Hafnium-Doped Mesoporous Silica as Efficient Lewis Acidic Catalyst for Friedel–Crafts Alkylation Reactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalysts Characterization
2.3. Catalysts Preparation
2.4. Catalytic Reactions
3. Results
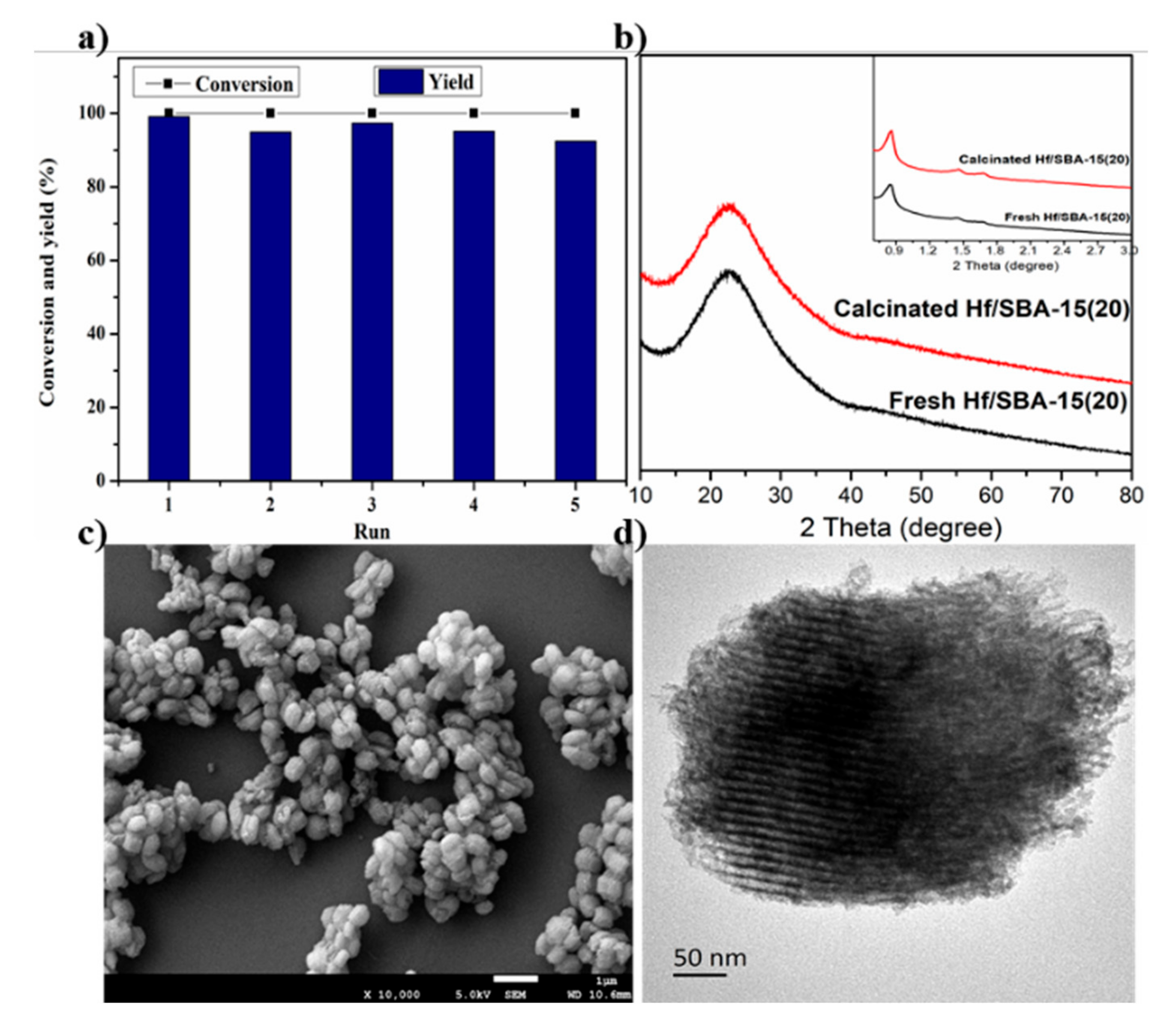
3.1. Catalysts Characterization
3.2. Catalyst Screening
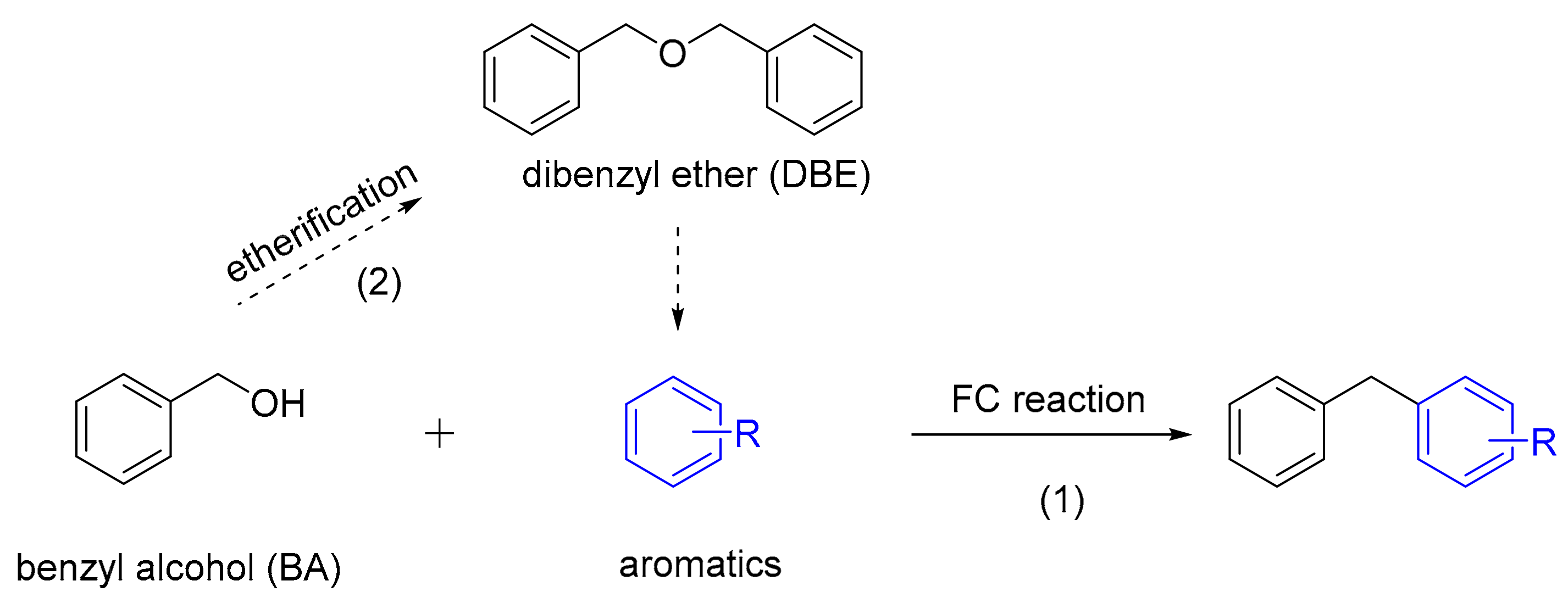
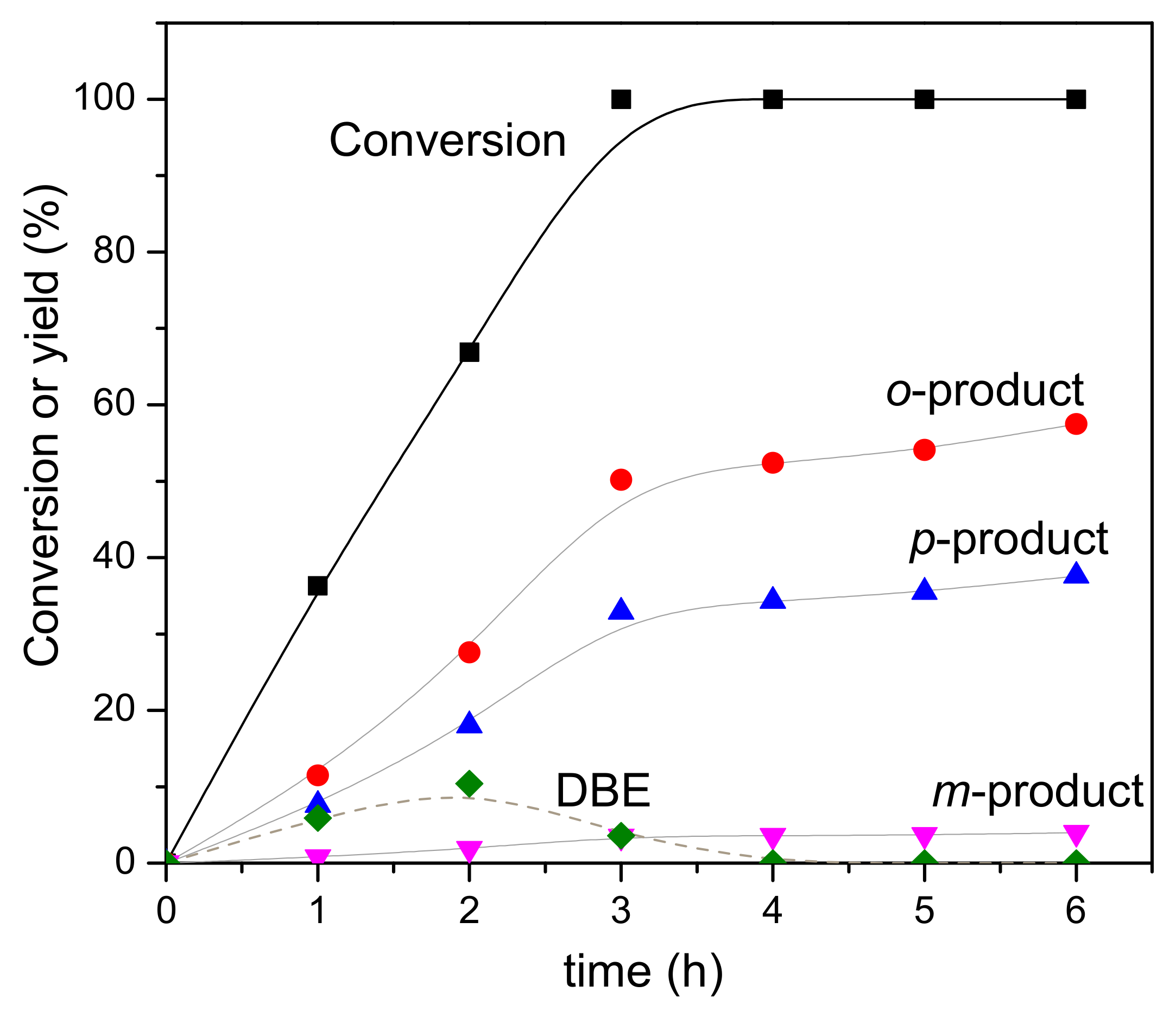
3.3. Reaction Pathway
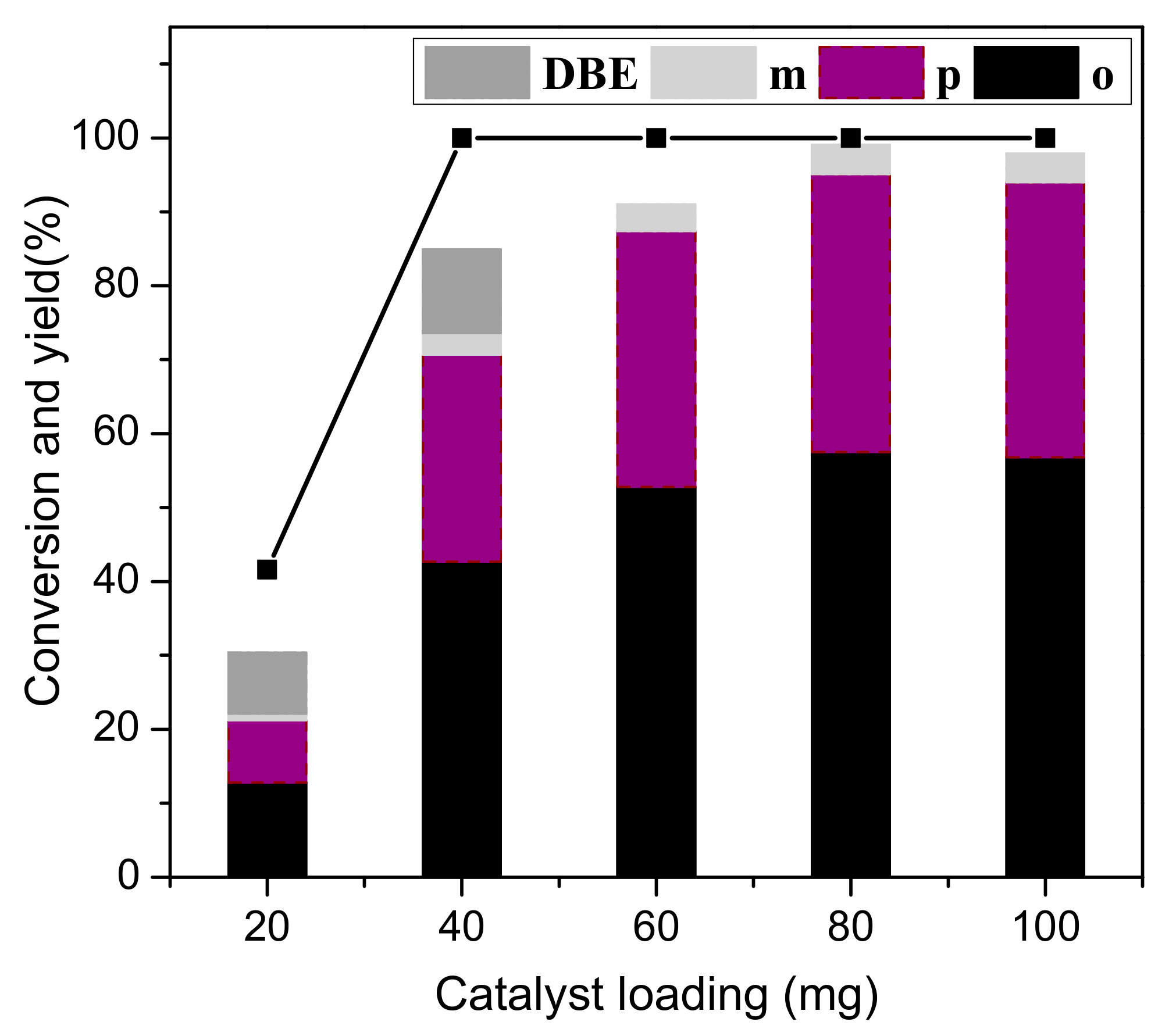
3.4. Effect of Catalyst Loading
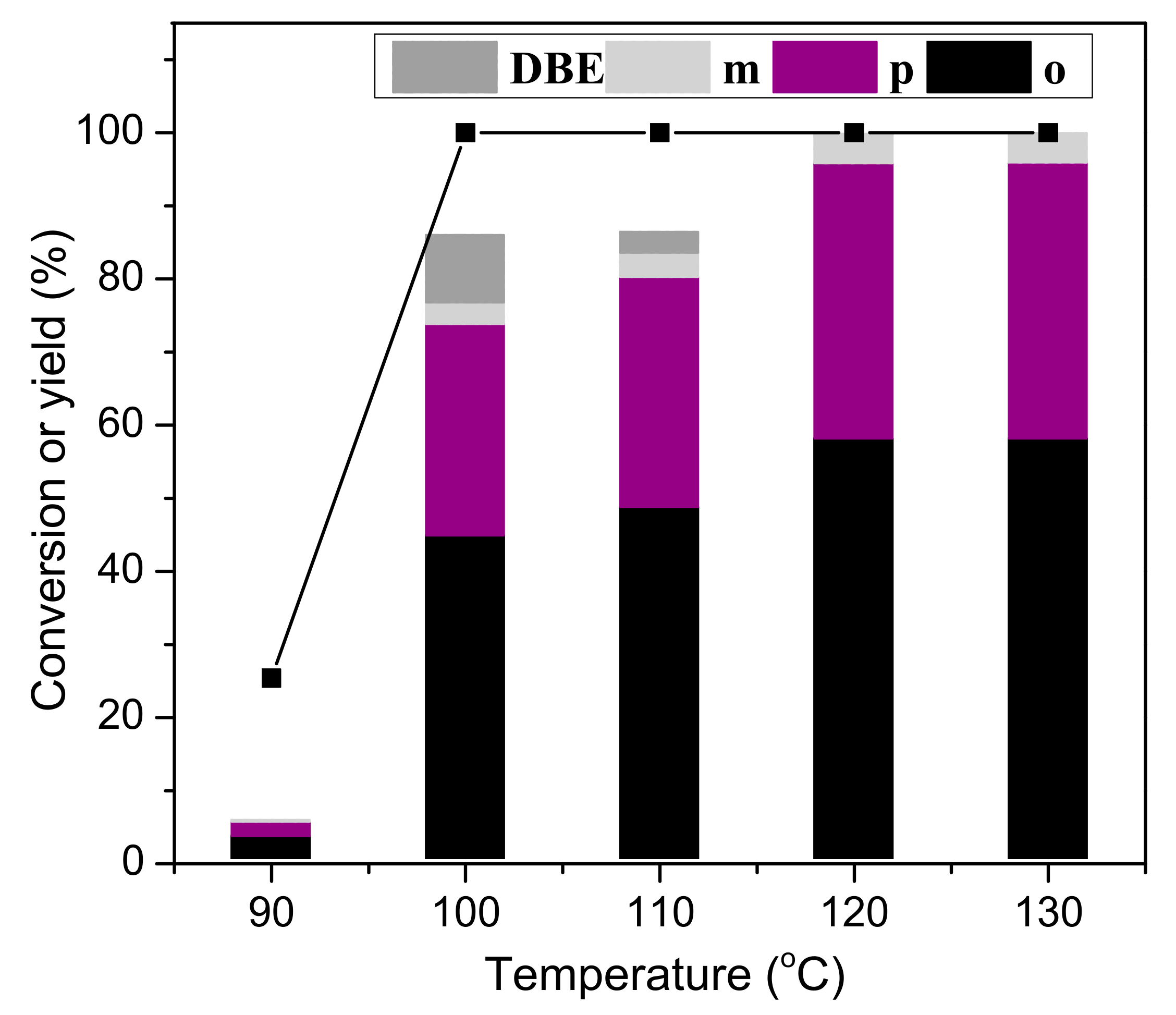
3.5. Effect of Reaction Temperature
3.6. Substrate Applications
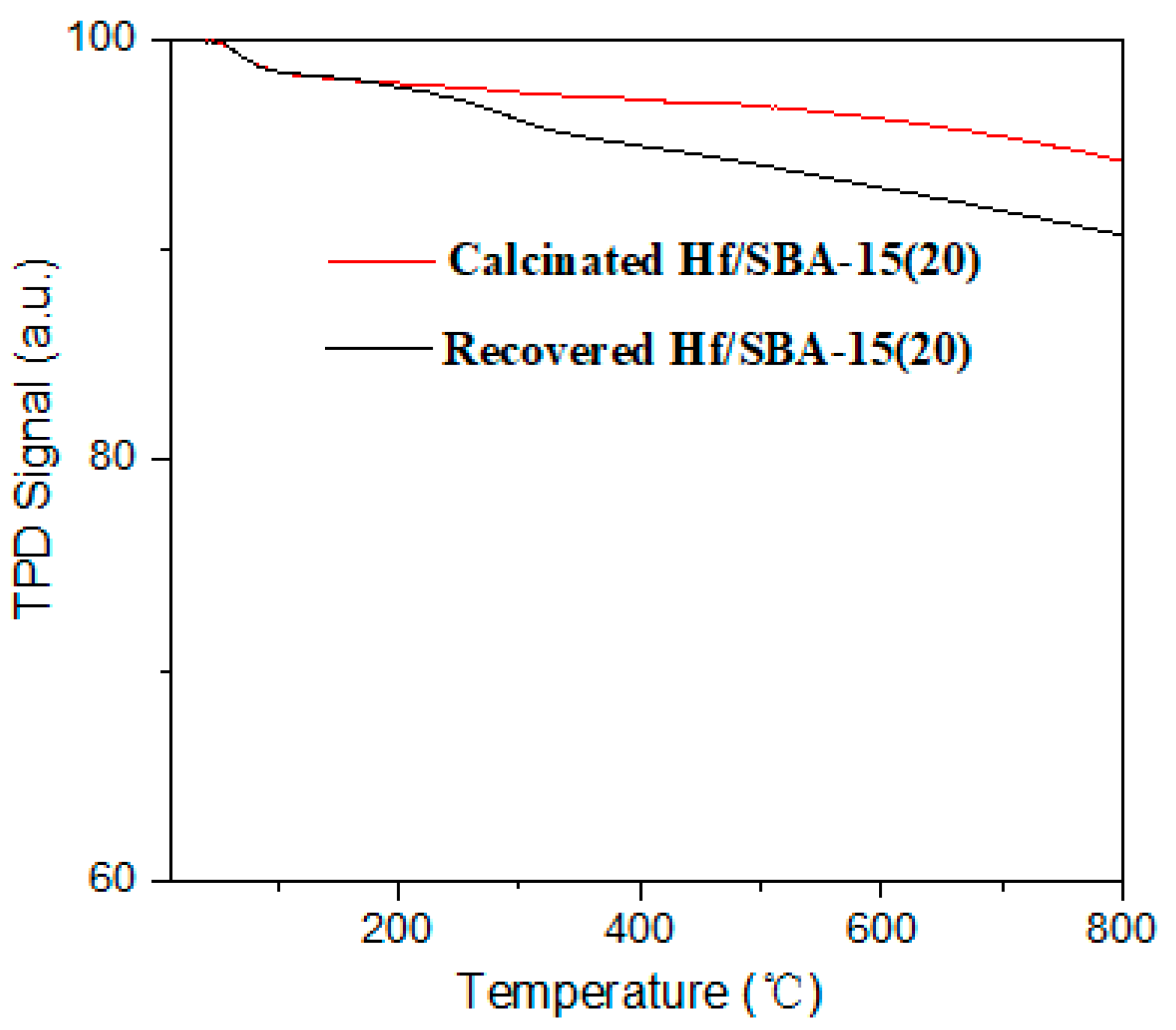
3.7. Catalyst Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bandini, M.; Melloni, A.; Umani-Ronchi, A. New Catalytic Approaches in the Stereoselective Friedel–Crafts Alkylation Reaction. Angew. Chem. Int. Ed. 2004, 43, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.J.; Milcent, T.; Crousse, B. Bisulfate Salt-Catalyzed Friedel–Crafts Benzylation of Arenes with Benzylic Alcohols. J. Org. Chem. 2018, 83, 14001–14009. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chuah, G.K.; Jaenicke, S. Room temperature synthesis of diphenylmethane over MCM-41 supported AlCl3 and other Lewis acids. Appl. Catal. A. Gen. 2001, 217, 1–9. [Google Scholar] [CrossRef]
- Arias, K.S.; Climent, M.J.; Corma, A.; Iborra, S. Synthesis of high-quality alkyl naphthenic kerosene by reacting an oil refinery with a biomass refinery stream. Energy Environ. Sci. 2015, 8, 317–331. [Google Scholar] [CrossRef]
- Yuan, B.; Li, Y.; Wang, Z.; Yu, F.; Xie, C.; Yu, S. A Novel Brønsted-Lewis acidic catalyst based on heteropoly phosphotungstates: Synthesis and catalysis in benzylation of p-xylene with benzyl alcohol. Mol. Catal. 2017, 443, 110–116. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Le, K.K.A.; Phan, T.D. MOF-5 as an efficient heterogeneous catalyst for Friedel-Crafts alkylation reactions. Appl. Catal. A Gen. 2010, 382, 246–253. [Google Scholar] [CrossRef]
- De la Cruz, M.C.H.; da Silva, J.F.C.; Lachter, E.R. Catalytic activity of niobium phosphate in the Friedel–Crafts reaction of anisole with alcohols. Catal. Today 2006, 118, 379–384. [Google Scholar] [CrossRef]
- Ruengsangtongkul, S.; Taprasert, P.; Sirion, U.; Jaratjaroonphong, J. Facile synthesis of nonsymmetrical heteroaryl-substituted triarylmethanes via the FeCl3·6H2O-catalyzed two-step Friedel-Crafts-type reaction. Org. Biomol. Chem. 2016, 14, 8493–8502. [Google Scholar] [CrossRef]
- Satam, J.R.; Jayaram, R.V. Liquid phase Friedel–Crafts benzylation of aromatics on a polymer-supported 12-tungstophosphoric acid catalyst. Catal. Commun. 2008, 9, 1937–1940. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, L.; Hu, J.; Li, W.; Wang, J. Effect of zinc salt on the synthesis of ZSM-5 for alkylation of benzene with ethanol. Catal. Commun. 2009, 10, 1615–1619. [Google Scholar] [CrossRef]
- Chaube, V.D. Benzylation of benzene to diphenylmethane using zeolite catalysts. Catal. Commun. 2004, 5, 321–326. [Google Scholar] [CrossRef]
- Taylor, S.F.R.; Sa, J.; Hardacre, C. Friedel–Crafts Alkylation of Aromatics with Benzyl Alcohol over Gold-Modified Silica. ChemCatChem 2011, 3, 119–121. [Google Scholar] [CrossRef]
- Kumar, C.R.; Rao, K.T.V.; Prasad, P.S.S.; Lingaiah, N. Tin exchanged heteropoly tungstate: An efficient catalyst for benzylation of arenes with benzyl alcohol. J. Mol. Catal. A Chem. 2011, 337, 17–24. [Google Scholar] [CrossRef]
- Mantri, K.; Komura, K.; Kubota, Y.; Sugi, Y. Friedel-Crafts alkylation of aromatics with benzyl alcohols catalyzed by rare earth metal triflates supported on MCM-41 mesoporous silica. J. Mol. Catal. A Chem. 2005, 236, 168–175. [Google Scholar] [CrossRef]
- Kaper, H.; Bouchmella, K.; Mutin, P.H.; Goettmann, F. High-Surface-Area SiO2-ZrO2 Mixed Oxides as catalysts for the Friedel-Crafts-Type alkylation of arenes with alcohols and tandem cyclopropanation reactions. ChemCatChem 2012, 4, 1813–1818. [Google Scholar] [CrossRef]
- Luo, Y.J.; Zhou, Y.H.; Huang, Y.B. A New Lewis Acidic Zr Catalyst for the Synthesis of Furanic Diesel Precursor from Biomass Derived Furfural and 2-Methylfuran. Catal. Lett. 2019, 149, 292–302. [Google Scholar] [CrossRef]
- Thunyaratchatanon, C.; Luengnaruemitchai, A.; Chaisuwan, T.; Chollacoop, N.; Chen, S.Y.; Yoshimura, Y. Synthesis and characterization of Zr incorporation into highly ordered mesostructured SBA-15 material and its performance for CO2, adsorption. Microporous Mesoporous Mater. 2017, 253, 18–28. [Google Scholar] [CrossRef]
- Cao, Z.; Fan, Z.; Chen, Y.; Li, M.; Shen, T.; Zhu, C.; Ying, H. Efficient preparation of 5-hydroxymethylfurfural from cellulose in a biphasic system over hafnyl phosphates. Appl. Catal. B Environ. 2019, 244, 170–177. [Google Scholar] [CrossRef]
- Feng, G.; Wang, J.; Boronat, M.; Li, Y.; Su, J.H.; Huang, J.; Ma, Y.; Yu, J. Radical-Facilitated Green Synthesis of Highly Ordered Mesoporous Silica Materials. J. Am. Chem. Soc. 2018, 140, 4770–4773. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Luo, Y.J.; Lin, Y.T.; Huang, Y.B. Enhanced Transfer Hydrogenation Activity of Zr-Doped Mesoporous Silica through Sol-Gel Method for the Reduction of Biomass-Derived Unsaturated Carbon-Oxygen Bonds. Chem. Sel. 2018, 3, 11071–11080. [Google Scholar] [CrossRef]
- Armelao, L.; Eisenmenger-Sittner, C.; Groenewolt, M.; Gross, S.; Sada, C.; Schubert, U.; Tondello, E.; Zattin, A. Zirconium and hafnium oxoclusters as molecular building blocks for highly dispersed ZrO2 or HfO2 nanoparticles in silica thin films. Mater. Chem. 2005, 15, 1838–1848. [Google Scholar] [CrossRef]
- Arafat, A.; Bamufleh, H.S. Fe2O3/TUD-1: An efficient catalyst for Friedel-Crafts alkylation of aromatics. J. Porous Mater. 2014, 21, 1091–1100. [Google Scholar] [CrossRef]
- Scott, S.L. A matter of life(time) and death. ACS Catal. 2018, 8, 8597–8599. [Google Scholar] [CrossRef]
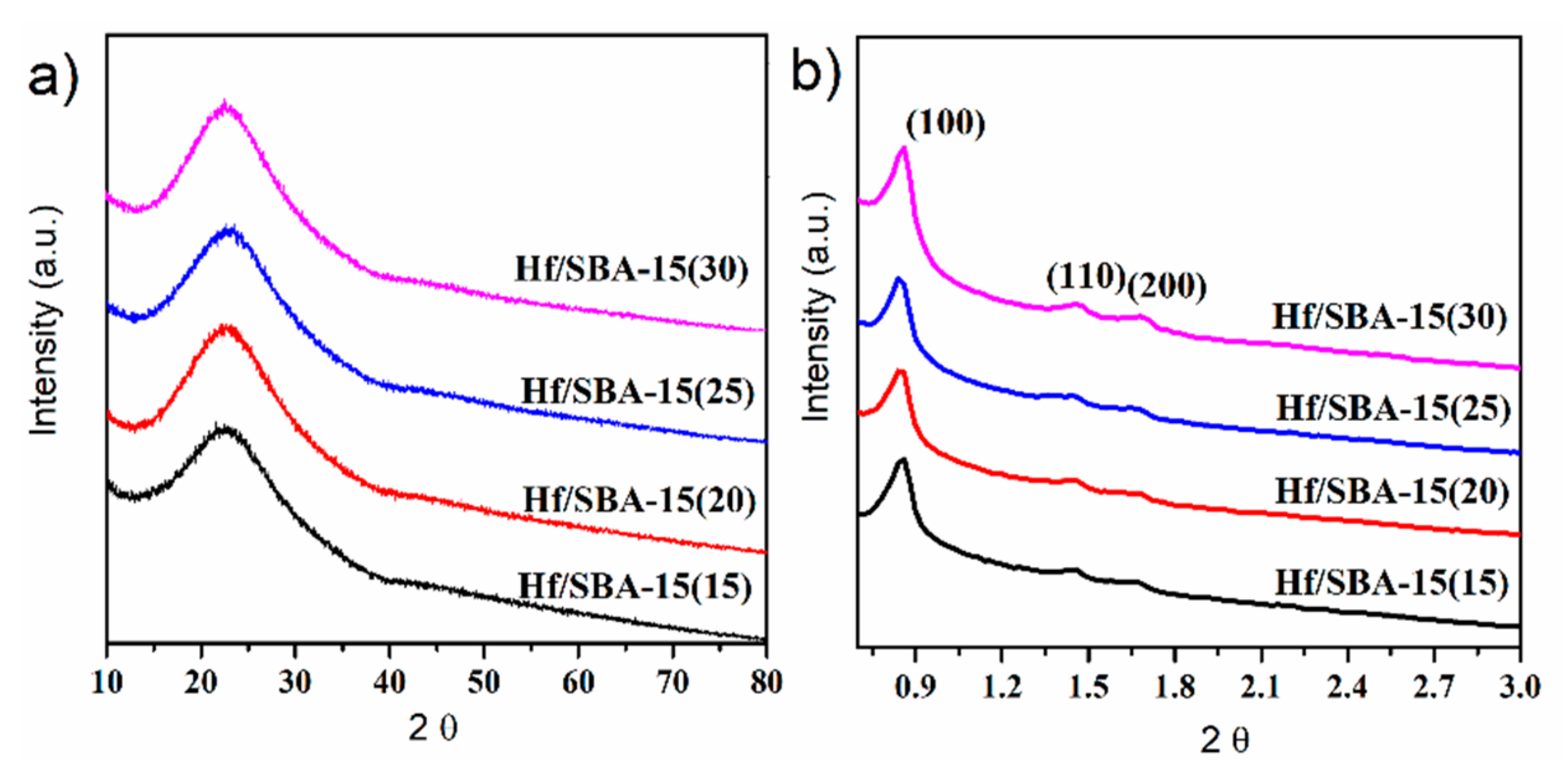

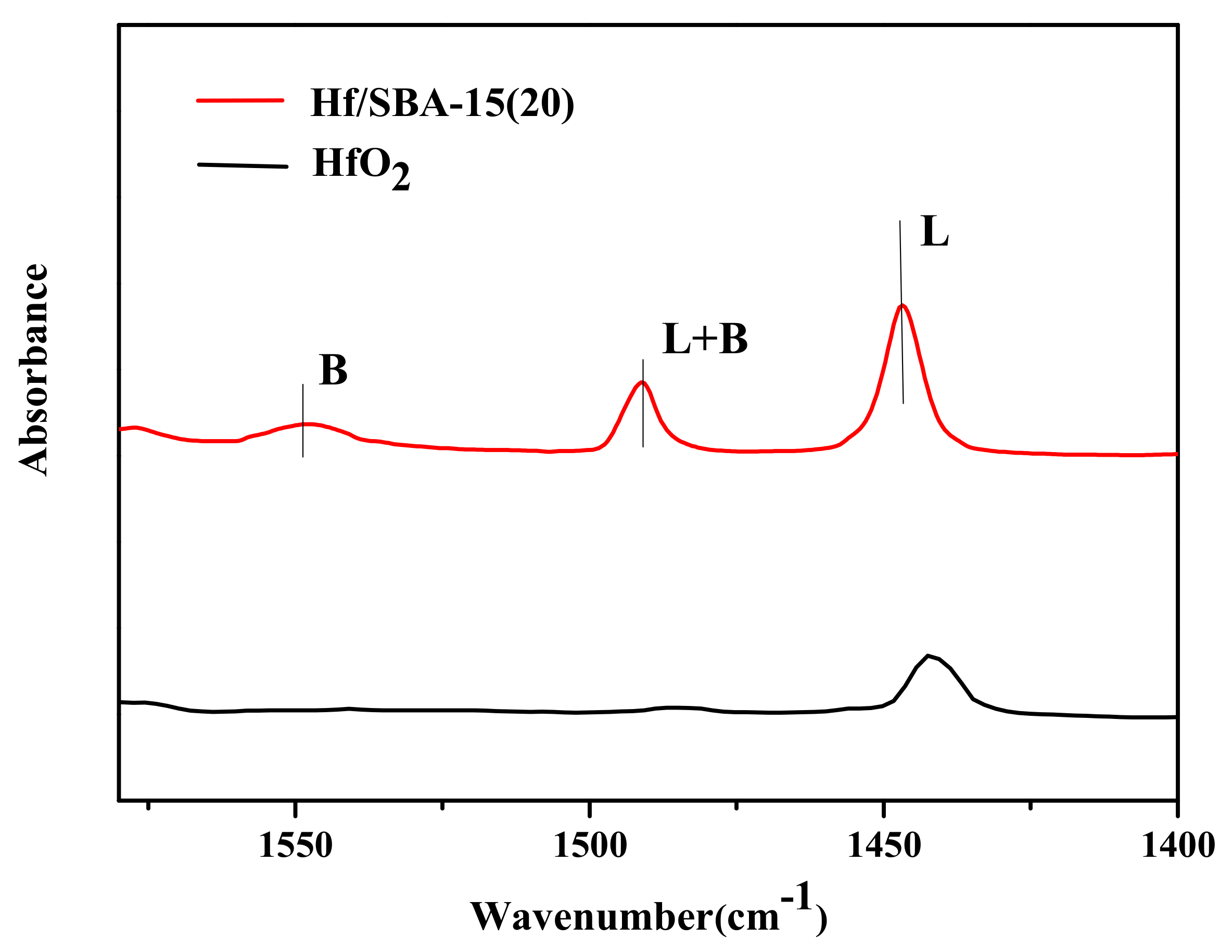
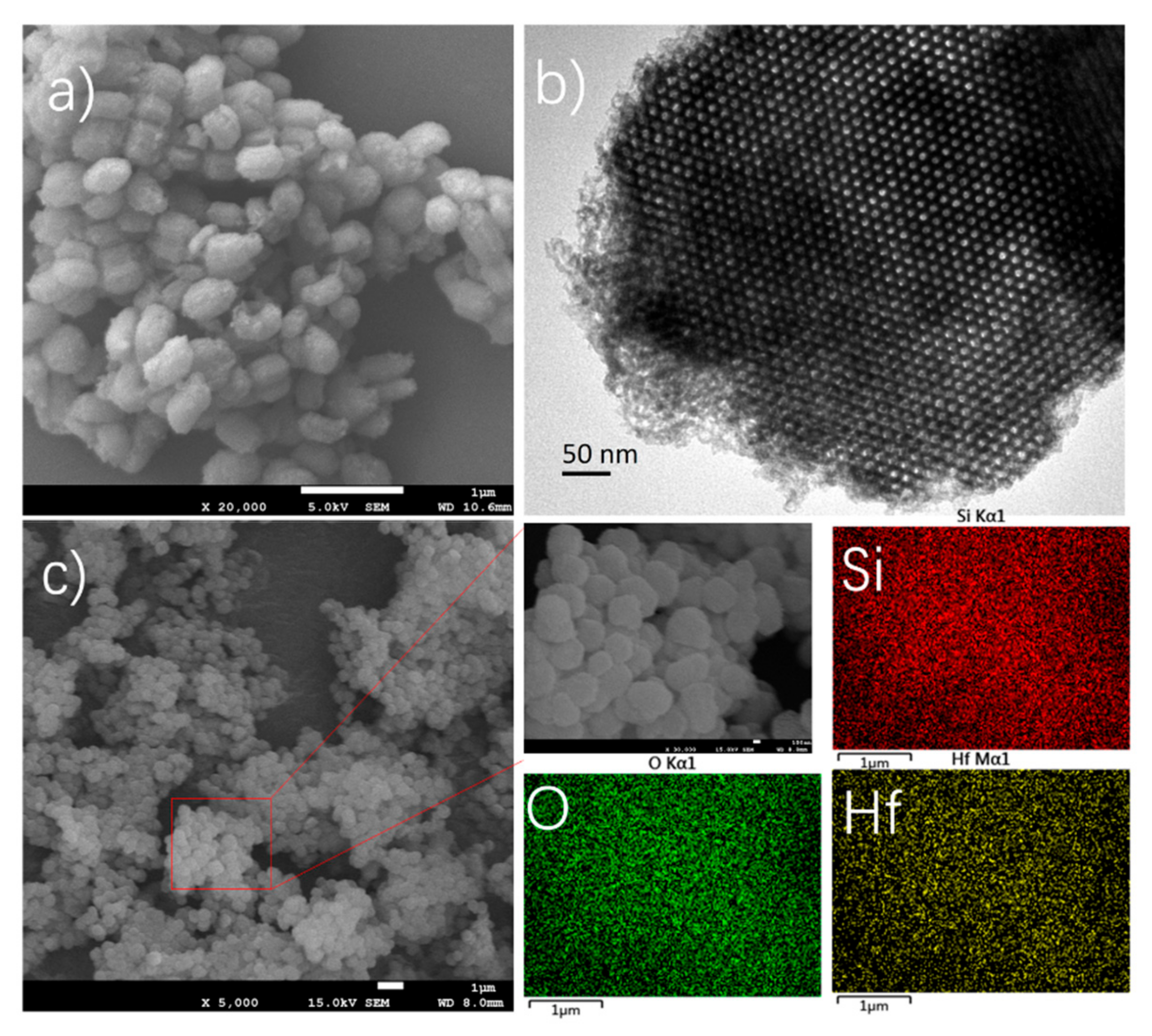
| Catalysts | Surface BET (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| Hf/SBA-15(15) | 624.4 | 1.1 | 10.3 |
| Hf/SBA-15(20) | 724.2 | 1.3 | 10.4 |
| Hf/SBA-15(25) | 774.7 | 1.3 | 10.3 |
| Hf/SBA-15(30) | 811.3 | 1.4 | 10.0 |
| Entry | Catalyst | Conversion (%) | BA Yield (%) | DBE Yield (%) | o/p/m |
|---|---|---|---|---|---|
| 1 | SBA-15 | 0 | 0 | - | - |
| 2 b | Zr/SBA-15(20) | 100 | 89.3 | - | 57:39:4 |
| 3 b | W/SBA-15(20) | 100 | 83.9 | - | 55:40:5 |
| 4 b | Fe/SBA-15(20) | 0 | 0 | - | - |
| 5 | Hf/SBA-15(15) | 100 | 92.4 | - | 57:39:4 |
| 6 c | Hf/SBA-15(20) | 100 | 99.1 (98.0) | - | 58:38:4 |
| 7 | Hf/SBA-15(25) | 100 | 94.2 | - | 57:38:5 |
| 8 | Hf/SBA-15(30) | 100 | 94.7 | - | 57:38:5 |
| 9 | HfCl4 | 0 | 0 | - | - |
| 10 | HfO2 | 0 | 0 | - | - |
| 11 | HfO(PO4)2 | 86.1 | 2.7 | 5.2 | 56:40:4 |
| 12 | HfO(OH)2 | 0 | 0 | - | - |
| 13 | Amberlyst-15 | 100 | 59 | - | 55:40:5 |
| 14 | HZSM-5 | 58.8 | 11.3 | 13.7 | 56:39:5 |
| 15 | H-β | 100 | 77.7 | - | 57:39:4 |
| Entry | Alcohol | Aromatics | Alcohol Conversion (%) | GC Yield (%) | Isolated Yield (%) | o/p/m c |
|---|---|---|---|---|---|---|
| 1 | 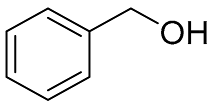 | 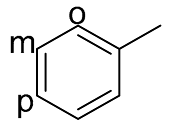 | 100 | 99.1 | 98.0 | 58:38:4 |
| 2 b |  |  | 100 | 65.8 | 63.1 | 76:22:1 |
| 3 | 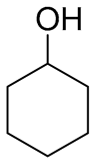 | 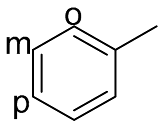 | 0 | 0 | - | - |
| 4 | 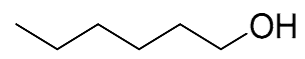 | 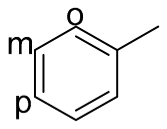 | 0 | 0 | - | - |
| 5 | 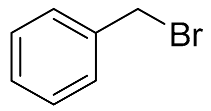 | 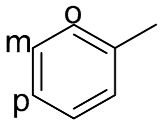 | 100 | 99.5 | 99.1 | 58:38:4 |
| 6 | 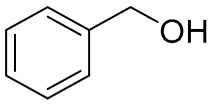 | 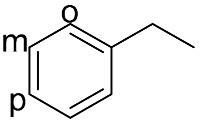 | 100 | 99.0 | 98.2 | 61:36:3 |
| 7 | 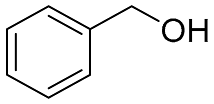 | 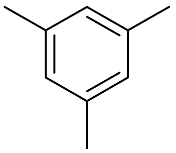 | 100 | 99.0 | 97.5 | - |
| 8 |  | 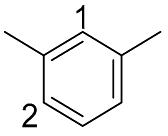 | 100 | 96.6 | 95.1 | 20:80 (1:2) |
| 9 | 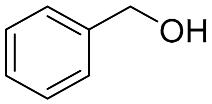 | 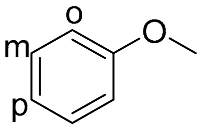 | 100 | 98.1 | 97.5 | 55:45:0 |
| 10 b | 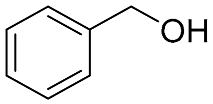 | 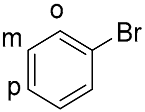 | 100 | 11.7 (31.2) | 10.5 (29.0) | 52:48:0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-B.; Luo, Y.-J.; Wang, F. Hafnium-Doped Mesoporous Silica as Efficient Lewis Acidic Catalyst for Friedel–Crafts Alkylation Reactions. Nanomaterials 2019, 9, 1128. https://doi.org/10.3390/nano9081128
Huang Y-B, Luo Y-J, Wang F. Hafnium-Doped Mesoporous Silica as Efficient Lewis Acidic Catalyst for Friedel–Crafts Alkylation Reactions. Nanomaterials. 2019; 9(8):1128. https://doi.org/10.3390/nano9081128
Chicago/Turabian StyleHuang, Yao-Bing, Yu-Jia Luo, and Fei Wang. 2019. "Hafnium-Doped Mesoporous Silica as Efficient Lewis Acidic Catalyst for Friedel–Crafts Alkylation Reactions" Nanomaterials 9, no. 8: 1128. https://doi.org/10.3390/nano9081128
APA StyleHuang, Y.-B., Luo, Y.-J., & Wang, F. (2019). Hafnium-Doped Mesoporous Silica as Efficient Lewis Acidic Catalyst for Friedel–Crafts Alkylation Reactions. Nanomaterials, 9(8), 1128. https://doi.org/10.3390/nano9081128





