with those of titania films prepared by various chemical and physical deposition techniques. For example, Roy et al. [
39] prepared nanocrystalline titania films from aqueous solution via hydrolysis of TiCl
4. Depending on the reaction conditions they obtained porous or dense films with rutile or anatase as dominating phase. Dynamic nanoindentation measurements revealed Young’s moduli of ~13.3 and ~24.5 GPa, respectively. These values are roughly one order of magnitude lower than Young’s moduli of crystalline titania layers deposited onto glass and silicon substrates by reactive pulse magnetron sputtering (170 GPa and 260 GPa for anatase and rutile, respectively) [
40]. Gaillard et al. [
41] studied titania films with different columnar microstructures prepared via physical vapor deposition (PVD). They showed that the elastic modulus decreased from 87.5 GPa to 16.5 GPa with increasing porosity and tilt angle of the columnar structure.