Luminescence and X-ray Absorption Properties of Uniform Eu3+:(H3O)Lu3F10 Nanoprobes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Nanoparticles
2.3. Characterization Techniques
3. Results and Discussion
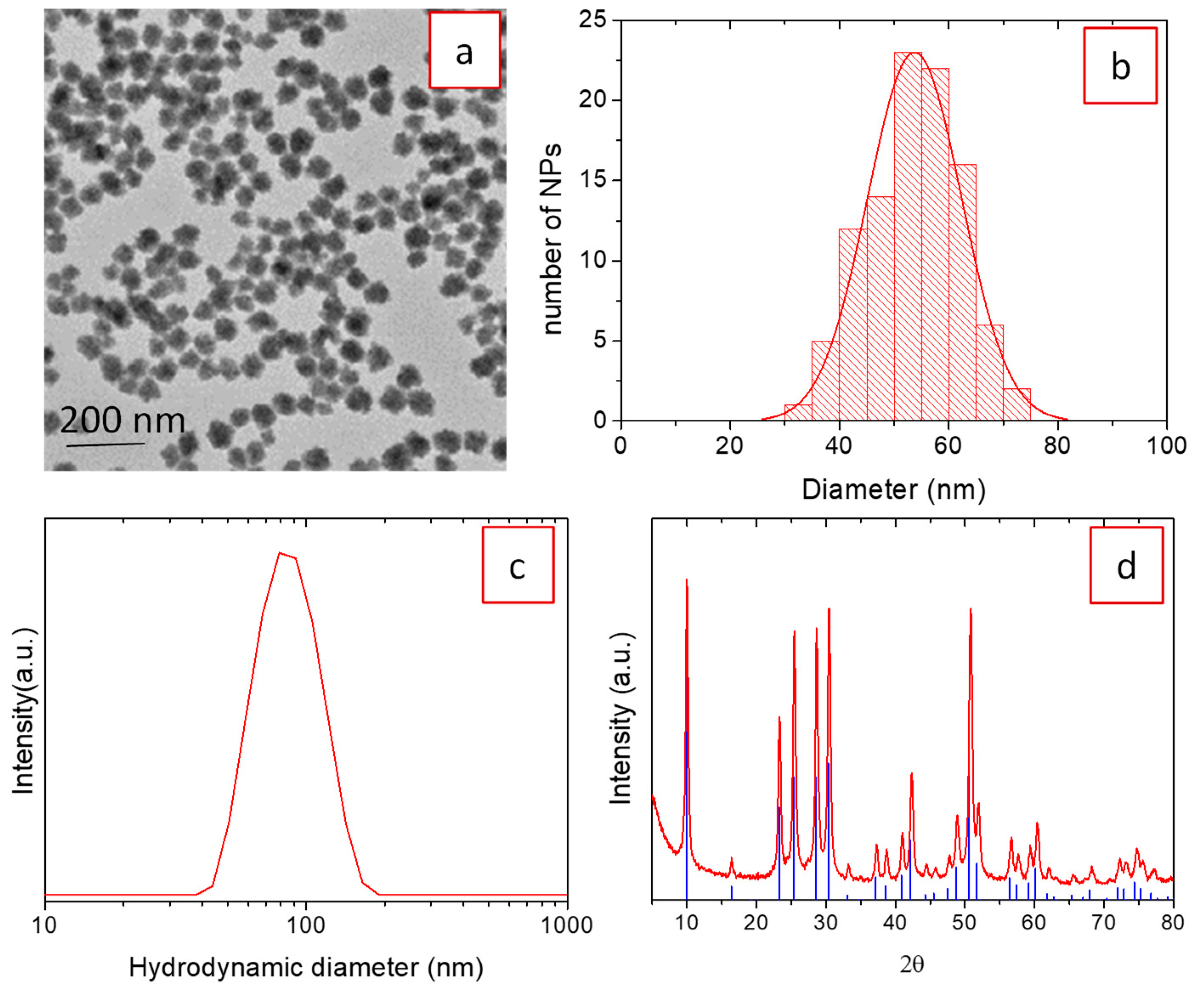
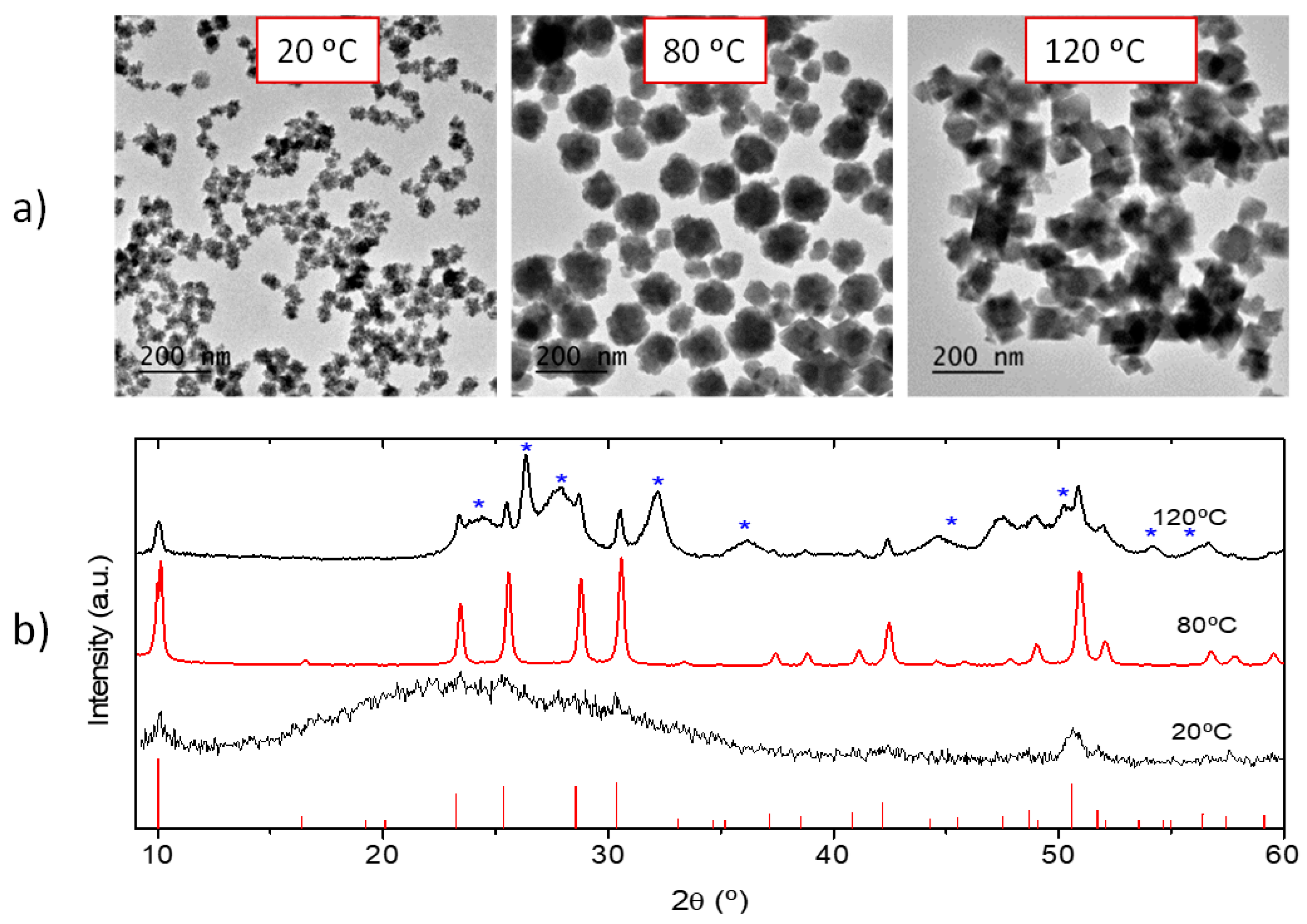
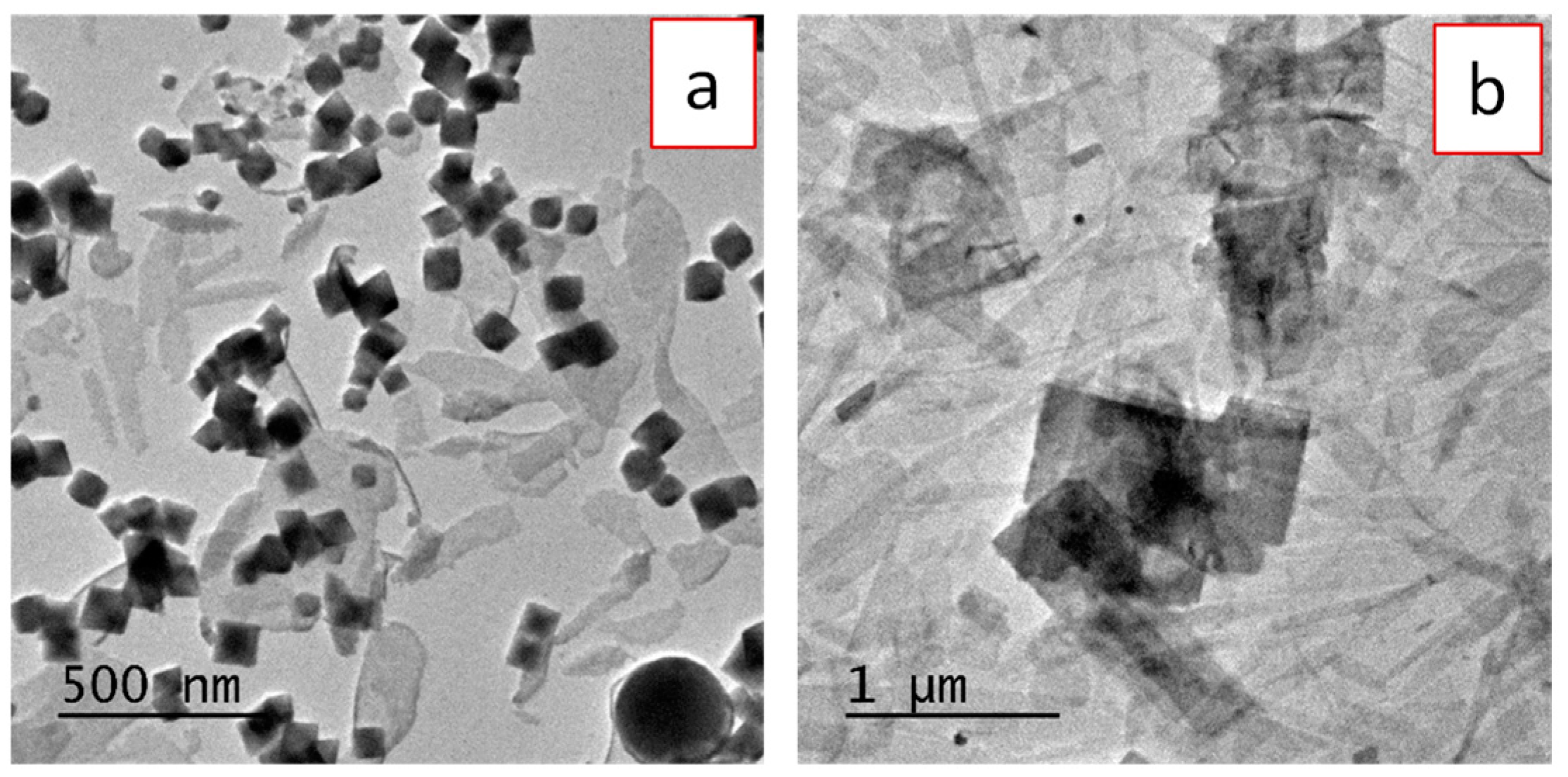
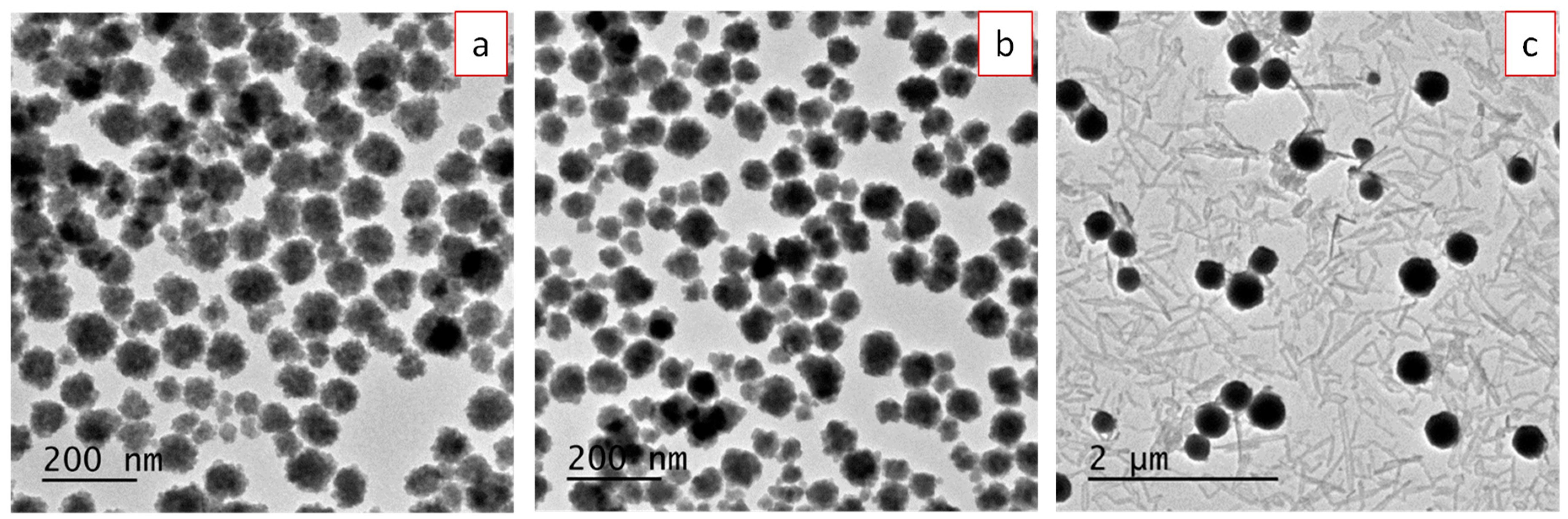
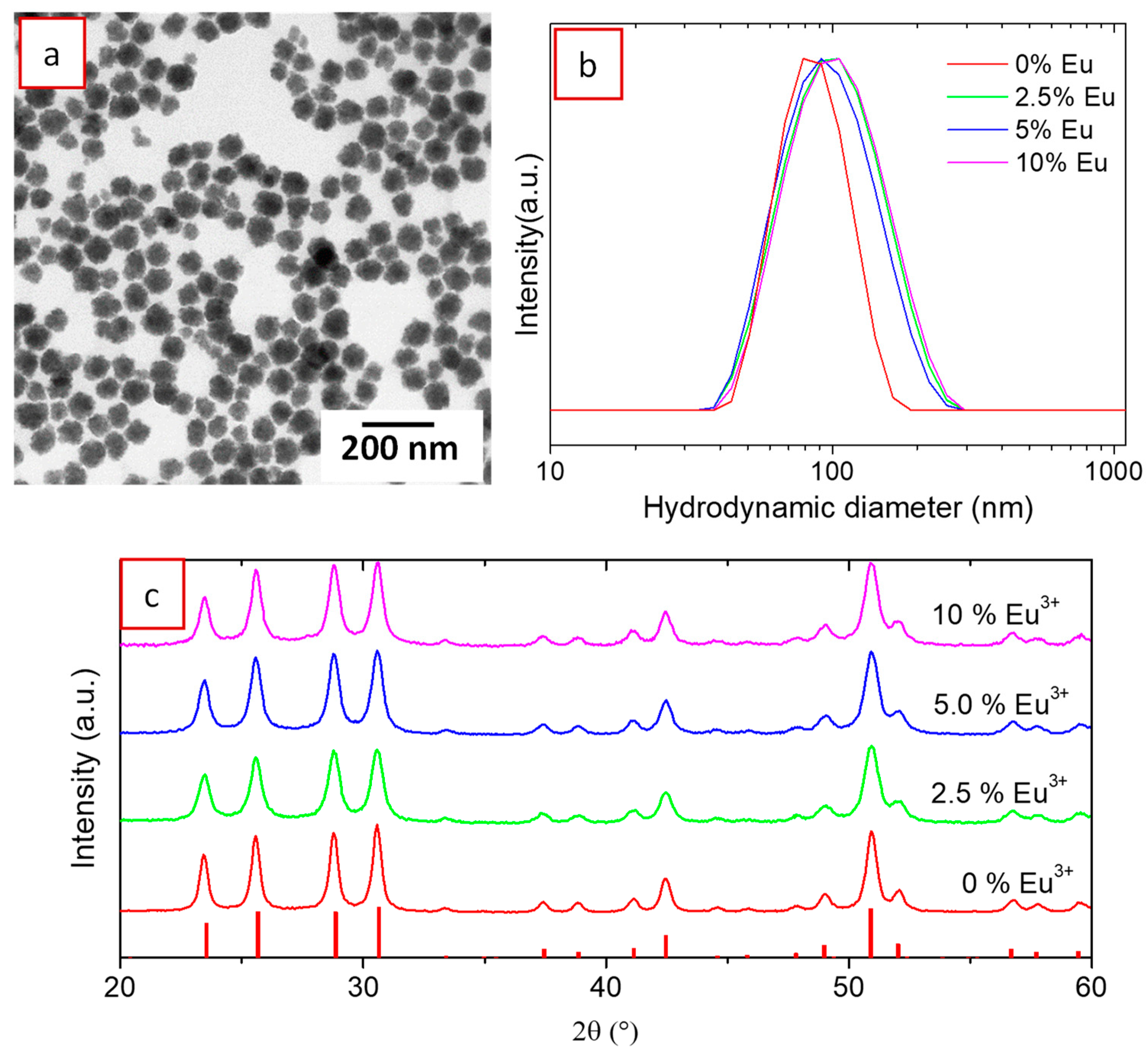
3.1. Synthesis, Morphology, and Crystal Structure of Undoped and Eu3+-Doped Lutetium Fluoride Nanoparticles
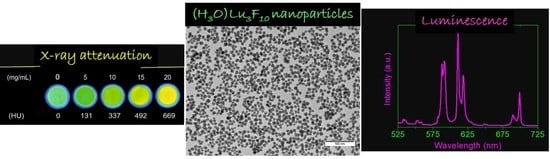
3.2. Luminescent Properties
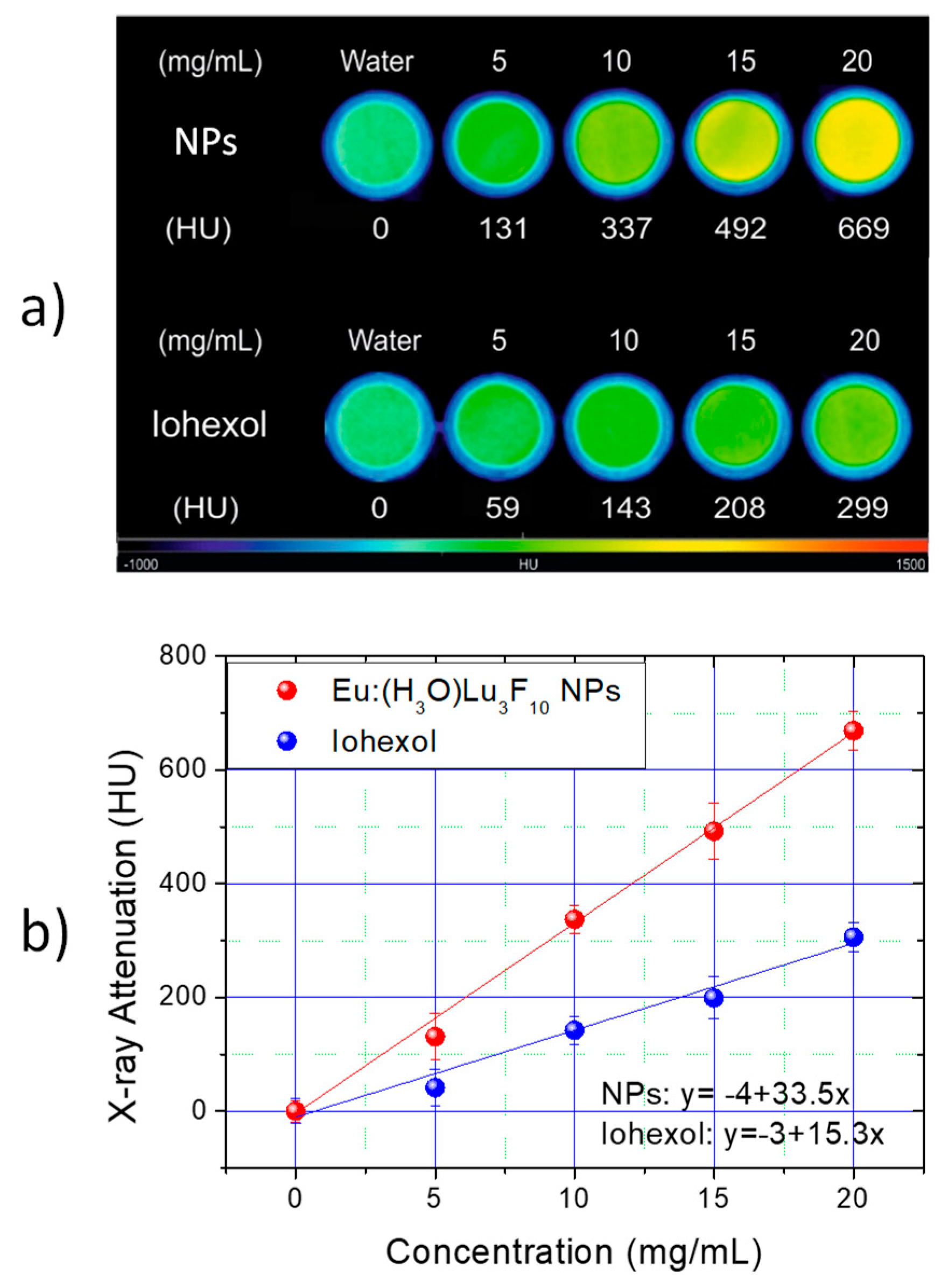
3.3. X-ray Attenuation Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Escudero, A.; Becerro, A.I.; Carrillo-Carrion, C.; Nuñez, N.O.; Zyuzin, M.V.; Laguna, M.; Gonzalez-Mancebo, D.; Ocaña, M.; Parak, W.J. Rare earth based nanostructured materials: Synthesis, functionalization, properties and bioimaging and biosensing applications. Nanophotonics 2017, 5, 881–921. [Google Scholar] [CrossRef]
- Chan, M.H.; Liu, R.S. Advanced sensing, imaging, and therapy nanoplatforms based on Nd3+-doped nanoparticle composites exhibiting upconversion induced by 808 nm near-infrared light. Nanoscale 2017, 9, 18153–18168. [Google Scholar] [CrossRef] [PubMed]
- Ge, K.; Zhang, C.; Sun, W.; Liu, H.; Jin, Y.; Li, Z.; Liang, X.J.; Jia, G.; Zhang, J. Up-Conversion Y2O3:Yb3+,Er3+ Hollow Spherical Drug Carrier with Improved Degradability for Cancer Treatment. ACS Appl. Mater. Interfaces 2016, 8, 25078–25086. [Google Scholar] [CrossRef]
- Naccache, R.; Yu, Q.; Capobianco, J.A. The Fluoride Host: Nucleation, Growth, and Upconversion of Lanthanide-Doped Nanoparticles. Adv. Opt. Mater. 2015, 3, 482–509. [Google Scholar] [CrossRef]
- Bazzi, R.; Flores, M.A.; Louis, C.; Lebbou, K.; Zhang, W.; Dujardin, C.; Roux, S.; Mercier, B.; Ledoux, G.; Bernstein, E.; et al. Synthesis and properties of europium-based phosphors on the nanometer scale: Eu2O3, Gd2O3:Eu, and Y2O3:Eu. J. Coll. Int. Sci. 2004, 273, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.I.; Lee, E.S.; Byeon, S.H. Assembly of layered rare-earth hydroxide nanosheets and SiO2 nanoparticles to fabricate multifunctional transparent films capable of combinatorial color generation. Adv. Funct. Mater. 2012, 22, 3562–3569. [Google Scholar] [CrossRef]
- Becerro, A.I.; Rodríguez-Liviano, S.; Fernández-Carrión, A.J.; Ocaña, M. A novel 3D architecture of GdPO4 nanophosphors with multicolor and white light emissions. Cryst. Growth Des. 2013, 13, 526–535. [Google Scholar] [CrossRef]
- Ilinskaya, A.N.; Dobrovolskaia, M.A. Nanoparticles and the blood coagulation system. Part II: Safety concerns. Nanomedicine 2013, 8, 969–998. [Google Scholar] [CrossRef]
- You, W.; Tu, D.; Zheng, W.; Shang, X.; Song, X.; Zhou, S.; Liu, Y.; Lia, R.; Chen, X. Large-scale synthesis of uniform lanthanide-doped NaREF4 upconversion/downshifting nanoprobes for bioapplications. Nanoscale 2018, 10, 11477–11484. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, P.P.; Luginina, A.A.; Kuznetsov, S.V.; Osiko, V.V. Nanofluorides. J. Fluor. Chem. 2011, 132, 1012–1039. [Google Scholar] [CrossRef]
- Gai, S.; Li, C.; Yang, P.; Jun, L. Recent Progress in Rare Earth Micro/Nanocrystals: Soft Chemical Synthesis, Luminescent Properties, and Biomedical Applications. Chem. Rev. 2014, 114, 2343–2389. [Google Scholar] [CrossRef]
- Becerro, A.I.; Gonzalez-Mancebo, D.; Ocaña, M. Uniform, luminescent Eu:LuF3 nanoparticles. J. Nanopart. Res. 2015, 17, 58. [Google Scholar] [CrossRef]
- Grzyb, T.; Runowski, M.; Szczeszak, A.; Liz, S. Structural, morphological and spectroscopic properties of Eu3+-doped rare earth fluorides synthesized by the hydrothermal method. J. Solid State Chem. 2013, 200, 76–83. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, T.; Jiang, J.; Geng, H.; Ning, Z.; Lai, X.; Bi, J.; Gao, D. Multicolor Tunable Luminescence Based on Tb3+/Eu3+ Doping through a Facile Hydrothermal Route. ACS Appl. Mater. Interfaces 2017, 9, 26184–26190. [Google Scholar] [CrossRef]
- González-Mancebo, D.; Becerro, A.I.; Rojas, T.C.; García-Martín, M.L.; de la Fuente, J.M.; Ocaña, M. HoF3 and DyF3 Nanoparticles as Contrast Agents for High-Field Magnetic Resonance Imaging. Part. Part. Syst. Char. 2017, 34, 1700116. [Google Scholar] [CrossRef]
- Becerro, A.I.; González-Mancebo, D.; Cantelar, E.; Cussó, F.; Stepien, G.; de la Fuente, J.M.; Ocaña, M. Ligand-Free Synthesis of Tunable Size Ln:BaGdF5 (Ln = Eu3+ and Nd3+) Nanoparticles: Luminescence, Magnetic Properties, and Biocompatibility. Langmuir 2016, 32, 411–420. [Google Scholar] [CrossRef]
- Li, C.; Xu, Z.; Yang, D.; Cheng, Z.; Hou, Z.; Ma, P.; Liana, H.; Lin, J. Well-dispersed KRE3F10 (RE = Sm–Lu, Y) nanocrystals: Solvothermal synthesis and luminescence properties. CrystEngComm 2012, 14, 670–678. [Google Scholar] [CrossRef]
- Orlovskii, Y.V.; Vanetsev, A.S.; Keevend, K.; Kaldvee, K.; Samsonova, E.V.; Puust, L.; del Rosal, B.; Jaque, D.; Ryabova, A.V.; Baranchikov, A.E.; et al. NIR fluorescence quenching by OH acceptors in the Nd3+ doped KY3F10 nanoparticles synthesized by microwave-hydrothermal treatment. J. Alloy Comp. 2016, 661, 312–321. [Google Scholar] [CrossRef]
- Mahalingam, V.; Vetrone, F.; Naccache, R.; Speghinib, A.; Capobianco, J.A. Structural and optical investigation of colloidal Ln3+/Yb3+ co-doped KY3F10 nanocrystals. J. Mater. Chem. 2009, 19, 3149–3152. [Google Scholar] [CrossRef]
- Bian, W.; Wang, T.; Guo, Y.; Yu, X.; Xu, X.; Qiu, J. Visible and near-infrared upconversion photoluminescence in lanthanide-doped KLu3F10 nanoparticles. CrystEngComm 2015, 17, 7332–7338. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Z.; Lei, P.; Liu, X.; Su, Y.; Dong, L.; Yao, S.; Zhou, L.; Song, S.; Feng, J.; et al. Cubic KLu3F10 nanocrystals: Mn2+ dopant, controlled synthesis and upconversion luminescence. Dalton Trans. 2015, 44, 17286–17292. [Google Scholar] [CrossRef]
- Le Berre, F.; Boucher, E.; Allain, M.; Courbion, G. Synthesis, stability and zeolitic behavior of d-ALn3F10,xH2O and c-ThLn2F10,H2O phases (Ln = lanthanide). J. Mater. Chem. 2000, 10, 2578–2586. [Google Scholar] [CrossRef]
- Andrrev, O.V.; Razumkova, I.A.; Boiko, A.N. Synthesis and thermal stability of rare earth compounds REF3, REF3·nH2O and (H3O)RE3F10·nH2O (RE = Tb − Lu, Y), obtained from sulphide precursors. J. Fluor. Chem. 2018, 207, 77–83. [Google Scholar] [CrossRef]
- Giri, J.; Sriharsha, T.; Bahadur, D. Optimization of parameters for the synthesis of nano-sized Co1−xZnxFe2O4, (0 ≤ x ≤ 0.8) by microwave refluxing. J. Mater. Chem. 2004, 14, 875–880. [Google Scholar] [CrossRef]
- Matijevic, E. Preparation and properties of uniform size colloids. Chem. Mater. 1993, 5, 412–426. [Google Scholar] [CrossRef]
- Zhao, Q.; Shao, B.; Lu, W.; Jia, Y.; Lv, W.; Jiao, M.; You, H. Ba2GdF7 Nanocrystals: Solution-Based Synthesis, Growth Mechanism, and Luminescence Properties. Cryst. Growth Des. 2014, 14, 1819–1826. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Q. Systematic studies for the novel synthesis of nano-structured lanthanide fluorides. Chem. Eng. J. 2014, 250, 190–197. [Google Scholar] [CrossRef]
- Jia, G.; You, H.; Song, Y.; Jia, J.; Zheng, Y.; Zhang, L.; Liu, K.; Zhang, H. Facile Chemical Conversion Synthesis and Luminescence Properties of Uniform Ln3+ (Ln = Eu, Tb)-doped NaLuF4 Nanowires and LuBO3 Microdisks. Inorg. Chem. 2009, 48, 10193–10201. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wei, Y.L.; Wei, R.F.; Yang, J.W.; Guo, H. Elaboration, Structure, and Luminescence of Eu3+-Doped BaLuF5-Based Transparent Glass-Ceramics. J. Am. Ceram. Soc. 2013, 96, 798–800. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Zhao, T.; Wang, Y.; Cui, T.; Sheng, Y.; Zheng, K.; Zhou, X.; You, H.; Zou, H. Facile synthesis and color-tunable properties of BaLuF5:Ce,Tb,Eu(Sm) submicrospheres via a facile ionic liquid/EG two-phase system. J. Colloid Interface Sci. 2017, 487, 281–288. [Google Scholar] [CrossRef]
- Sayed, F.N.; Grover, V.; Dubey, K.A.; Sudarsan, V.; Pandey, B.N.; Asthana, A.; Vatsa, R.K.; Tyagi, A.K. Multicolored and white-light phosphors based on doped GdF3 nanoparticles and their potential bio-applications. J. Colloid Interface Sci. 2012, 367, 161–170. [Google Scholar] [CrossRef]
- Lusic, H.; Grinstaff, M.W. X-ray-computed tomography contrast agents. Chem. Rev. 2013, 113, 1641–1666. [Google Scholar] [CrossRef]

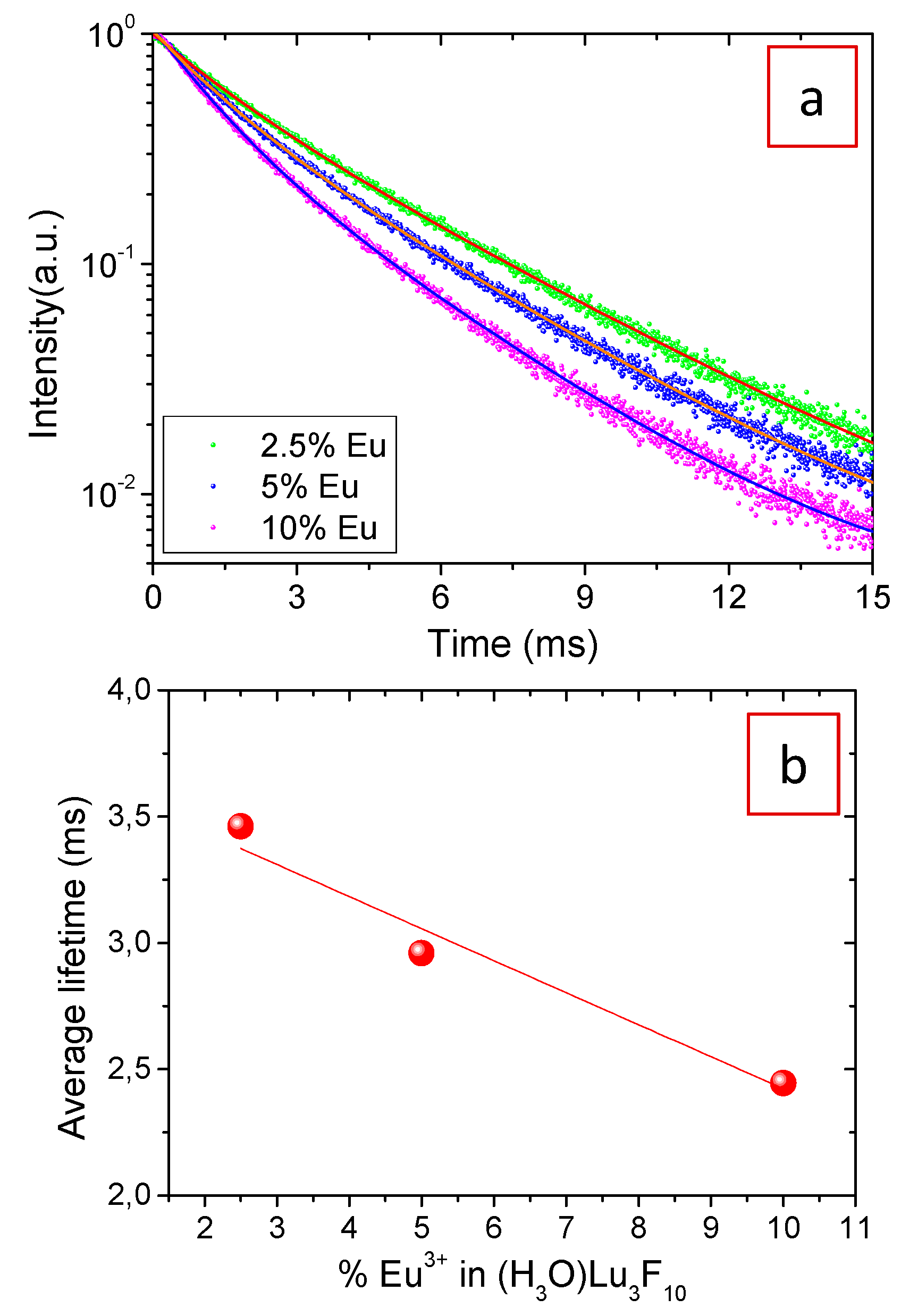
| % Eu3+ | τ1 (ms) | I1 (%) | τ2 (ms) | I2 (%) | <τ> (ms) |
|---|---|---|---|---|---|
| 2.5 | 3.90 | 63 | 1.54 | 37 | 3.46 |
| 5 | 3.47 | 55 | 1.30 | 45 | 2.96 |
| 10 | 3.08 | 43 | 1.15 | 57 | 2.44 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Mancebo, D.; Becerro, A.I.; Corral, A.; Balcerzyk, M.; Ocaña, M. Luminescence and X-ray Absorption Properties of Uniform Eu3+:(H3O)Lu3F10 Nanoprobes. Nanomaterials 2019, 9, 1153. https://doi.org/10.3390/nano9081153
González-Mancebo D, Becerro AI, Corral A, Balcerzyk M, Ocaña M. Luminescence and X-ray Absorption Properties of Uniform Eu3+:(H3O)Lu3F10 Nanoprobes. Nanomaterials. 2019; 9(8):1153. https://doi.org/10.3390/nano9081153
Chicago/Turabian StyleGonzález-Mancebo, Daniel, Ana Isabel Becerro, Ariadna Corral, Marcin Balcerzyk, and Manuel Ocaña. 2019. "Luminescence and X-ray Absorption Properties of Uniform Eu3+:(H3O)Lu3F10 Nanoprobes" Nanomaterials 9, no. 8: 1153. https://doi.org/10.3390/nano9081153
APA StyleGonzález-Mancebo, D., Becerro, A. I., Corral, A., Balcerzyk, M., & Ocaña, M. (2019). Luminescence and X-ray Absorption Properties of Uniform Eu3+:(H3O)Lu3F10 Nanoprobes. Nanomaterials, 9(8), 1153. https://doi.org/10.3390/nano9081153