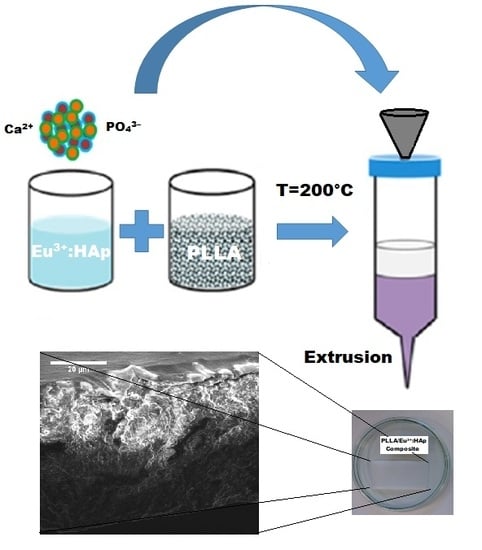
The Comprehensive Approach to Preparation and Investigation of the Eu3+ Doped Hydroxyapatite/poly(L-lactide) Nanocomposites: Promising Materials for Theranostics Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
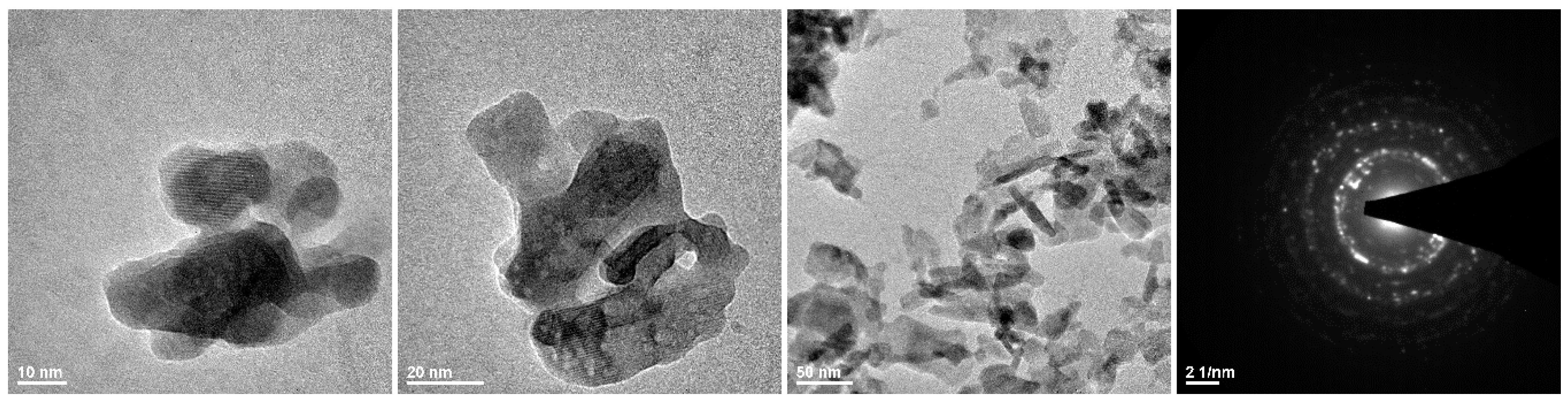
2.2. Synthesis of HAp and Eu3+-Doped HAp Powders
2.3. Preparation of the PLLA/Eu3+-Doped Hydroxyapatite Composites
2.4. Characterization
3. Results
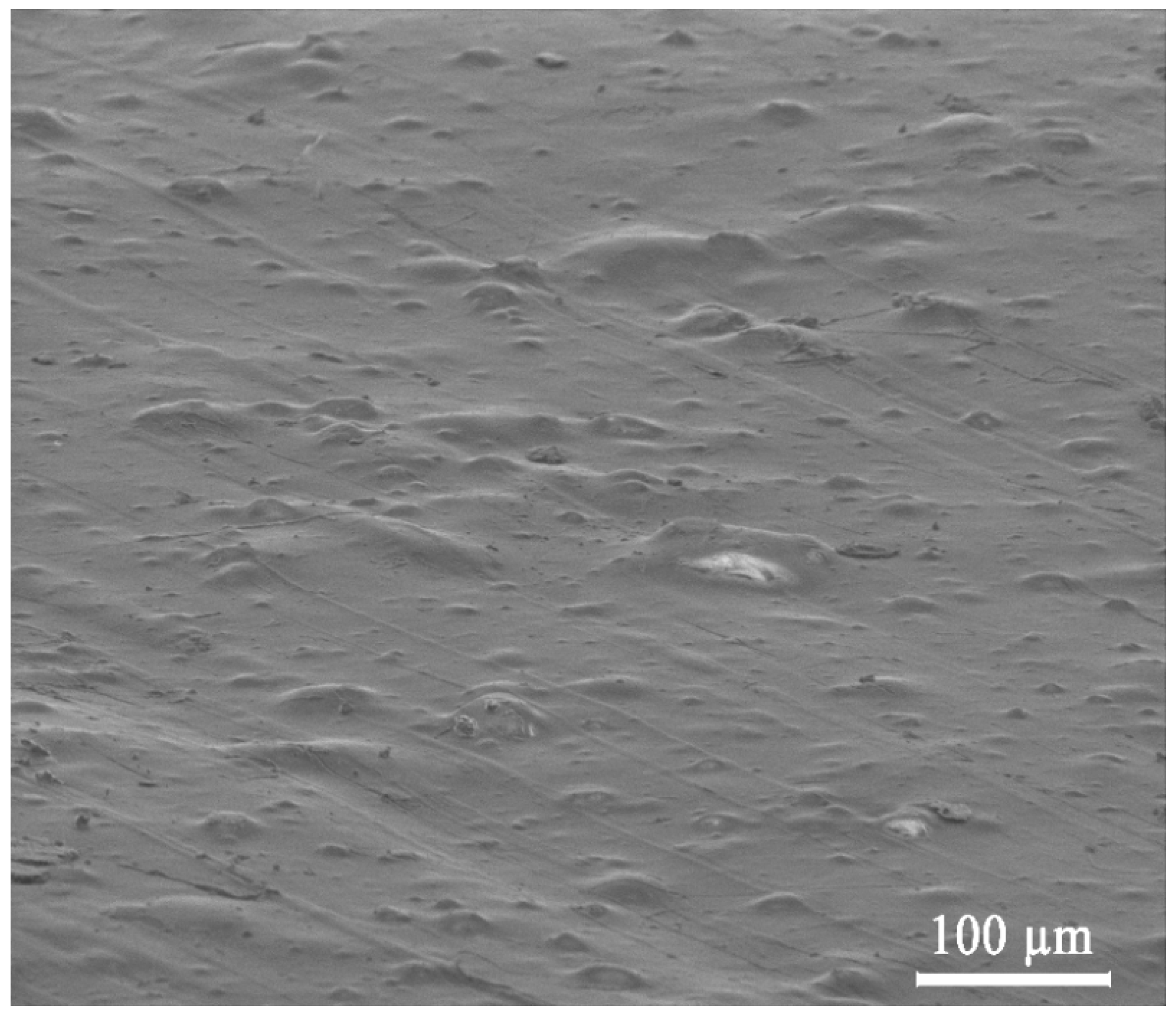
3.1. Morphology
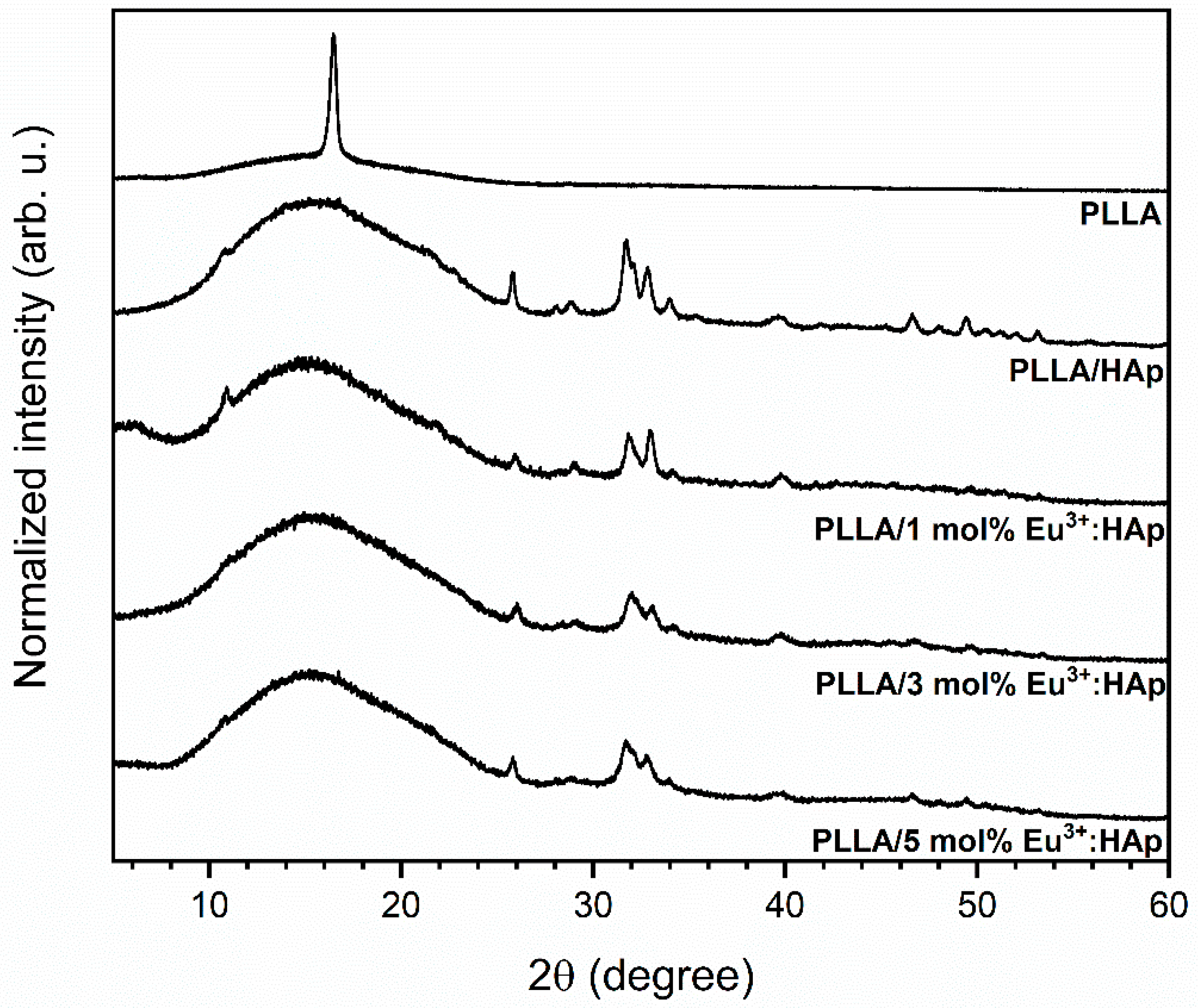
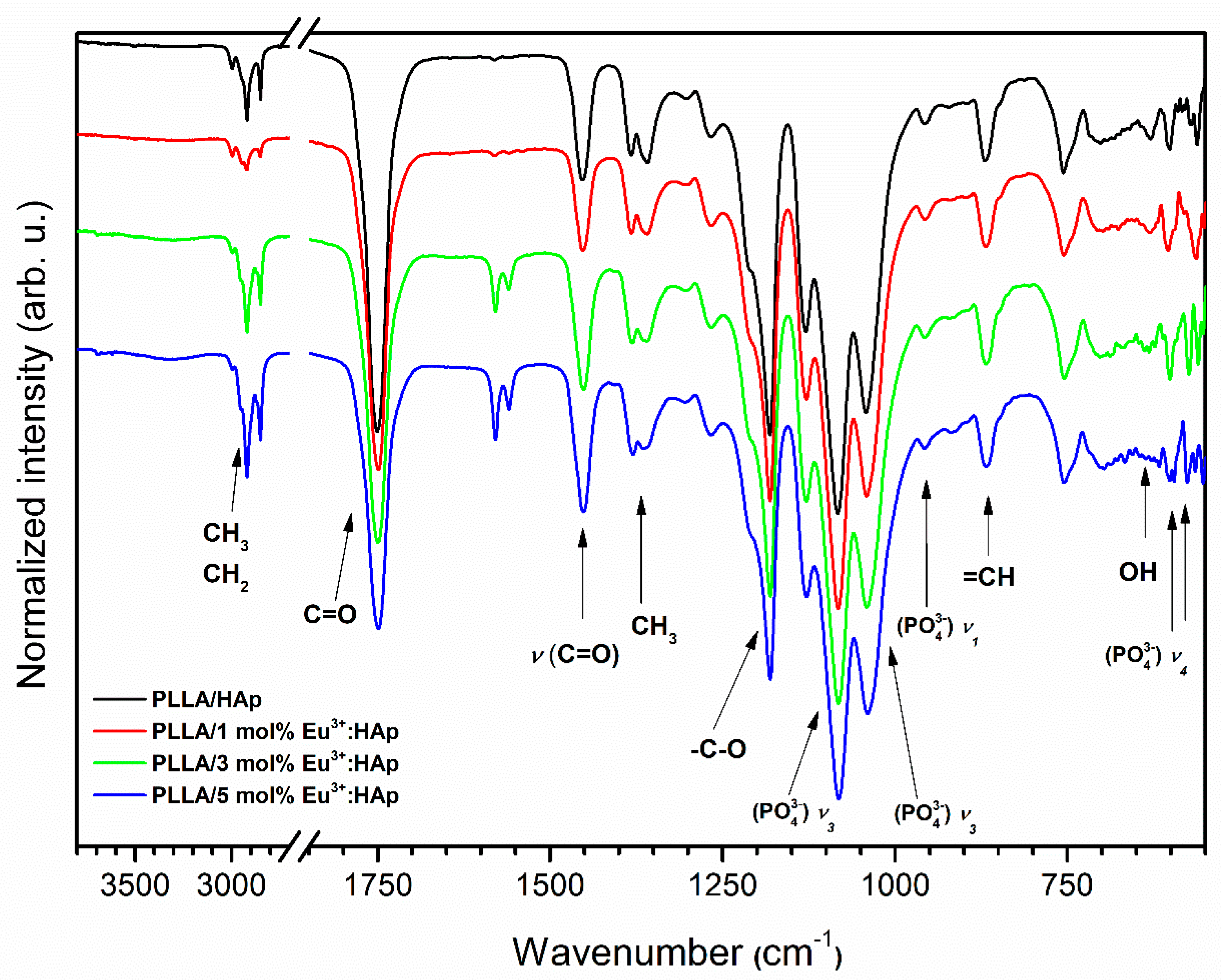
3.2. Structural Analysis of Eu3+-Doped Composites
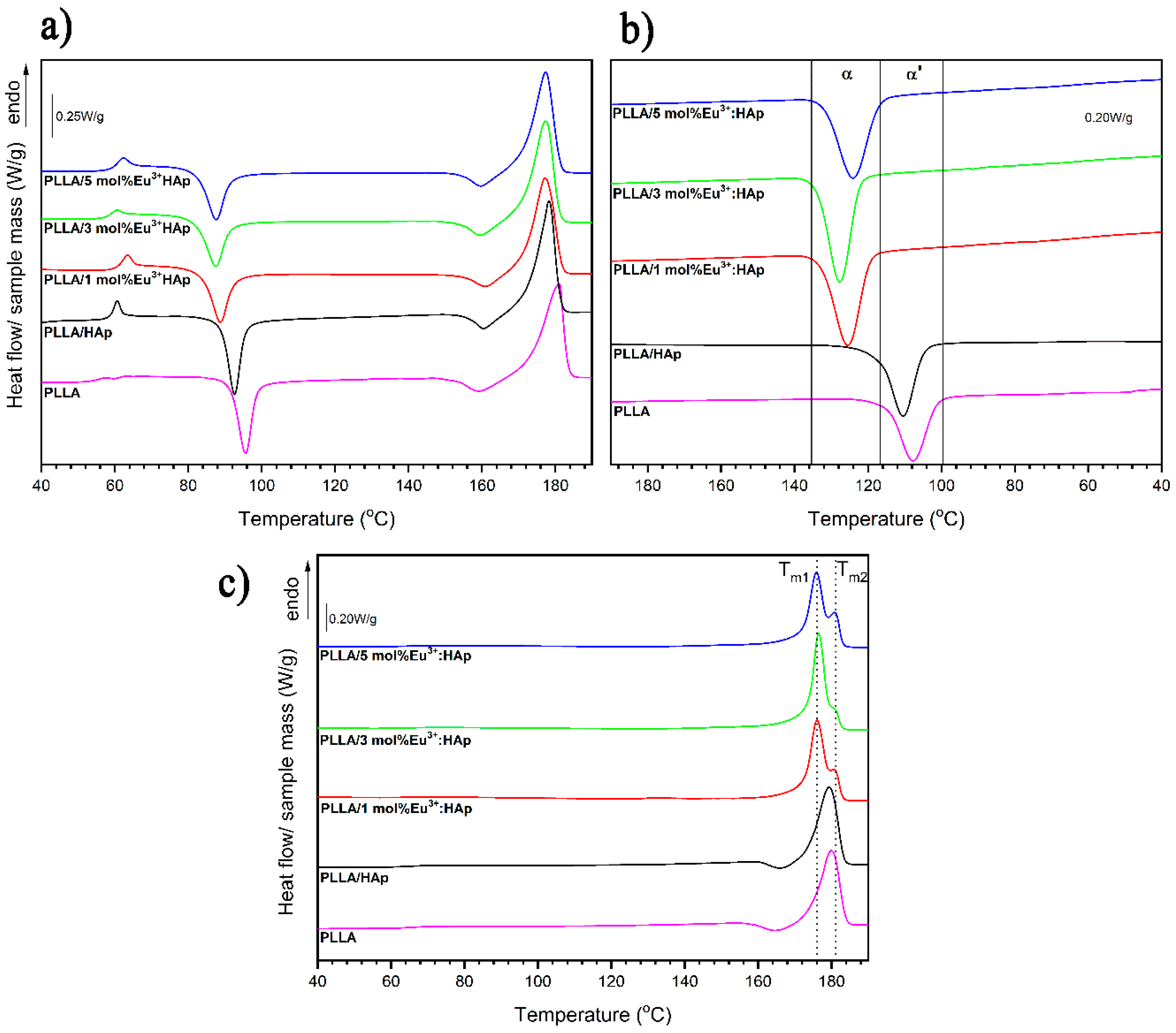
3.3. Thermal Properties
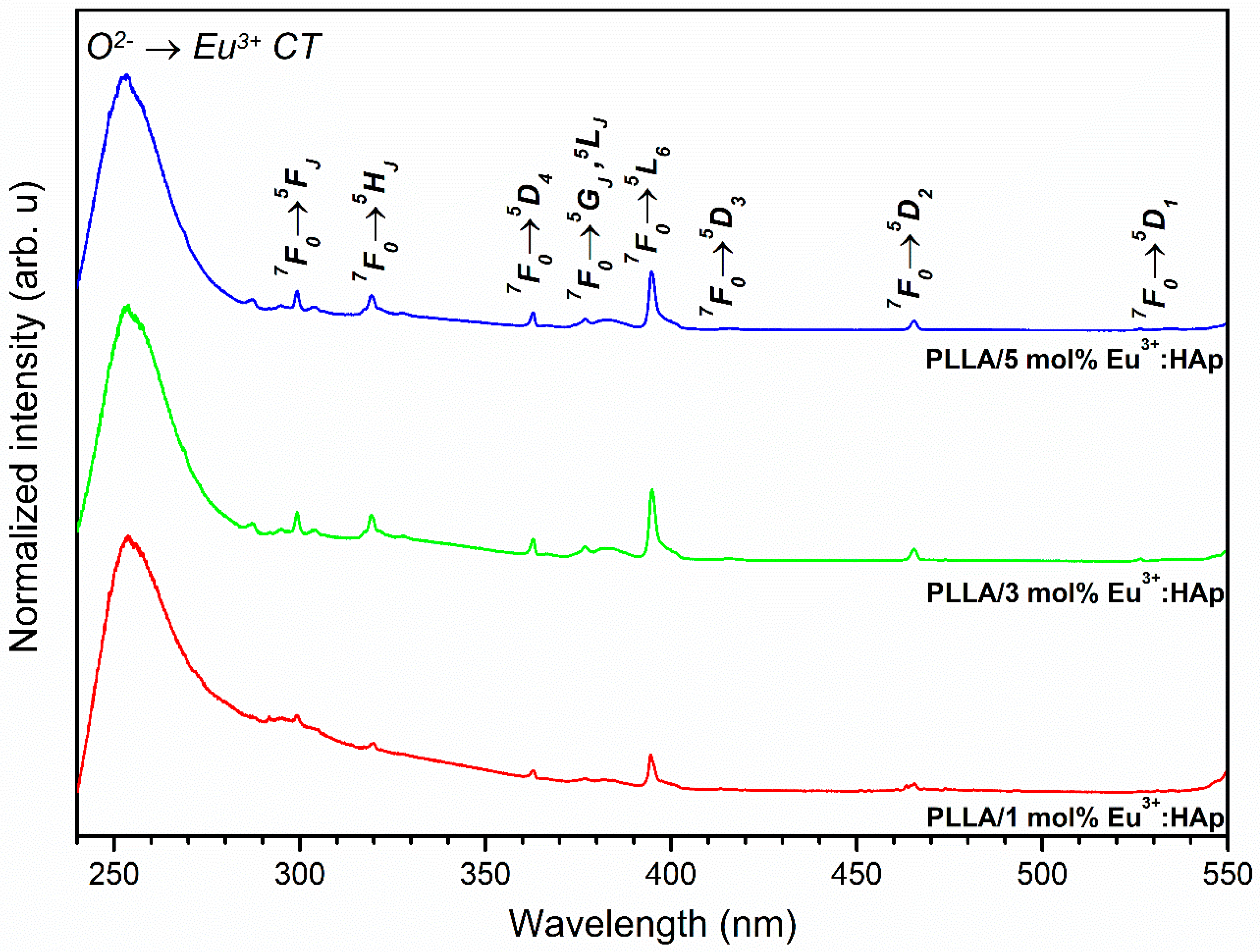
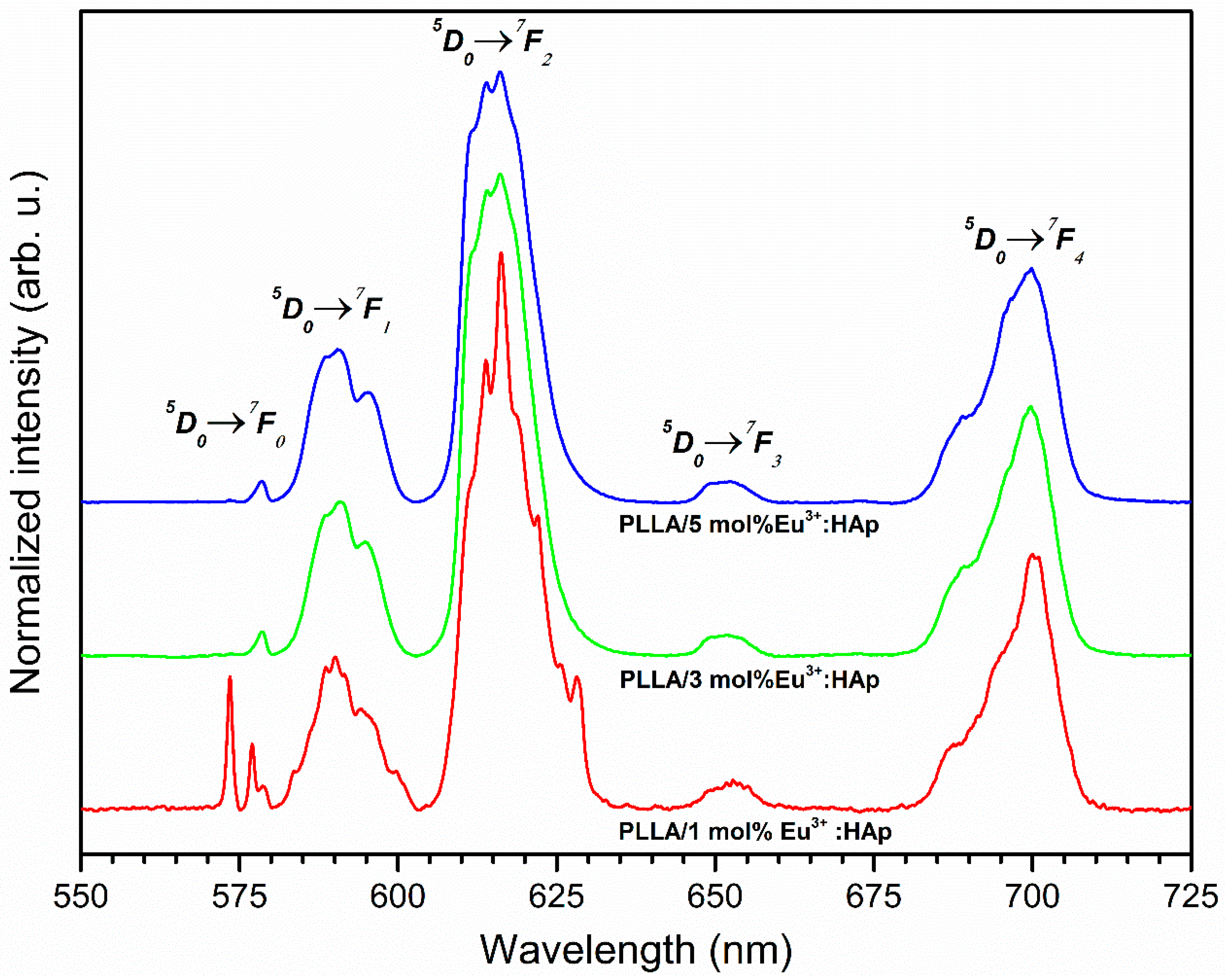
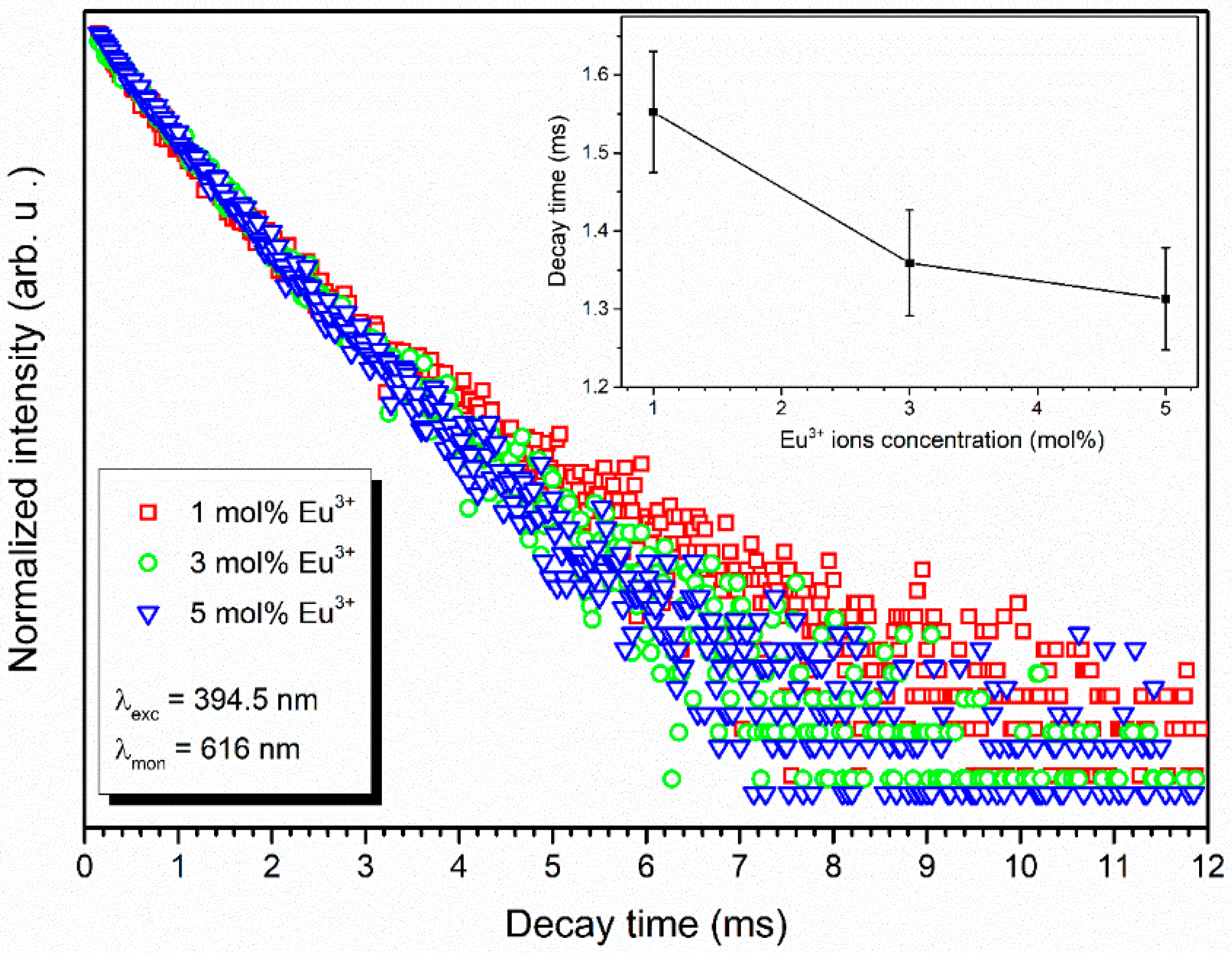
3.4. Spectroscopic Properties
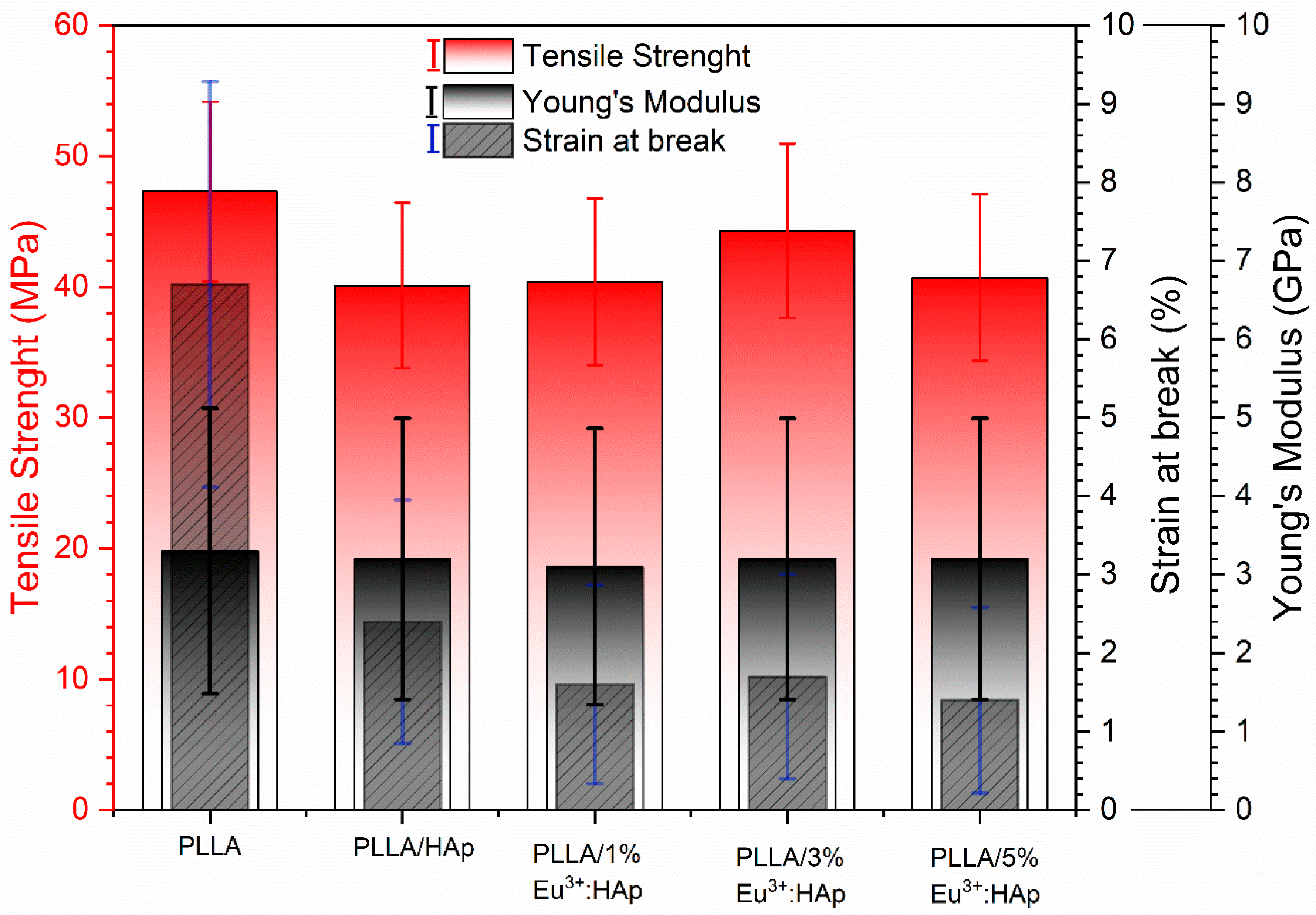
3.5. Tensile Properties
4. Discussion
4.1. Thermal Properties
4.2. Spectroscopic Properties
4.3. Tensile Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Terukina, T.; Saito, H.; Tomita, Y.; Hattori, Y.; Otsuka, M. Development and effect of a sustainable and controllable simvastatin-releasing device based on PLGA microspheres/carbonate apatite cement composite: In vitro evaluation for use as a drug delivery system from bone-like biomaterial. J. Drug Deliv. Sci. Technol. 2017, 37, 74–80. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactic acid) stereocomplexes: A decade of progress. Adv. Drug Deliv. Rev. 2016, 107, 97–135. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Pirosa, A.; Lupi, G.; Erba, P.A.; Giachi, G.; Chiellini, F. Design and fabrication of novel polymeric biodegradable stents for small caliber blood vessels by computer-aided wet-spinning. Biomed. Mater. 2017, 12, 035011. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pyda, M.; Cebe, P. Electrospun fibers of poly(L-lactic acid) containing lovastatin with potential applications in drug delivery. J. Appl. Polym. Sci. 2017, 134, 45287. [Google Scholar] [CrossRef]
- Ji, X.; Yang, W.; Wang, T.; Mao, C.; Guo, L.; Xiao, J.; He, N. Coaxially electrospun core/shell structured poly(L-lactide) acid/chitosan nanofibers for potential drug carrier in tissue engineering. J. Biomed. Nanotechnol. 2013, 9, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.W.; Prasad, G.; Wang, S.C.; Wu, J.L.; Lu, S.G. Enhancement of the Oil Absorption Capacity of Poly(Lactic Acid) Nano Porous Fibrous Membranes Derived via a Facile Electrospinning Method. Appl. Sci. 2019, 9, 1014. [Google Scholar] [CrossRef]
- le Phuong, H.A.; Ayob, N.A.I.; Blanford, C.F.; Rawi, N.F.M.; Szekely, G. Nonwoven Membrane Supports from Renewable Resources: Bamboo Fiber Reinforced Poly(Lactic Acid) Composites. ACS Sustain. Chem. Eng. 2019, 7, 11885–11893. [Google Scholar] [CrossRef]
- Muller, J.; González-Martínez, C.; Chiralt, A. Combination of Poly(lactic) Acid and Starch for Biodegradable Food Packaging. Materials 2017, 10, 952. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, L.; Xiao, P.; Huang, Y.; Chen, P.; Wang, X.; Gu, J.; Zhang, J.; Chen, T. Biodegradable PLA Nonwoven Fabric with Controllable Wettability for Efficient Water Purification and Photocatalysis Degradation. ACS Sustain. Chem. Eng. 2018, 6, 2445–2452. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Venugopal, J.; Ramakrishna, S. Electrospun nanostructured scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 2884–2893. [Google Scholar] [CrossRef]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates in dentistry. J. Mater. Sci. Mater. Med. 2013, 24, 1335–1363. [Google Scholar] [CrossRef]
- Pietrasik, J.; Szustakiewicz, K.; Zaborski, M.; Haberko, K. Hydroxyapatite: An environmentally friendly filler for elastomers. Mol. Cryst. Liq. Cryst. 2008, 483, 172–178. [Google Scholar] [CrossRef]
- Szustakiewicz, K.; Stępak, B.; Antończak, A.J.; Maj, M.; Gazińska, M.; Kryszak, B.; Pigłowski, J. Femtosecond laser-induced modification of PLLA/hydroxypatite composite. Polym. Degrad. Stab. 2018, 149, 152–161. [Google Scholar] [CrossRef]
- Smieszek, A.; Marycz, K.; Szustakiewicz, K.; Kryszak, B.; Targonska, S.; Zawisza, K.; Watras, A.; Wiglusz, R.J. New approach to modification of poly (l-lactic acid) with nano-hydroxyapatite improving functionality of human adipose-derived stromal cells (hASCs) through increased viability and enhanced mitochondrial activity. Mater. Sci. Eng. C 2019, 98, 213–226. [Google Scholar] [CrossRef]
- Wei, Y.; He, Y.; Li, X.; Chen, H.; Deng, X. Cellular uptake and delivery-dependent effects of Tb3+-doped hydroxyapatite nanorods. Molecules 2017, 22, 1043. [Google Scholar] [CrossRef]
- Curtin, C.M.; Cunniffe, G.M.; Lyons, F.G.; Bessho, K.; Dickson, G.R.; Duffy, G.P.; O’Brien, F.J. Innovative collagen nano-hydroxyapatite scaffolds offer a highly efficient non-viral gene delivery platform for stem cell-mediated bone formation. Adv. Mater. 2012, 24, 749–754. [Google Scholar] [CrossRef]
- Yuan, Q.; Wu, J.; Qin, C.; Xu, A.; Zhang, Z.; Lin, S.; Ren, X.; Zhang, P. Spin-coating synthesis and characterization of Zn-doped hydroxyapatite/polylactic acid composite coatings. Surf. Coat. Technol. 2016, 307, 461–469. [Google Scholar] [CrossRef]
- Wang, X.; Song, G.; Lou, T. Fabrication and characterization of nano-composite scaffold of PLLA/silane modified hydroxyapatite. Med. Eng. Phys. 2010, 32, 391–397. [Google Scholar] [CrossRef]
- Ajdukovic, Z. Repair of Bone Tissue Affected by Osteoporosis with Hydroxyapatite-Poly-L-lactide (HAp-PLLA) With and Without Blood Plasma. J. Biomater. Appl. 2005, 20, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Nishida, Y.; Domura, R.; Sakai, R.; Okamoto, M.; Arakawa, S.; Ishiki, R.; Salick, M.R.; Turng, L.S. Fabrication of PLLA/HA composite scaffolds modified by DNA. Polymer 2015, 56, 73–81. [Google Scholar] [CrossRef]
- Park, K.; Kim, J.J. Effect of Surface-activated PLLA Scaffold on Apatite Formation in Simulated Body Fluid. J. Bioact. Compat. Polym. 2010, 25, 27–39. [Google Scholar] [CrossRef]
- Sabir, M.I.; Xu, X.; Li, L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Junchao, W.; Yanfeng, D.; Yiwang, C.; Xuesi, C. Mechanical and thermal properties of polypeptide modified hydroxyapatite/poly(L-lactide) nanocomposites. Sci. China Chem. 2011, 54, 431–437. [Google Scholar] [CrossRef]
- Wilberforce, S.I.J.; Finlayson, C.E.; Best, S.M.; Cameron, R.E. The influence of hydroxyapatite (HA) microparticles (m) and nanoparticles (n) on the thermal and dynamic mechanical properties of poly-L-lactide. Polymer 2011, 52, 2883–2890. [Google Scholar] [CrossRef]
- Cho, Y.S.; Hong, M.W.; Jeong, H.-J.; Lee, S.J.; Kim, Y.Y.; Cho, Y.S. The fabrication of well-interconnected polycaprolactone/hydroxyapatite composite scaffolds, enhancing the exposure of hydroxyapatite using the wire-network molding technique. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2315–2325. [Google Scholar] [CrossRef]
- Duan, B.; Wang, M.; Zhou, W.Y.; Cheung, W.L.; Li, Z.Y.; Lu, W.W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010, 6, 4495–4505. [Google Scholar] [CrossRef]
- Szustakiewicz, K.; Gazińska, M.; Kryszak, B.; Grzymajło, M.; Pigłowski, J.; Wiglusz, R.J.; Okamoto, M. The influence of hydroxyapatite content on properties of poly(L-lactide)/hydroxyapatite porous scaffolds obtained using thermal induced phase separation technique. Eur. Polym. J. 2019, 113, 313–320. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Zhang, C.L.; Wang, J.F.; Lu, C.H.; Zhuang, Z.; Wang, X.P.; Fang, Q.F. Fabrication and in vitro investigation of nanohydroxyapatite, chitosan, poly(L-lactic acid) ternary biocomposite. J. Appl. Polym. Sci. 2013, 127, 2152–2159. [Google Scholar] [CrossRef]
- Niu, X.; Feng, Q.; Wang, M.; Guo, X.; Zheng, Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J. Control. Release 2009, 134, 111–117. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, H.; Geng, H.; Liu, D.; Zhang, X.; Cai, Q.; Yang, X. Dual functional polylactide–hydroxyapatite nanocomposites for bone regeneration with nano- silver being loaded via reductive polydopamine. RSC Adv. 2016, 6, 91349–91360. [Google Scholar] [CrossRef]
- Popa, C.L.; Ciobanu, C.S.; Voicu, G.; Vasile, E.; Chifiriuc, M.C.; Iconaru, S.L.; Predoi, D. Influence of Thermal Treatment on the Antimicrobial Activity of Silver-Doped Biological Apatite. Nanoscale Res. Lett. 2015, 10, 502. [Google Scholar] [CrossRef]
- Mocanu, A.; Furtos, G.; Rapuntean, S.; Horovitz, O.; Flore, C.; Garbo, C.; Danisteanu, A.; Rapuntean, G.; Prejmerean, C. Tomoaia-Cotisel, Synthesis; Characterization and antimicrobial effects of composites based on multi-substituted hydroxyapatite and silver nanoparticles. Appl. Surf. Sci. 2014, 298, 225–235. [Google Scholar] [CrossRef]
- Banerjee, S.; Bagchi, B.; Bhandary, S.; Kool, A.; Hoque, N.A.; Thakur, P.; Das, S. A facile vacuum assisted synthesis of nanoparticle impregnated hydroxyapatite composites having excellent antimicrobial properties and biocompatibility. Ceram. Int. 2017, 44, 1066–1077. [Google Scholar] [CrossRef]
- Marques, C.F.; Olhero, S.; Abrantes, J.C.C.; Marote, A.; Ferreira, S.; Vieira, S.I.; Ferreira, J.M.F. Biocompatibility and antimicrobial activity of biphasic calcium phosphate powders doped with metal ions for regenerative medicine. Ceram. Int. 2017, 43, 15719–15728. [Google Scholar] [CrossRef]
- Hickey, D.J.; Ercan, B.; Sun, L.; Webster, T.J. Adding MgO nanoparticles to hydroxyapatite-PLLA nanocomposites for improved bone tissue engineering applications. Acta Biomater. 2015, 14, 175–184. [Google Scholar] [CrossRef]
- Nikolaev, A.; Kolesnikov, I.; Frank-Kamenetskaya, O.; Kuz’mina, M. Europium concentration effect on characteristics and luminescent properties of hydroxyapatite nanocrystalline powders. J. Mol. Struct. 2017, 1149, 323–331. [Google Scholar] [CrossRef]
- Wiglusz, R.J.; Kedziora, A.; Lukowiak, A.; Doroszkiewicz, W.; Strek, W. Hydroxyapatites and Europium(III) doped hydroxyapatites as a carrier of silver nanoparticles and their antimicrobial activity. J. Biomed. Nanotechnol. 2018, 8, 605–612. [Google Scholar] [CrossRef]
- Tesch, A.; Wenisch, C.; Herrmann, K.-H.; Reichenbach, J.R.; Warncke, P.; Fischer, D.; Müller, F.A. Luminomagnetic Eu3+- and Dy3+-doped hydroxyapatite for multimodal imaging. Mater. Sci. Eng. C 2017, 81, 422–431. [Google Scholar] [CrossRef]
- Syamchand, S.S.; Sony, G. Europium enabled luminescent nanoparticles for biomedical applications. J. Lumin. 2015, 165, 190–215. [Google Scholar] [CrossRef]
- Chen, M.H.; Yoshioka, T.; Ikoma, T.; Hanagata, N.; Lin, F.H.; Tanaka, J. Photoluminescence and doping mechanism of theranostic Eu3+/Fe3+ dual-doped hydroxyapatite nanoparticles. Sci. Technol. Adv. Mater. 2014, 15, 055005. [Google Scholar] [CrossRef]
- Hasna, K.; Kumar, S.S.; Komath, M.; Varma, M.R.; Jayaraj, M.K.; Kumar, K.R. Synthesis of chemically pure, luminescent Eu3+ doped HAp nanoparticles: A promising fluorescent probe for in vivo imaging applications. Phys. Chem. Chem. Phys. 2013, 15, 8106. [Google Scholar] [CrossRef]
- Szustakiewicz, K.; Gazińska, M.; Kiersnowski, A.; Pigłowski, J. Polyamide 6/organomontmorillonite nanocomposites based on waste materials. Polimery 2011, 56, 397–400. [Google Scholar] [CrossRef]
- Balan, E.; Delattre, S.; Roche, D.; Segalen, L.; Morin, G.; Guillaumet, M.; Blanchard, M.; Lazzeri, M.; Brouder, C.; Salje, E.K.H. Line-broadening effects in the powder infrared spectrum of apatite. Phys. Chem. Miner. 2011, 38, 111–122. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S.; Tábi, T.; Kovács, J. Comparison of injection moulded, natural fibre-reinforced composites with PP and PLA as matrices. J. Thermoplast. Compos. Mater. 2012, 25, 927–948. [Google Scholar] [CrossRef]
- Ignjatović, N.; Savić, V.; Najman, S.; Plavšić, M.; Uskoković, D. A study of HAp/PLLA composite as a substitute for bone powder, using FT-IR spectroscopy. Biomaterials 2011, 22, 571–575. [Google Scholar] [CrossRef]
- Rasselet, D.; Ruellan, A.; Guinault, A.; Miquelard-Garnier, G.; Sollogoub, C.; Fayolle, B. Oxidative degradation of polylactide (PLA) and its effects on physical and mechanical properties. Eur. Polym. J. 2014, 50, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Androsch, R.; Schick, C.; di Lorenzo, M.L. Melting of conformationally disordered crystals (α′ phase) of poly(L-lactic acid). Macromol. Chem. Phys. 2014, 215, 1134–1139. [Google Scholar] [CrossRef]
- Wilberforce, S.I.J.; Finlayson, C.E.; Best, S.M.; Cameron, R.E. A comparative study of the thermal and dynamic mechanical behaviour of quenched and annealed bioresorbable poly-L-lactide/α-tricalcium phosphate nanocomposites. Acta Biomater. 2011, 7, 2176–2184. [Google Scholar] [CrossRef]
- Sobierajska, P.; Pazik, R.; Zawisza, K.; Renaudin, G.; Nedelec, J.-M.; Wiglusz, R.J. Effect of lithium substitution on the charge compensation, structural and luminescence properties of nanocrystalline Ca10(PO4)6F2 activated with Eu3+ ions. CrystEngComm 2016, 18, 3447–3455. [Google Scholar] [CrossRef]
- Zawisza, K.; Strzep, A.; Wiglusz, R.J. Influence of annealing temperature on the spectroscopic properties of hydroxyapatite analogues doped with Eu3+. New J. Chem. 2017, 41, 9990–9999. [Google Scholar] [CrossRef]
- Zawisza, K.; Wiglusz, R.J. Preferential site occupancy of Eu3+ ions in strontium hydroxyapatite nanocrystalline - Sr10(PO4)6(OH)2 - Structural and spectroscopic characterisation. Dalton Trans. 2017, 46, 3265–3275. [Google Scholar] [CrossRef]
- Zawisza, K.; Sobierajska, P.; Renaudin, G.; Nedelec, J.-M.; Wiglusz, R.J. Effects of crystalline growth on structural and luminescence properties of Ca(10−3x)Eu2x(PO4)6F2 nanoparticles fabricated by using a microwave driven hydrothermal process. CrystEngComm 2017, 19, 6936–6949. [Google Scholar] [CrossRef]
- Armentano, I.; Puglia, D.; Luzi, F.; Arciola, C.R.; Morena, F.; Martino, S.; Torre, L. Nanocomposites based on biodegradable polymers. Materials 2018, 11, 795. [Google Scholar] [CrossRef]
| Symbol | PLLA (wt.%) | Hap (wt.%) |
|---|---|---|
| HAp | 0 | 100 |
| 1 mol% Eu3+:HAp | 0 | 100 |
| 3 mol% Eu3+:HAp | 0 | 100 |
| 5 mol% Eu3+:HAp | 0 | 100 |
| PLLA | 100 | 0 |
| PLLA/HAp | 90 | 10 |
| PLLA/1 mol% Eu3+:HAp | 90 | 10 |
| PLLA/3 mol% Eu3+:HAp | 90 | 10 |
| PLLA/5 mol% Eu3+:HAp | 90 | 10 |
| x mol% Eu3+:HAp Powders | ||||||||
| Sample | Arad (s−1) | Anrad (s−1) | Atot (s−1) | T (ms) | Ω2 (10−20 cm2) | Ω4 (10−20 cm2) | η (%) | R |
| 1 mol% Eu3+ | 160.05 | 305.07 | 465.12 | 2.15 | 4.0441 | 1.0793 | 34.41 | 2.874 |
| 3 mol% Eu3+ | 179.94 | 391.49 | 571.43 | 1.75 | 4.6633 | 1.3196 | 31.49 | 3.314 |
| 5 mol% Eu3+ | 155.55 | 387.93 | 543.48 | 1.84 | 3.7878 | 1.2680 | 28.62 | 2.692 |
| PLLA/x mol% Eu3+:HAp Composites | ||||||||
| 1 mol% Eu3+ | 230.30 | 414.86 | 645.16 | 1.55 | 4.9835 | 4.5359 | 35.70 | 3.542 |
| 3 mol% Eu3+ | 218.28 | 517.02 | 735.29 | 1.36 | 4.5349 | 4.5455 | 29.69 | 3.223 |
| 5 mol% Eu3+ | 201.85 | 561.50 | 763.36 | 1.31 | 4.0362 | 4.3208 | 26.44 | 2.868 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szyszka, K.; Targonska, S.; Gazinska, M.; Szustakiewicz, K.; Wiglusz, R.J. The Comprehensive Approach to Preparation and Investigation of the Eu3+ Doped Hydroxyapatite/poly(L-lactide) Nanocomposites: Promising Materials for Theranostics Application. Nanomaterials 2019, 9, 1146. https://doi.org/10.3390/nano9081146
Szyszka K, Targonska S, Gazinska M, Szustakiewicz K, Wiglusz RJ. The Comprehensive Approach to Preparation and Investigation of the Eu3+ Doped Hydroxyapatite/poly(L-lactide) Nanocomposites: Promising Materials for Theranostics Application. Nanomaterials. 2019; 9(8):1146. https://doi.org/10.3390/nano9081146
Chicago/Turabian StyleSzyszka, Katarzyna, Sara Targonska, Malgorzata Gazinska, Konrad Szustakiewicz, and Rafal J. Wiglusz. 2019. "The Comprehensive Approach to Preparation and Investigation of the Eu3+ Doped Hydroxyapatite/poly(L-lactide) Nanocomposites: Promising Materials for Theranostics Application" Nanomaterials 9, no. 8: 1146. https://doi.org/10.3390/nano9081146
APA StyleSzyszka, K., Targonska, S., Gazinska, M., Szustakiewicz, K., & Wiglusz, R. J. (2019). The Comprehensive Approach to Preparation and Investigation of the Eu3+ Doped Hydroxyapatite/poly(L-lactide) Nanocomposites: Promising Materials for Theranostics Application. Nanomaterials, 9(8), 1146. https://doi.org/10.3390/nano9081146