Prolonged Release and Functionality of Interleukin-10 Encapsulated within PLA-PEG Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line
2.2. Stimulants
2.3. Stimulation of Macrophages
2.4. Preparation of Nanoparticles
2.5. Encapsulation Efficiency
2.6. Zeta-Sizer, Zeta-Potential, and Polydispersity Index (PDI) Measurements
2.7. UV Visible Spectra (UV-Vis)
2.8. Fourier Transform-Infrared Spectrometry (FT-IR)
2.9. Differential Scanning Calorimetry (DSC)
2.10. In Vitro Release of Encapsulated-IL-10
2.11. In Vitro Stimulation of Macrophages with Encapsulated Nanoparticles
2.12. Cytokines Measurement
2.13. RNA Extraction and Quantitative Real Time-PCR (qRT-PCR)
2.14. Statistical Analysis
3. Results
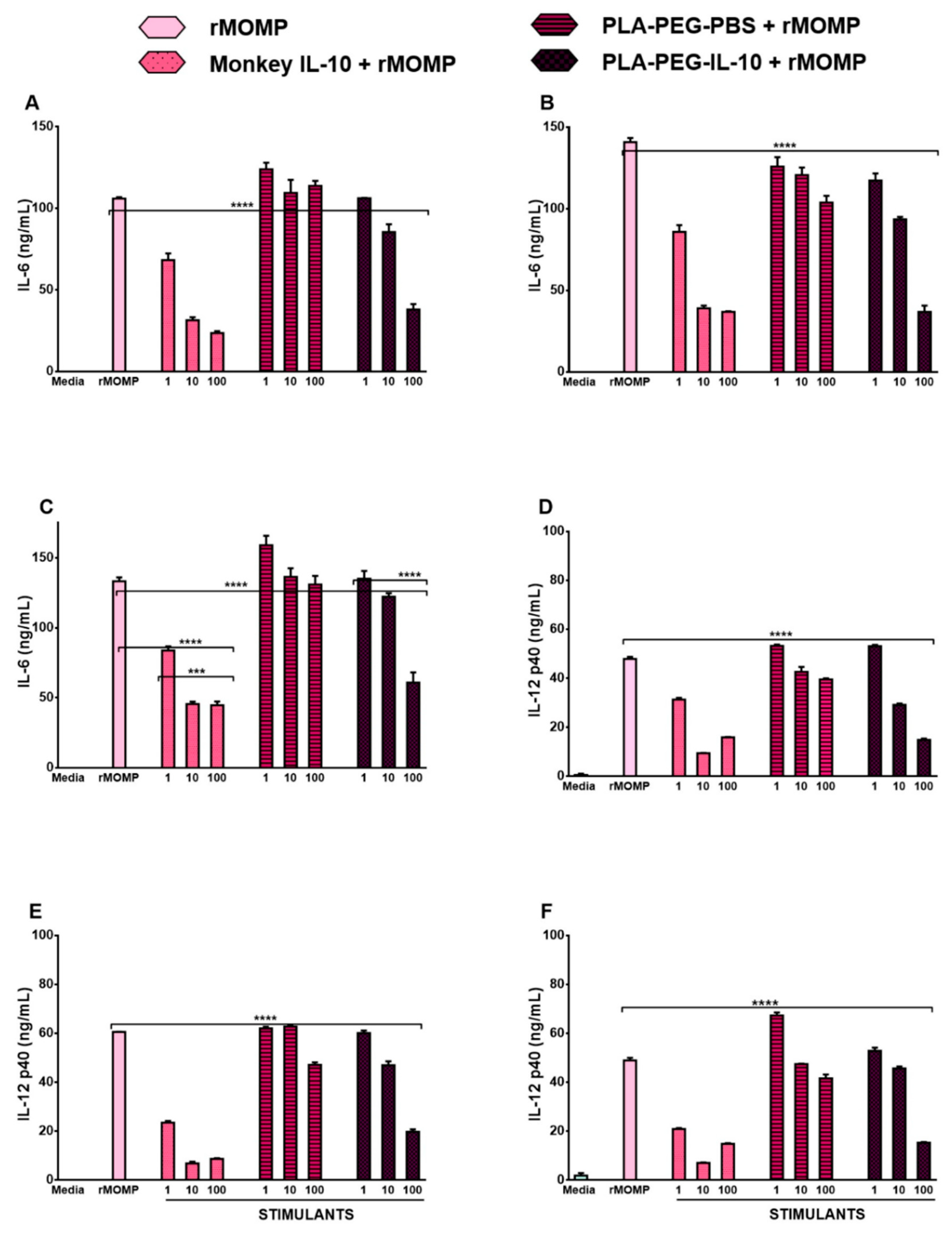
3.1. Monkey IL-10 Is Efficacious at Suppressing Chlamydial-Induced Inflammatory Cytokines
3.2. Chlamydia rMOMP Triggers Enhanced mRNA Gene Transcripts of SOCS3 and STAT1 Alone or Combined with Mouse IL-10 in Macrophages
3.3. Physical-Structural Characterization of Monkey Recombinant IL-10 Encapsulated in PLA-PEG Nanoparticles
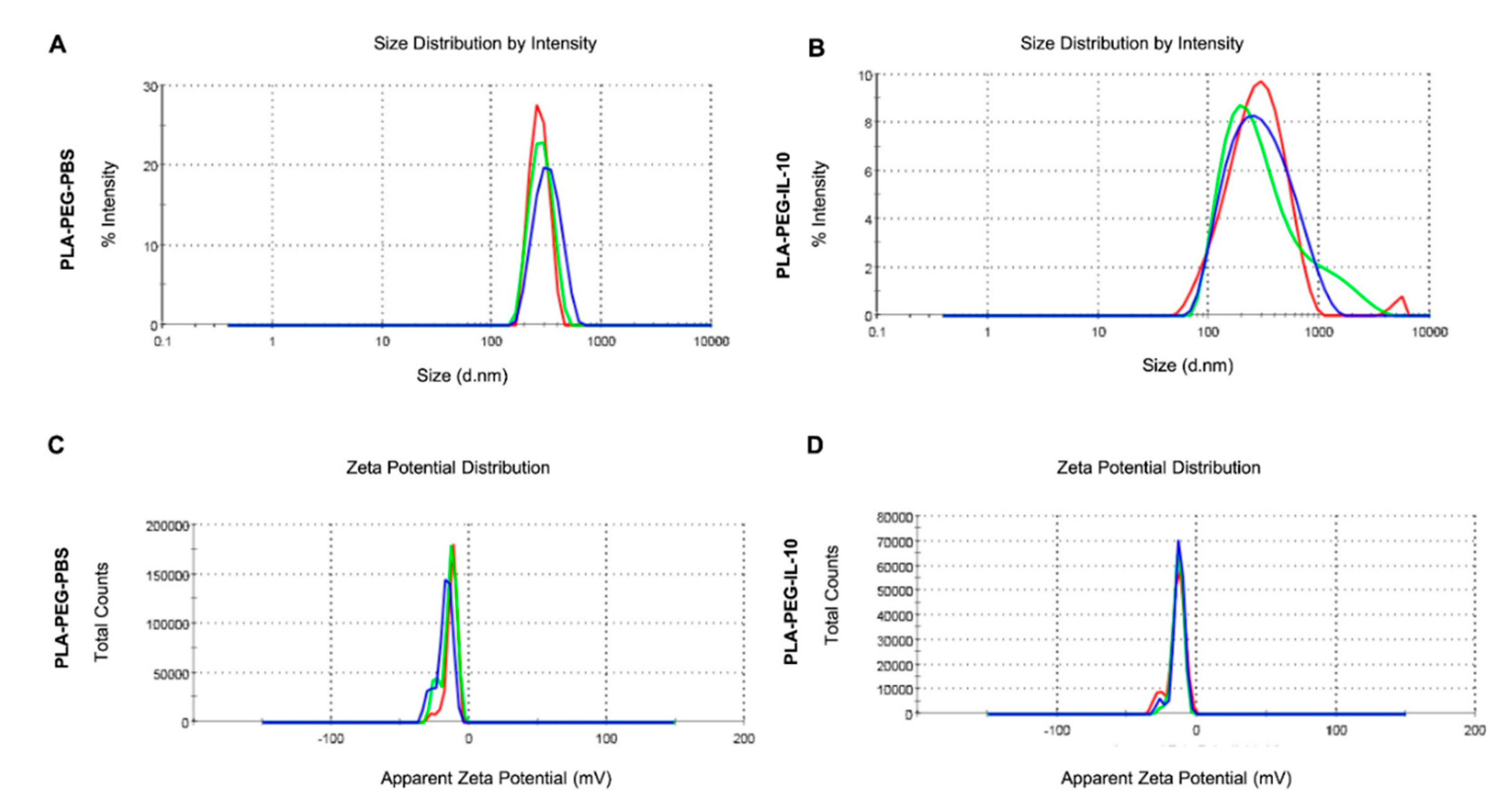
3.3.1. Encapsulation Efficiency, Zeta-Sizing, Zeta-Potential, and PDI
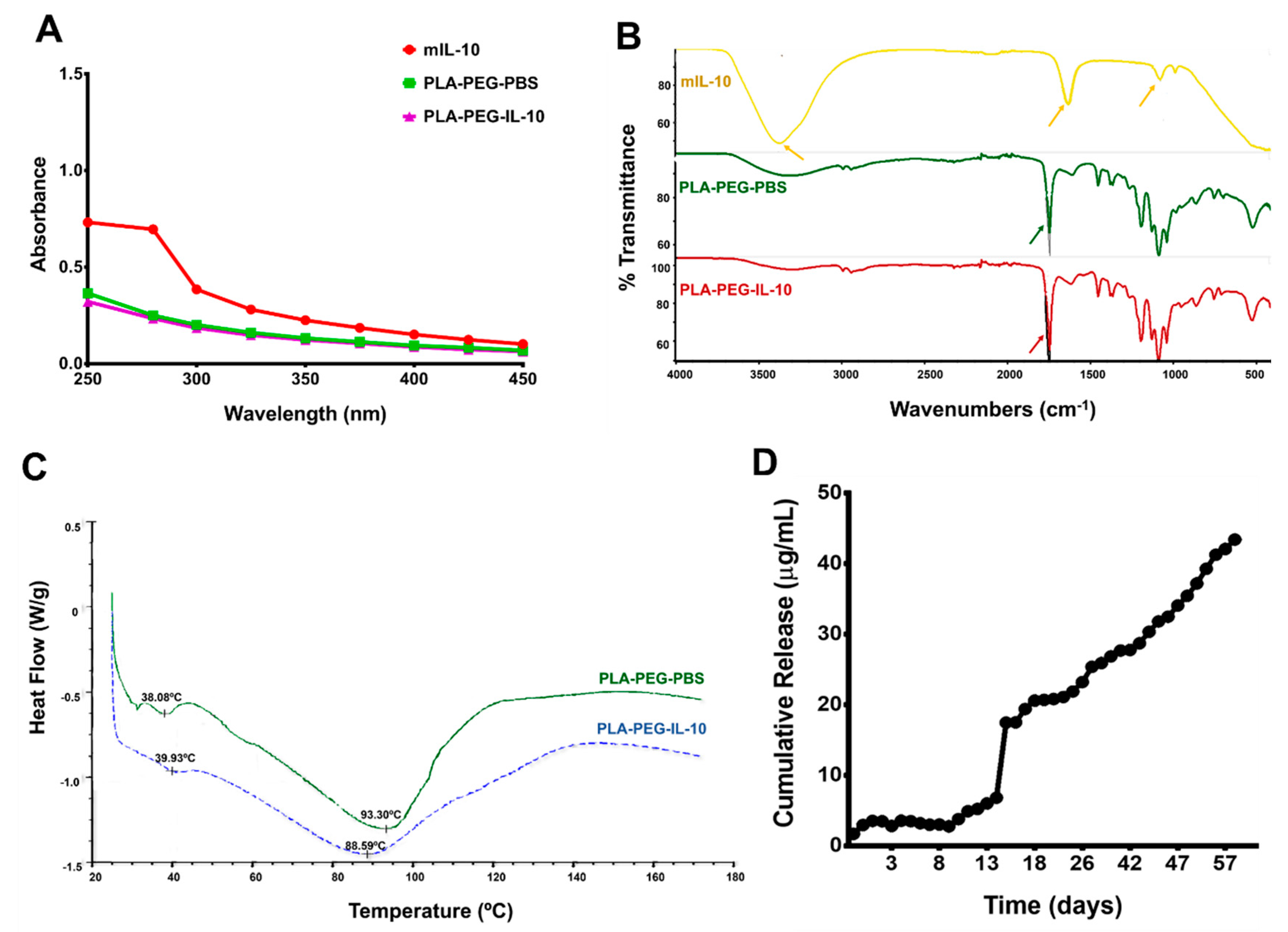
3.3.2. Spectrometry, Differential Scanning Calorimetry (DSC), and In Vitro Release of Encapsulated IL-10
3.4. In Vitro Bioactivity of PLA-PEG-IL-10
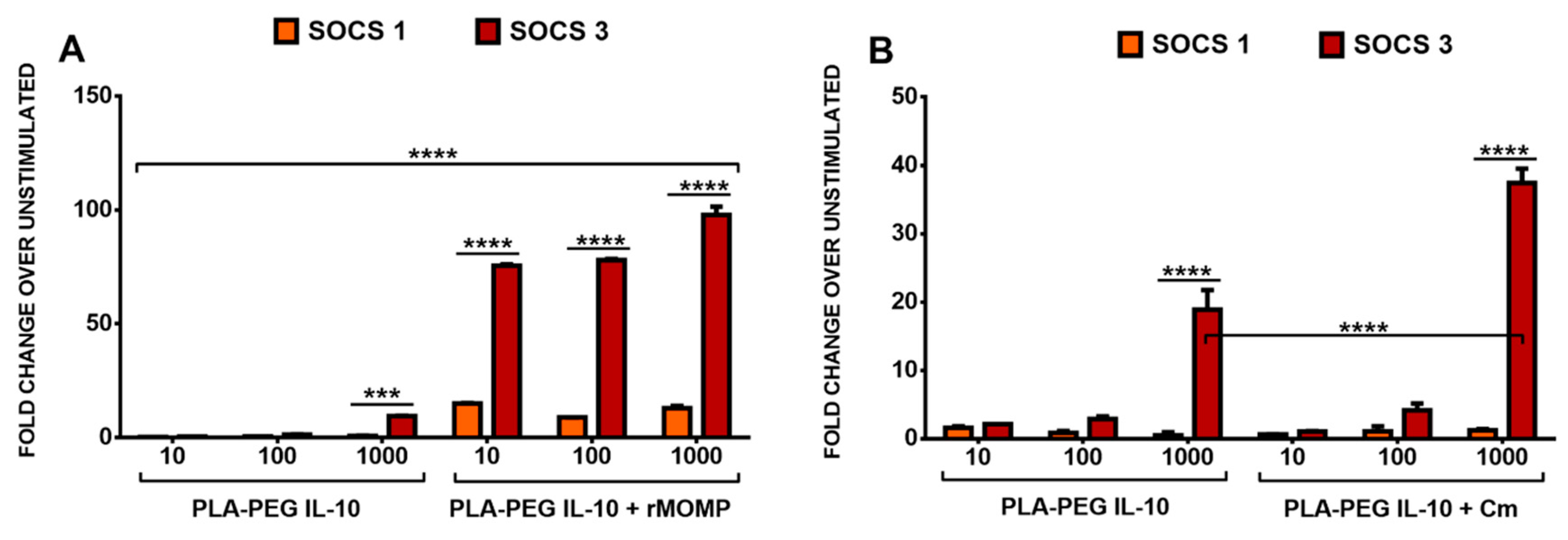
3.5. Differential SOCS1 and SOCS3 Expressions in Response to the Stimulation of Macrophages with rMOMP and Encapsulated IL-10
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malhotra, M.; Sood, S.; Mukherjee, A.; Muralidhar, S.; Bala, M. Genital Chlamydia trachomatis: An update. Indian J. Med. Res. 2013, 138, 303–316. [Google Scholar] [PubMed]
- Finethy, R.; Coers, J. Sensing the enemy, containing the threat: Cell-autonomous immunity to Chlamydia trachomatis. FEMS Microbiol. Rev. 2016, 40, 875–893. [Google Scholar] [CrossRef] [PubMed]
- Redgrove, K.A.; McLaughlin, E.A. The Role of the Immune Response in Chlamydia trachomatis Infection of the Male Genital Tract: A Double-Edged Sword. Front. Immunol. 2014, 5, 534. [Google Scholar] [CrossRef] [PubMed]
- Yilma, A.N.; Singh, S.R.; Fairley, S.J.; Taha, M.A.; Dennis, V.A. The anti-inflammatory cytokine, interleukin-10, inhibits inflammatory mediators in human epithelial cells and mouse macrophages exposed to live and UV-inactivated Chlamydia trachomatis. Mediat. Inflamm. 2012, 2012, 520174. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Asadullah, K.; Sterry, W.; Volk, H.D. Interleukin-10 therapy—review of a new approach. Pharm. Rev. 2003, 55, 241–269. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Schmidt, J.; Londono, D.; Bai, Y.; Quandt, J.; Hornung, R.; Marques, A.; Martin, R.; Cadavid, D. Role of interleukin 10 during persistent infection with the relapsing fever Spirochete Borrelia turicatae. Am. J. Pathol. 2007, 170, 251–262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stober, C.B.; Lange, U.G.; Roberts, M.T.; Alcami, A.; Blackwell, J.M. IL-10 from regulatory T cells determines vaccine efficacy in murine Leishmania major infection. J. Immunol. 2005, 175, 2517–2524. [Google Scholar] [CrossRef]
- Sharma, S.; Yang, B.; Xi, X.; Grotta, J.C.; Aronowski, J.; Savitz, S.I. IL-10 directly protects cortical neurons by activating PI-3 kinase and STAT-3 pathways. Brain Res. 2011, 1373, 189–194. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Lalani, I.; Bhol, K.; Ahmed, A.R. Interleukin-10: Biology, role in inflammation and autoimmunity. Ann. Allergy Asthma Immunol. 1997, 79, 469–483. [Google Scholar] [CrossRef]
- Chen, K.S.; Wang, P.H.; Yang, S.F.; Lin, D.B.; Lin, Y.J.; Kuo, D.Y.; Lin, L.Y.; Wu, M.T.; Lin, C.W.; Lee, S.; et al. Significant elevation of a Th2 cytokine, interleukin-10, in pelvic inflammatory disease. Clin. Chem. Lab. Med. 2008, 46, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Li, J.L.; Wang, D.G.; Zhou, D. Targeting IL-10 in auto-immune diseases. Cell Biochem. Biophys. 2014, 70, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Beebe, A.M.; Cua, D.J.; de Waal Malefyt, R. The role of interleukin-10 in autoimmune disease: Systemic lupus erythematosus (SLE) and multiple sclerosis (MS). Cytokine Growth Factor Rev. 2002, 13, 403–412. [Google Scholar] [CrossRef]
- Asadullah, K.; Docke, W.D.; Sabat, R.V.; Volk, H.D.; Sterry, W. The treatment of psoriasis with IL-10: Rationale and review of the first clinical trials. Expert Opin. Investig. Drugs 2000, 9, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Asadullah, K.; Sterry, W.; Stephanek, K.; Jasulaitis, D.; Leupold, M.; Audring, H.; Volk, H.D.; Docke, W.D. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: A new therapeutic approach. J. Clin. Investig. 1998, 101, 783–794. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 18. [Google Scholar] [CrossRef]
- Cho, H.; Gao, J.; Kwon, G.S. PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol-gels for drug delivery. J. Control. Release 2016, 240, 191–201. [Google Scholar] [CrossRef]
- Irache, J.M.; Esparza, I.; Gamazo, C.; Agueros, M.; Espuelas, S. Nanomedicine: Novel approaches in human and veterinary therapeutics. Vet. Parasitol. 2011, 180, 47–71. [Google Scholar] [CrossRef]
- Shalgunov, V.; Zaytseva-Zotova, D.; Zintchenko, A.; Levada, T.; Shilov, Y.; Andreyev, D.; Dzhumashev, D.; Metelkin, E.; Urusova, A.; Demin, O.; et al. Comprehensive study of the drug delivery properties of poly(l-lactide)-poly(ethylene glycol) nanoparticles in rats and tumor-bearing mice. J. Control. Release 2017, 261, 31–42. [Google Scholar] [CrossRef]
- Essa, S.; Louhichi, F.; Raymond, M.; Hildgen, P. Improved antifungal activity of itraconazole-loaded PEG/PLA nanoparticles. J. Microencapsul. 2013, 30, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.A.; Singh, S.R.; Dennis, V.A. Biodegradable PLGA85/15 nanoparticles as a delivery vehicle for Chlamydia trachomatis recombinant MOMP-187 peptide. Nanotechnology 2012, 23, 325101. [Google Scholar] [CrossRef] [PubMed]
- Fairley, S.J.; Singh, S.R.; Yilma, A.N.; Waffo, A.B.; Subbarayan, P.; Dixit, S.; Taha, M.A.; Cambridge, C.D.; Dennis, V.A. Chlamydia trachomatis recombinant MOMP encapsulated in PLGA nanoparticles triggers primarily T helper 1 cellular and antibody immune responses in mice: A desirable candidate nanovaccine. Int. J. Nanomed. 2013, 8, 2085–2099. [Google Scholar] [CrossRef]
- Dixit, S.; Sahu, R.; Verma, R.; Duncan, S.; Giambartolomei, G.H.; Singh, S.R.; Dennis, V.A. Caveolin-mediated endocytosis of the Chlamydia M278 outer membrane peptide encapsulated in poly(lactic acid)-Poly(ethylene glycol) nanoparticles by mouse primary dendritic cells enhances specific immune effectors mediated by MHC class II and CD4(+) T cells. Biomaterials 2018, 159, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Singh, S.R.; Yilma, A.N.; Agee, R.D., II; Taha, M.; Dennis, V.A. Poly(lactic acid)-poly(ethylene glycol) nanoparticles provide sustained delivery of a Chlamydia trachomatis recombinant MOMP peptide and potentiate systemic adaptive immune responses in mice. Nanomedicine 2014, 10, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Cambridge, C.D.; Singh, S.R.; Waffo, A.B.; Fairley, S.J.; Dennis, V.A. Formulation, characterization, and expression of a recombinant MOMP Chlamydia trachomatis DNA vaccine encapsulated in chitosan nanoparticles. Int. J. Nanomed. 2013, 8, 1759–1771. [Google Scholar] [CrossRef]
- Gautam, A.; Dixit, S.; Embers, M.; Gautam, R.; Philipp, M.T.; Singh, S.R.; Morici, L.; Dennis, V.A. Different patterns of expression and of IL-10 modulation of inflammatory mediators from macrophages of Lyme disease-resistant and -susceptible mice. PLoS ONE 2012, 7, e43860. [Google Scholar] [CrossRef] [PubMed]
- Yilma, A.N.; Singh, S.R.; Dixit, S.; Dennis, V.A. Anti-inflammatory effects of silver-polyvinyl pyrrolidone (Ag-PVP) nanoparticles in mouse macrophages infected with live Chlamydia trachomatis. Int. J. Nanomed. 2013, 8, 2421–2432. [Google Scholar] [CrossRef]
- Yilma, A.N.; Singh, S.R.; Morici, L.; Dennis, V.A. Flavonoid naringenin: A potential immunomodulator for Chlamydia trachomatis inflammation. Mediat. Inflamm. 2013, 2013, 102457. [Google Scholar] [CrossRef] [PubMed]
- Finney, S.J.; Leaver, S.K.; Evans, T.W.; Burke-Gaffney, A. Differences in lipopolysaccharide- and lipoteichoic acid-induced cytokine/chemokine expression. Intensive Care Med. 2012, 38, 324–332. [Google Scholar] [CrossRef]
- Meng, F.; Lowell, C.A. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J. Exp. Med. 1997, 185, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Sriram, S. Mechanism of inhibition of LPS-induced IL-12p40 production by IL-10 and TGF-beta in ANA-1 cells. J. Leukoc. Biol. 1998, 64, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, J.; Zheng, M.; Gong, W.; Xue, X.; Li, W.; Zhang, L. Identification of immunodominant linear B-cell epitopes within the major outer membrane protein of Chlamydia trachomatis. Acta Biochim. Biophys. Sin. (Shanghai) 2010, 42, 771–778. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kubo, M.; Hanada, T.; Yoshimura, A. Suppressors of cytokine signaling and immunity. Nat. Immunol. 2003, 4, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Dennis, V.A.; Jefferson, A.; Singh, S.R.; Ganapamo, F.; Philipp, M.T. Interleukin-10 anti-inflammatory response to Borrelia burgdorferi, the agent of Lyme disease: A possible role for suppressors of cytokine signaling 1 and 3. Infect. Immun. 2006, 74, 5780–5789. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Fan, R.; Zhao, S.; Wang, Y. Over-expression of SOCS-3 gene promotes IL-10 production by JEG-3 trophoblast cells. Placenta 2009, 30, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.B.; Kim, A.; Kang, K.; Kim, H.; Lim, J.S. NDRG2-mediated Modulation of SOCS3 and STAT3 Activity Inhibits IL-10 Production. Immune Netw. 2010, 10, 219–229. [Google Scholar] [CrossRef]
- Danafar, H.; Davaran, S.; Rostamizadeh, K.; Valizadeh, H.; Hamidi, M. Biodegradable m-PEG/PCL Core-Shell Micelles: Preparation and Characterization as a Sustained Release Formulation for Curcumin. Adv. Pharm. Bull. 2014, 4, 501–510. [Google Scholar] [CrossRef]
- Condotta, S.A.; Richer, M.J. The immune battlefield: The impact of inflammatory cytokines on CD8+ T-cell immunity. PLOS Pathog. 2017, 13, e1006618. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, R.; Ranjan, P.; Kumar, A. Interleukin-10: A Compelling Therapeutic Target in Patients with Irritable Bowel Syndrome. Clin. Ther. 2017, 39, 632–643. [Google Scholar] [CrossRef]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.Z.; Zeng, Z.W.; Zhou, G.L.; Wang, J.J.; Li, F.Z.; Wang, A.M. Recent advances in PEG-PLA block copolymer nanoparticles. Int. J. Nanomed. 2010, 5, 1057–1065. [Google Scholar] [CrossRef]
- Kim, K.; Yu, M.; Zong, X.; Chiu, J.; Fang, D.; Seo, Y.S.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Control of degradation rate and hydrophilicity in electrospun non-woven poly(d,l-lactide) nanofiber scaffolds for biomedical applications. Biomaterials 2003, 24, 4977–4985. [Google Scholar] [CrossRef]
- Manickavasagam, D.; Novak, K.; Oyewumi, M.O. Therapeutic Delivery of Simvastatin Loaded in PLA-PEG Polymersomes Resulted in Amplification of Anti-inflammatory Effects in Activated Microglia. AAPS J. 2017, 20, 18. [Google Scholar] [CrossRef]
- Danhier, F.; Lecouturier, N.; Vroman, B.; Jerome, C.; Marchand-Brynaert, J.; Feron, O.; Preat, V. Paclitaxel-loaded PEGylated PLGA-based nanoparticles: In vitro and in vivo evaluation. J. Control. Release 2009, 133, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Gryparis, E.C.; Hatziapostolou, M.; Papadimitriou, E.; Avgoustakis, K. Anticancer activity of cisplatin-loaded PLGA-mPEG nanoparticles on LNCaP prostate cancer cells. Eur. J. Pharm. Biopharm. 2007, 67, 8. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Jin, C.; Lv, W.; Ding, Q.; Han, X. Developing a highly stable PLGA-mPEG nanoparticle loaded with cisplatin for chemotherapy of ovarian cancer. PLoS ONE 2011, 6, e25433. [Google Scholar] [CrossRef]
- Jia, L.; Zheng, J.J.; Jiang, S.M.; Huang, K.H. Preparation, physicochemical characterization and cytotoxicity in vitro of gemcitabine-loaded PEG-PDLLA nanovesicles. World J. Gastroenterol. 2010, 16, 1008–1013. [Google Scholar] [CrossRef]
- Cheng, J.; Teply, B.A.; Sherifi, I.; Sung, J.; Luther, G.; Gu, F.X.; Levy-Nissenbaum, E.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials 2007, 28, 869–876. [Google Scholar] [CrossRef]
- Carvalho, V.; Castanheira, P.; Faria, T.Q.; Goncalves, C.; Madureira, P.; Faro, C.; Domingues, L.; Brito, R.M.; Vilanova, M.; Gama, M. Biological activity of heterologous murine interleukin-10 and preliminary studies on the use of a dextrin nanogel as a delivery system. Int. J. Pharm. 2010, 400, 234–242. [Google Scholar] [CrossRef]
- Hami, Z.; Amini, M.; Ghazi-Khansari, M.; Rezayat, S.M.; Gilani, K. Doxorubicin-conjugated PLA-PEG-Folate based polymeric micelle for tumor-targeted delivery: Synthesis and in vitro evaluation. Daru 2014, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Rafat, M.; Cleroux, C.A.; Fong, W.G.; Baker, A.N.; Leonard, B.C.; O’Connor, M.D.; Tsilfidis, C. PEG-PLA microparticles for encapsulation and delivery of Tat-EGFP to retinal cells. Biomaterials 2010, 31, 3414–3421. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, R.; Abdollahi, M.; Zadeh, E.E.; Khodabakhshi, K.; Badeli, A.; Bagheri, H.; Hosseinkhani, S. mPEG-PLA and PLA-PEG-PLA nanoparticles as new carriers for delivery of recombinant human Growth Hormone (rhGH). Sci. Rep. 2018, 8, 9854. [Google Scholar] [CrossRef] [PubMed]
- Asadullah, K.; Docke, W.D.; Ebeling, M.; Friedrich, M.; Belbe, G.; Audring, H.; Volk, H.D.; Sterry, W. Interleukin 10 treatment of psoriasis: Clinical results of a phase 2 trial. Arch. Derm. 1999, 135, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.; Castanheira, P.; Madureira, P.; Ferreira, S.A.; Costa, C.; Teixeira, J.P.; Faro, C.; Vilanova, M.; Gama, M. Self-assembled dextrin nanogel as protein carrier: Controlled release and biological activity of IL-10. Biotechnol. Bioeng. 2011, 108, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [(14)C]-doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef] [PubMed]
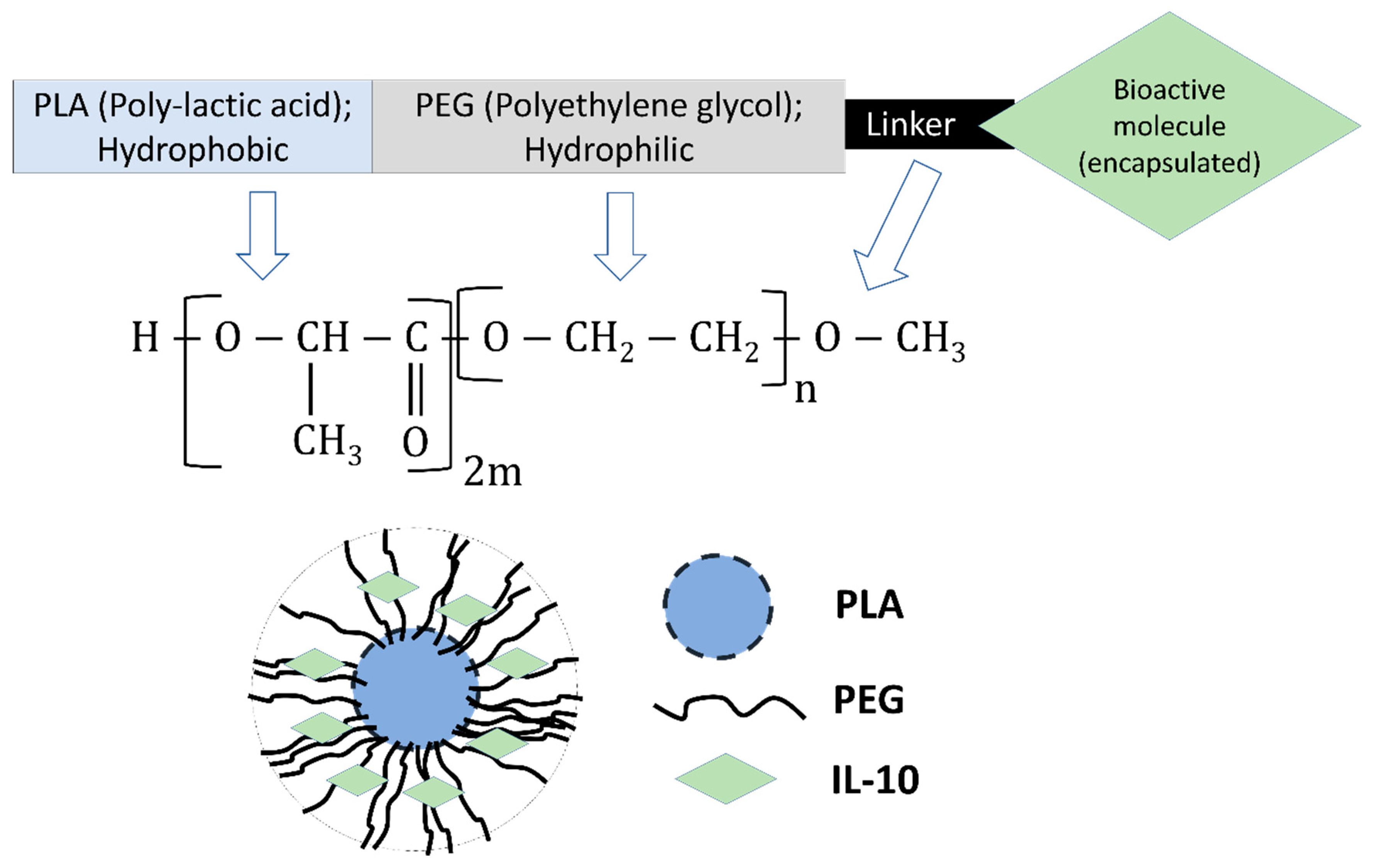
| Nanoparticles | Zeta-Sizer (nm) | Zeta-Potential (mV) | PDI | Encapsulation Efficiency |
|---|---|---|---|---|
| PLA-PEG-PBS | 265.5 | −12.9 | 0.225 | - |
| PLA-PEG-IL-10 | 238.2 | −14.2 | 0.256 | ~77% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duncan, S.A.; Dixit, S.; Sahu, R.; Martin, D.; Baganizi, D.R.; Nyairo, E.; Villinger, F.; Singh, S.R.; Dennis, V.A. Prolonged Release and Functionality of Interleukin-10 Encapsulated within PLA-PEG Nanoparticles. Nanomaterials 2019, 9, 1074. https://doi.org/10.3390/nano9081074
Duncan SA, Dixit S, Sahu R, Martin D, Baganizi DR, Nyairo E, Villinger F, Singh SR, Dennis VA. Prolonged Release and Functionality of Interleukin-10 Encapsulated within PLA-PEG Nanoparticles. Nanomaterials. 2019; 9(8):1074. https://doi.org/10.3390/nano9081074
Chicago/Turabian StyleDuncan, Skyla A., Saurabh Dixit, Rajnish Sahu, David Martin, Dieudonné R. Baganizi, Elijah Nyairo, Francois Villinger, Shree R. Singh, and Vida A. Dennis. 2019. "Prolonged Release and Functionality of Interleukin-10 Encapsulated within PLA-PEG Nanoparticles" Nanomaterials 9, no. 8: 1074. https://doi.org/10.3390/nano9081074
APA StyleDuncan, S. A., Dixit, S., Sahu, R., Martin, D., Baganizi, D. R., Nyairo, E., Villinger, F., Singh, S. R., & Dennis, V. A. (2019). Prolonged Release and Functionality of Interleukin-10 Encapsulated within PLA-PEG Nanoparticles. Nanomaterials, 9(8), 1074. https://doi.org/10.3390/nano9081074





