Functionalized Gold Nanoparticles as Contrast Agents for Proton and Dual Proton/Fluorine MRI
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Synthesis of NP-C8TEG/C8-DO3AGd
2.3. Synthesis of NP-C6OF-PEG
2.4. Synthesis of NP-C6OF-PEG/C6OF-DO3AGd
2.5. Determination of the Au/Gd Ratio
2.6. Determination of Nanoparticles’ 19F Relaxation Times
2.7. Determination of T1 and T2 Values with the 7 T Preclinical Scanner
2.7.1. 1H Experiments
2.7.2. 19F Experiments
2.8. Dynamic Light Scattering
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marson, D.; Guida, F.; Şologan, M.; Boccardo, S.; Pengo, P.; Perissinotto, F.; Iacuzzi, V.; Pellizzoni, E.; Polizzi, S.; Casalis, L.; et al. Mixed Fluorinated/Hydrogenated Self-Assembled Monolayer-Protected Gold Nanoparticles: In Silico and In Vitro Behavior. Small 2019, 15, 1900323. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Uzun, O.; Hu, Y.; Hu, Y.; Han, H.-S.; Watson, N.; Chen, S.; Irvine, D.J.; Stellacci1, F. Surface Structure-Regulated Cell Membrane Penetration by Monolayer Protected Nanoparticles. Nat. Mater. 2008, 7, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Pengo, P.; Şologan, M.; Pasquato, L.; Guida, F.; Pacor, S.; Tossi, A.; Stellacci, F.; Marson, D.; Boccardo, S.; Pricl, S.; et al. Gold nanoparticles with patterned surface monolayers for nanomedicine: Current perspectives. Eur. Biophys. J. 2017, 46, 749–771. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ali, M.R.K.; Chen, K.; Fang, N.; El-Sayed, M.A. Gold nanoparticles in biological optical imaging. Nano Today 2019, 24, 120–140. [Google Scholar] [CrossRef]
- Washner, J.; Gale, E.M.; Rodriguez-Rodriguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, W.; Luo, K.; Song, H.; Lan, F.; Wu, Y.; Gu, Z. Superparamagnetic Iron Oxide Nanoparticles as MRI contrast agents for Non-invasive Stem Cell Labeling and Tracking. Theranostics 2013, 3, 595–615. [Google Scholar] [CrossRef] [PubMed]
- Lux, J.; Sherry, D. Advances in gadolinium-based MRI contrast agent designs for monitoring biological processes in vivo. Curr. Opin. Chem. Biol. 2018, 45, 121–130. [Google Scholar] [CrossRef]
- Burai, L.; Hietapelto, V.; Király, R.; Tóth, É.; Brücher, E. Stability constants and 1H relaxation effects of ternary complexes formed between Gd-DTPAt, Gd-DTPA-BMA, Gd-DOTA, and Gd-EDTA and citrate, phosphate, and carbonate ions. Magn. Reson. Med. 1997, 38, 146–150. [Google Scholar] [CrossRef]
- Idée, J.M.; Port, M.; Raynal, I.; Schaefer, M.; Le Greneur, S.; Corot, C. Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: A review. Fundam. Clin. Pharmacol. 2006, 20, 563–576. [Google Scholar] [CrossRef]
- Wood, M.L.; Hardy, P.A. Proton relaxation enhancement. J. Magn. Reson. Imaging 1993, 3, 149–156. [Google Scholar] [CrossRef]
- Marangoni, V.S.; Neumann, O.; Henderson, L.; Kaffes, C.C.; Zhang, H.; Runmin Zhang, R.; Bishnoi, S.; Ayala-Orozco, C.; Zucolotto, V.; Bankson, J.A.; et al. Enhancing T1 magnetic resonance imaging contrast with internalized gadolinium(III) in a multilayer nanoparticle. Proc. Natl. Acad. Sci. USA 2017, 114, 6960–6965. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.; Neumann, O.; Kaffes, C.; Zhang, R.; Marangoni, V.; Ravoori, M.K.; Kundra, V.; Bankson, J.; Nordlander, P.; Halas, N.J. Routes to Potentially Safer T1 Magnetic Resonance Imaging Contrast in a Compact Plasmonic Nanoparticle with Enhanced Fluorescence. ACS Nano 2018, 12, 8214–8223. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, R.J.; Rammohan, N.; Rotz, M.W.; MacRenaris, K.W.; Preslar, A.T.; Meade, T.J. Gd(III)-Dithiolane Gold Nanoparticles for T1-Weighted Magnetic Resonance Imaging of the Pancreas. Nano Lett. 2016, 16, 3202–3209. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Goerner, F.L.; Snyder, C.; Morelli, J.N.; Hao, D.; Hu, D.; Li, X.; Runge, V.M. T1 Relaxivities of Gadolinium-Based Magnetic Resonance Contrast Agents in Human Whole Blood at 1.5, 3, and 7 T. Investig. Radiol. 2015, 50, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Moriggi, L.; Cannizzo, C.; Dumas, E.; Mayer, C.; Ulianov, A.; Helm, L. Gold Nanoparticles Functionalized with Gadolinium Chelates as High-Relaxivity MRI Contrast Agents. J. Am. Chem. Soc. 2009, 131, 10828–10829. [Google Scholar] [CrossRef] [PubMed]
- Milne, M.; Gobbo, P.; McVicar, N.; Workentin, M.; Hudson, R. Water-soluble gold nanoparticles (AuNP) functionalized with a gadolinium(III) chelate via Michael addition for use as a MRI contrast agent. J. Mater. Chem. B 2013, 1, 5628–5635. [Google Scholar] [CrossRef]
- Irure, A.; Marradi, M.; Arnaiz, B.; Genicio, N.; Padro, D.; Penadés, S. Sugar/gadolinium-loaded gold nanoparticles for labelling and imaging cells by magnetic resonance imaging. Biomater. Sci. 2013, 1, 658–668. [Google Scholar] [CrossRef]
- Rotz, M.W.; Culver, K.S.B.; Parigi, G.; MacRenaris, K.W.; Luchinat, C.; Odom, T.W.; Meade, T.J. High Relaxivity Gd(III)DNA Gold Nanostars: Investigation of Shape Effects on Proton Relaxation. ACS Nano 2015, 9, 3385–3396. [Google Scholar] [CrossRef]
- Culver, K.B.S.; Shin, Y.J.; Rotz, M.W.; Meade, T.J.; Hersam, M.C.; Odom, T.W. Shape-Dependent Relaxivity of Nanoparticle-Based T1 Magnetic Resonance Imaging Contrast Agents. J. Phys. Chem. C 2016, 120, 22103–22109. [Google Scholar] [CrossRef]
- Chen, J.; Lanza, G.M.; Wickline, S.A. Quantitative magnetic resonance fluorine imaging: Today and tomorrow. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 431–440. [Google Scholar] [CrossRef]
- Srinivas, M.; Heerschap, A.; Ahrens, E.T.; Figdor, C.G.; de Vries, I.J.M. 19F MRI for quantitative in vivo cell tracking. Trends Biotechnol. 2010, 28, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.L.; Srivastava, K.; Pierre, V.C. Fluorinated Paramagnetic Complexes: Sensitive and Responsive Probes for Magnetic Resonance Spectroscopy and Imaging. Front. Chem. 2018, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.C.; Edwards, P.G.; Paiseyc, S.J. Fluorinated contrast agents for magnetic resonance imaging; a review of recent developments. RSC Adv. 2011, 1, 1415–1425. [Google Scholar] [CrossRef]
- Janjic, J.M.; Ahrens, E.T. Fluorine-containing nanoemulsions for MRI cell tracking. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Janjic, J.M.; Srinivas, M.; Kadayakkara, D.K.; Ahrens, E.T. Self-delivering nanoemulsions for dual fluorine-19 MRI and fluorescence detection. J. Am. Chem. Soc. 2008, 130, 2832–2841. [Google Scholar] [CrossRef]
- Tirotta, I.; Mastropietro, A.; Cordiglieri, C.; Gazzera, L.; Baggi, F.; Baselli, G.; Bruzzone, M.G.; Zucca, I.; Cavallo, G.; Terraneo, G.; et al. A Superfluorinated molecular probe for highly sensitive in vivo (19)F-MRI. J. Am. Chem. Soc. 2014, 136, 8524–8527. [Google Scholar] [CrossRef]
- Tirotta, I.; Dichiarante, V.; Pigliacelli, C.; Cavallo, G.; Terraneo, G.; Baldelli Bombelli, F.; Metrangolo, P.; Resnati, G. 19F Magnetic Resonance Imaging (MRI): From Design of Materials to Clinical Applications. Chem. Rev. 2015, 115, 1106–1129. [Google Scholar] [CrossRef]
- Boccalon, M.; Franchi, P.; Lucarini, M.; Delgrado, J.J.; Souza, F.; Stellacci, F.; Zucca, I.; Scotti, A.; Spreafico, R.; Pengo, P.; et al. Gold nanoparticles protected by fluorinated ligands for 19F MRI. Chem. Commun. 2013, 49, 8794–8796. [Google Scholar] [CrossRef]
- Bidoggia, S.; Milocco, F.; Polizzi, S.; Canton, P.; Saccani, A.; Sanavio, B.; Krol, S.; Stellacci, F.; Pengo, P.; Pasquato, L. Fluorinated and Charged Hydrogenated Alkanethiolates Grafted on Gold: Expanding the Diversity of Mixed-Monolayer Nanoparticles for Biological Applications. Bioconj. Chem. 2017, 28, 43–52. [Google Scholar] [CrossRef]
- Boccalon, M.; Bidoggia, S.; Romano, F.; Gualandi, L.; Franchi, P.; Lucarini, M.; Pengo, P.; Pasquato, L. Gold nanoparticles as drug carriers: A contribution to the quest for basic principles for monolayer design. J. Mater. Chem. B 2015, 3, 432–439. [Google Scholar] [CrossRef]
- Pengo, P.; Pasquato, L. Gold nanoparticles protected by fluorinated ligands: Syntheses, properties and applications. J. Fluor. Chem. 2015, 177, 2–10. [Google Scholar] [CrossRef]
- Lee, H.; Price, R.R.; Holburn, G.E.; Partain, C.L.; Adams, M.D.; Cacheris, W.P. In vivo fluorine-19 MR imaging: Relaxation enhancement with Gd-DTPA. J. Magn. Reson. Imaging 1994, 4, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Li, V.; Chang, A.Y.; William, T. A noncovalent, fluoroalkyl coating monomer for phosphonate-covered nanoparticles. Tetrahedron 2013, 69, 7741–7745. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Dawsey, A.; Siriwardena-Mahanama, B.; Allen, M.; William, T. A (Fluoroalkyl)Guanidine Modulates the Relaxivity of a Phosphonate-Containing T1-Shortening Contrast Agent. J. Fluor. Chem. 2014, 168, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.L.; Brown, A.; Blackburn, O.; Tropiano, M.; Faulkner, S.; Beer, P.; Davis, J. Ligation driven 19F relaxation enhancement in self-assembled Ln(III) complexes. Chem. Commun. 2015, 51, 2918–2920. [Google Scholar] [CrossRef] [PubMed]
- Kislukhin, A.A.; Xu, H.; Adams, S.R.; Narsinh, K.H.; Tsien, R.Y.; Ahrens, E.T. Paramagnetic fluorinated nanoemulsions for sensitive cellular fluorine-19 magnetic resonance imaging. Nat. Mater. 2016, 15, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-X.; Feng, Y.; Bruce, Y.; Yu, Y. Fluorinated paramagnetic chelates as potential multi-chromic 19F tracer agents. Chem. Commun. 2011, 47, 7233–7235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Au nanoparticles target cancer. Nano Today 2007, 2, 18–29. [Google Scholar] [CrossRef]
- Pengo, P.; Polizzi, S.; Battagliarin, M.; Pasquato, L.; Scrimin, P. Synthesis, characterization and properties of water-soluble gold nanoparticles with tunable core size. J. Mater. Chem. 2003, 13, 2471–2478. [Google Scholar] [CrossRef]
- Suk, J.S.; Xua, Q.; Kima, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Hermann, P.; Kotek, J.; Kubíček, V.; Ivan Lukeš, I. Gadolinium(III) complexes as MRI contrast agents: Ligand design and properties of the complexes. Dalton Trans. 2008, 3027–3047. [Google Scholar] [CrossRef] [PubMed]


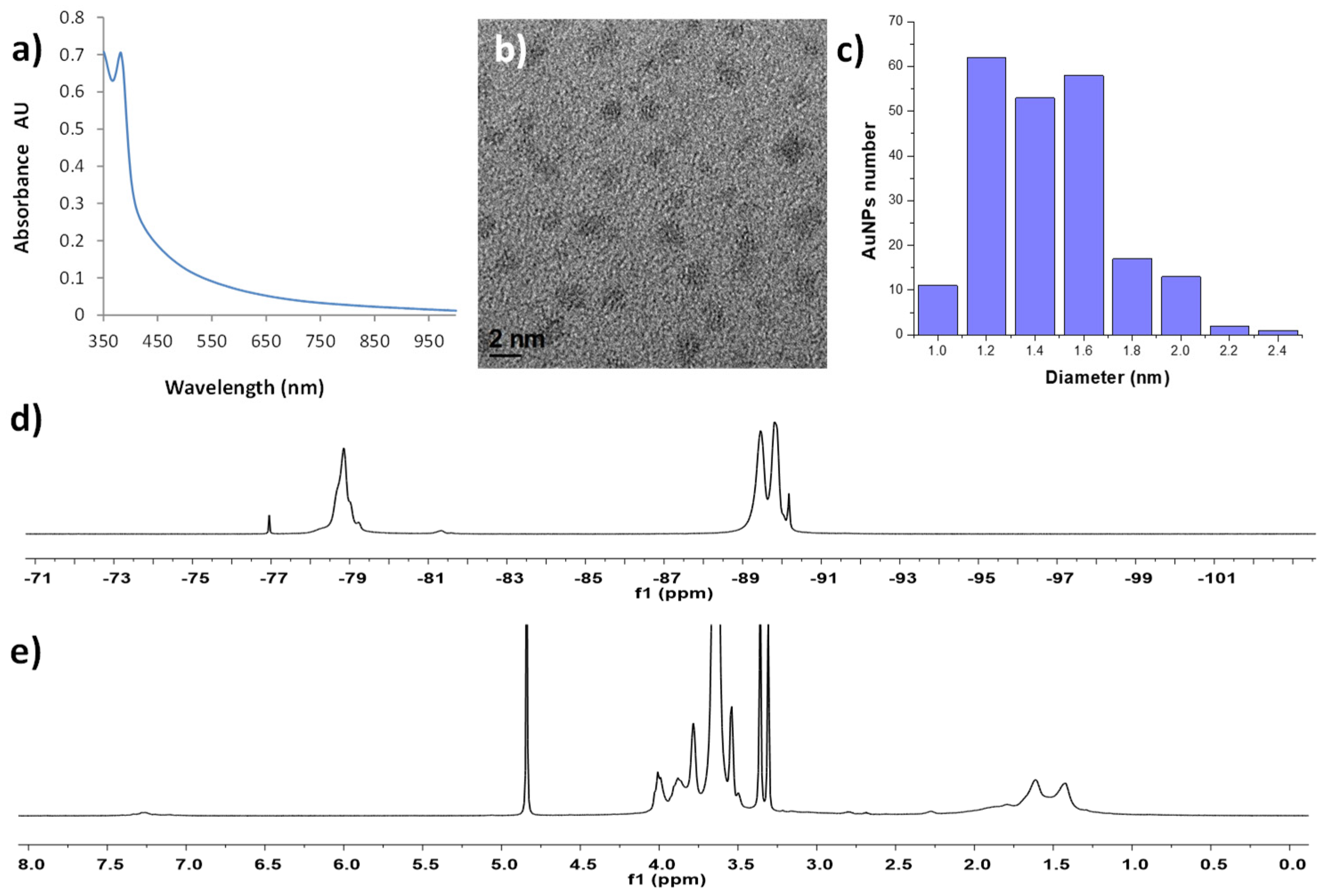
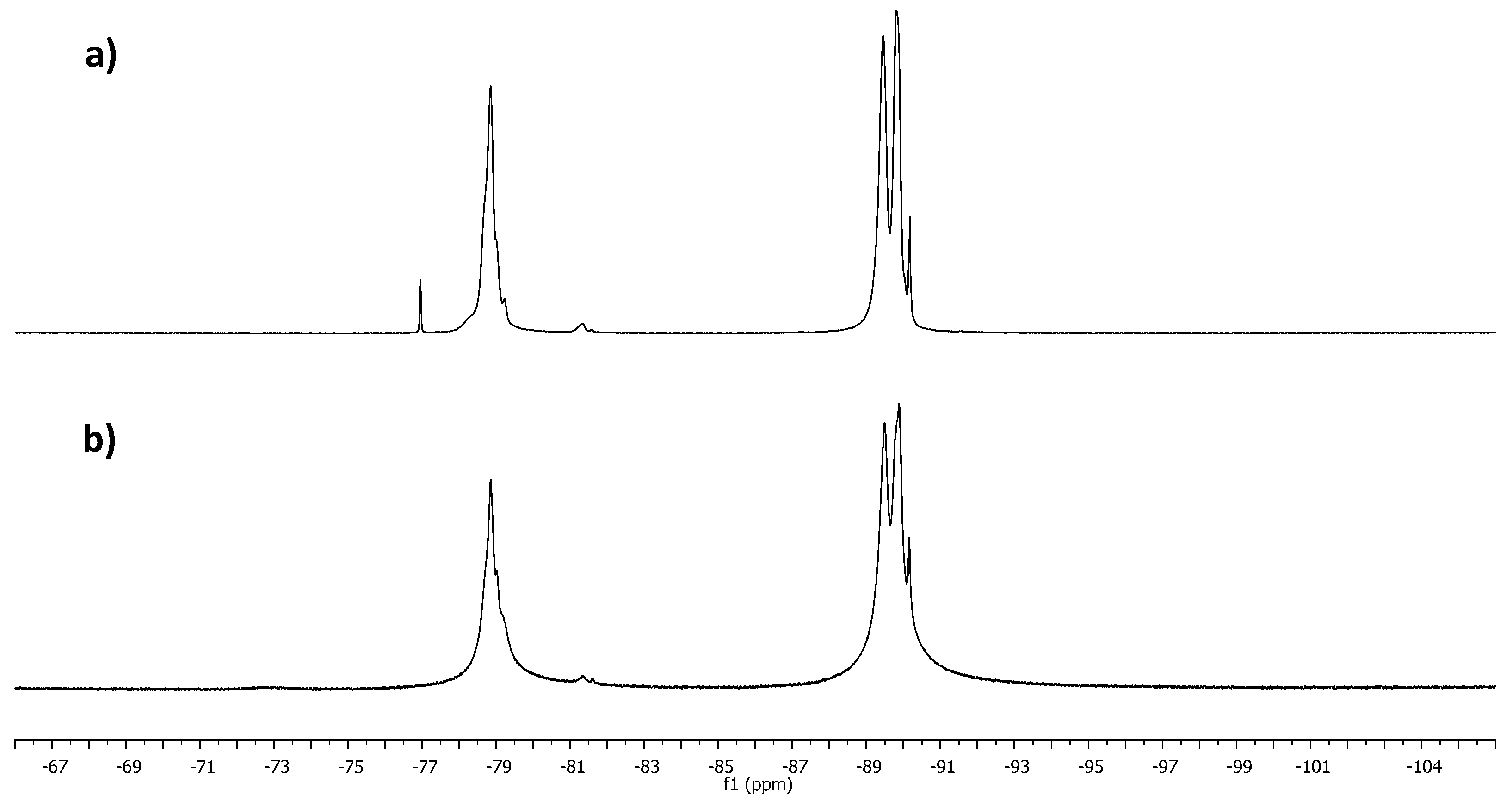

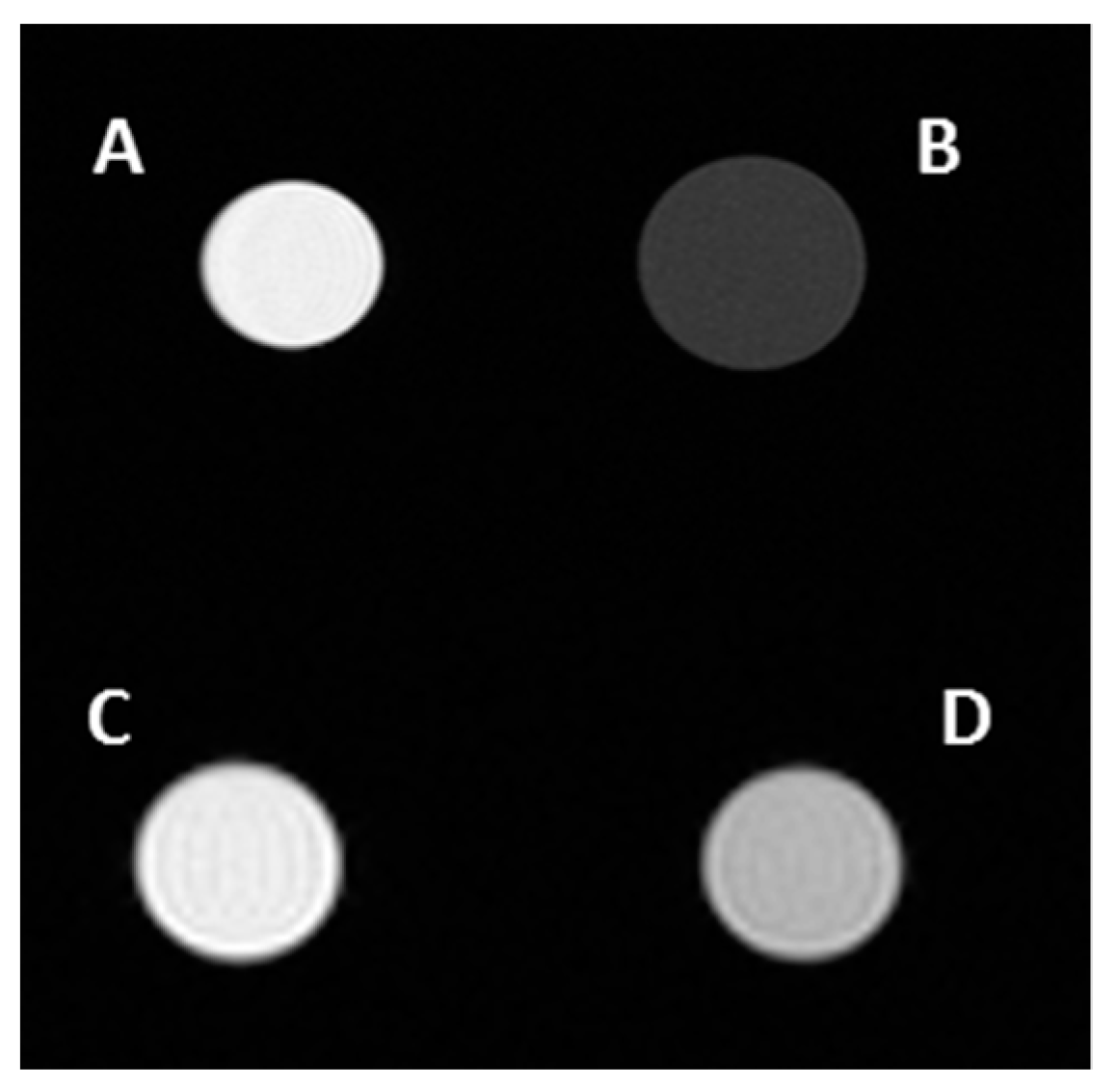
| Sample | T1 (ms) | T2 (ms) | r1 (s−1 mM−1) | r2 (s−1 mM−1) |
|---|---|---|---|---|
| NP-C8TEG/C8-DO3AGd-a | 33.55 ± 0.04 | 6.43 ± 0.07 | 3.2 | 16.9 |
| NP-C8TEG/C8-DO3AGd-b | 14.49 ± 0.06 | 3.17 ± 0.06 | 4.9 | 22.5 |
| Sample | T1 a (ms) | T2 a (ms) | T1 b (ms) |
|---|---|---|---|
| HS-C6OF-DO3A | 627 | 157 | - |
| NP-C6OF-PEG | 719 | 165 | 409 ± 24 |
| NP-C6OF-PEG/C6OF-DO3AGd | 258 | 33 | 233 ± 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şologan, M.; Padelli, F.; Giachetti, I.; Aquino, D.; Boccalon, M.; Adami, G.; Pengo, P.; Pasquato, L. Functionalized Gold Nanoparticles as Contrast Agents for Proton and Dual Proton/Fluorine MRI. Nanomaterials 2019, 9, 879. https://doi.org/10.3390/nano9060879
Şologan M, Padelli F, Giachetti I, Aquino D, Boccalon M, Adami G, Pengo P, Pasquato L. Functionalized Gold Nanoparticles as Contrast Agents for Proton and Dual Proton/Fluorine MRI. Nanomaterials. 2019; 9(6):879. https://doi.org/10.3390/nano9060879
Chicago/Turabian StyleŞologan, Maria, Francesco Padelli, Isabella Giachetti, Domenico Aquino, Mariangela Boccalon, Gianpiero Adami, Paolo Pengo, and Lucia Pasquato. 2019. "Functionalized Gold Nanoparticles as Contrast Agents for Proton and Dual Proton/Fluorine MRI" Nanomaterials 9, no. 6: 879. https://doi.org/10.3390/nano9060879
APA StyleŞologan, M., Padelli, F., Giachetti, I., Aquino, D., Boccalon, M., Adami, G., Pengo, P., & Pasquato, L. (2019). Functionalized Gold Nanoparticles as Contrast Agents for Proton and Dual Proton/Fluorine MRI. Nanomaterials, 9(6), 879. https://doi.org/10.3390/nano9060879





