Investigation of Optimum Mg Doping Content and Annealing Parameters of Cu2MgxZn1−xSnS4 Thin Films for Solar Cells
Abstract
:1. Introduction
2. Experimental Method
2.1. Sample Preparation
2.2. Materials Characterization
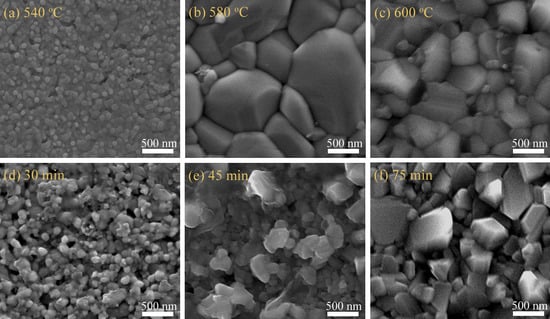
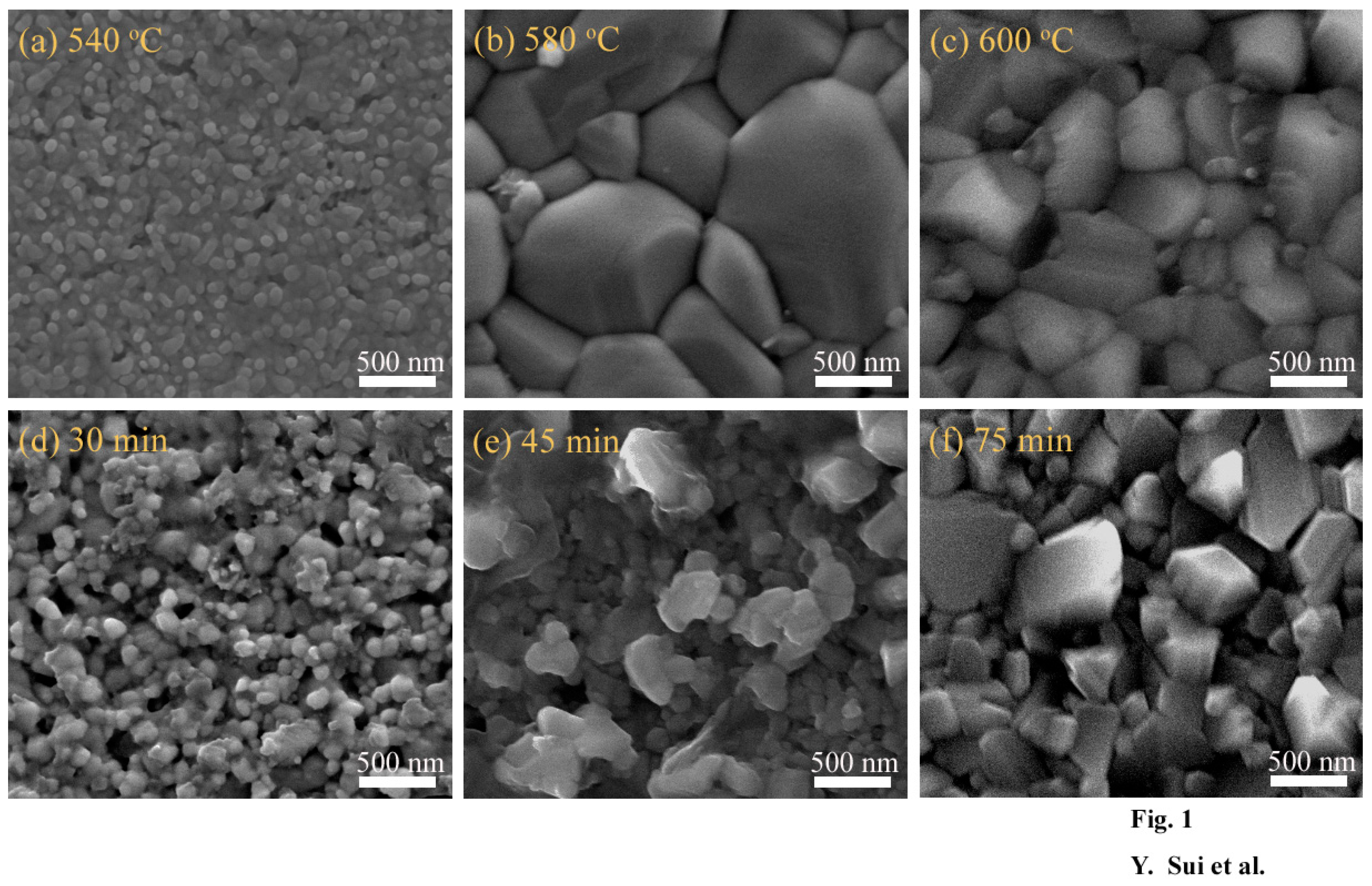
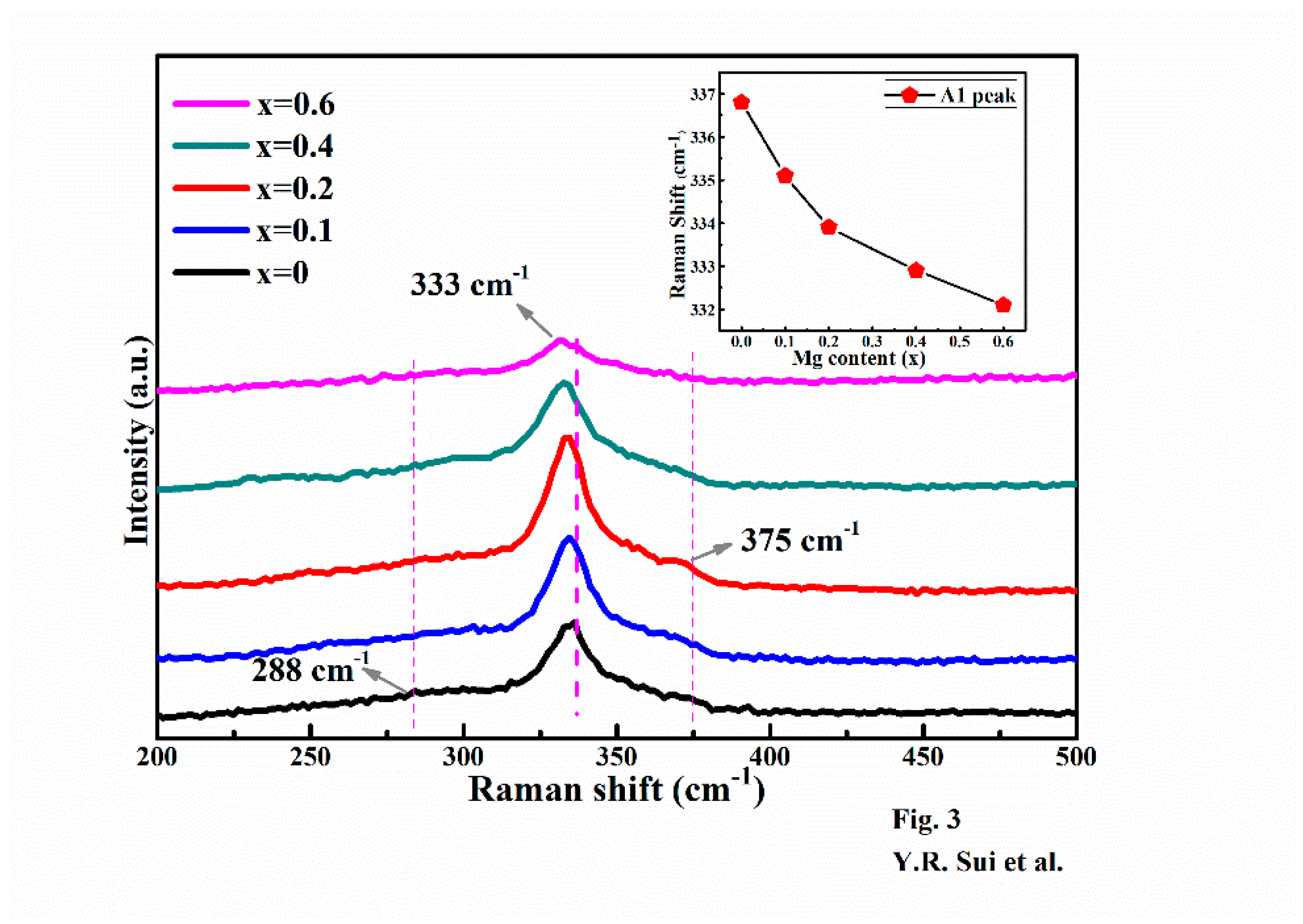
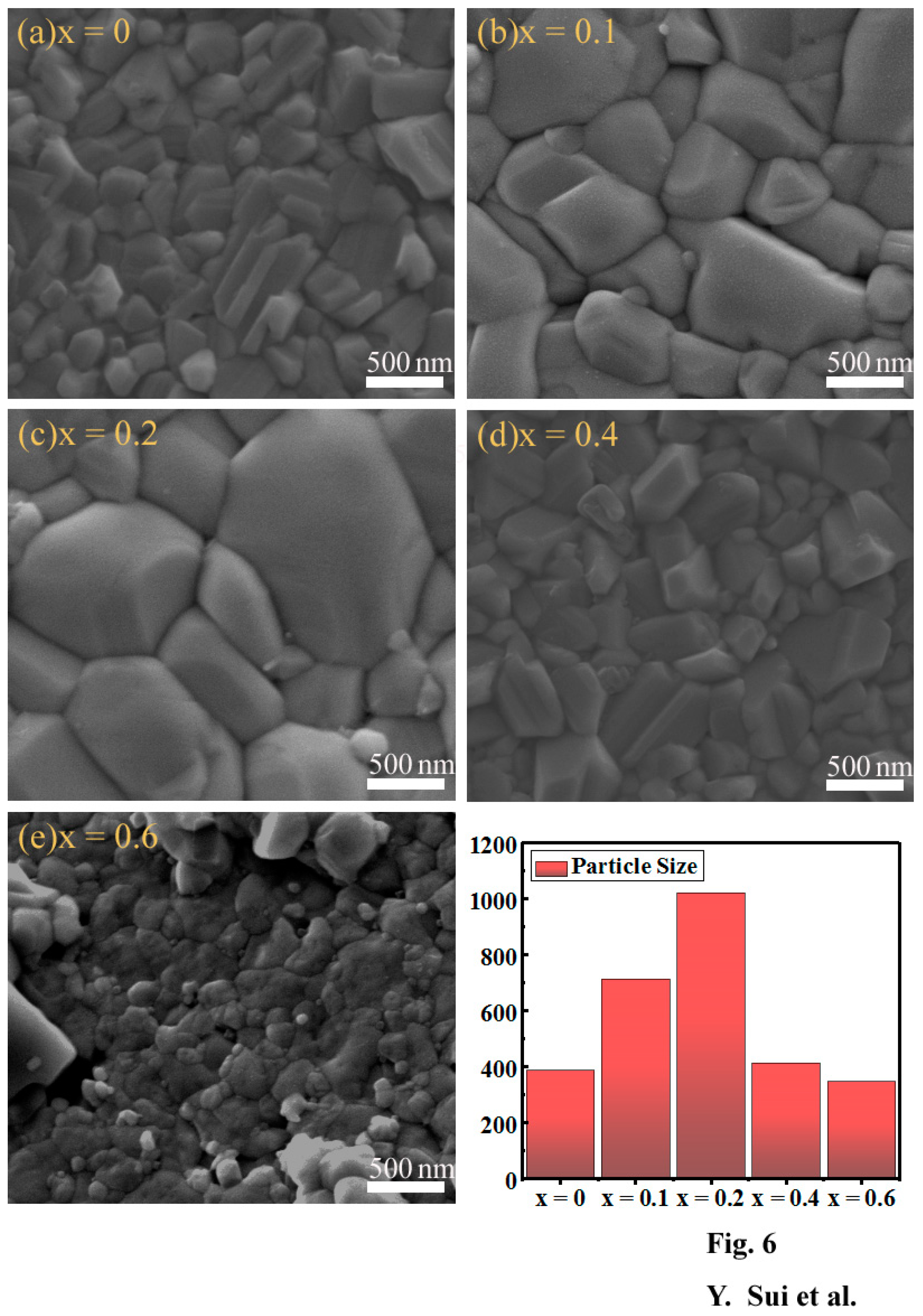
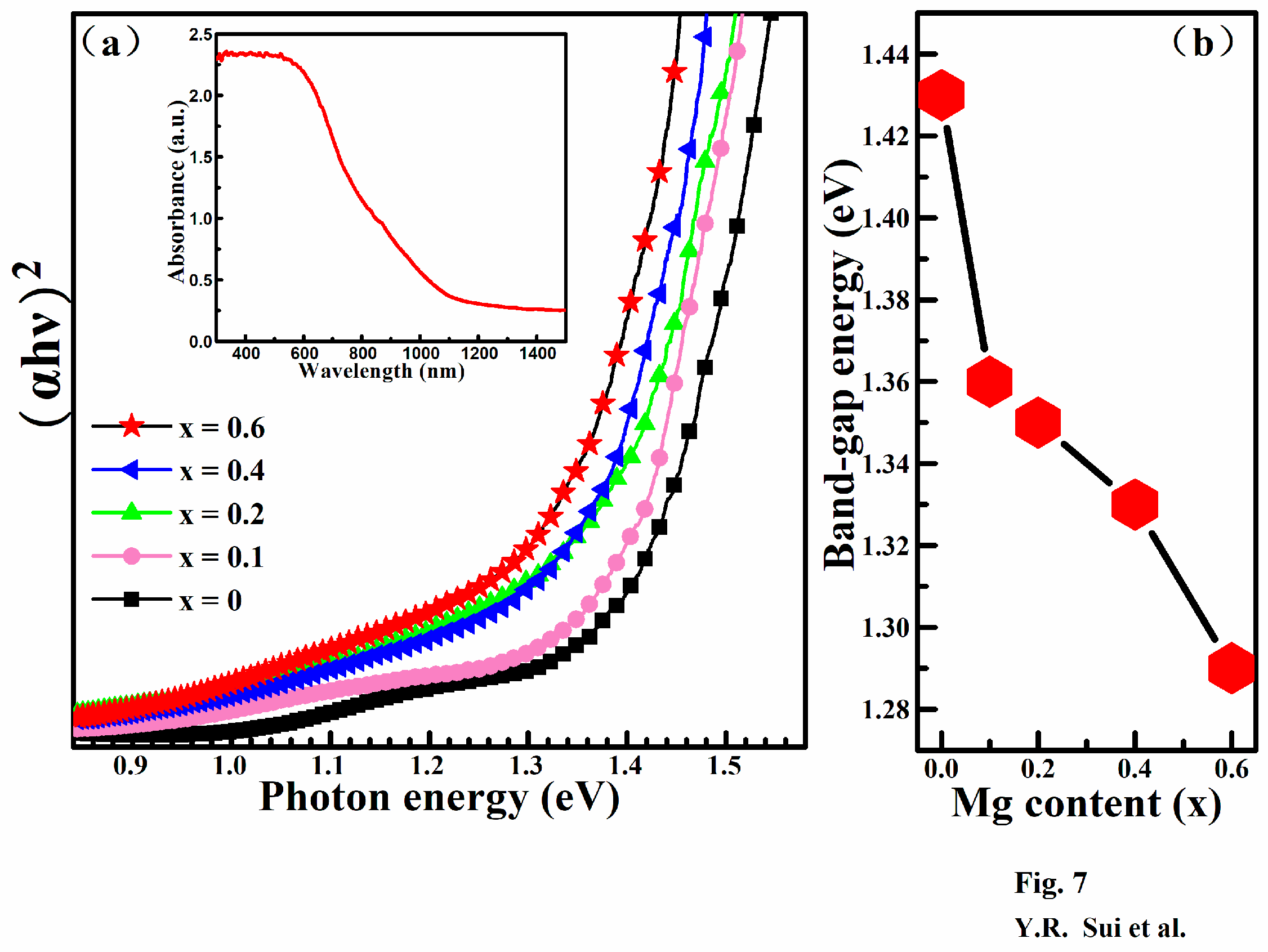
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lokhande, A.C.; Chalapathy, R.B.V.; He, M.; Jo, E.; Gang, M.; Pawar, S.A.; Lokhande, C.D.; Kim, J.H. Development of Cu2SnS3 (CTS) thin film solar cells by physical techniques: A status review. Sol. Energy Mater. Sol. Cells 2016, 153, 84–107. [Google Scholar] [CrossRef]
- Khadka, D.B.; Kim, J.H. Band gap engineering of alloyed Cu2ZnGexSn1−xQ4 (Q = S,Se) films for solar cell. J. Phys. Chem. C 2015, 119, 1706–1713. [Google Scholar] [CrossRef]
- Friedlmeier, T.M.; Wieser, N.; Walter, T.; Dittrich, H.; Schock, H.W. Hetero-junctions based on Cu2ZnSnS4 and Cu2ZnSnSe4 thin films. In Proceedings of the 14th European PVSEC and Exhibition, Barcelona, Spain, 30 June–4 July 1997; pp. 1242–1245. [Google Scholar]
- Yan, C.; Huang, J.; Sun, K.; Johnston, S.; Zhang, Y.; Sun, H.; Pu, A.; He, M.; Liu, F.; Eder, K.; et al. Cu2ZnSnS4 solar cells with over 10% power conversion efficiency enabled by heterojunction heat treatment. Nat. Energy 2018, 3, 764–772. [Google Scholar] [CrossRef]
- Yan, C.; Sun, K.W.; Huang, J.L.; Johnston, S.; Liu, F.Y.; Veettil, B.P.; Sun, K.; Pu, A.; Zhou, F.Z.; Stride, J.A.; et al. Xiaojing hao beyond 11% efficient sulfide kesterite Cu2ZnxCd1−xSnS4 Solar cell: Effects of cadmium alloying. ACS Energy Lett. 2017, 2, 930–936. [Google Scholar] [CrossRef]
- Jackson, P.; Hariskos, D.; Wuerz, R.; Kiowski, O.; Bauer, A.; Friedlmeier, T.M.; Powalla, M. Properties of Cu(In,Ga)Se2 solar cells with new record efficiencies up to 21.7%. Phys. Status Solidi 2015, 9, 28–31. [Google Scholar]
- Mitzi, D.B.; Gunawan, O.; Todorov, T.K.; Barkhouse, D.A.R. Prospects and performance limitations for Cu–Zn–Sn–S–Se photovoltaic technology. Philos. Trans. R. Soc. 2013, 371, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, G.; Zhao, W.; Tian, Q.; Huang, L.; Pan, D. Solution-Processed Highly Efficient Cu2ZnSnSe4 Thin Film Solar Cells by Dissolution of Elemental Cu, Zn, Sn, and Se Powders. ACS Appl. Mater. Interfaces 2015, 7, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Miskin, C.; Yang, W.; Hages, C.; Carter, N.; Joglekar, C.; Stach, E.; Agrawal, R. 9.0% efficient Cu2ZnSn(S,Se)4 solar cells from selenized nanopartcle inks. Prog. Photovolt. 2015, 23, 654–659. [Google Scholar] [CrossRef]
- Hages, C.; Levcenco, S.; Miskin, C.; Alsmeier, J.; Abou-Ras, D.; Wilks, R.; Bär, M.; Unold, T.; Agrawal, R. Improved performance of ge-alloyed CZTGeSSe thin-film solar cells through control of elemental losses. Prog. Photovolt. 2015, 23, 376–384. [Google Scholar] [CrossRef]
- Lokhande, A.C.; Gurav, K.V.; Jo, E.J.; Lokhande, C.D.; Kim, J.H. Chemical synthesis of Cu2SnS3 (CTS) nanoparticles: A status review. J. Alloys Compd. 2016, 656, 295–310. [Google Scholar] [CrossRef]
- Woo, K.; Kim, Y.; Yang, W.; Kim, K.; Kim, I.; Oh, Y.; Kim, J.; Moon, J. band-gap-graded Cu2ZnSn(S1-x, Sex)4 solar cells fabricated by an ethanol-based, particulate precursor ink route. Sci. Rep. 2013, 3, 3069. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Katahara, J.K.; Braly, I.L.; Hillhouse, H.W. 8% efficient Cu2ZnSn(S,Se)4 solar cells from redox equilibrated simple precursors in DMSO. Adv. Energy Mater. 2014, 4, 1301823. [Google Scholar] [CrossRef]
- Duan, H.; Yang, W.; Bob, B.; Hsu, C.; Lei, B.; Yang, Y. The Role of Sulfur in Solution-Processed Cu2ZnSn(S,Se)4 and its effect on defect properties. Adv. Funct. Mater. 2013, 23, 1466–1471. [Google Scholar] [CrossRef]
- Todorov, T.; Reuter, K.; Mitzi, D. High-efficiency solar cell with earth-abundant liquid-processed absorber. Adv. Mater. 2010, 22, E156–E159. [Google Scholar] [CrossRef]
- Fu, J.; Tian, Q.W.; Zhou, Z.J.; Kou, D.X.; Meng, Y.N.; Zhou, W.H.; Wu, S.X. Improving the performance of solution-processed Cu2ZnSn(S,Se)4 photovoltaic materials by Cd2+ substitution. Chem. Mater. 2016, 28, 16. [Google Scholar] [CrossRef]
- Xiao, Z.Y.; Li, Y.F.; Yao, B.; Deng, R.; Ding, Z.H.; Wu, T. Bandgap engineering of Cu2CdxZn1−xSnS4 alloy for photovoltaic applications: A complementary experimental and first-principles study. J. Appl. Phys. 2013, 114, 18. [Google Scholar] [CrossRef]
- Hsieh, Y.T.; Han, Q.; Jiang, C.; Song, T.B.; Chen, H.; Meng, L.; Zhou, H.; Yang, Y. Efficiency enhancement of Cu2ZnSn(S,Se)4 solar cells via alkali metals doping. Adv. Energy Mater. 2016, 6, 1502386. [Google Scholar] [CrossRef]
- Sutter-Fella, C.M.; Stuckelberger, J.A.; Hagendorfer, H.; La Mattina, F.; Kranz, L.; Nishiwaki, S.; Uhl, A.R.; Romanyuk, Y.E.; Tiwari, A.N. Sodium assisted sintering of chalcogenides and its application to solution process Cu2ZnSn(S,Se)4 thin film solar cells. Chem. Mater. 2014, 26, 1420–1425. [Google Scholar] [CrossRef]
- Yuan, M.; Mitzi, D.B.; Liu, W.; Kellock, A.J.; Chey, S.J.; Deline, V.R. Optimization of CIGS-based PV device through antimony doping. Chem. Mater. 2010, 22, 285–287. [Google Scholar] [CrossRef]
- Johnson, M.; Baryshev, S.V.; Thimsen, E.; Manno, M.; Zhang, X.; Veryovkin, I.V.; Leighton, C.; Aydil, E.S. Alkali metal-enhanced grain growth in Cu2ZnSnS4 thin films. Energy Environ. Sci. 2014, 7, 1931–1938. [Google Scholar] [CrossRef]
- Yuan, M.; Mitzi, D.B.; Gunawan, O.; Kellock, A.J.; Chey, S.J.; Deline, V.R. Antimony assisted low-temperature processing of CuIn1-xGaxSe2-ySy solar cells. Thin Solid Films 2010, 519, 852–856. [Google Scholar] [CrossRef]
- Tai, K.F.; Fu, D.C.; Chiam, S.Y.; Huan, C.H.A.; Batabyal, S.K.; Wong, L.H. Antimony doping in solution-processed Cu2ZnSn(S,Se)4 solar cells. ChemSusChem 2015, 8, 3504–3511. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Du, Q.; Wang, R.; Jiang, G.; Liu, W.; Zhu, C. Synthesis of new earth-abundant kesterite cu2mgsns4 nanoparticles by hot-injection method. Chem. Lett. 2014, 43, 1149–1151. [Google Scholar] [CrossRef]
- Zhang, Y.; Sui, Y.R.; Wu, Y.J.; Jiang, D.Y.; Wang, Z.W.; Wang, F.Y.; Lv, S.Q.; Yao, B.; Yang, L.L. Synthesis and investigation of environmental protection and earth-abundant kesterite Cu2MgxZn1-xSn(S,Se)4 thin films for Solar Cells. Ceram. Int. 2018, 44, 15249–15255. [Google Scholar] [CrossRef]
- Kishor Kumar, Y.B.; Suresh Babu, G.; Uday Bhaskar, P.; Sundar Raja, V. Preparation and characterization of spray-deposited Cu2ZnSnS4 thin films. Sol. Energy Mater. Sol. Cells 2009, 93, 1230–1237. [Google Scholar] [CrossRef]
- Sui, Y.R.; Wu, Y.J.; Zhang, Y.; Wang, F.Y.; Gao, Y.B.; Lv, S.Q.; Wang, Z.W.; Sun, Y.F.; Wei, M.B.; Yao, B.; et al. Synthesis of simple, low cost and benign sol–gel Cu2InxZn1-xSnS4 alloy thin films: Influence of different rapid thermal annealing conditions and their photovoltaic solar cells. RSC Adv. 2018, 8, 9038–9048. [Google Scholar] [CrossRef]
- Sui, Y.R.; Wu, Y.J.; Zhang, Y.; Wang, Z.W.; Wei, M.B.; Yao, B. Indium effect on structural, optical and electrical properties of Cu2InxZn1-xSnS4 alloy thin films for Solar Cell. Superlattices Microstruct. 2017, 111, 579–590. [Google Scholar] [CrossRef]
- Kuo, D.H.; Wubet, W. Mg dopant in Cu2ZnSnSe4: An n-type former and a promoter of electrical mobility up to120 cm2 V−1 s−1. J. Solid State Chem. 2014, 215, 122–127. [Google Scholar] [CrossRef]
- Ibraheam, A.S.; Al-Douri, Y.; Hashim, U.; Ghezzar, M.R.; Addou, A.; Ahmed, W.K. Cadmium effect on optical properties of Cu2Zn1-xCdxSnS4 quinternary alloys nanostructures. Sol. Energy 2015, 114, 39–50. [Google Scholar] [CrossRef]
- Kevin, P.; Malik, M.A.; Mcadams, S.; O’Brien, P. Synthesis of nanoparticulate alloys of the composition Cu2Zn1-xFexSnS4: Structural, optical, and magnetic properties. J. Am. Chem. Soc. 2015, 137, 15086–15089. [Google Scholar] [CrossRef]
- Walsh, A.; Chen, S.; Wei, S.H.; Gong, X.G. Kesterite thin-film solar cells: Advances in materials modelling of Cu2ZnSnS4. Adv. Energy Mater. 2012, 2, 400–409. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Yao, B.; Li, Y.F.; Ding, Z.H.; Xiao, Z.Y.; Yang, G.; Liu, R.J.; Liu, K.S.; Sun, Y.M. Effect of Cd content and sulfurization on structures and properties of Cd doped Cu2SnS3 thin films. J. Alloys Compd. 2017, 721, 92–99. [Google Scholar] [CrossRef]
- Khare, A.; Himmetoglu, B.; Johnson, M.; Norris, D.J.; Cococcioni, M.; Aydil, E.S. Calculation of the lattice dynamics and Raman spectra of copper zinc tin chalcogenides and comparison to experiments. J. Appl. Phys. 2012, 118, 083707. [Google Scholar] [CrossRef]
- Dumcenco, D.; Huang, Y.S. The vibrational properties study of kesterite Cu2ZnSnS4 single crystals by using polarization dependent Raman spectroscopy. Opt. Mater. 2013, 35, 419–425. [Google Scholar] [CrossRef]
- Tong, Z.; Yan, C.; Su, Z.; Zeng, F.; Yang, J.; Li, Y.; Jiang, L.; Lai, Y.; Liu, F. Effects of potassium doping on solution processed kesterite Cu2ZnSnS4 thin film solar cells. Appl. Phys. Lett. 2014, 105, 223903. [Google Scholar] [CrossRef]
- Tsega, M.; Dejene, F.B.; Kuo, D.H. Morphological evolution and structural properties of Cu2ZnSn(S,Se)4 thin films deposited from single ceramic target by a one-step sputtering process and selenization without H2Se. J. Alloys Compd. 2015, 642, 140–147. [Google Scholar] [CrossRef]
- Chen, H.Y.; Yu, S.M.; Shin, D.W.; Yoo, J.B. Solvothermal synthesis and characterization of chalcopyrite CuInSe2 nanoparticles. Nanoscale Res. Lett. 2010, 5, 217–223. [Google Scholar] [CrossRef]
- Rondiya, S.; Wadnerkar, N.; Jadhav, Y.; Jadkar, S.; Haram, S.; Kabir, M. Structural, electronic, and optical properties of Cu2NiSnS4: A combined experimental and theoretical study toward photovoltaic applications. Chem. Mater. 2017, 29, 3133–3142. [Google Scholar] [CrossRef]
- Li, Y.F.; Yao, B.; Lu, Y.M.; Wei, Z.P.; Gai, Y.Q.; Zheng, C.J.; Li, B.H.; Shen, D.Z.; Fan, X.W.; Tang, Z.K. Realization of p-type conduction in undoped MgxZn1-xO thin films by controlling Mg content. Appl. Phys. Lett. 2007, 91, 2115. [Google Scholar] [CrossRef]
- Chen, S.; Walsh, A.; Luo, Y.; Yang, J.H.; Gong, X.G.; Wei, S.H. Wurtzite-derived poly types of kesterite and stannite quaternary chalcogenide semiconductors. Phys. Rev. B 2010, 82, 195203. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Zhang, P.; Yuan, X.; Huang, F.; Zhang, W. Structural properties and quasiparticle band structures of cu-based quaternary semiconductors for photovoltaic applications. J. Appl. Phys. 2012, 111, 0637091–0637096. [Google Scholar] [CrossRef]
- Son, D.H.; Kim, D.H.; Park, S.N.; Yang, K.J.; Nam, D.; Cheong, H.; Kang, J.K. Growth and device characteristics of CZTSSe thin-film solar cells with 8.03% efficiency. Chem. Mater. 2015, 27, 5180–5188. [Google Scholar] [CrossRef]
- Shu, Q.; Yang, J.H.; Chen, S.; Huang, B.; Xiang, H.; Gong, X.G.; Wei, S.H. Cu2Zn(Sn,Ge)Se4 and Cu2Zn(Sn,Si)Se4 alloys as photovoltaic materials: Structural and electronic properties. Phys. Rev. B 2013, 87, 1152081–1152085. [Google Scholar] [CrossRef]
- Zhou, H.; Song, T.; Hsu, W.; Luo, S.; Ye, S.; Duan, H.; Hsu, C.; Yang, W.; Yang, Y. Rational defect passivation of Cu2ZnSn(S,Se)4 photovoltaics with solution-processed Cu2ZnSnS4:Na nanocrystals. J. Am. Chem. Soc. 2013, 135, 15998–16001. [Google Scholar] [CrossRef] [PubMed]
- Laemmle, A.; Wuerz, R.; Powalla, M. Efficiency enhancement of Cu(In,Ga)Se2 thin-film solar cells by a post-deposition treatment with potassium fluoride. Phys. Status Solidi RRL 2013, 7, 631–634. [Google Scholar] [CrossRef]


| Sample | ρ (Ω.cm) | n (cm−3) | μ (cm2 V−1 S−1) | Type |
|---|---|---|---|---|
| A1 | 9.43 × 100 | 4.12 × 1015 | 3.70 × 100 | p |
| A2 | 1.16 × 10−1 | 3.29 × 1018 | 1.01 × 10−1 | p |
| A3 | 1.53 × 100 | 3.79 × 1017 | 7.87 × 10−1 | p |
| B1 | 7.99 × 101 | 3.21 × 1014 | 6.02 × 100 | p |
| B2 | 8.97 × 100 | 3.79 × 1015 | 2.02 × 100 | P |
| B3 | 4.53 × 100 | 4.62 × 1016 | 9.32 × 10−1 | P |
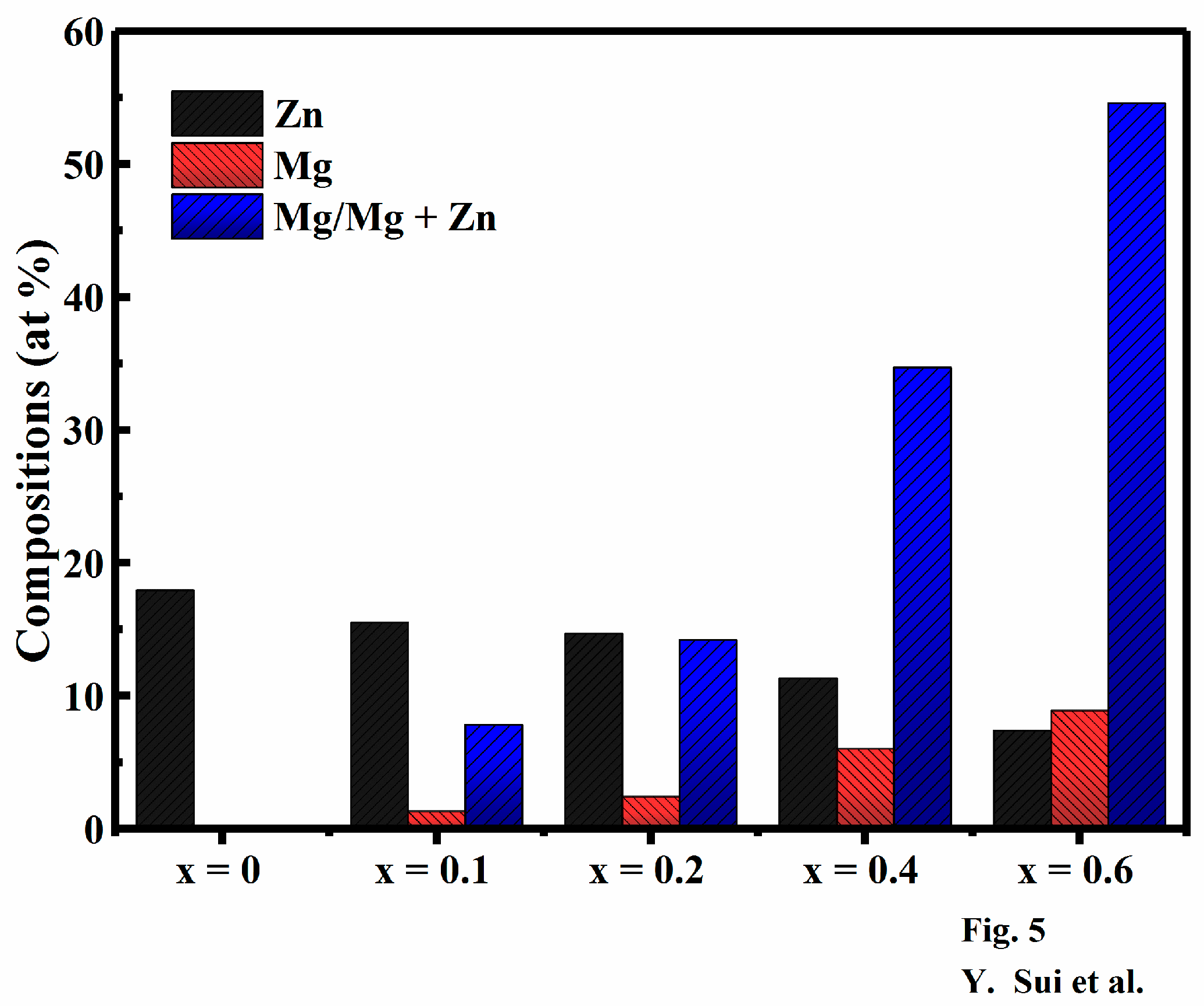
| Sample | Cu (at %) | Zn (at %) | Mg (at %) | Sn (at %) | S (at %) | Mg/(Mg + Zn) (at %) |
|---|---|---|---|---|---|---|
| x = 0 | 25.07 | 17.95 | 0 | 10.30 | 46.98 | 0 |
| x = 0.1 | 25.34 | 15.51 | 1.31 | 10.03 | 47.81 | 7.79 |
| x = 0.2 | 25.52 | 14.66 | 2.43 | 10.61 | 46.78 | 14.22 |
| x = 0.4 | 25.31 | 11.32 | 6.02 | 10.32 | 47.03 | 34.72 |
| x = 0.6 | 25.08 | 7.39 | 8.89 | 10.77 | 47.87 | 54.61 |
| Sample | ρ (Ω.cm) | n (cm−3) | μ (cm2 V−1 S−1) | Type |
|---|---|---|---|---|
| x = 0 | 3.73 × 101 | 6.95 × 1016 | 2.63 × 100 | p |
| x = 0.1 | 3.21 × 101 | 2.46 × 1017 | 3.23 × 10−1 | p |
| x = 0.2 | 1.16 × 10−1 | 3.29 × 1018 | 1.01 × 10−1 | p |
| x = 0.4 | 1.92 × 10−1 | 1.21 × 1018 | 1.22 × 100 | p |
| x = 0.6 | 1.12 × 100 | 2.02 × 1017 | 1.43 × 100 | p |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, Y.; Zhang, Y.; Jiang, D.; He, W.; Wang, Z.; Wang, F.; Yao, B.; Yang, L. Investigation of Optimum Mg Doping Content and Annealing Parameters of Cu2MgxZn1−xSnS4 Thin Films for Solar Cells. Nanomaterials 2019, 9, 955. https://doi.org/10.3390/nano9070955
Sui Y, Zhang Y, Jiang D, He W, Wang Z, Wang F, Yao B, Yang L. Investigation of Optimum Mg Doping Content and Annealing Parameters of Cu2MgxZn1−xSnS4 Thin Films for Solar Cells. Nanomaterials. 2019; 9(7):955. https://doi.org/10.3390/nano9070955
Chicago/Turabian StyleSui, Yingrui, Yu Zhang, Dongyue Jiang, Wenjie He, Zhanwu Wang, Fengyou Wang, Bin Yao, and Lili Yang. 2019. "Investigation of Optimum Mg Doping Content and Annealing Parameters of Cu2MgxZn1−xSnS4 Thin Films for Solar Cells" Nanomaterials 9, no. 7: 955. https://doi.org/10.3390/nano9070955
APA StyleSui, Y., Zhang, Y., Jiang, D., He, W., Wang, Z., Wang, F., Yao, B., & Yang, L. (2019). Investigation of Optimum Mg Doping Content and Annealing Parameters of Cu2MgxZn1−xSnS4 Thin Films for Solar Cells. Nanomaterials, 9(7), 955. https://doi.org/10.3390/nano9070955