Thermal Flow Self-Assembled Anisotropic Chemically Derived Graphene Aerogels and Their Thermal Conductivity Enhancement
Abstract
1. Introduction
2. Materials and Methods
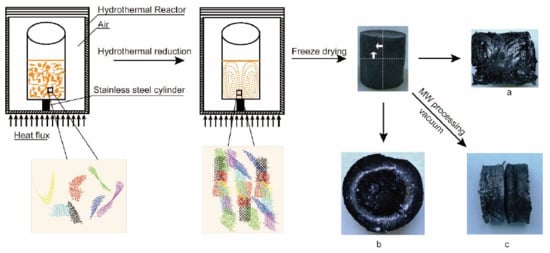
2.1. Preparation of Anisotropic Chemically Derived Graphene Aerogels (AGAs)
2.2. Performance and Structure Characterization
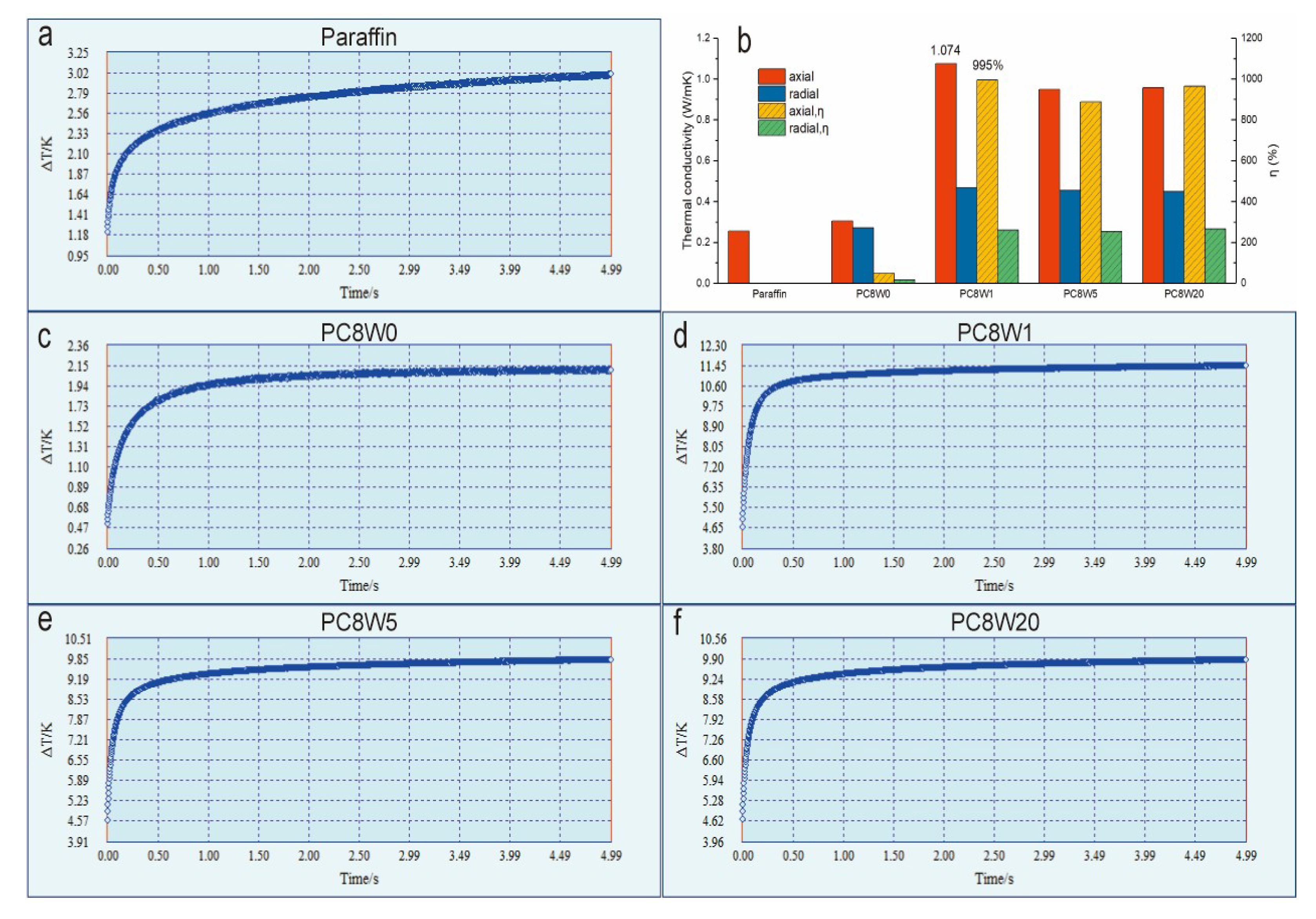
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhuang, Y.T.; Jiang, R.; Wu, D.F.; Yu, Y.L.; Wang, J.H. Selenocarrageenan-inspired hybrid graphene hydrogel as recyclable adsorbent for efficient scavenging of dyes and Hg2+ in water environment. J. Colloid Interface Sci. 2019, 540, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.W.; Wei, Y.F.; Liu, C.B.; Ma, J.H.; Liu, H.; Wei, S.D.; Deng, W.; Xiang, J.N.; Luo, S.L. Ultrafast and deep removal of arsenic in high-concentration wastewater: A superior bulk adsorbent of porous Fe2O3 nanocubes-impregnated graphene aerogel. Chemosphere 2019, 222, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Feng, P.; Chen, J.; Sun, Z.M.; Zhao, B.X. Electrically conductive hydrogels for flexible energy storage systems. Prog. Polym. Sci. 2019, 88, 220–240. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, K.; Yan, Y.; Cai, Z.H.; Lin, S.X.; Hu, X.B. Graphene Aerogels Enhanced Phase Change Materials prepared by one-pot method with high thermal conductivity and large latent energy storage. Sol. Energy Mater. Sol. Cells 2018, 185, 487–493. [Google Scholar] [CrossRef]
- Yang, J.; Qi, G.Q.; Liu, Y.; Bao, R.Y.; Liu, Z.Y.; Yang, W.; Xie, B.H.; Yang, M.B. Hybrid graphene aerogels/phase change material composites: Thermal conductivity, shape-stabilization and light-to-thermal energy storage. Carbon 2016, 100, 693–702. [Google Scholar] [CrossRef]
- Yuan, Y.; Lv, H.P.; Xu, Q.J.; Liu, H.M.; Wang, Y.G. A few-layered MoS2 nanosheets/nitrogen-doped graphene 3D aerogel as a high performance and long-term stability supercapacitor electrode. Nanoscale 2019, 11, 4318–4327. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, F.; Qiu, W.J.; Xu, Z.X.; Xu, J.G.; Wen, Y.C. General Synthesis of Uniform Three-Dimensional Metal Oxides/Reduced Graphene Oxide Aerogels by a Nucleation-Inducing Growth Strategy for High-Performance Lithium Storage. Acs Sustainable Chem. Eng. 2019, 7, 847–857. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, G.Z.; Li, F.B.; Zhang, L.; Wang, S.T.; Zhao, H.H.; Ge, Q.; Li, H.J. Large-area superelastic graphene aerogels based on a room-temperature reduction self-assembly strategy for sensing and particulate matter (PM2.5 and PM10) capture. Nanoscale 2019, 11, 10372–10380. [Google Scholar] [CrossRef]
- Xiao, Z.J.; Zhou, W.Y.; Zhang, N.; Zhang, Q.; Xia, X.G.; Gu, X.G.; Wang, Y.C.; Xie, S.S. All-Carbon Pressure Sensors with High Performance and Excellent Chemical Resistance. Small 2019, 15. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.F.; Han, S.; Yang, R.Z.; Min, P.; Yu, Z.Z. High-quality graphene aerogels for thermally conductive phase change composites with excellent shape stability. J. Mater. Chem. A 2018, 6, 5880–5886. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhou, M.; Huang, F.; Lin, T.; Wan, D. Effect of graphene aerogel on thermal behavior of phase change materials for thermal management. Sol. Energy Mater. Sol. Cells 2013, 113, 195–200. [Google Scholar] [CrossRef]
- Li, A.; Dong, C.; Dong, W.J.; Atinafu, D.G.; Gao, H.Y.; Chen, X.; Wang, G. Hierarchical 3D Reduced Graphene Porous-Carbon-Based PCMs for Superior Thermal Energy Storage Performance. Acs Appl. Mater. Interfaces 2018, 10, 32093–32101. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.F.; Han, S.; Zhang, Y.T.; Min, P.; Koratkar, N.; Yu, Z.Z. Air-dried, high-density graphene hybrid aerogels for phase change composites with exceptional thermal conductivity and shape stability. J. Mater. Chem. A 2016, 4, 18067–18074. [Google Scholar] [CrossRef]
- Xu, Y.; Fleischer, A.S.; Feng, G. Reinforcement and shape stabilization of phase-change material via graphene oxide aerogel. Carbon 2017, 114, 334–346. [Google Scholar] [CrossRef]
- Wang, X.L.; Cheng, X.M.; Li, Y.Y.; Li, G.; Xu, J. Self-assembly of three-dimensional 1-octadecanol/graphene thermal storage materials. Sol. Energy 2019, 179, 128–134. [Google Scholar] [CrossRef]
- Xu, Y.X.; Sheng, K.X.; Li, C.; Shi, G.Q. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. Acs Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H.; Baumann, T.F. Synthesis of Graphene Aerogel with High Electrical Conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef] [PubMed]
- Min, P.; Liu, J.; Li, X.F.; An, F.; Liu, P.F.; Shen, Y.X.; Koratkar, N.; Yu, Z.Z. Thermally Conductive Phase Change Composites Featuring Anisotropic Graphene Aerogels for Real-Time and Fast-Charging Solar-Thermal Energy Conversion. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Li, X.H.; Liu, P.F.; Li, X.F.; An, F.; Min, P.; Liao, K.N.; Yu, Z.Z. Vertically aligned, ultralight and highly compressive all-graphitized graphene aerogels for highly thermally conductive polymer composites. Carbon 2018, 140, 624–633. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Zhang, F.; Medarametla, S.P.; Li, H.; Zhou, C.; Lin, D. 3D Printing of Graphene Aerogels. Small 2016, 12, 1702–1708. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhães-Paniago, R.; Pimenta, M.A. General equation for the determination of the crystallite size L a of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Takai, K.; Ohki, Y.; Taomoto, A.; Suenaga, K.; Iijima, S. Structure and electronic properties of a nongraphitic disordered carbon system and its heat-treatment effects. Phys. Rev. B 2003, 67, 214202. [Google Scholar] [CrossRef]
- Garcia-Bordeje, E.; Victor-Roman, S.; Sanahuja-Parejo, O.; Benito, A.M.; Maser, W.K. Control of the microstructure and surface chemistry of graphene aerogels via pH and time manipulation by a hydrothermal method. Nanoscale 2018, 10, 3526–3539. [Google Scholar] [CrossRef] [PubMed]
- Zielke, U.; Hüttinger, K.J.; Hoffman, W.P. Surface-oxidized carbon fibers: I. Surface structure and chemistry. Carbon 1996, 34, 983–998. [Google Scholar] [CrossRef]
- Lin, Y.; Su, D. Fabrication of Nitrogen-Modified Annealed Nanodiamond with Improved Catalytic Activity. ACS Nano 2014, 8, 7823–7833. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, J.; Nanse, G.; Fioux, P.; Bagreev, A.; Broshnik, A.; Strelko, V. Chemical transformation during the carbonisation in air and the pyrolysis under argon of a vinylpyridine–divinylbenzene copolymer by X-ray photoelectron spectroscopy. Appl. Surf. Sci. 1999, 147, 153–174. [Google Scholar] [CrossRef]
- Arrigo, R.; Hävecker, M.; Wrabetz, S.; Blume, R.; Lerch, M.; McGregor, J.; Parrott, E.P.J.; Zeitler, J.A.; Gladden, L.F.; Knop-Gericke, A.; et al. Tuning the Acid/Base Properties of Nanocarbons by Functionalization via Amination. J. Am. Chem. Soc. 2010, 132, 9616–9630. [Google Scholar] [CrossRef]
- Mu, B.Y.; Li, M. Fabrication and thermal properties of tetradecanol/graphene aerogel form-stable composite phase change materials. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Xie, Y.S.; Xu, S.; Xu, Z.L.; Wu, H.C.; Deng, C.; Wang, X.W. Interface-mediated extremely low thermal conductivity of graphene aerogel. Carbon 2016, 98, 381–390. [Google Scholar] [CrossRef]
- Canc¸ado, L.G.; Pimenta, M.A.; Neves, B.R.A.; Dantas, M.S.S.; Jorio, A. Influence of the atomic structure on the Raman spectra of graphite edges. Phys. Rev. Lett. 2004, 93, 247401. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Zeng, F.; Marconnet, A.; Nguyen, S.T.; Lim, C.Y.H.; Hai, M.D. Effects of heat treatment on the thermal properties of highly nanoporous graphene aerogels using the infrared microscopy technique. Int. J. Heat Mass Transfer 2014, 76, 122–127. [Google Scholar]
- Timo, S. An electrical method for the measurement of the thermal and electrical conductivity of reduced graphene oxide nanostructures. Nanotechnology 2009, 40. [Google Scholar]
- Yue, C.W.; Feng, J.; Feng, J.Z.; Jiang, Y.G. Low-thermal-conductivity nitrogen-doped graphene aerogels for thermal insulation. Rsc Adv. 2016, 6, 9396–9401. [Google Scholar] [CrossRef]
- Tang, G.Q.; Jiang, Z.G.; Li, X.F.; Zhang, H.B.; Dasari, A.; Yu, Z.Z. Three dimensional graphene aerogels and their electrically conductive composites. Carbon 2014, 77, 592–599. [Google Scholar] [CrossRef]
- El Sachat, A.; Koenemann, F.; Menges, F.; Del Corro, E.; Garrido, J.A.; Torres, C.M.S.; Alzina, F.; Gotsmann, B. Crossover from ballistic to diffusive thermal transport in suspended graphene membranes. 2d Mater. 2019, 6. [Google Scholar] [CrossRef]
- Jung, G.S.; Yeo, J.; Tian, Z.T.; Qin, Z.; Buehler, M.J. Unusually low and density-insensitive thermal conductivity of three-dimensional gyroid graphene. Nanoscale 2017, 9, 13477–13484. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.Q.; Yang, W.B.; He, F.F.; Xie, C.Q.; Zhang, K.; Fan, J.H.; Wu, J.Y. Paraffin/carbon aerogel phase change materials with high enthalpy and thermal conductivity. Fullerenes Nanotubes Carbon Nanostruct. 2017, 25, 512–518. [Google Scholar] [CrossRef]
- Ye, S.B.; Zhang, Q.L.; Hu, D.D.; Feng, J.C. Core-shell-like structured graphene aerogel encapsulating paraffin: shape-stable phase change material for thermal energy storage. J. Mater. Chemi. A 2015, 3, 4018–4025. [Google Scholar] [CrossRef]
- Yang, J.; Qi, G.-Q.; Bao, R.-Y.; Yi, K.; Li, M.; Peng, L.; Cai, Z.; Yang, M.-B.; Wei, D.; Yang, W. Hybridizing graphene aerogel into three-dimensional graphene foam for high-performance composite phase change materials. Energ. Storage Mater. 2018, 13, 88–95. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Guo, Y.; Jiang, Z.; He, F.; Xie, C.; Fan, J.; Wu, J.; Zhang, K. Novel segregated-structure phase change materials composed of paraffin@graphene microencapsules with high latent heat and thermal conductivity. J. Mater. Sci. 2018, 53, 2566–2575. [Google Scholar] [CrossRef]
- Guo, Y.L.; Yang, W.B.; He, F.F.; Xie, C.Q.; Fan, J.H.; Wu, J.Y.; Zhang, K. Electrostatic interaction-based self-assembly of paraffin@graphene microcapsules with remarkable thermal conductivity for thermal energy storage. Fullerenes Nanotubes Carbon Nanostruct. 2019, 27, 120–127. [Google Scholar] [CrossRef]
- Cao, Q.; He, F.; Xie, C.; Fan, J.; Wu, J.; Zhang, K.; Yang, Z.; Yang, W. Paraffin-based shape-stable phase change materials with graphene/carbon nanotube three-dimensional network structure. Fullerenes, Nanotubes Carbon Nanostruct. 2019, 27, 492–497. [Google Scholar] [CrossRef]



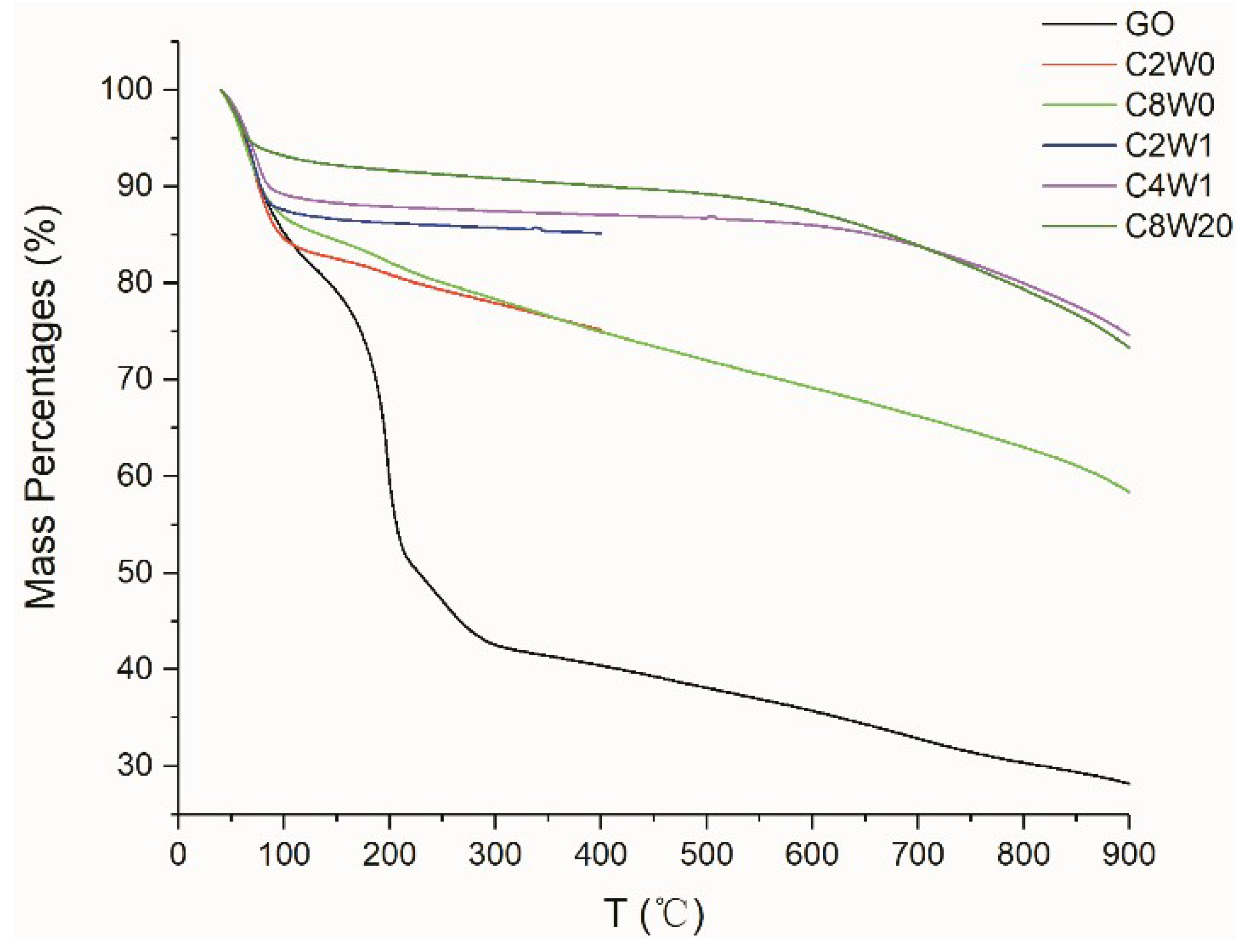
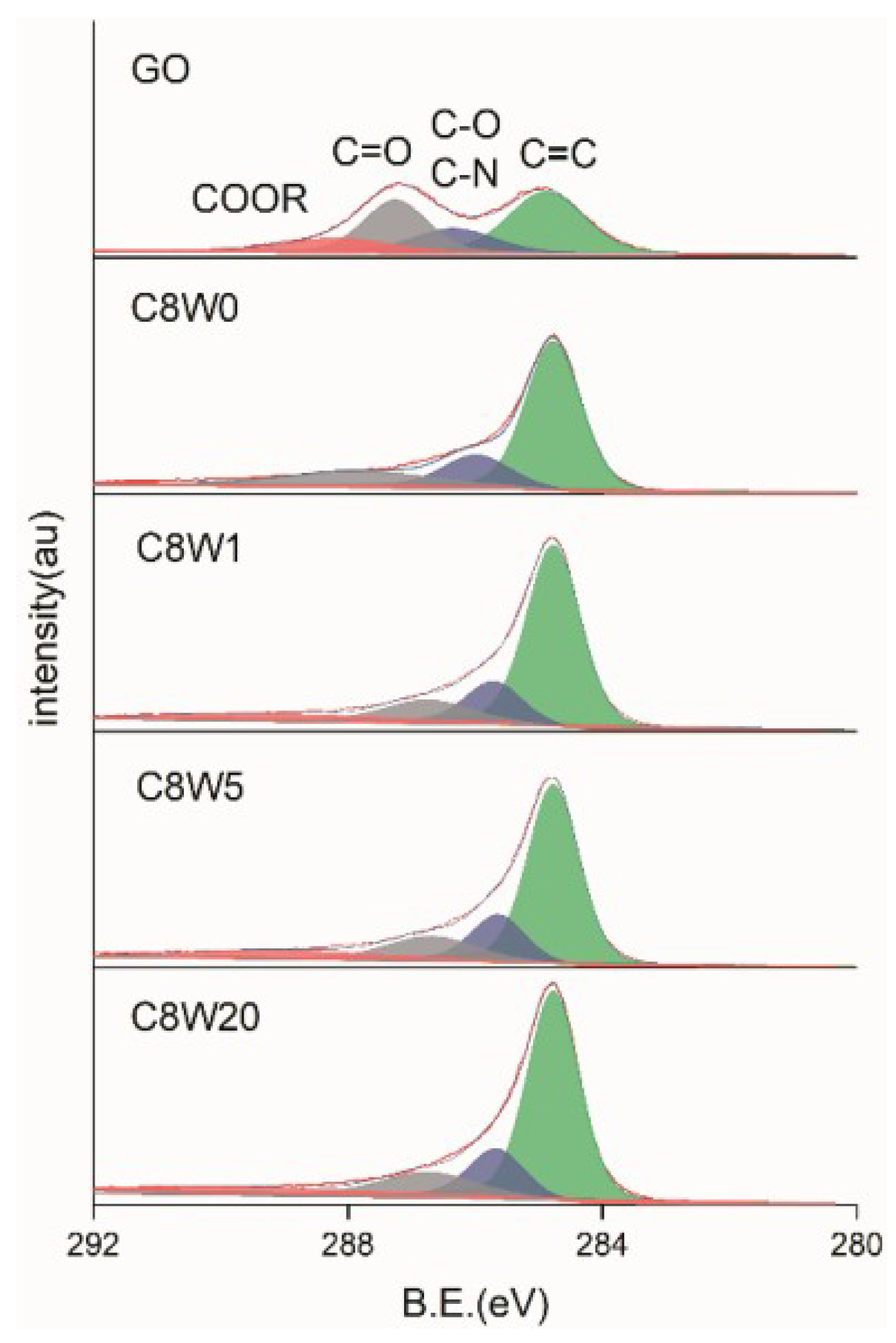
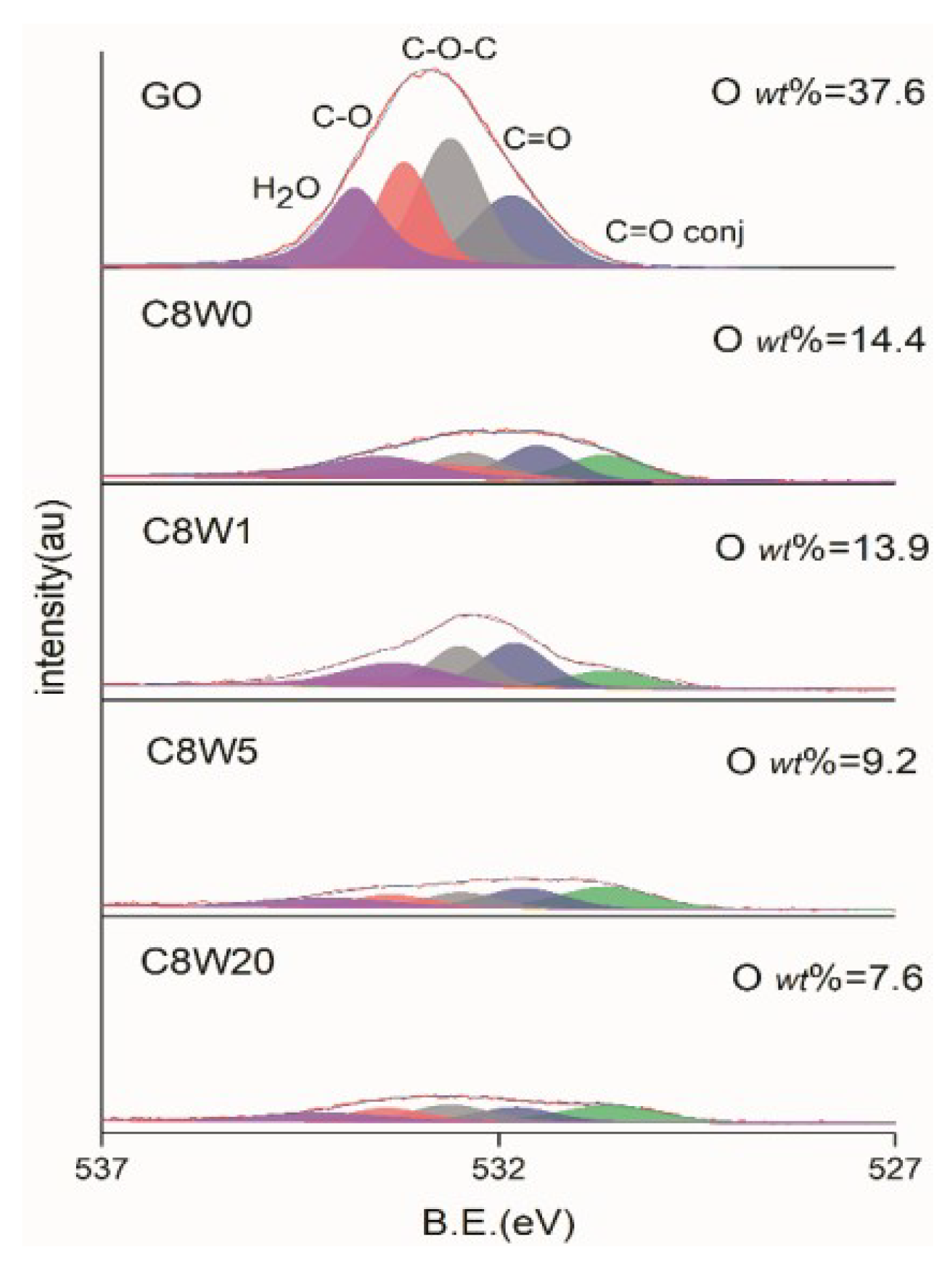
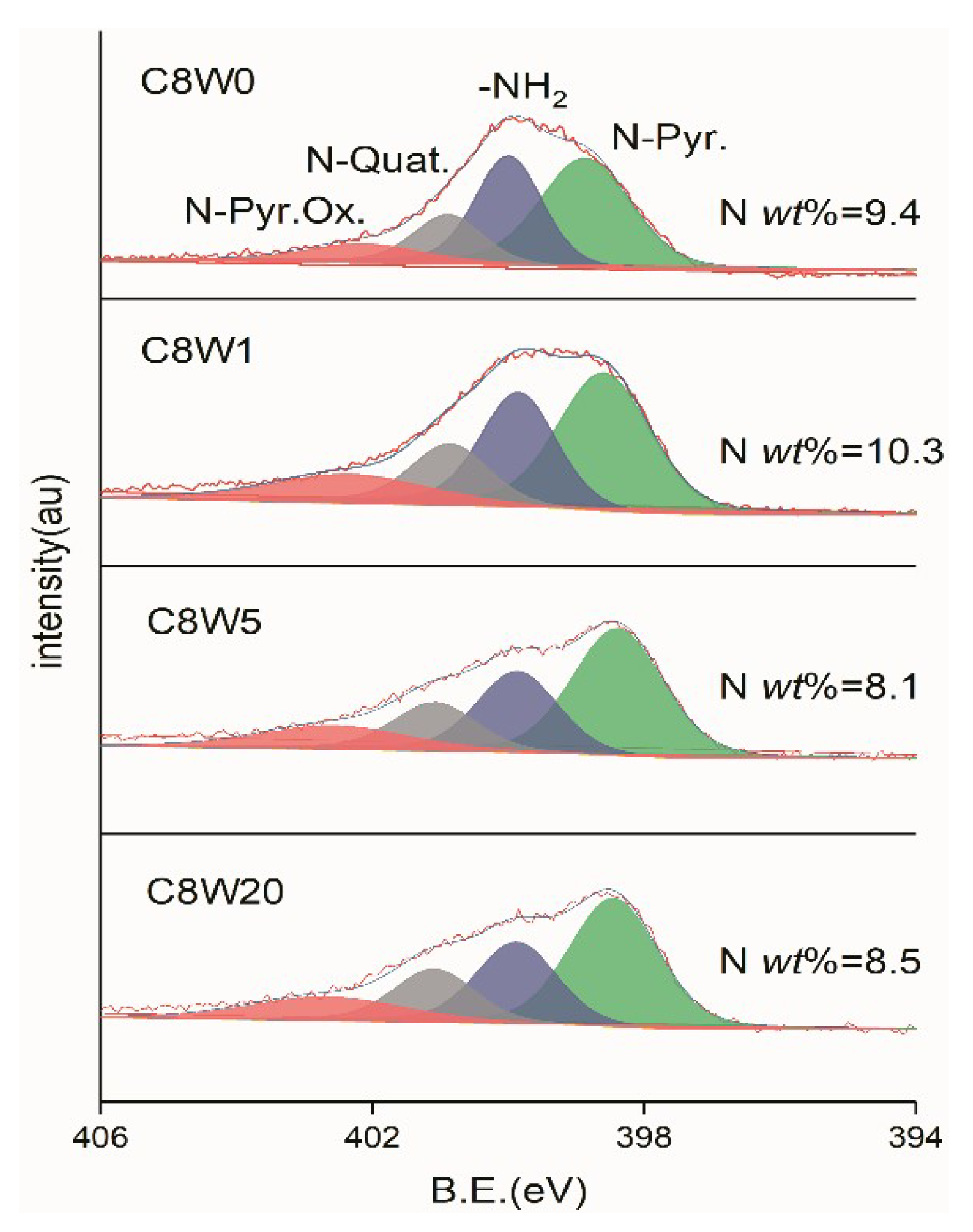
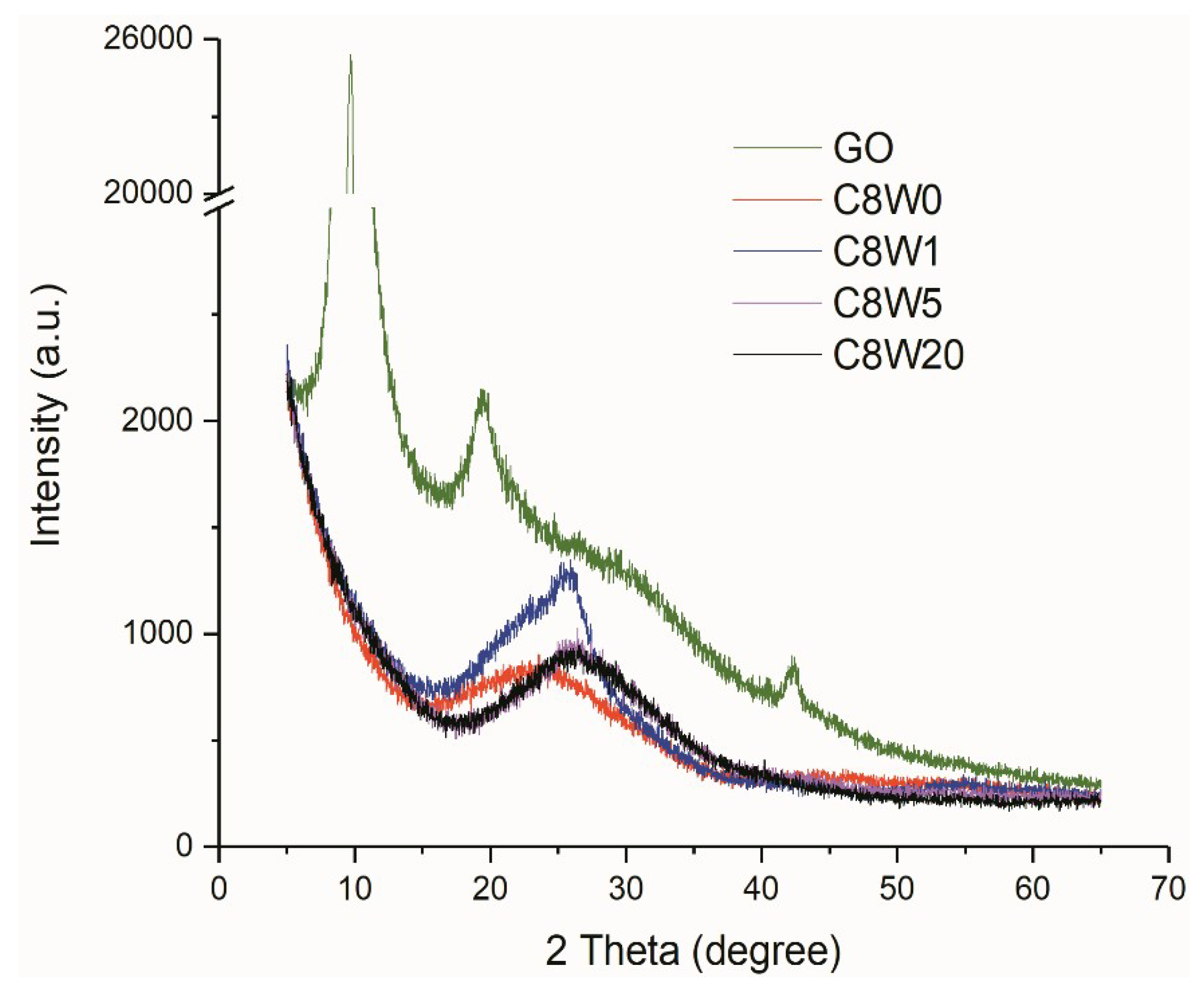
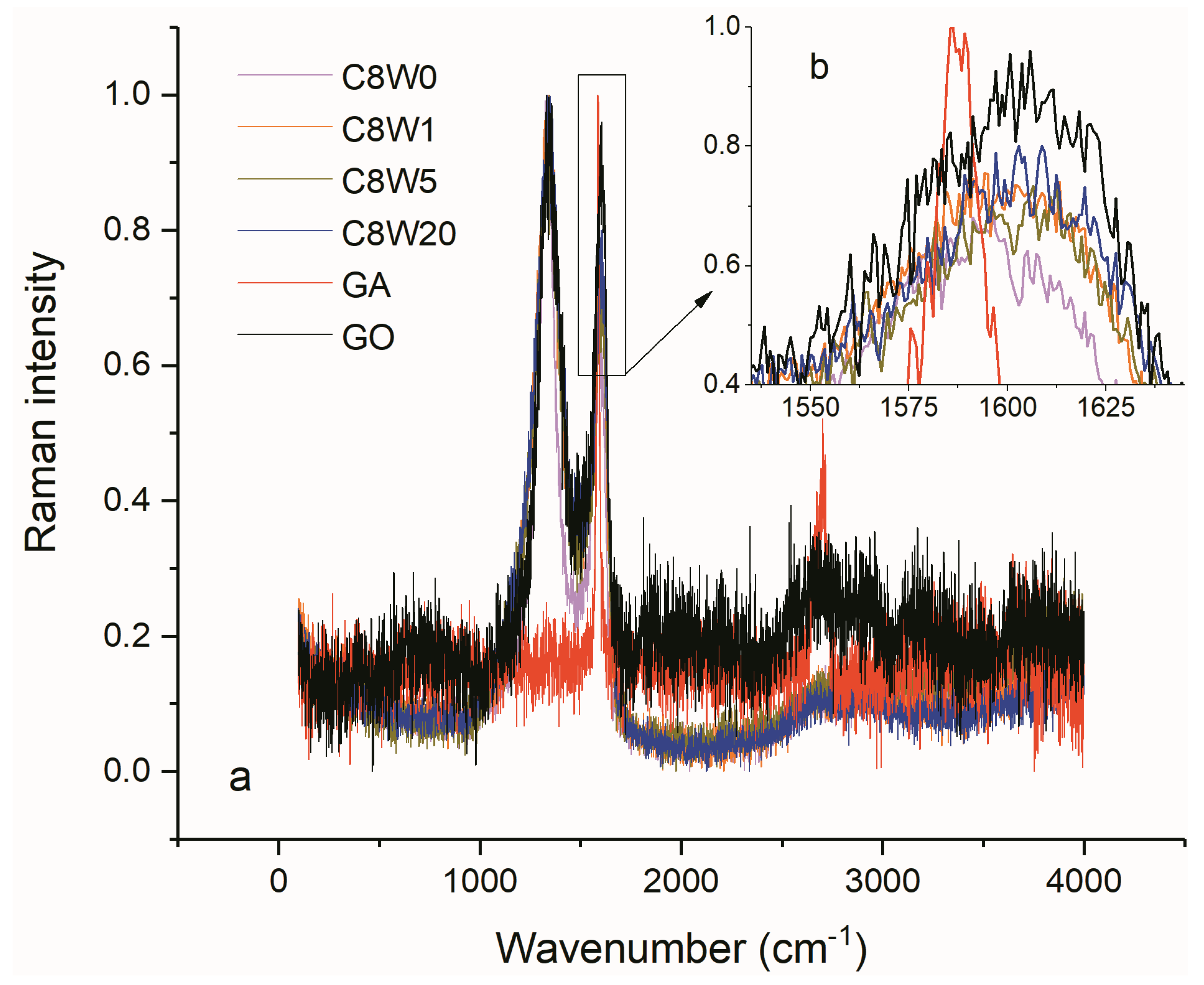
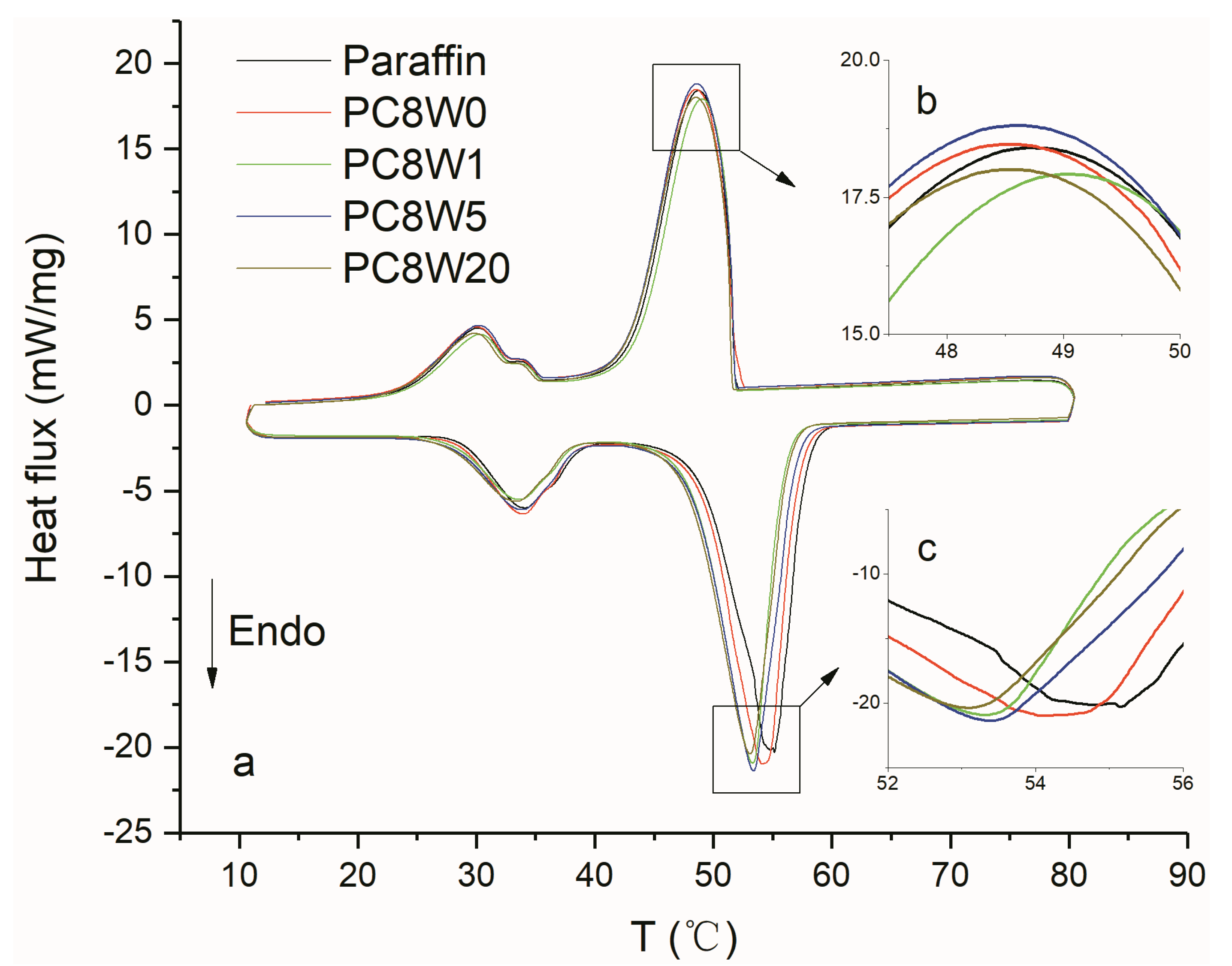
| Sample Number | GO Concentration (mg/mL) | Microwave Processing Time (min) |
|---|---|---|
| C2W0 | 2 | 0 |
| C2W1 | 2 | 1 |
| C4W1 | 4 | 1 |
| C8W0 | 8 | 0 |
| C8W1 | 8 | 1 |
| C8W5 | 8 | 5 |
| C8W20 | 8 | 20 |
| C1s | O1s | N1s | |||
|---|---|---|---|---|---|
| Species | Range(eV) | Species | Range(eV) | Species | Range(eV) |
| C=C | 284.6 | C=O,conj [28] | 530.7 | N-Pyr. | 398.5 |
| C-O,C-N | 286.3 | C=O | 531.5 | -NH2 | 400.0 |
| C=O | 287.2 | C-O-C | 532.6 | N-Quat. | 401.2 |
| COOR | 288.9 | C-O | 533.5 | N-Pyr. Ox. | 402.5 |
| H2O | 534.0 | ||||
| Samples | Lc (nm) | La (nm) |
|---|---|---|
| C8W1 | 4.0 | 31.0 |
| C8W5 | 2.4 | 30.4 |
| C8W20 | 1.4 | 30.0 |
| Samples | Paraffin | PC8W0 | PC8W1 | PC8W5 | PC8W20 |
|---|---|---|---|---|---|
| Supercooling degree (°C) | 6.1 | 5.5 | 4.2 | 4.8 | 4.5 |
| Reduce degree (°C) | - | 0.6 | 1.9 | 1.3 | 1.6 |
| Samples | Loading | k, W/(mK) | Preparation Methods | η,year |
|---|---|---|---|---|
| Graphene/Paraffin | 3 wt% | 0.274 | rGO | 27% [41] |
| GAs/Paraffin | 2 wt% | 2.111 | rGO + Annealing (1000 °C, Ar,30 min.) | 1144% [40] |
| Graphene foam /Paraffin | 2.54 vol% | 1.82 | rGO + CVD | 226% [42] |
| Graphene/Paraffin | 0.4 vol% | 0.418 | rGO | 586% [43] |
| Graphene/Paraffin | 8 wt% | 1.73 | - a | 162% [44] |
| GO/CNTs/Paraffin | 1.314 wt% | 0.813 | rGO + CNT | 605% [45] |
| AGAs/Paraffin | 0.32 vol% | 1.074 | rGO + Wave(800 W,vacuum,1 min.), Anisotropy | 995%, this work |
| GAs/Epoxy resin | 0.75 vol% | 6.57 | rGO + Annealing (2800 °C, Ar, 2 h), Anisotropy | 5890% [19] |
| GAs/Octadecanoic acid | 20 vol% | 2.635 | rGO + Annealing (1000 °C, Ar, 30 min.) | 66.7% [11] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zhang, B.; Valdiserri, P.; Huang, X.; Yin, G.; Cui, Y. Thermal Flow Self-Assembled Anisotropic Chemically Derived Graphene Aerogels and Their Thermal Conductivity Enhancement. Nanomaterials 2019, 9, 1226. https://doi.org/10.3390/nano9091226
Huang J, Zhang B, Valdiserri P, Huang X, Yin G, Cui Y. Thermal Flow Self-Assembled Anisotropic Chemically Derived Graphene Aerogels and Their Thermal Conductivity Enhancement. Nanomaterials. 2019; 9(9):1226. https://doi.org/10.3390/nano9091226
Chicago/Turabian StyleHuang, Jinhui, Buning Zhang, Paolo Valdiserri, Xue Huang, Guoqiang Yin, and Yingde Cui. 2019. "Thermal Flow Self-Assembled Anisotropic Chemically Derived Graphene Aerogels and Their Thermal Conductivity Enhancement" Nanomaterials 9, no. 9: 1226. https://doi.org/10.3390/nano9091226
APA StyleHuang, J., Zhang, B., Valdiserri, P., Huang, X., Yin, G., & Cui, Y. (2019). Thermal Flow Self-Assembled Anisotropic Chemically Derived Graphene Aerogels and Their Thermal Conductivity Enhancement. Nanomaterials, 9(9), 1226. https://doi.org/10.3390/nano9091226