Preparation, Characterization and Adsorption Potential of Grainy Halloysite-CNT Composites for Anthracene Removal from Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hal-CNT Composite Preparation
2.3. Halloysite-CNT Composite Characterization
2.4. Kinetic and Adsorption Study
3. Results
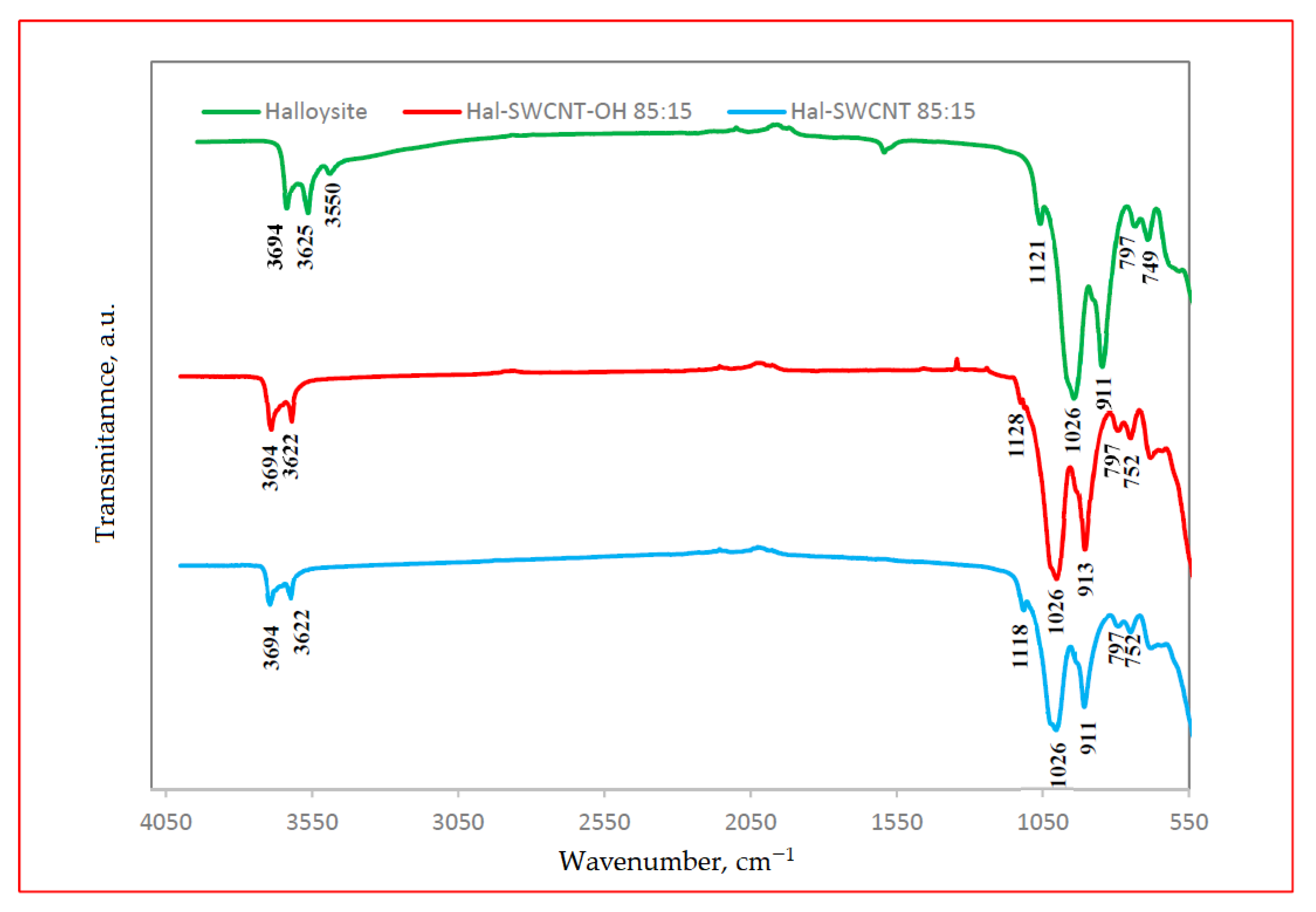
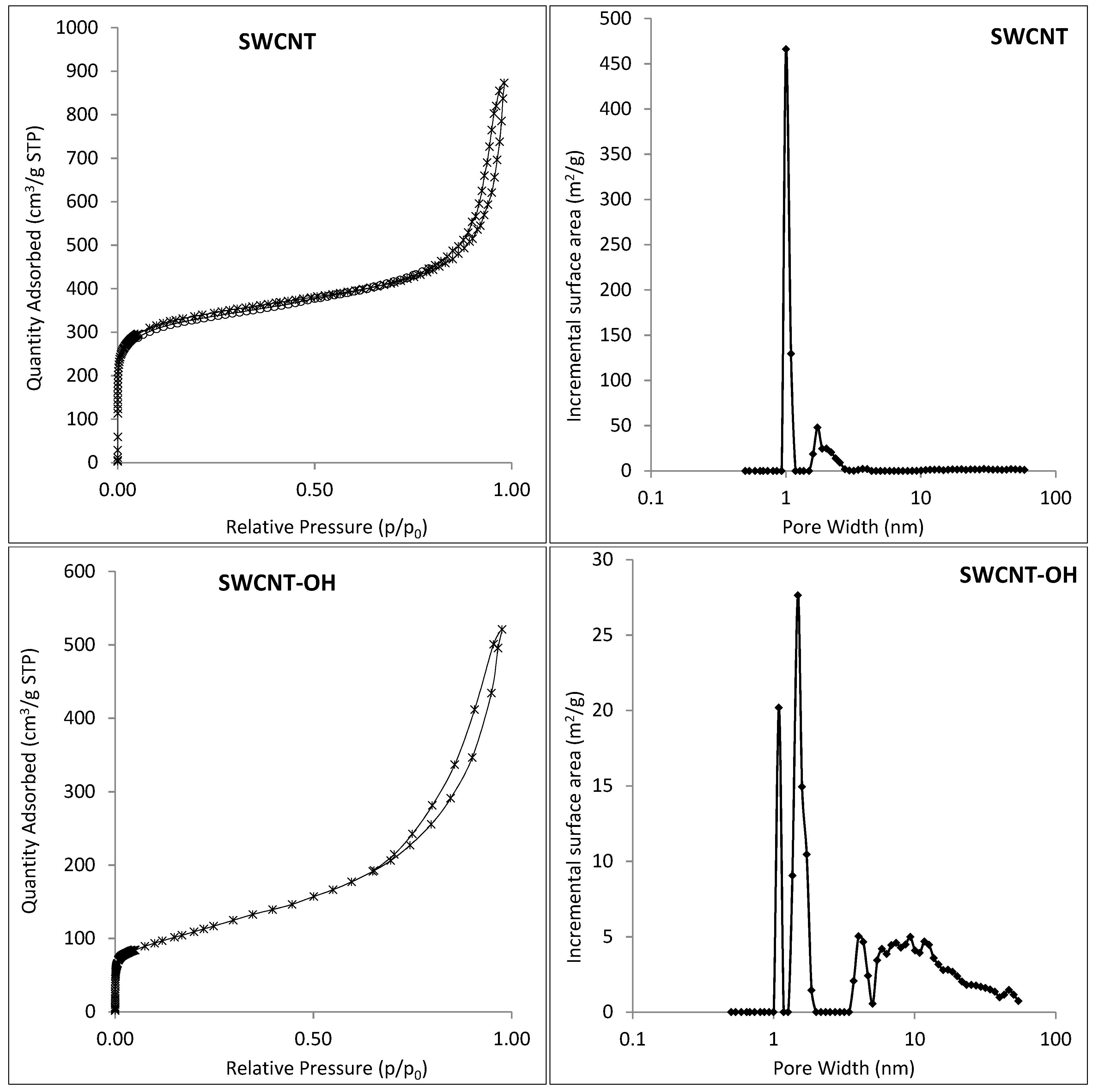
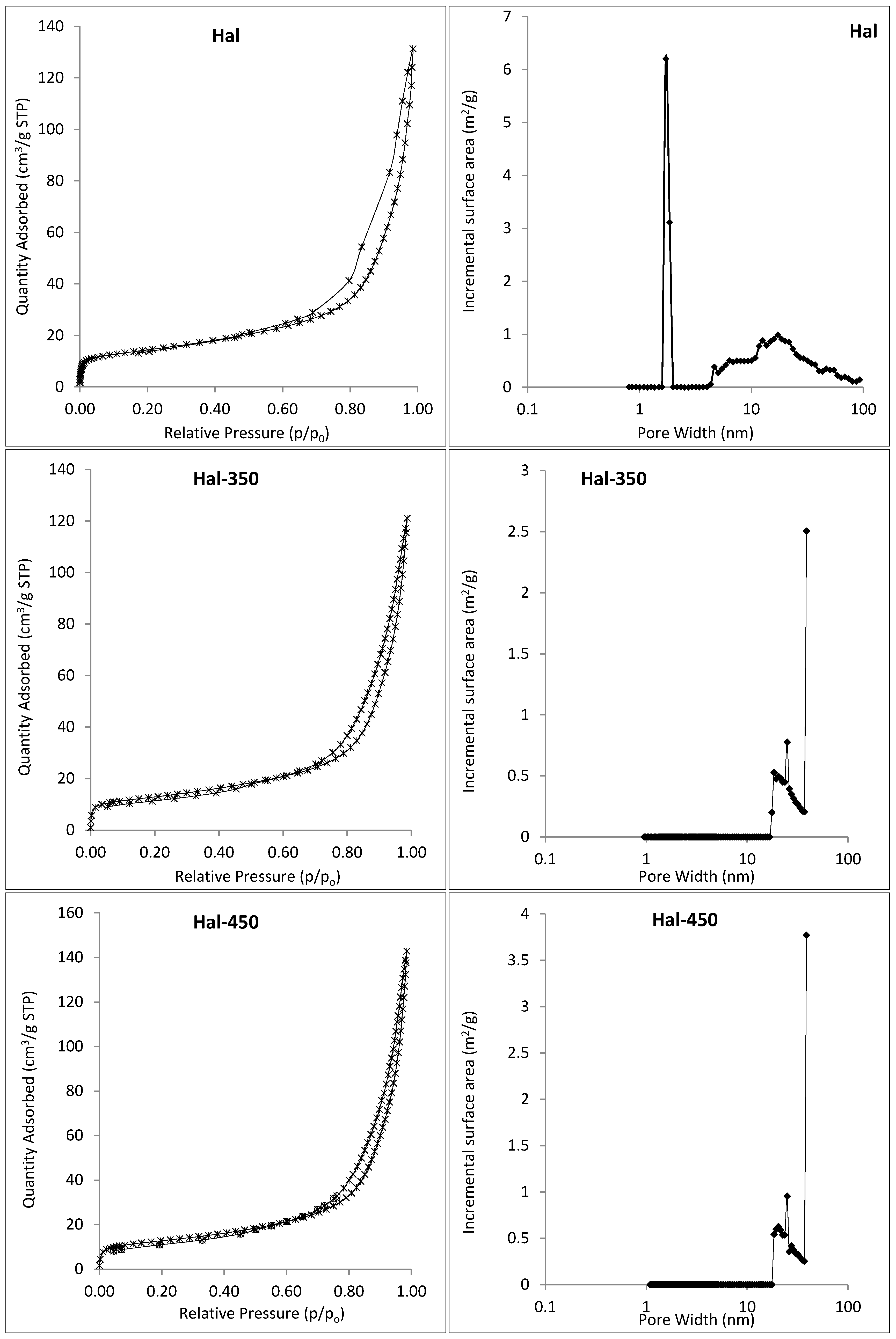
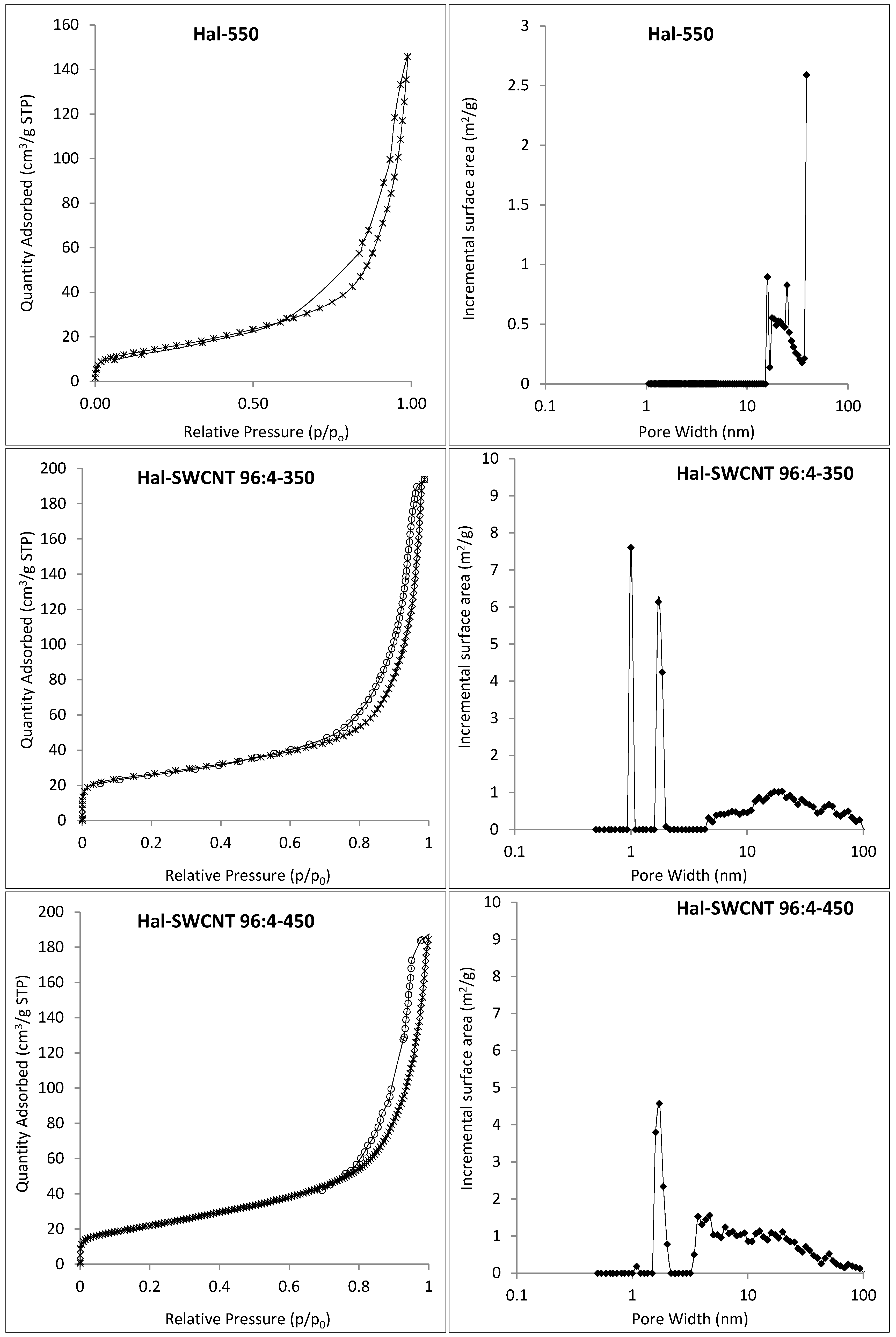
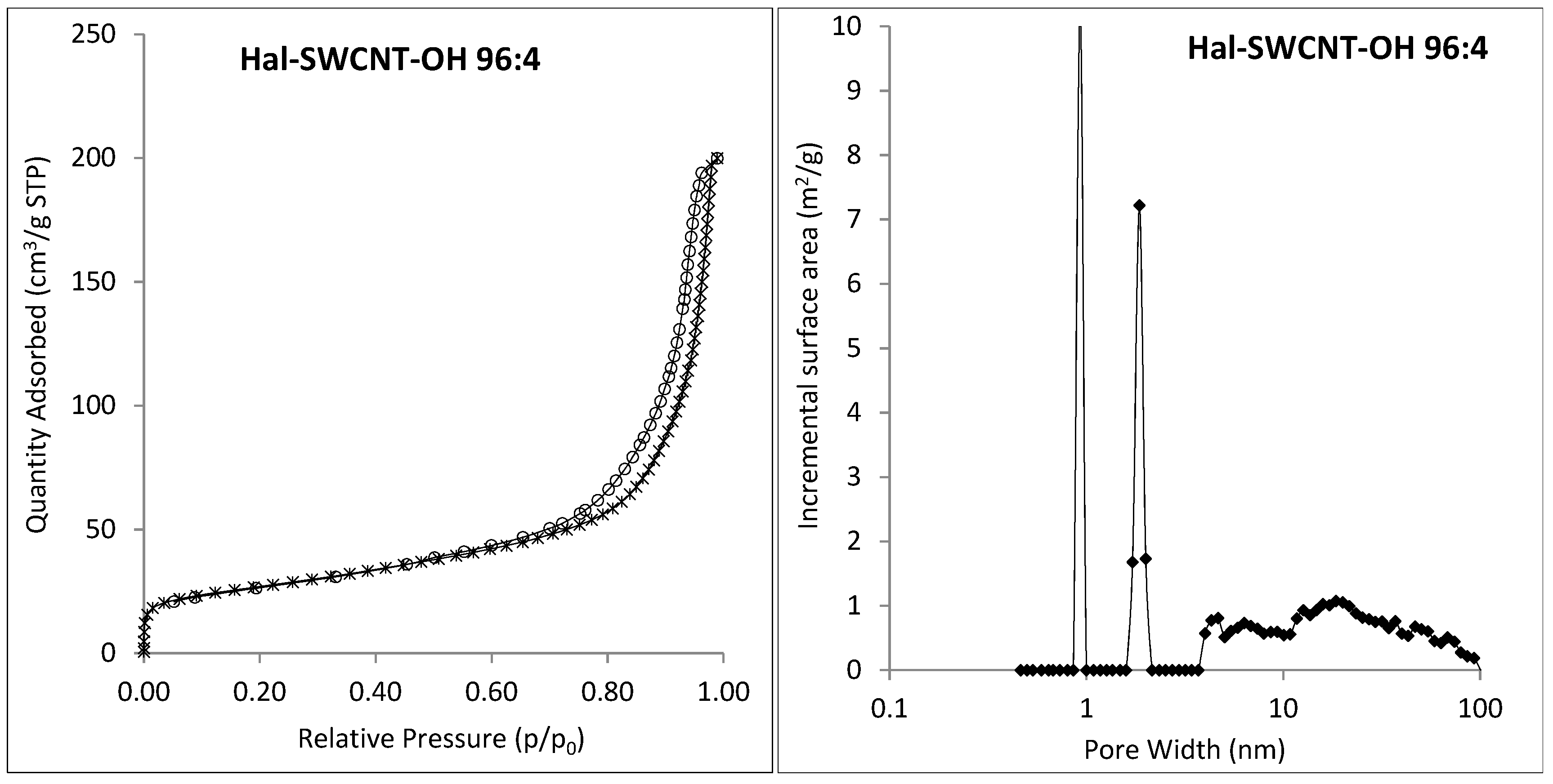
3.1. Characterization of Hal-CNT Composites
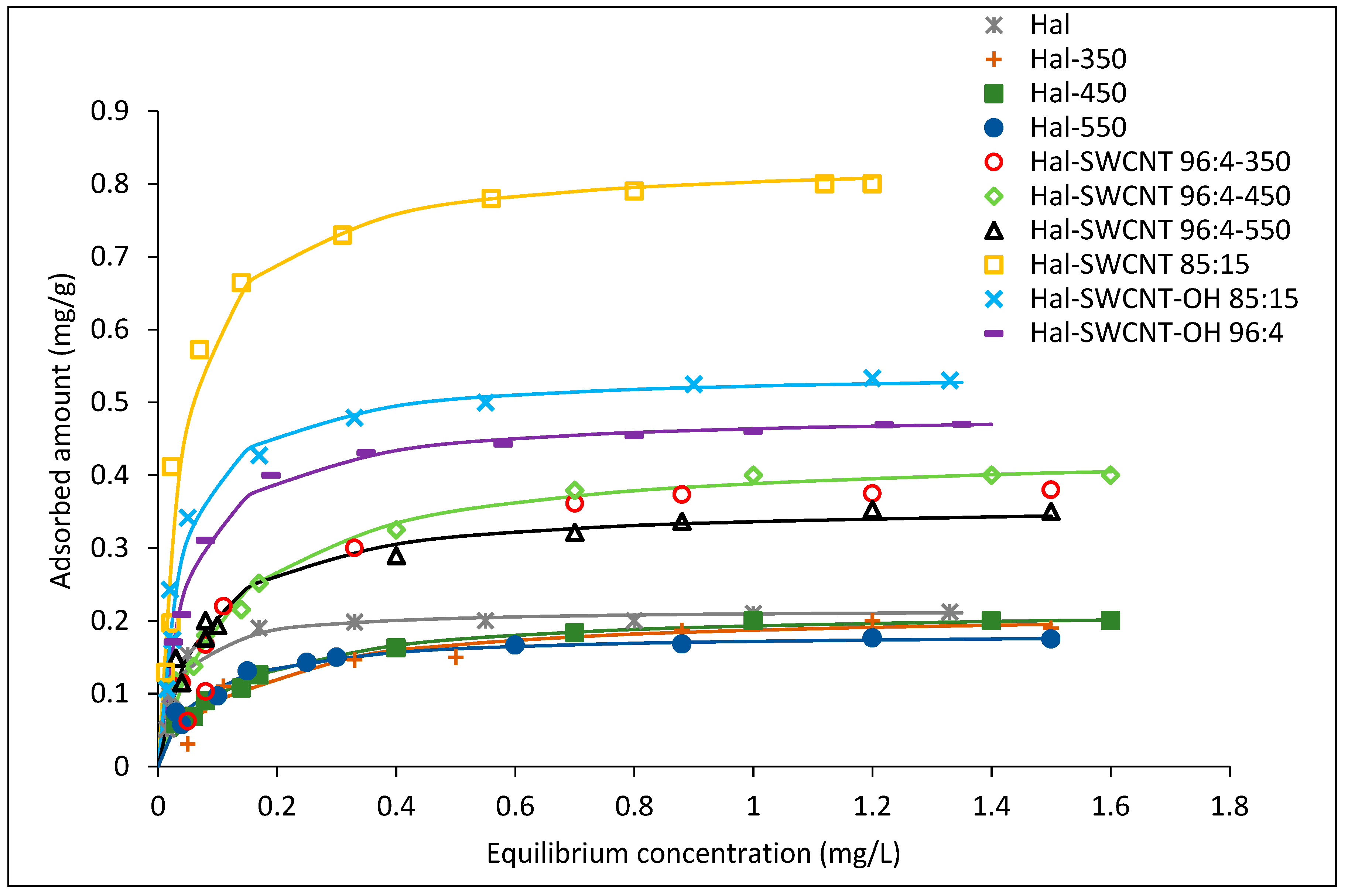
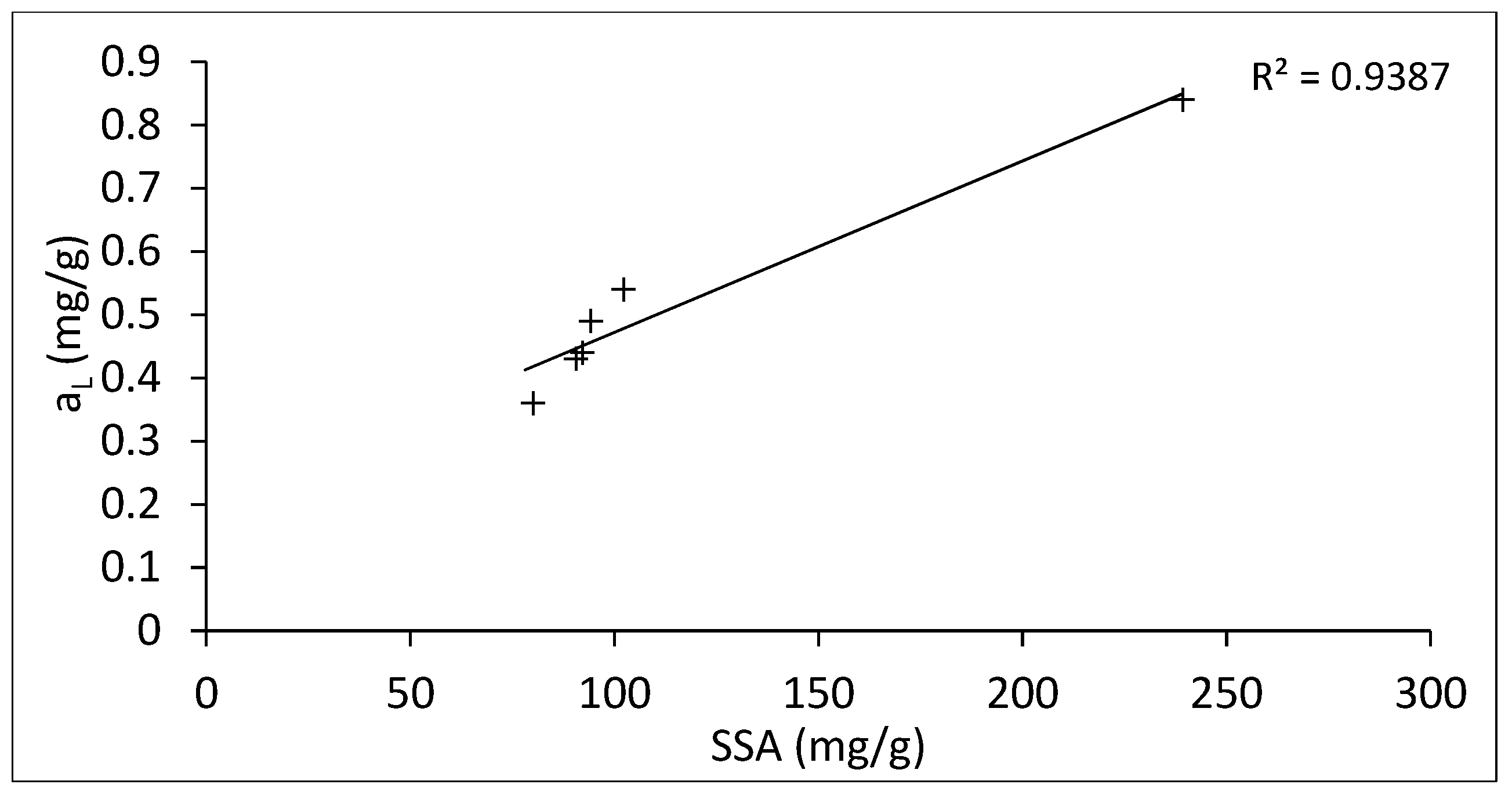
3.2. Adsorption Isotherms
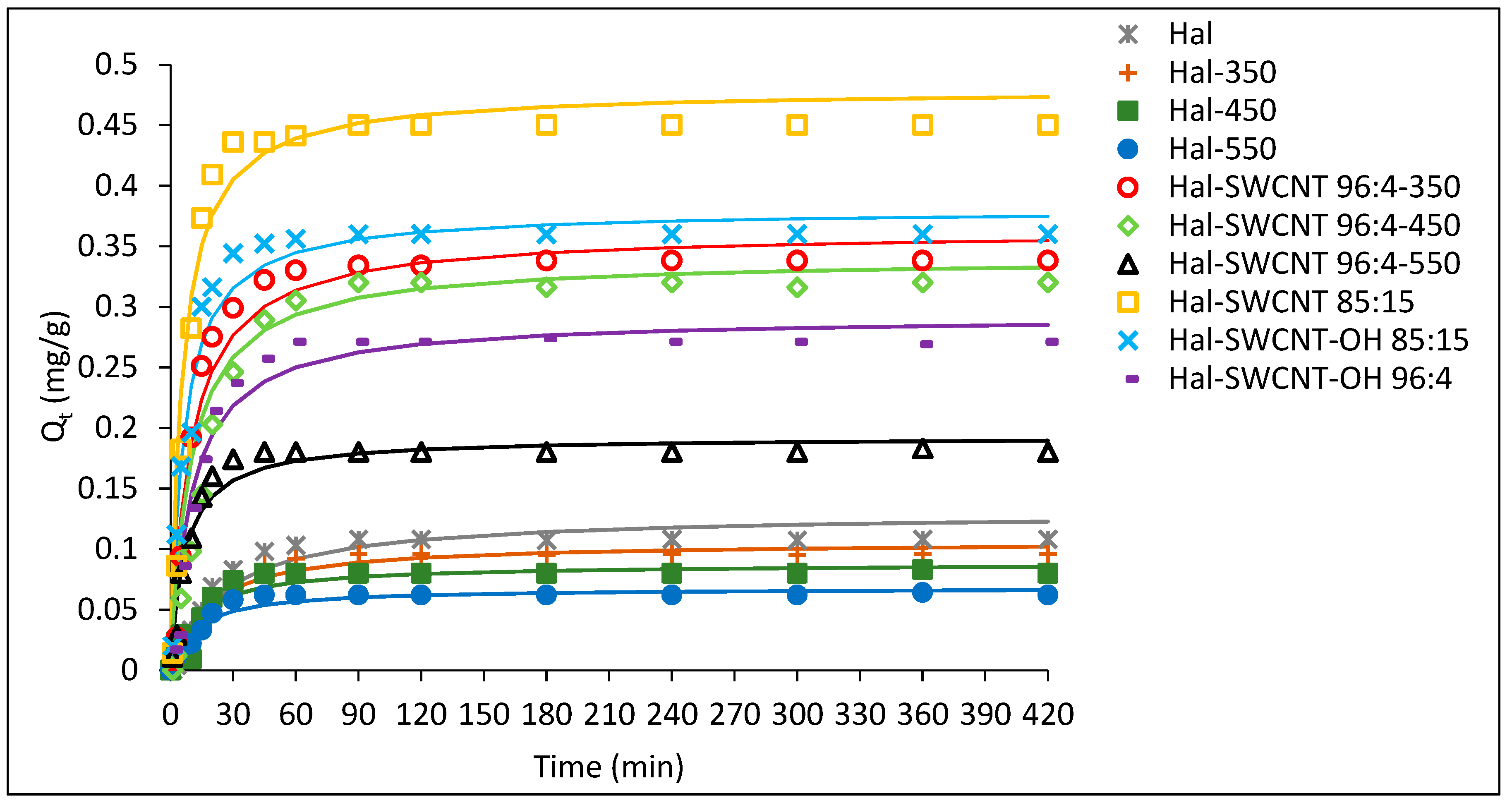
3.3. Adsorption Kinetics
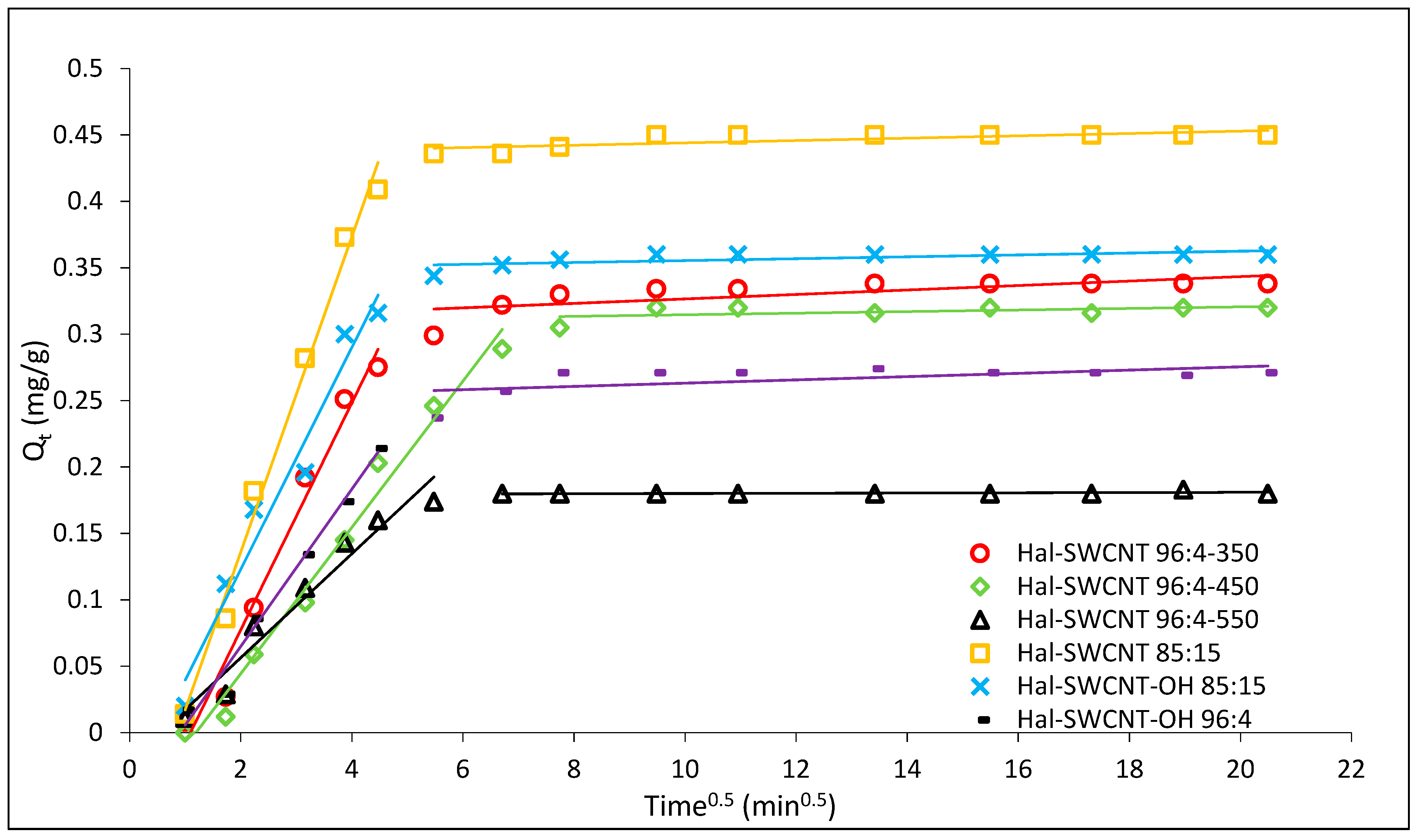
3.4. Adsorption Rate-Controlling Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Directive 2013/39/EU of the European Parliament and of The Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:226:0001:0017:EN:PDF (accessed on 14 June 2019).
- Meng, Y.; Liu, X.; Lu, S.; Zhang, T.; Jin, B.; Wang, Q.; Tang, Z.; Liu, Y.; Guo, X.; Zhou, J.; et al. A review on occurrence and risk of polycyclic aromatic hydrocarbons (PAHs) in lakes of China. Sci. Total Environ. 2019, 651, 2497–2506. [Google Scholar] [PubMed]
- Tan, X.; Yu, X.; Cai, L.; Wang, J.; Peng, J. Microplastics and associated PAHs in surface water from the Feilaixia Reservoir in the Beijiang River, China. Chemosphere 2019, 221, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Vecchiato, M.; Turetta, C.; Patti, B.; Barbante, C.; Piazza, R.; Bonato, T.; Gambaro, A. Distribution of fragrances and PAHs in the surface seawater of the Sicily Channel, Central Mediterranean. Sci. Total Environ. 2018, 634, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhao, S.; Shi, Y.; Fan, X.; Wang, T. Formation of environmentally persistent free radicals during the transformation of anthracene in different soils: Roles of soil characteristics and ambient conditions. J. Hazard. Mater. 2019, 362, 214–223. [Google Scholar]
- Bohdziewicz, J.; Liszczyk, G. Evaluation of effectiveness of bisphenol A removal on domestic and foreign activated carbons. Ecol. Chem. Eng. S 2013, 20, 371–379. [Google Scholar]
- Kamińska, G.; Dudziak, M.; Kudelk, E.; Bohdziewicz, J. Single and competitive adsorption of OMPs by carbon nanotubes—Mechanism and fitting models. In Proceedings of the International Conference on Advances in Energy Systems and Environmental Engineering (ASEE17), Wrocław, Poland, 2–5 July 2017; Kaźmierczak, B., Kutyłowska, M., Piekarska, K., Jouhara, H., Danielewicz, J., Eds.; E3S Web of Conferences. EDP Sciences: Les Ulis, France, 2017; Volume 22. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Agrawal, S.; Rachna. Water purification by using adsorbents: A review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Gisi, S.D.; Lofranom, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar]
- Ren, X.; Chen, C.; Nagatsu, M.; Wang, X. Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem. Eng. J. 2011, 170, 395–410. [Google Scholar] [CrossRef]
- Fernandes, M.F.R.; Buzaglo, M.; Regev, O.; Furó, I.; Marques, E. Mechanical agitation induces counterintuitive aggregation of pre-dispersed carbon nanotubes. J. Colloid Interface Sci. 2017, 493, 398–404. [Google Scholar] [CrossRef]
- Chen, Q.; Saltiel, C.; Manickavasagam, S.; Schadler, R.S.; Siegel, R.W.; Yang, H. Aggregation behavior of single-walled carbon nanotubes in dilute aqueous suspension. J. Colloid Interface Sci. 2004, 280, 91–97. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B. Desorption of polycyclic aromatic hydrocarbons from carbon nanomaterials in water. Environ. Pollut. 2007, 145, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y.; Presser, V. Carbon Nanomaterials; Taylor & Francis: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2006. [Google Scholar]
- Xu, L.; Li, J.; Zhang, M. Adsorption characteristics of a novel carbon-nanotube-based composite adsorbent toward organic pollutants. Ind. Eng. Chem. Res. 2015, 54, 2379–2384. [Google Scholar] [CrossRef]
- Boncel, S.; Kyzioł-Komasińska, J.; Krzyrzewska, I.; Czupioł, J. Interactions of carbon nanotubes with aqueous/aquatic media containing organic/inorganic contaminants and selected organisms of aquatic ecosystems-a review. Chemosphere 2015, 136, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Kim, T.; Vellingiri, K.; Tsang, D.C.W.; Shon, J.R.; Ki-Hyun, K.; Kumar, S. Recycling and regeneration of carbonaceous and porous materials through thermal or solvent treatment. Chem. Eng. J. 2019, 364, 514–529. [Google Scholar] [CrossRef]
- Li, Q.; Qi, J.; Gao, C. Chemical regeneration of spent powdered activated carbon used in decolorization of sodium salicylate for the pharmaceutical industry. J. Clean. Prod. 2015, 86, 424–431. [Google Scholar] [CrossRef]
- Shan, D.; Deng, S.; Zhao, T.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of regenerable granular carbon nanotubes by a simple heating filtration method for efficient removal of typical pharmaceuticals. Chem. Eng. J. 2016, 294, 353–361. [Google Scholar] [CrossRef]
- Kamińska, G.; Bohdziewicz, J.; Calvo, J.I.; Prádanos, P.; Palacio, L.; Hernández, A. Fabrication and characterization of polyethersulfone nanocomposite for the removal of endocrine disrupting micropollutants from wastewater. Mechanisms and performance. J. Membr. Sci. 2015, 493, 66–79. [Google Scholar] [CrossRef]
- Manawi, Y.; Kochkodan, V.; Hussein, M.A.; Khaleel, M.A.; Khraisheh, M.; Hilal, N. Can carbon-based nanomaterials revolutionize membrane fabrication for water treatment and desalination? Desalination 2016, 391, 69–88. [Google Scholar] [CrossRef]
- Wei, H.; Deng, S.; Huang, Q.; Nie, Y.; Wang, B.; Huang, J.; Yu, G. Regenerable granular carbon nanotubes/alumina hybrid adsorbents for diclofenac sodium and carbamazepine removal from aqueous solution. Water Res. 2013, 47, 4139–4147. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Baytak, S. Synthesis of bentonite–carbon nanotube nanocomposite and its adsorption of rhodamine dye from water. Arab. J. Sci. Eng. 2016, 41, 4775–4785. [Google Scholar] [CrossRef]
- Shaban, M.; Hassouna, M.; Nasief, F.M.; AbuKhadra, M.R. Adsorption properties of kaolinite-based nanocomposites for Fe and Mn pollutants from aqueous solutions and raw ground water: Kinetics and equilibrium studies. Environ. Sci. Pollut. Res. Int. 2017, 24, 22954–22966. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, S. Synthesis of clay-CNTs nanocomposite. J. Nanostruct. Chem. 2013, 3, 1–24. [Google Scholar] [CrossRef]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Nazli, Z.-i-H.; Bhatti, H.N.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Kamińska, G. Removal of organic micropollutants by grainy bentonite-activated carbon adsorbent in fixed bed column. Water 2018, 10, 1791. [Google Scholar] [CrossRef]
- Szczepanik, B. Photocatalytic degradation of organic contaminants over clay-TiO2 nanocomposites: A review. Appl. Clay Sci. 2017, 141, 227–239. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Mittal, A.; Usman, M.; Mittal, Y.; Yu, G.; Núñez-Delgadofez, A.; Kornaros, M. A review on halloysite-based adsorbents to remove pollutants in water and wastewater. J. Mol. Liq. 2018, 269, 855–868. [Google Scholar] [CrossRef]
- Kamble, R.; Ghag, M.; Gaikawad, S.; Panda, B.K. Halloysite nanotubes and applications: A review. J. Adv. Sci. Res. 2012, 3, 25–29. [Google Scholar]
- Xiao, J.; Xie, S.; Jing, Y.; Yao, Y.; Wang, X.; Jia, Y. Preparation of halloysite @graphene oxide composite and its application for high-efficient decontamination of U(VI) from aqueous solution. J. Mol. Liq. 2016, 220, 304–310. [Google Scholar] [CrossRef]
- Bohdziewicz, J.; Dudziak, M.; Kamińska, G.; Kudlek, E. Chromatographic determination and toxicological potential evaluation of selected micropollutants in aquatic environment - analytical problems. Desalin. Water. Treat. 2016, 57, 1361–1369. [Google Scholar] [CrossRef]
- Kamińska, G.; Bohdziewicz, J. Potential of various materials for adsorption of micropollutants from wastewater. Environ. Prot. Eng. 2016, 4, 161–178. [Google Scholar]
- Bohdziewicz, J.; Kamińska, G. Kinetics and equilibrium of the sorption of bisphenol A by carbon nanotubes from wastewater. Water Sci. Technol. 2013, 68, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2005. [Google Scholar]
- Du, P.; Yuan, P.; Liu, D.; Wang, S.; Song, H.; Guo, H. Calcination induced changes in structure, morphology, and porosity of allophane. Appl. Clay Sci. 2018, 158, 211–218. [Google Scholar] [CrossRef]
- Shu, Z.; Chen, Y.; Zhou, J.; Li, T.; Sheng, Z.; Tao, C.; Wang, Y. Preparation of halloysite-derived mesoporous silica nanotube with enlarged specific surface area for enhanced dye adsorption. Appl. Clay Sci. 2016, 132–133, 114–121. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]
- Szczepanik, B.; Rogala, P.; Słomkiewicz, P.M.; Banaś, D.; Kubala-Kukuś, A.; Stabrawa, I. Synthesis, characterization and photocatalytic activity of TiO2-halloysite and Fe2O3-halloysite nanocomposites for photodegradation of chloroanilines in water. Appl. Clay Sci. 2017, 149, 118–126. [Google Scholar] [CrossRef]
- Frost, R.L.; Vassallo, A.M. The dihydroxylation of the kaolinite clay minerals using infrared emission spectroscopy. Clays Clay Miner. 1996, 44, 635–651. [Google Scholar] [CrossRef]
- Donohue, M.D.; Aranovich, G.L. Classification of Gibbs adsorption isotherms. Adv. Colloid Interface Sci. 1998, 76–77, 137–152. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption hysteresis loops and the characterization of nanoporous materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Fan, C.; Nguyen, V.; Zeng, Y.; Phadungbut, P.; Horikawa, T.; Do, D.D.; Nicholson, D. Novel approach to the characterization of the pore structure and surface chemistry of porous carbon with Ar, N2, H2O and CH3OH adsorption. Microporous Mesoporous Mater. 2015, 209, 79–89. [Google Scholar] [CrossRef]
- Yu, D.; Wang, J.; Hu, W.; Guo, R. Preparation and controlled release behavior of halloysite/2-mercaptobenzothiazole nanocomposite with calcined halloysite as nanocontainer. Mater. Design 2017, 129, 103–110. [Google Scholar] [CrossRef]
- Alam, N.; Mokaya, R. Strongly acidic mesoporous aluminosilicates prepared via hydrothermal restructuring of a crystalline layered silicate. J. Mater. Chem. A 2015, 3, 7799–7809. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, F.; Jiang, Y.; Li, F.; Wei, C. The effect of calcination temperature on the structure and activity of TiO2/SiO2 composite catalysts derived from titanium sulfate and fly ash acid sludge. Colloid Surf. A 2018, 554, 81–85. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, X.; Zheng, S.; Sun, Z.; Liu, S.; Li, H. Influence of calcination temperature on the structural, adsorption and photocatalytic properties of TiO2 nanoparticles supported on natural zeolite. Powder Technol. 2015, 274, 88–97. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.-P.; Charlet, L.; Szenknect, S.; Barthés, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, D.S. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurements of specific surface areas of solids. J. Chem. Soc. 1960, 10, 3973–3999. [Google Scholar] [CrossRef]
- Viotti, P.V.; Moreira, W.M.; Andro dos Santos, O.A.; Bergamasco, R.; Vieira, A.M.S.; Vieira, M.F. Diclofenac removal from water by adsorption on Moringa oleifera pods and activated carbon: Mechanism, kinetic and equilibrium study. J. Clean. Prod. 2019, 219, 809–817. [Google Scholar] [CrossRef]
- Hüffer, T.; Endo, S.; Metzelder, F.; Schroth, S.; Schmidt, T.C. Prediction of sorption of aromatic and aliphatic organic compounds by carbon nanotubes using poly-parameter linear free-energy relationships. Water Res. 2014, 59, 295–303. [Google Scholar] [CrossRef]
- Liu, F.; Fan, J.; Wang, S.; Ma, G. Adsorption of natural organic matter analogues by multi-walled carbon nanotubes: Comparison with powdered activated carbon. Chem. Eng. J. 2013, 219, 450–458. [Google Scholar] [CrossRef]
- Sui, Q.; Huang, J.; Liu, Y.; Chang, X.; Deng, S.; Xie, T.; Yu, G. Rapid removal of bisphenol A on highly ordered mesoporous carbon. J. Environ. Sci. 2011, 23, 177–182. [Google Scholar] [CrossRef]
- Strachowski, P.; Bystrzejewski, M. Comparative studies of sorption of phenolic compounds onto carbon-encapsulated iron nanoparticles, carbon nanotubes and activated carbon. Colloid. Surf. A 2015, 467, 113–123. [Google Scholar] [CrossRef]
- Deng, L.; Yuan, P.; Liu, D.; Annabi-Bergaya, F.; Zhou, J.; Chen, F.; Liu, Z. Effects of microstructure of clay minerals, montmorillonite, kaolinite and halloysite, on their benzene adsorption behaviors. Appl. Clay Sci. 2017, 143, 184–191. [Google Scholar] [CrossRef]
- Szczepanik, B.; Słomkiewicz, P.; Garnuszek, M.; Czech, K. Adsorption of chloroanilines from aqueous solutions on the modified halloysite. Appl. Clay Sci. 2014, 101, 206–264. [Google Scholar] [CrossRef]
- Zhu, Q.; Moggridge, G.D.; D’Agostino, C. Adsorption of pyridine from aqueous solutions by polymeric adsorbents MN 200 and MN 500. Part 2: Kinetics and diffusion analysis. Chem. Eng. J. 2016, 306, 1223–1233. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, F.; Liu, X.; Yang, L.; Jiang, X.; Yang, J.; Shi, W. Multi-walled carbon nanotubes as sorbent for recovery of endocrine disrupting compound-bisphenol F from wastewater. Chem. Eng. J. 2013, 218, 238–246. [Google Scholar] [CrossRef]
- Kumar, A.K.; Mohan, S.V. Removal of natural and synthetic endocrine disrupting estrogens by multiwalled carbon nanotubes (MWCNT) as adsorbent: Kinetic and mechanism evaluation. Sep. Purif. Technol. 2012, 87, 22–30. [Google Scholar] [CrossRef]
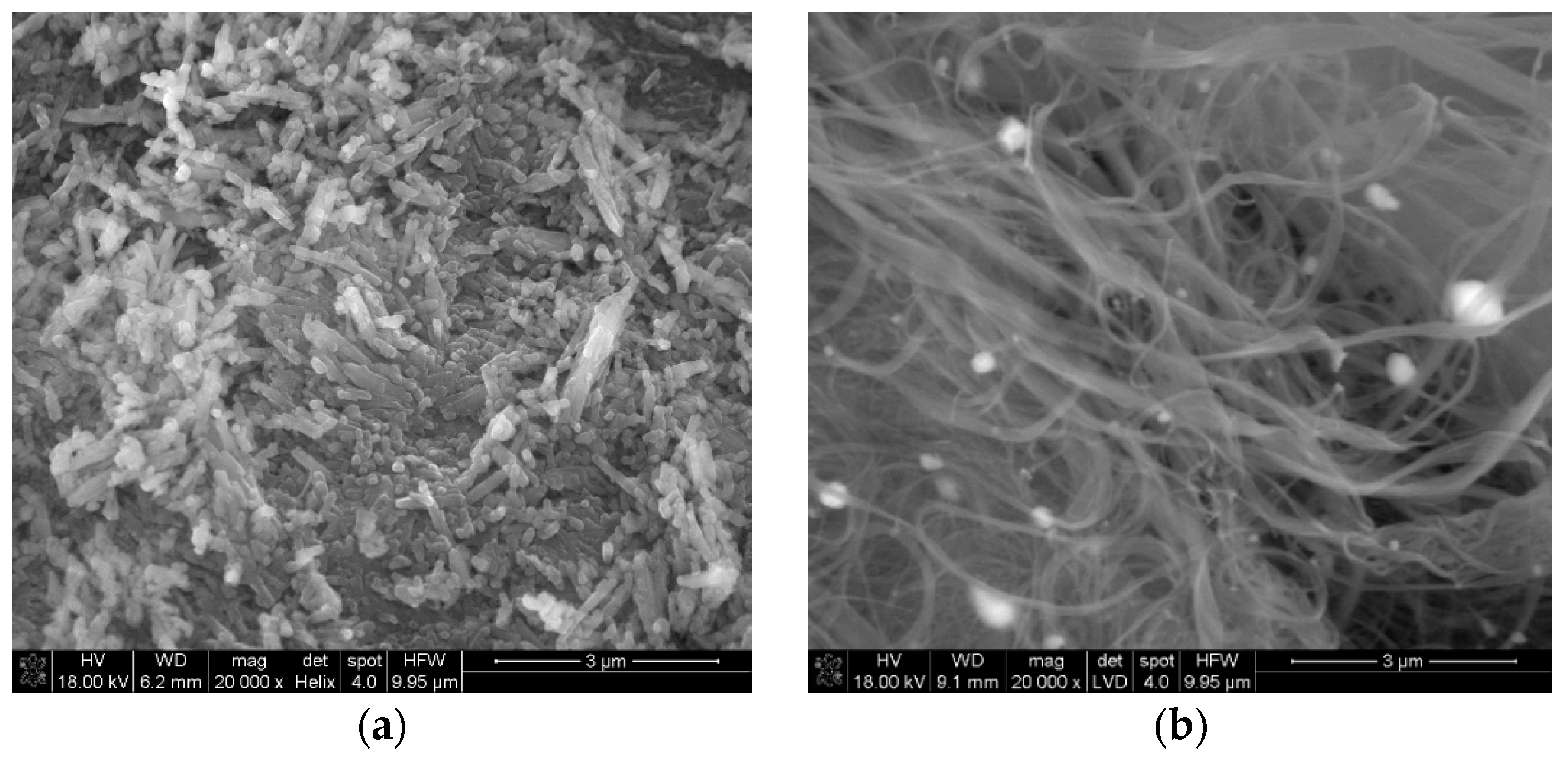

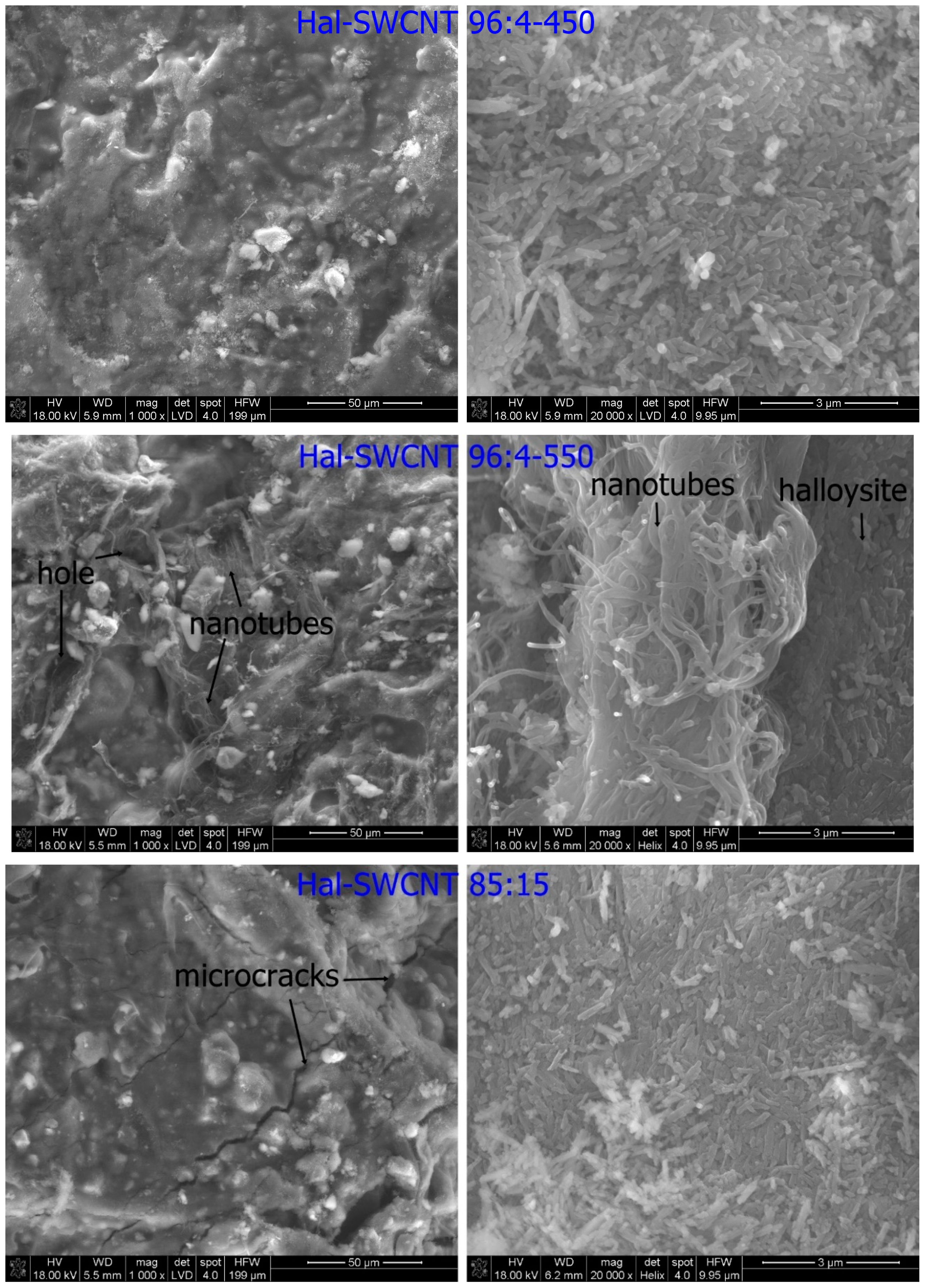
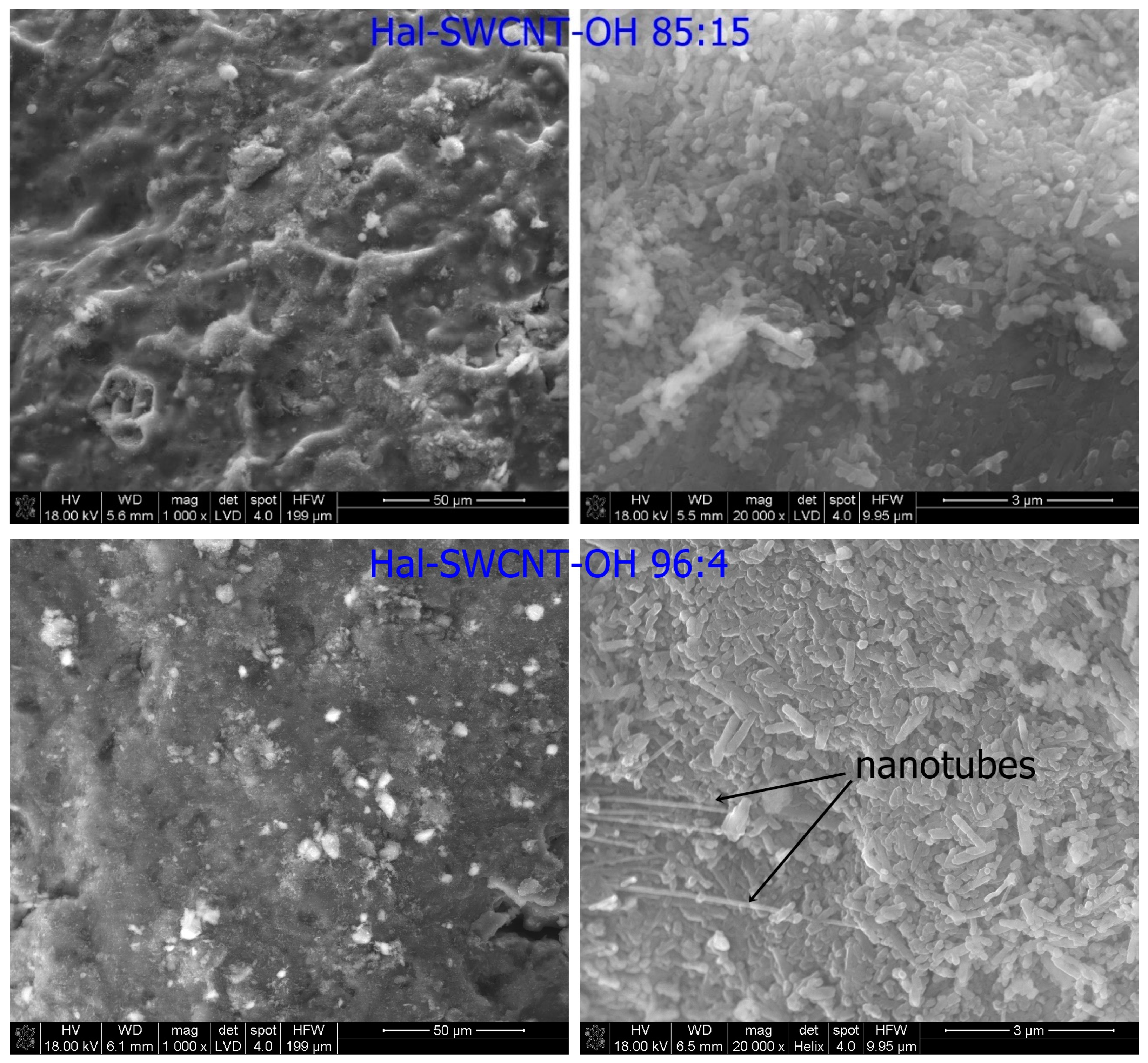
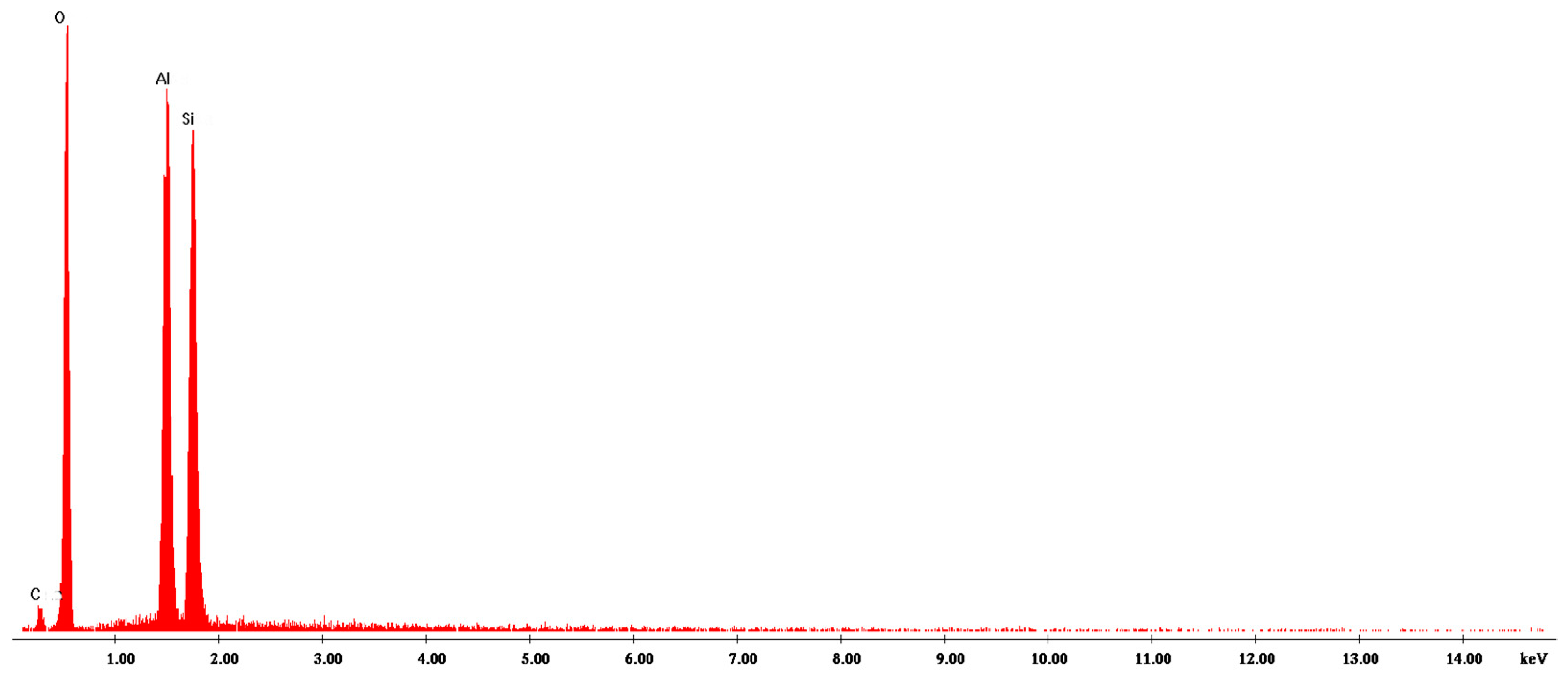

| Name | Outer Diameter 1 (nm) | Length 1 (µm) | Purity 1 (wt%) | OH Content 1 (%) |
|---|---|---|---|---|
| SWCNT | <2 | 5–30 | 95 | not applicable |
| SWCNT-OH | <2 | 5–30 | 90 | 3.7 |
| Compound | Chemical Structure | Molecular Mass 1 (g/mol) | log Kow 1 (-) | Solubility in Water 20 °C 1 (mg/L) |
|---|---|---|---|---|
| ANT |  | 178.55 | 4.45 | 0.043 |
| Symbol | Carbon Nanotubes Type | Halloysite to CNT Weight Ratio (%) | Calcination Temperature (°C) |
|---|---|---|---|
| Hal-350 | - | - | 350 |
| Hal-450 | - | - | 450 |
| Hal-550 | - | - | 550 |
| Hal-SWCNT 96:4-350 | SWCNT | 96:4 | 350 |
| Hal-SWCNT 96:4-450 | SWCNT | 96:4 | 450 |
| Hal-SWCNT 96:4-550 | SWCNT | 96:4 | 550 |
| Hal-SWCNT 85:15 | SWCNT | 85:15 | 450 |
| Hal-SWCNT-OH 85:15 | SWCNT-OH | 85:15 | 450 |
| Hal-SWCNT-OH 96:4 | SWCNT-OH | 96:4 | 450 |
| Adsorbent | BET Specific Surface Area (m2/g) | Total Volume in Pores (cm3/g) | Total Area in Pores (m2/g) |
|---|---|---|---|
| SWCNT | 1124.6 | 0.88 | 945.6 |
| SWCNT-OH | 410.63 | 0.6 | 257.52 |
| Hal | 50.36 | 0.19 | 32.69 |
| Hal-350 | 45.15 | 0.06 | 45.86 |
| Hal-450 | 44.89 | 0.08 | 50.18 |
| Hal-550 | 54.16 | 0.07 | 56.55 |
| Hal-SWCNT 96:4-350 | 90.56 | 0.25 | 55.64 |
| Hal-SWCNT 96:4-450 | 92.15 | 0.23 | 54.39 |
| Hal-SWCNT 96:4-550 | 80.09 | 0.27 | 47.24 |
| Hal-SWCNT 85:15 | 239.22 | 0.33 | 153.12 |
| Hal-SWCNT-OH 85:15 | 102.24 | 0.29 | 68.28 |
| Hal-SWCNT-OH 96:4 | 94.20 | 0.26 | 62.12 |
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
(mg/g) | (L/mg) | R2 (-) | ((mg/g)·(L/mg)n) | (-) | R2 (-) | |
| Hal | 0.21 | 32.30 | 0.9592 | 0.21 | 5.01 | 0.8171 |
| Hal-350 | 0.21 | 7.21 | 0.9548 | 0.19 | 3.05 | 0.9045 |
| Hal-450 | 0.22 | 8.32 | 0.9871 | 0.19 | 3.52 | 0.9518 |
| Hal-550 | 0.18 | 14.47 | 0.9633 | 0.17 | 4.51 | 0.8805 |
| Hal-SWCNT 96:4-350 | 0.43 | 6.67 | 0.9531 | 0.37 | 2.92 | 0.8905 |
| Hal-SWCNT 96:4-450 | 0.44 | 8.24 | 0.9867 | 0.38 | 3.52 | 0.9464 |
| Hal-SWCNT 96:4-550 | 0.36 | 13.84 | 0.9609 | 0.34 | 4.07 | 0.9596 |
| Hal-SWCNT 85:15 | 0.84 | 24.78 | 0.9519 | 0.84 | 4.38 | 0.8174 |
| Hal-SWCNT-OH 85:15 | 0.54 | 26.29 | 0.9646 | 0.54 | 4.53 | 0.8701 |
| Hal-SWCNT-OH 96:4 | 0.49 | 20.87 | 0.9963 | 0.47 | 5.06 | 0.8819 |
| Sample | Pseudo-Second-Order Equation Parameters | |||||
|---|---|---|---|---|---|---|
(g/(mg·min)) | (mg/g) | (mg/g) | (min) | (mg/(g·min)) | R2 | |
| Hal | 0.23 | 0.108 | 0.11 | 18.27 | 0.006 | 0.9438 |
| Hal-350 | 0.2 | 0.096 | 0.1 | 17.61 | 0.006 | 0.9686 |
| Hal-450 | 0.088 | 0.083 | 0.09 | 16.27 | 0.006 | 0.870 |
| Hal-550 | 0.268 | 0.06 | 0.055 | 8.76 | 0.007 | 0.9498 |
| Hal-SWCNT 96:4-350 | 0.296 | 0.35 | 0.36 | 9.33 | 0.039 | 0.964 |
| Hal-SWCNT 96:4-450 | 0.31 | 0.32 | 0.34 | 9.49 | 0.036 | 0.972 |
| Hal-SWCNT 96:4-550 | 0.757 | 0.18 | 0.19 | 6.96 | 0.027 | 0.960 |
| Hal-SWCNT 85:15 | 0.479 | 0.45 | 0.38 | 5.49 | 0.087 | 0.970 |
| Hal-SWCNT-OH 85:15 | 0.428 | 0.36 | 0.38 | 6.16 | 0.062 | 0.967 |
| Hal-SWCNT-OH 96:4 | 0.292 | 0.27 | 0.34 | 10.07 | 0.029 | 0.972 |
| Sample | (mg/g·min0.5) | (mg/g) |
|---|---|---|
| Hal-SWCNT 96:4-350 | 0.013 | 0.143 |
| Hal-SWCNT 96:4-450 | 0.015 | 0.09 |
| Hal-SWCNT 96:4-550 | 0.006 | 0.12 |
| Hal-SWCNT 85:15 | 0.015 | 0.229 |
| Hal-SWCNT-OH 85:15 | 0.012 | 0.18 |
| Hal-SWCNT-OH 96:4 | 0.01 | 0.113 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamińska, G.; Dudziak, M.; Kudlek, E.; Bohdziewicz, J. Preparation, Characterization and Adsorption Potential of Grainy Halloysite-CNT Composites for Anthracene Removal from Aqueous Solution. Nanomaterials 2019, 9, 890. https://doi.org/10.3390/nano9060890
Kamińska G, Dudziak M, Kudlek E, Bohdziewicz J. Preparation, Characterization and Adsorption Potential of Grainy Halloysite-CNT Composites for Anthracene Removal from Aqueous Solution. Nanomaterials. 2019; 9(6):890. https://doi.org/10.3390/nano9060890
Chicago/Turabian StyleKamińska, Gabriela, Mariusz Dudziak, Edyta Kudlek, and Jolanta Bohdziewicz. 2019. "Preparation, Characterization and Adsorption Potential of Grainy Halloysite-CNT Composites for Anthracene Removal from Aqueous Solution" Nanomaterials 9, no. 6: 890. https://doi.org/10.3390/nano9060890
APA StyleKamińska, G., Dudziak, M., Kudlek, E., & Bohdziewicz, J. (2019). Preparation, Characterization and Adsorption Potential of Grainy Halloysite-CNT Composites for Anthracene Removal from Aqueous Solution. Nanomaterials, 9(6), 890. https://doi.org/10.3390/nano9060890






