Multiwalled Carbon Nanotube Reinforced Bio-Based Benzoxazine/Epoxy Composites with NIR-Laser Stimulated Shape Memory Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Benzoxazine Monomer
2.3. Preparation of V-fa/ECO Copolymer Reinforced with MWCNTs
2.4. Characterization Methods
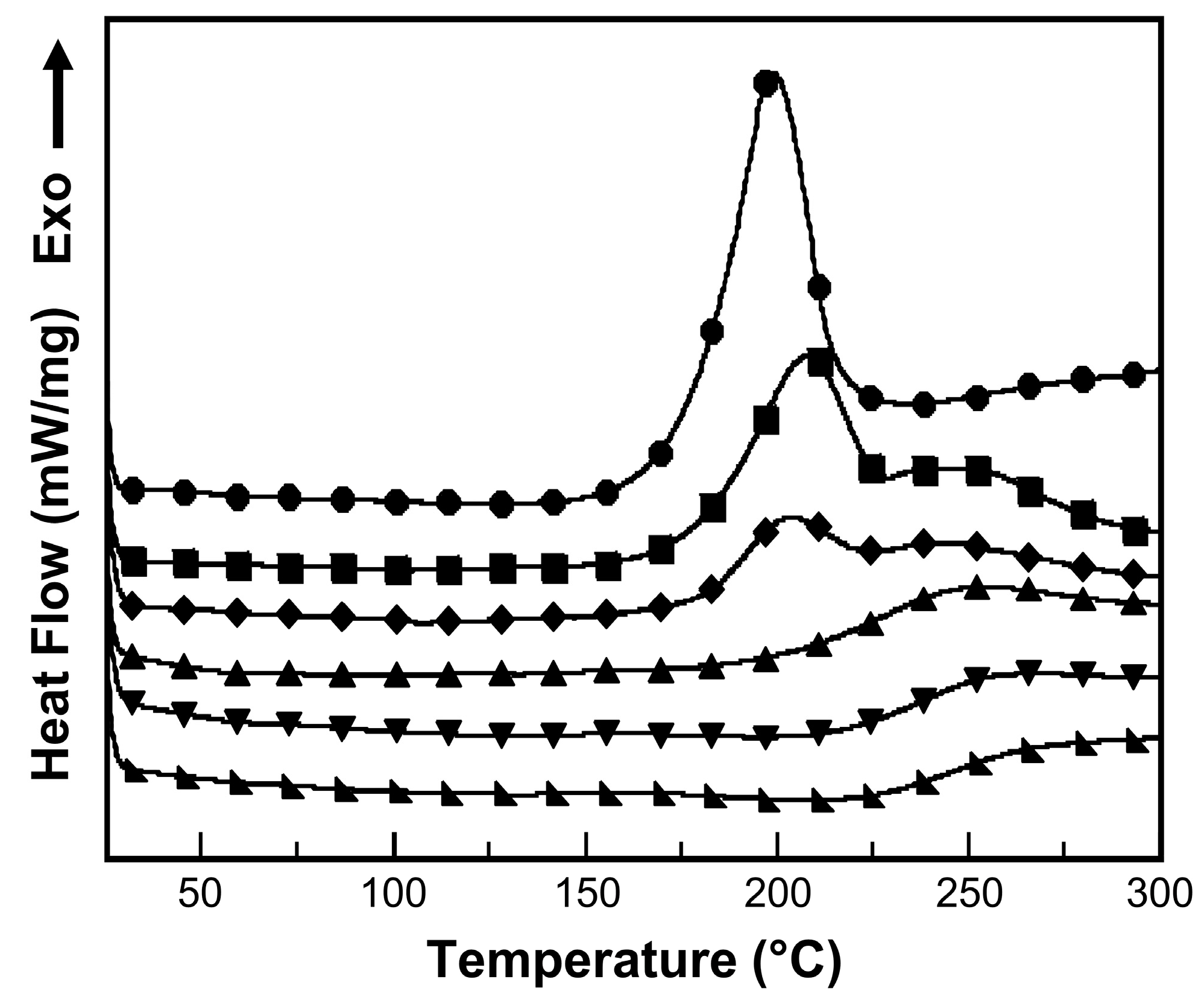
2.4.1. Differential Scanning Calorimetry
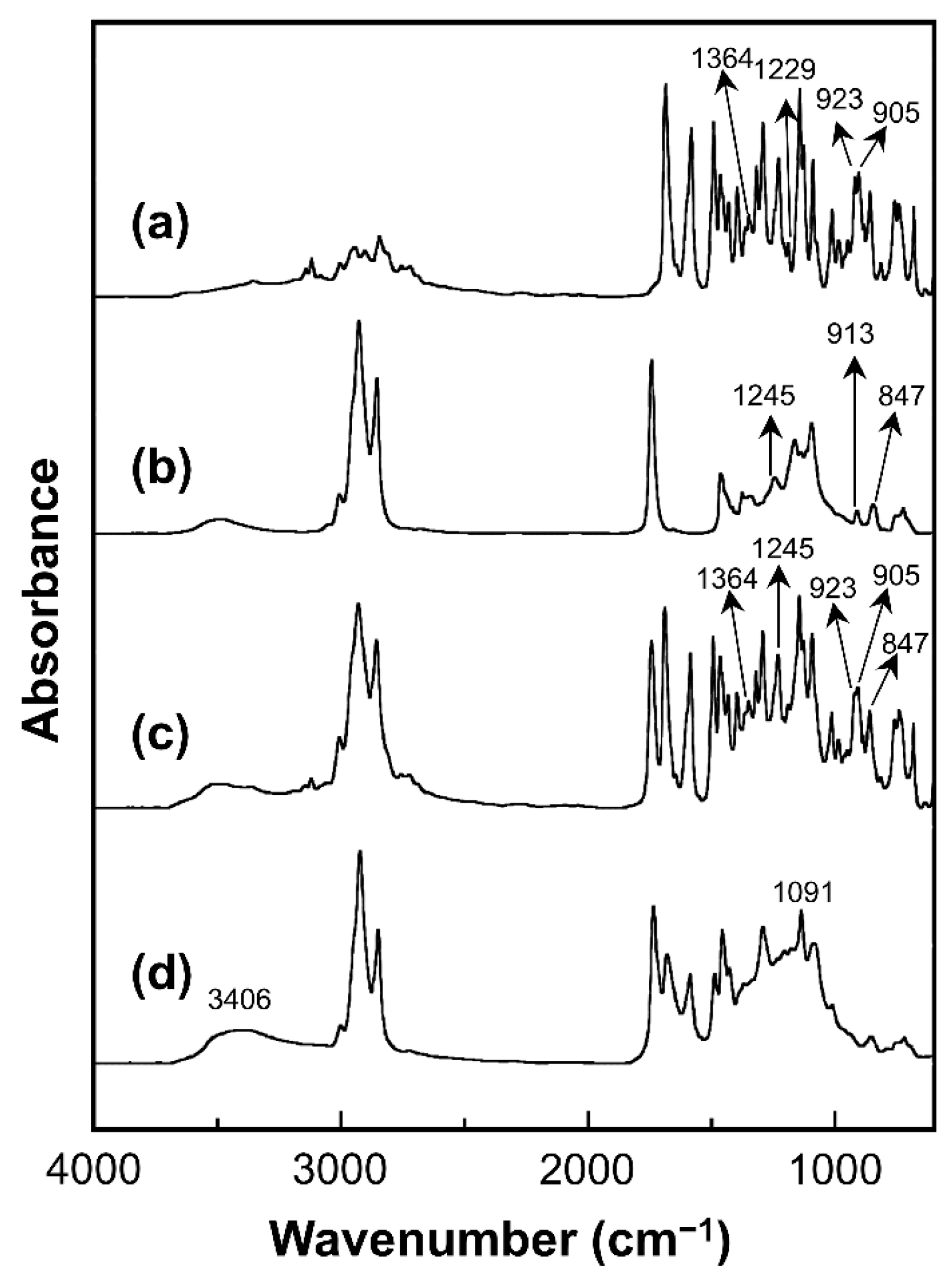
2.4.2. FT-IR Spectroscopy
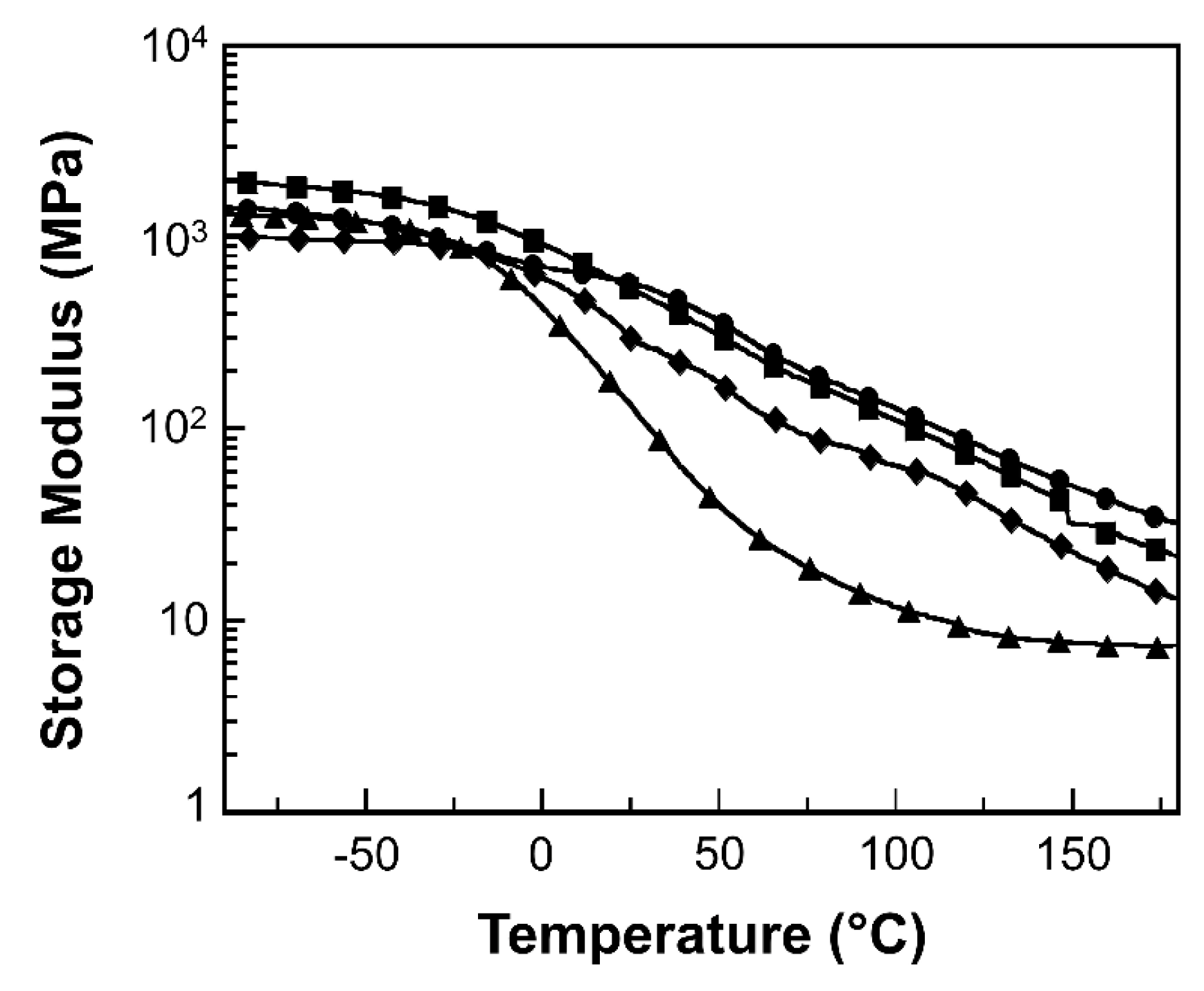
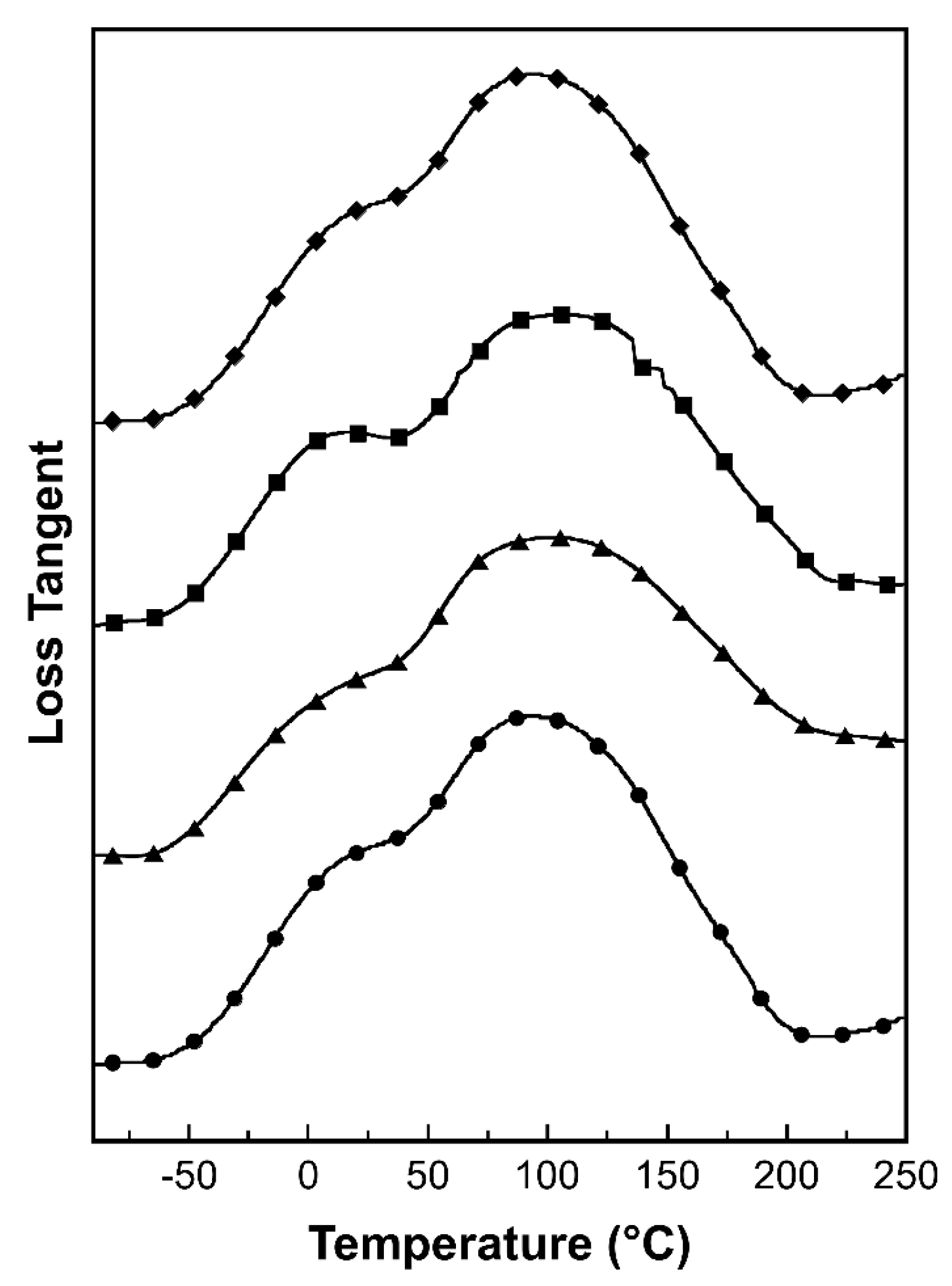
2.4.3. Dynamic Mechanical Analysis
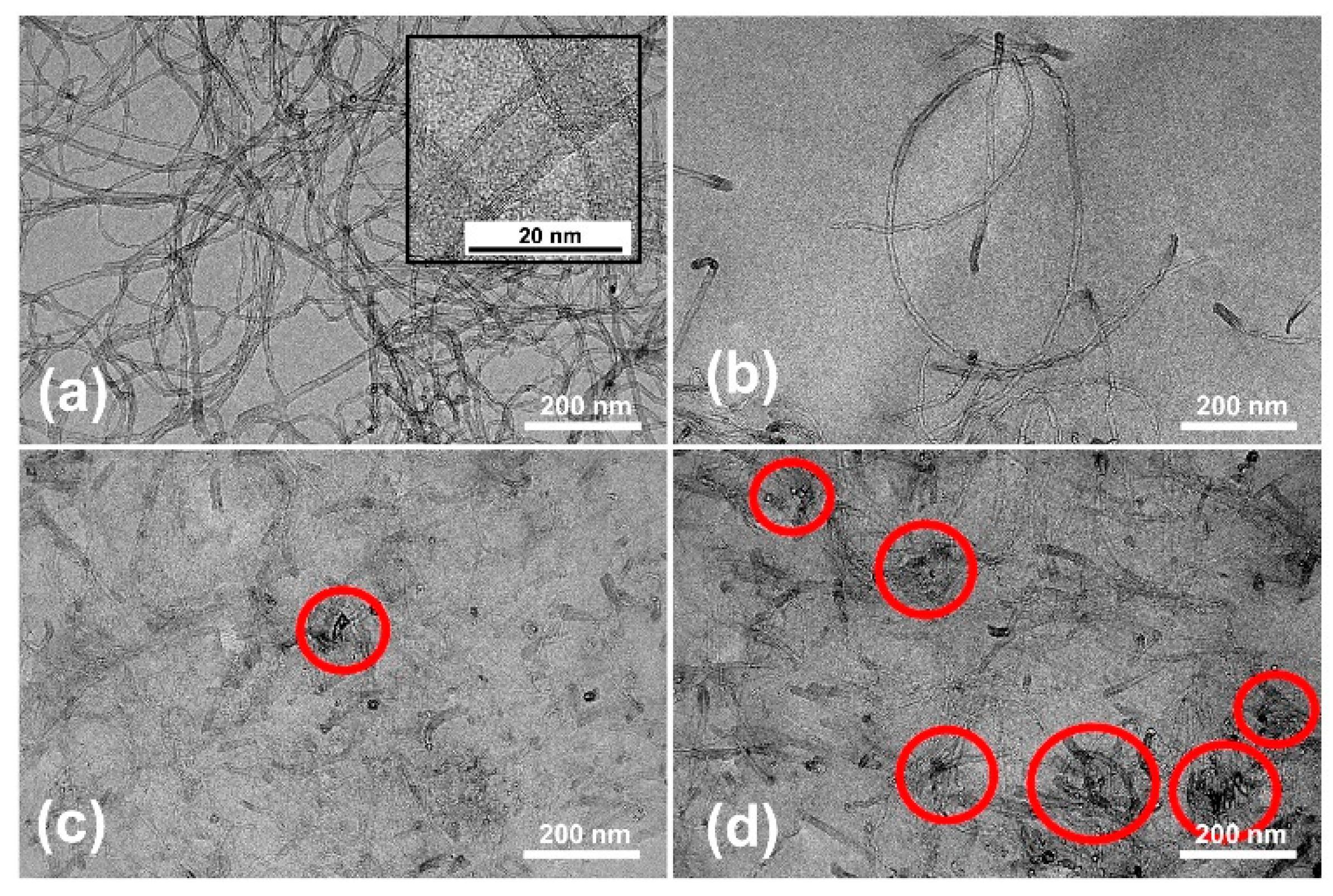
2.4.4. Transmission Electron Microscopy
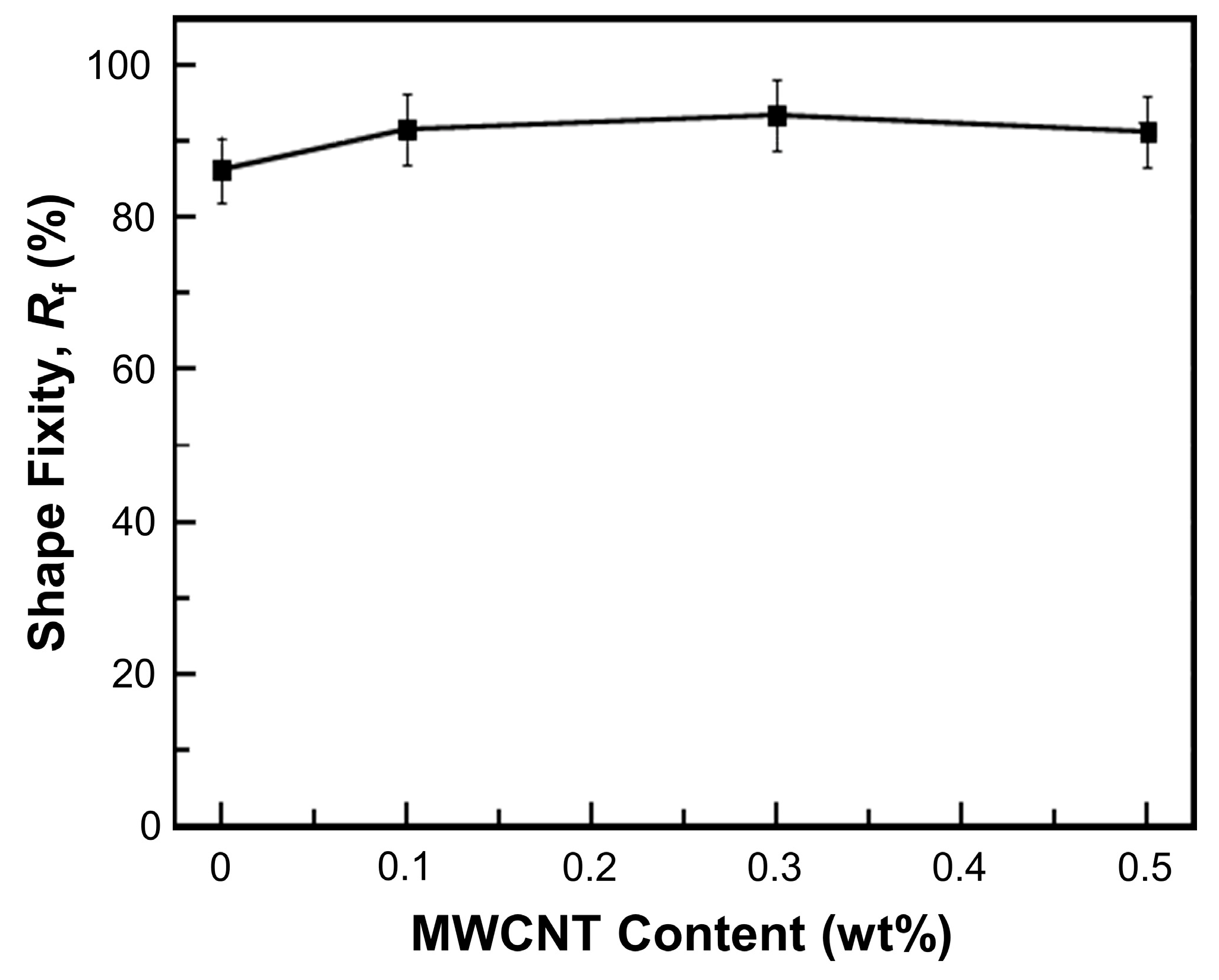
2.4.5. Shape Memory Performances
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matar, S.; Hatch, L.F. Primary Raw Materials for Petrochemicals. In Chemistry of Petrochemical Processes; Elsevier: Amsterdam, The Netherlands, 2001; pp. 1–28. [Google Scholar]
- Pathak, C.; Mandalia, H.C. Petroleum industries: Environmental pollution effects, management and treatment methods. Int. J. Sep. Environ. Sci. 2012, 1, 55–62. [Google Scholar]
- Thirukumaran, P.; Shakilaparveen, A.; Sarojadevi, M. Eugenol-Based Polybenzoxazines. In Advanced and Emerging Polybenzoxazine Science and Technology; Ishida, H., Froimowicz, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 523–531. [Google Scholar]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Niaounakis, M. Biopolymers: Processing and Products; William Andrew: Amsterdam, The Netherlands, 2014; pp. 447–458. [Google Scholar]
- Arslan, M.; Kiskan, B.; Yagci, Y. Ring-Opening Polymerization of 1,3-Benzoxazines via Borane Catalyst. Polymers 2018, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H. Process for Preparation of Benzoxazine Compounds in Solventless Systems. U.S. Patent US5543516A, 6 August 1996. [Google Scholar]
- Rimdusit, S.; Bangsen, W.; Kasemsiri, P. Chemorheology and thermomechanical characteristics of benzoxazine-urethane copolymers. J. Appl. Polym. Sci. 2011, 121, 3669–3678. [Google Scholar] [CrossRef]
- Ishida, H. Overview and Historical Background of Polybenzoxazine Research. In Handbook of Benzoxazine Resins; Elsevier: Amsterdam, The Netherlands, 2011; pp. 3–81. [Google Scholar]
- Trejo-Machin, A.; Puchot, L.; Verge, P. Design and Synthesis of Bio-Based Benzoxazines. In Paint and Coatings Industry; IntechOpen: London, UK, 2018; pp. 53–69. [Google Scholar]
- Rimdusit, S.; Jubsilp, C.; Tiptipakorn, S. Alloys and Composites of Polybenzoxazines; Springer Science and Business Media LLC: Larkspur, CA, USA, 2013; pp. 29–46. [Google Scholar]
- Rimdusit, S.; Ishida, H. Synergism and multiple mechanical relaxations observed in ternary systems based on benzoxazine, epoxy, and phenolic resins. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 1687–1698. [Google Scholar] [CrossRef]
- Rimdusit, S.; Kunopast, P.; Dueramae, I. Thermomechanical properties of arylamine-based benzoxazine resins alloyed with epoxy resin. Polym. Eng. Sci. 2011, 51, 1797–1807. [Google Scholar] [CrossRef]
- Tanpitaksit, T. Effects of benzoxazine resin on property enhancement of shape memory epoxy: A dual function of benzoxazine resin as a curing agent and a stable network segment. Express Polym. Lett. 2015, 9, 824–837. [Google Scholar] [CrossRef]
- Kiskan, B.; Yagci, Y. Benzoxazine resins as smart materials and future perspectives. In Thermosets; Elsevier: Amsterdam, The Netherlands, 2018; pp. 543–576. [Google Scholar]
- Kohlmeyer, R.R.; Lor, M.; Chen, J. Remote, Local, and Chemical Programming of Healable Multishape Memory Polymer Nanocomposites. Nano Lett. 2012, 12, 2757–2762. [Google Scholar] [CrossRef]
- Sini, N.K.; Bijwe, J.; Varma, I.K. Renewable Benzoxazine Monomer from Vanillin: Synthesis, Characterization, and Studies on Curing Behavior. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 7–11. [Google Scholar] [CrossRef]
- Van, A.; Chiou, K.; Ishida, H. Use of renewable resource vanillin for the preparation of benzoxazine resin and reactive monomeric surfactant containing oxazine ring. Polymers 2014, 55, 1443–1451. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Shakila, A.; Muthusamy, S. Synthesis and characterization of novel bio-based benzoxazines from eugenol. RSC Adv. 2014, 4, 7959. [Google Scholar] [CrossRef]
- Lochab, B.; Varma, I.K.; Bijwe, J. Thermal behaviour of cardanol-based benzoxazines. J. Therm. Anal. Calorim. 2010, 102, 769–774. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.; Liu, X.; Sudo, A.; Endo, T. Synthesis and copolymerization of fully bio-based benzoxazines from guaiacol, furfurylamine and stearylamine. Green Chem. 2012, 14, 2799–2806. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, C.; Sun, J.; Huang, S.; Liu, X.; Endo, T. Synthesis and thermal properties of a bio-based polybenzoxazine with curing promoter. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2016–2023. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Parveen, A.S.; Sarojadevi, M. Synthesis and Copolymerization of Fully Biobased Benzoxazines from Renewable Resources. ACS Sustain. Chem. Eng. 2014, 2, 2790–2801. [Google Scholar] [CrossRef]
- Ni, J.; Tao, F.; Du, H.; Xu, P. Mimicking a natural pathway for de novo biosynthesis: Natural vanillin production from accessible carbon sources. Sci. Rep. 2015, 5, 13670. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Chou, C.-I. High performance benzoxazine monomers and polymers containing furan groups. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5267–5282. [Google Scholar] [CrossRef]
- Wei, Z.G.; Sandstroröm, R.; Miyazaki, S. Shape-memory materials and hybrid composites for smart systems: Part I Shape-memory materials. J. Mater. Sci. 1998, 33, 3743–3762. [Google Scholar] [CrossRef]
- Akabori, S.; Habata, Y.; Nakazawa, M.; Yamada, Y.; Shindo, Y.; Sugimura, T.; Sato, S. The novel syntheses of photoreversible cyclobutanocrown ethers by the intramolecular photoaddition of α, ω-dicinnamoyl polyethylene glycol derivatives. Chem. Chem. Ind. Bull. Chem. Soc. Jpn. 1987, 60, 3453–3455. [Google Scholar] [CrossRef]
- Camacho-Lopez, M.; Finkelmann, H.; Palffy-Muhoray, P.; Shelley, M. Fast liquid-crystal elastomer swims into the dark. Nat. Mater. 2004, 3, 307–310. [Google Scholar] [CrossRef]
- Finkelmann, H.; Nishikawa, E.; Pereira, G.G.; Warner, M. A New Opto-Mechanical Effect in Solids. Phys. Rev. Lett. 2001, 87, 015501. [Google Scholar] [CrossRef] [PubMed]
- Lendlein, A.; Jiang, H.; Jünger, O.; Langer, R. Light-induced shape-memory polymers. Nature 2005, 434, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Honda, K. Photoreversible reactions of polymers containing cinnamylideneacetate derivatives and the model compounds. J. Polym. Sci. Polym. Chem. Ed. 1977, 15, 2685–2689. [Google Scholar] [CrossRef]
- Rimdusit, S.; Lohwerathama, M.; Hemvichian, K.; Kasemsiri, P.; Dueramae, I. Shape memory polymers from benzoxazine-modified epoxy. Smart Mater. Struct. 2013, 22, 75033. [Google Scholar] [CrossRef]
- Prathumrat, P.; Tiptipakorn, S.; Rimdusit, S. Multiple-shape memory polymers from benzoxazine–urethane copolymers. Smart Mater. Struct. 2017, 26, 65025. [Google Scholar] [CrossRef]
- Likitaporn, C.; Mora, P.; Tiptipakorn, S.; Rimdusit, S. Recovery stress enhancement in shape memory composites from silicon carbide whisker-filled benzoxazine-epoxy polymer alloy. J. Intell. Mater. Syst. Struct. 2018, 29, 388–396. [Google Scholar] [CrossRef]
- Yabe, H.; Fujii, R.; Nishi, Y.; Oguri, K.; Uchida, H.-H.; Matsumura, Y.; Uchida, H. Magnetic field induced shape memory effect of Fe-Pd alloy thin film. J. Adv. Sci. 2000, 12, 44–45. [Google Scholar] [CrossRef]
- Ohtsuka, M.; Sanada, M.; Matsumoto, M.; Itagaki, K. Magnetic-field induced shape memory effect in Ni2MnGa sputtered films. Mater. Sci. Eng. A 2004, 378, 377–383. [Google Scholar] [CrossRef]
- Han, X.J.; Dong, Z.Q.; Fan, M.M.; Liu, Y.; Li, J.H.; Wang, Y.F.; Yuan, Q.J.; Li, B.J.; Zhang, S. pH-Induced Shape-Memory Polymers. Macromol. Rapid Commun. 2012, 33, 1055–1060. [Google Scholar] [CrossRef]
- Meng, Q.; Hu, J. A review of shape memory polymer composites and blends. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1661–1672. [Google Scholar] [CrossRef]
- Basit, A.; L’Hostis, G.; Durand, B. Multi-shape memory effect in shape memory polymer composites. Mater. Lett. 2012, 74, 220–222. [Google Scholar] [CrossRef]
- Habault, D.; Zhang, H.; Zhao, Y. Light-triggered self-healing and shape-memory polymers. Chem. Soc. Rev. 2013, 42, 7244. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.H.; Yoo, H.J.; Mahapatra, S.S.; Kim, Y.A.; Cho, J.W. The synergistic effect of the combined thin multi-walled carbon nanotubes and reduced graphene oxides on photothermally actuated shape memory polyurethane composites. J. Colloid Interface Sci. 2014, 432, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Koerner, H.; Price, G.; Pearce, N.A.; Alexander, M.; Vaia, R.A. Remotely actuated polymer nanocomposites—stress-recovery of carbon-nanotube-filled thermoplastic elastomers. Nat. Mater. 2004, 3, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.; Ding, X.; Singh, R.; Kraft, R.A.; Levi-Polyachenko, N.; Rylander, M.N.; Szot, C.; Buchanan, C.; Whitney, J.; Fisher, J.; et al. Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proc. Natl. Acad. Sci. USA 2009, 106, 12897–12902. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Image Processing and Analysis in Java. Available online: https://imagej.nih.gov/ij (accessed on 5 May 2019).
- Hernandez, N.L.P.; Bonon, A.J.; Bahú, J.O.; Barbosa, M.I.R.; Maciel, M.R.W.; Filho, R.M. Epoxy monomers obtained from castor oil using a toxicity-free catalytic system. J. Mol. Catal. A Chem. 2017, 426, 550–556. [Google Scholar] [CrossRef]
- Ishida, H.; Allen, D.J. Mechanical characterization of copolymers based on benzoxazine and epoxy. Polymer 1996, 37, 4487–4495. [Google Scholar] [CrossRef]
- Kimura, H.; Matsumoto, A.; Hasegawa, K.; Ohtsuka, K.; Fukuda, A. Epoxy resin cured by bisphenol A based benzoxazine. J. Appl. Polym. Sci. 1998, 68, 1903–1910. [Google Scholar] [CrossRef]
- Rimdusit, S.; Ishida, H. Development of new class of electronic packaging materials based on ternary systems of benzoxazine, epoxy, and phenolic resins. Polymers 2000, 41, 7941–7949. [Google Scholar] [CrossRef]
- Kasemsiri, P.; Lorwanishpaisarn, N.; Pongsa, U.; Ando, S. Reconfigurable Shape Memory and Self-Welding Properties of Epoxy Phenolic Novolac/Cashew Nut Shell Liquid Composites Reinforced with Carbon Nanotubes. Polymers 2018, 10, 482. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-F.; Kuo, S.-W.; Lin, C.-H.; Chen, H.-G.; Liao, C.-S.; Hung, P.-R. Benzoxazine as a reactive noncovalent dispersant for carbon nanotubes. RSC Adv. 2014, 4, 36012–36016. [Google Scholar] [CrossRef]
- Ling, Y.; Li, W.; Wang, B.; Gan, W.; Zhu, C.; Brady, M.A.; Wang, C. Epoxy resin reinforced with nanothin polydopamine-coated carbon nanotubes: A study of the interfacial polymer layer thickness. RSC Adv. 2016, 6, 31037–31045. [Google Scholar] [CrossRef]
- Ma, P.C.; Kim, J.-K.; Tang, B.Z. Effects of silane functionalization on the properties of carbon nanotube/epoxy nanocomposites. Compos. Sci. Technol. 2007, 67, 2965–2972. [Google Scholar] [CrossRef]
- Teng, C.-C.; Ma, C.-C.M.; Huang, Y.-W.; Yuen, S.-M.; Weng, C.-C.; Chen, C.-H.; Su, S.-F. Effect of MWCNT content on rheological and dynamic mechanical properties of multiwalled carbon nanotube/polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1869–1875. [Google Scholar] [CrossRef]
- Kord, B.; Jamshidi, M.; Hosseinihashemi, S.K. Effect of Multi-Walled Carbon Nanotubes on Viscoelastic Properties of PP/Reed Flour Composites. J. Polym. Environ. 2017, 25, 1313–1320. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, C.; Huang, W.M. Programming of Shape-Memory Polymers: The Temperature Memory Effect and Triple/Multiple-Shape-Memory Effect in Polymers. In Shape-Memory Polymer Device Design; Safranski, D.L., Griffis, J.C., Eds.; William Andrew: Amsterdam, The Netherlands, 2017; pp. 113–137. [Google Scholar]
- Zhao, P.; Zhou, Q.; Deng, Y.; Zhu, R.; Gu, Y. A novel benzoxazine/epoxy blend with multiphase structure. RSC Adv. 2014, 4, 238–242. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, Q.; Liu, X.; Zhu, R.Q.; Ran, Q.C.; Gu, Y. Phase separation in benzoxazine/epoxy resin blending systems. Polym. J. 2013, 45, 637–644. [Google Scholar] [CrossRef]
- Rahman, M.M.; Zainuddin, S.; Hosur, M.; Malone, J.; Salam, M.; Kumar, A.; Jeelani, S. Improvements in mechanical and thermo-mechanical properties of e-glass/epoxy composites using amino functionalized MWCNTs. Compos. Struct. 2012, 94, 2397–2406. [Google Scholar] [CrossRef]
- Gu, S.; Yan, B.; Liu, L.; Ren, J. Carbon nanotube–polyurethane shape memory nanocomposites with low trigger temperature. Eur. Polym. J. 2013, 49, 3867–3877. [Google Scholar] [CrossRef]
- Abdullah, S.A.; Jumahat, A.; Abdullah, N.R.; Frormann, L. Determination of Shape Fixity and Shape Recovery Rate of Carbon Nanotube-filled Shape Memory Polymer Nanocomposites. Procedia Eng. 2012, 41, 1641–1646. [Google Scholar] [CrossRef]
- Yang, Q.-S.; He, X.-Q.; Liu, X.; Leng, F.-F.; Mai, Y.-W. The effective properties and local aggregation effect of CNT/SMP composites. Compos. Part B Eng. 2012, 43, 33–38. [Google Scholar] [CrossRef]
- Herath, H.; Epaarachchi, J.; Islam, M.; Al-Azzawi, W.; Leng, J.; Zhang, F. Structural performance and photothermal recovery of carbon fibre reinforced shape memory polymer. Compos. Sci. Technol. 2018, 167, 206–214. [Google Scholar] [CrossRef]
- Meng, Q.; Hu, J.; Zhu, Y. Shape-memory polyurethane/multiwalled carbon nanotube fibers. J. Appl. Polym. Sci. 2007, 106, 837–848. [Google Scholar] [CrossRef]
- Hua, D.; Zhang, X.; Ji, Z.; Yan, C.; Yu, B.; Li, Y.; Wang, X.; Zhou, F. 3D printing of shape changing composites for constructing flexible paper-based photothermal bilayer actuators. J. Mater. Chem. C 2018, 6, 2123–2131. [Google Scholar] [CrossRef]
- Yu, K.; Liu, Y.J.; Liu, Y.; Peng, H.X.; Leng, J.S. Mechanical and shape recovery properties of shape memory polymer composite embedded with cup-stacked carbon nanotubes. J. Intell. Mater. Syst. Struct. 2014, 25, 1264–1275. [Google Scholar] [CrossRef]
- Yu, K.; Liu, Y.; Leng, J. Shape memory polymer/CNT composites and their microwave induced shape memory behaviors. RSC Adv. 2014, 4, 2961–2968. [Google Scholar] [CrossRef]
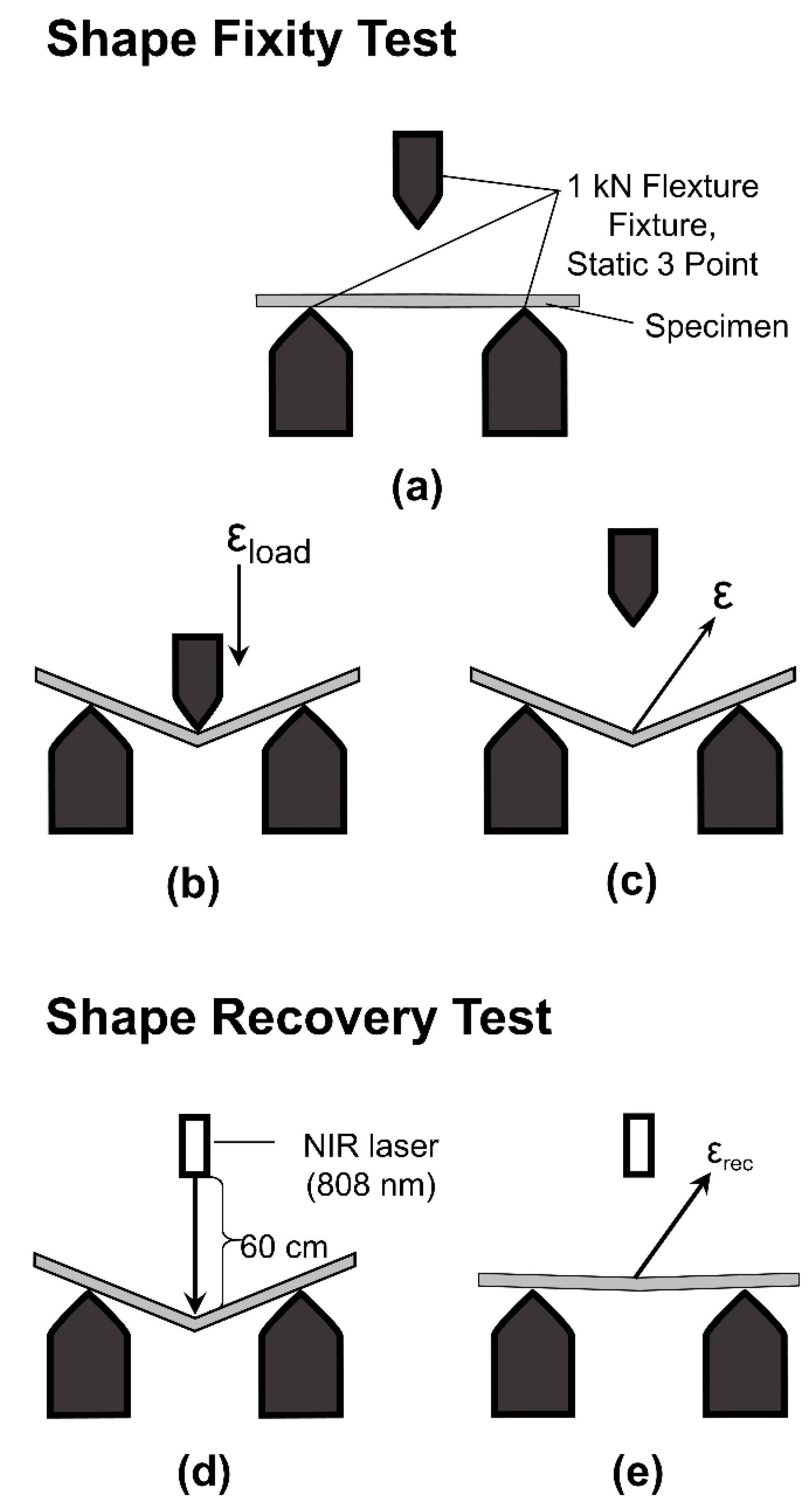


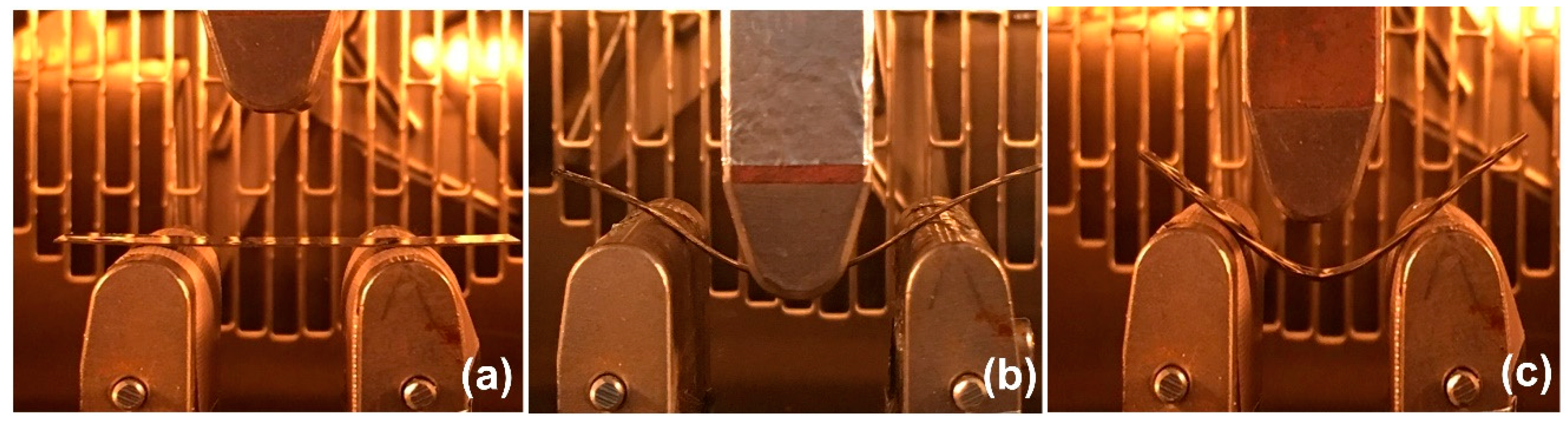
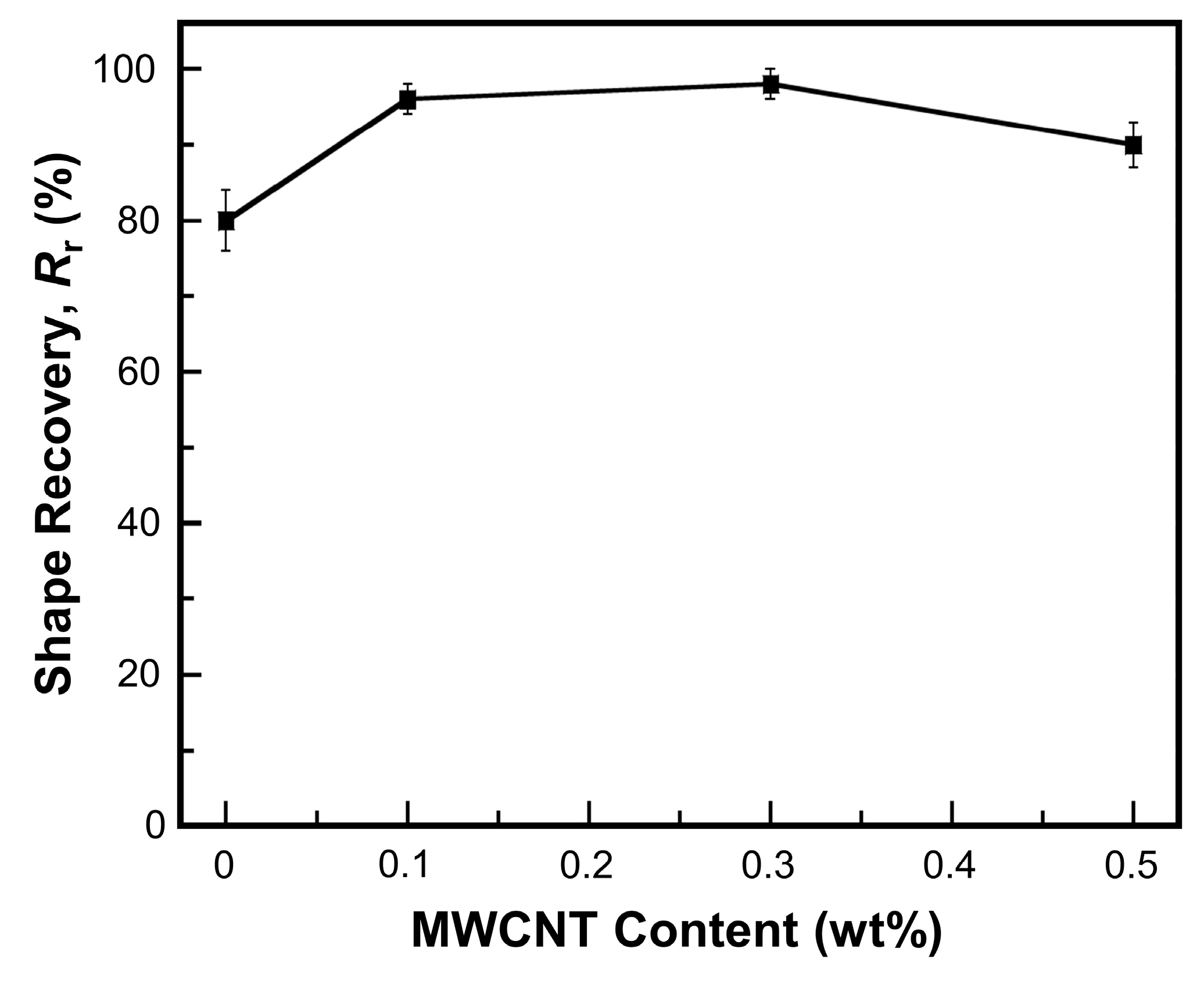
 ) NIR laser triggering and (
) NIR laser triggering and ( ) thermal heating of MWCNT reinforced V-fa/ECO composites at 0, 0.1, 0.3, and 0.5 wt% of MWCNTs.
) thermal heating of MWCNT reinforced V-fa/ECO composites at 0, 0.1, 0.3, and 0.5 wt% of MWCNTs.
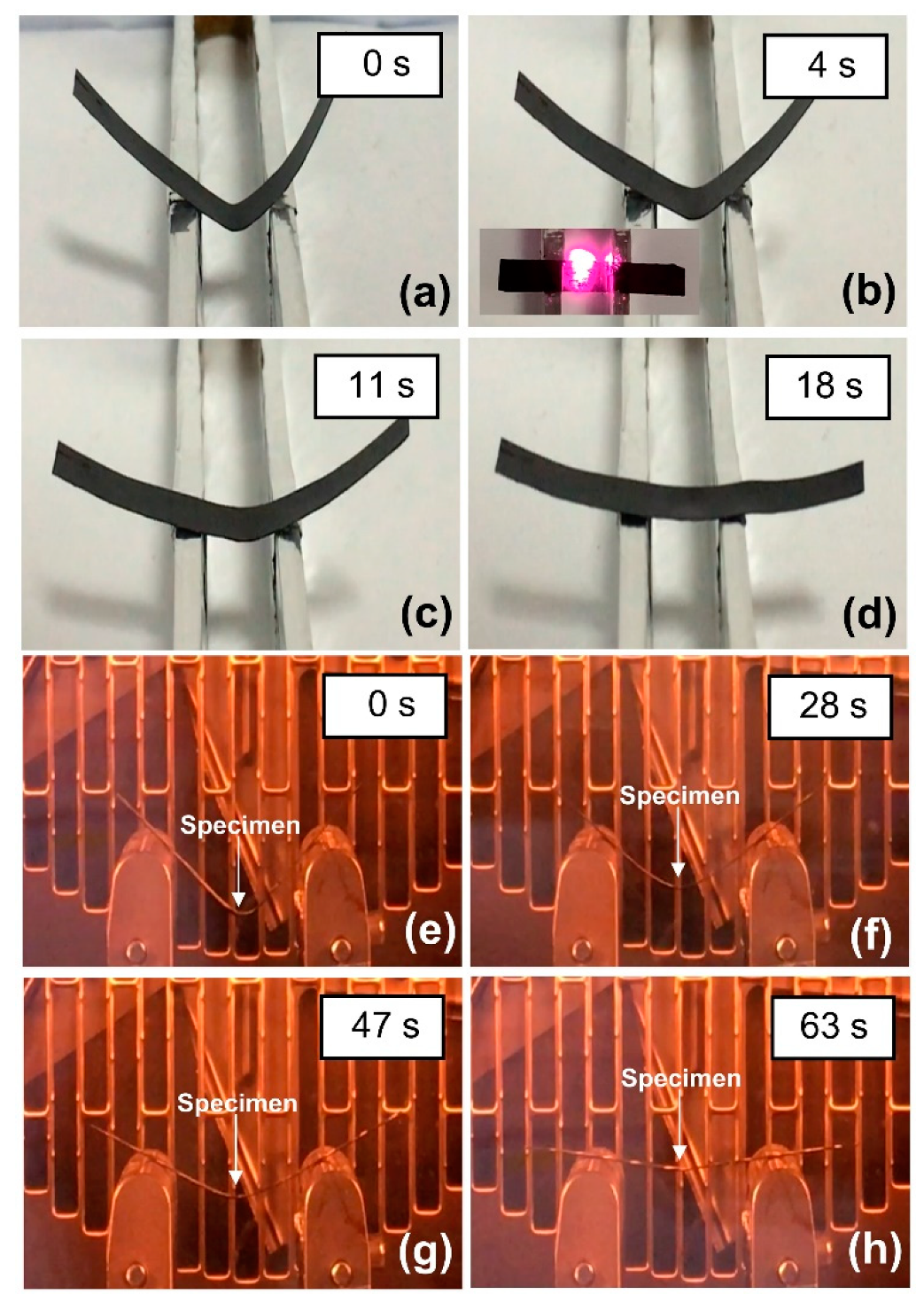
 ) NIR laser triggering and (
) NIR laser triggering and ( ) thermal heating of MWCNT reinforced V-fa/ECO composites at 0, 0.1, 0.3, and 0.5 wt% of MWCNTs.
) thermal heating of MWCNT reinforced V-fa/ECO composites at 0, 0.1, 0.3, and 0.5 wt% of MWCNTs.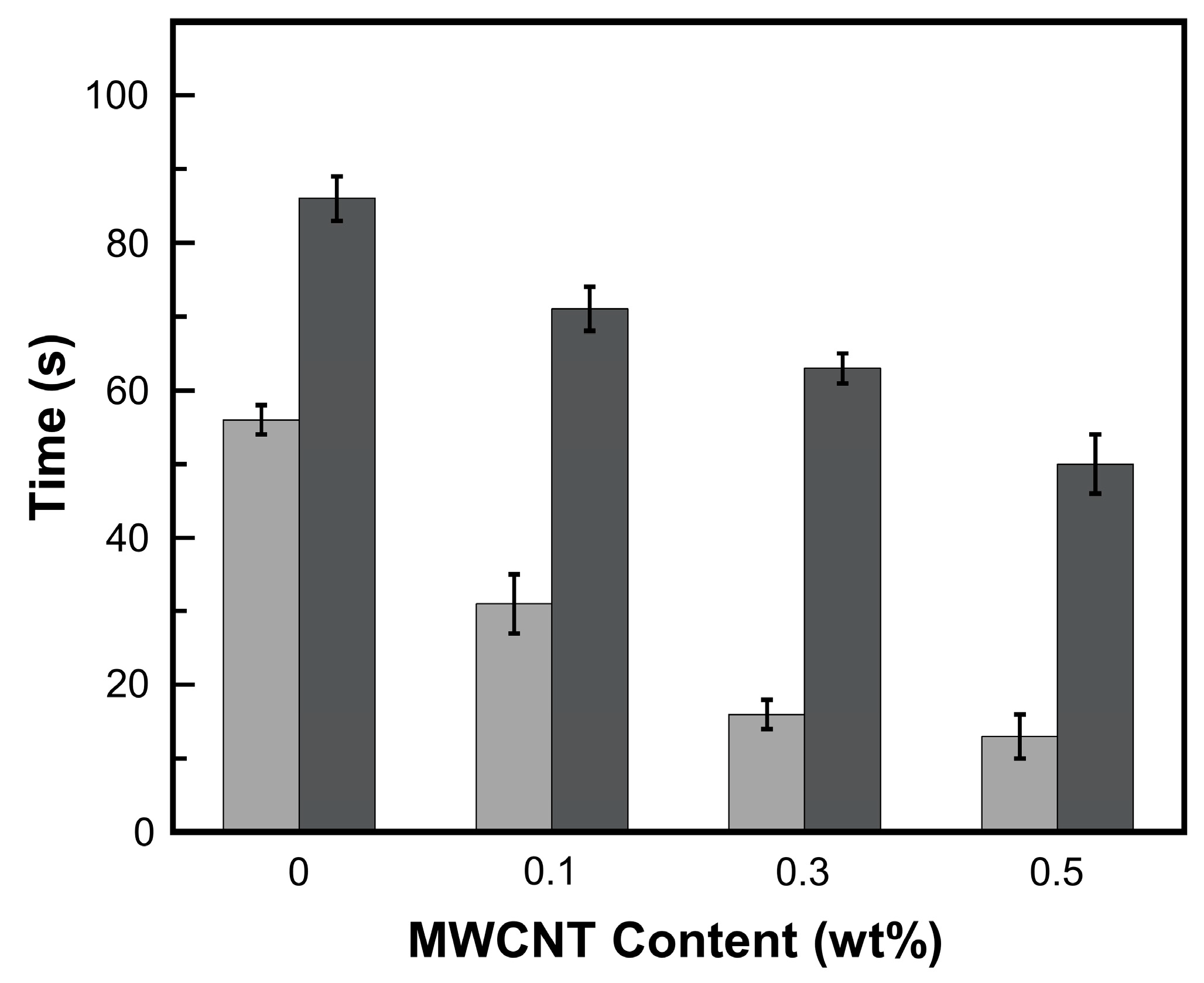

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasomsin, W.; Parnklang, T.; Sapcharoenkun, C.; Tiptipakorn, S.; Rimdusit, S. Multiwalled Carbon Nanotube Reinforced Bio-Based Benzoxazine/Epoxy Composites with NIR-Laser Stimulated Shape Memory Effects. Nanomaterials 2019, 9, 881. https://doi.org/10.3390/nano9060881
Prasomsin W, Parnklang T, Sapcharoenkun C, Tiptipakorn S, Rimdusit S. Multiwalled Carbon Nanotube Reinforced Bio-Based Benzoxazine/Epoxy Composites with NIR-Laser Stimulated Shape Memory Effects. Nanomaterials. 2019; 9(6):881. https://doi.org/10.3390/nano9060881
Chicago/Turabian StylePrasomsin, Wassika, Tewarak Parnklang, Chaweewan Sapcharoenkun, Sunan Tiptipakorn, and Sarawut Rimdusit. 2019. "Multiwalled Carbon Nanotube Reinforced Bio-Based Benzoxazine/Epoxy Composites with NIR-Laser Stimulated Shape Memory Effects" Nanomaterials 9, no. 6: 881. https://doi.org/10.3390/nano9060881
APA StylePrasomsin, W., Parnklang, T., Sapcharoenkun, C., Tiptipakorn, S., & Rimdusit, S. (2019). Multiwalled Carbon Nanotube Reinforced Bio-Based Benzoxazine/Epoxy Composites with NIR-Laser Stimulated Shape Memory Effects. Nanomaterials, 9(6), 881. https://doi.org/10.3390/nano9060881





