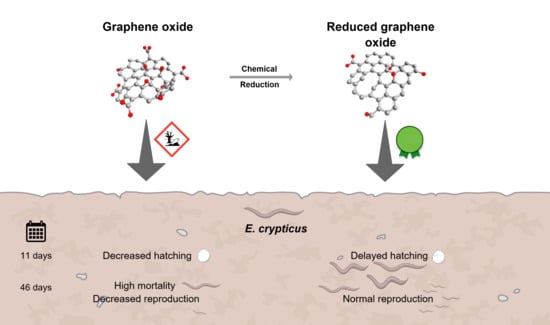
Graphene-Based Nanomaterials in Soil: Ecotoxicity Assessment Using Enchytraeus crypticus Reduced Full Life Cycle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Nanomaterials and Characterization
2.2. Test Organisms
2.3. Test Soil and Spiking Procedure
2.4. Exposure Experimental Design
2.5. Data Analysis
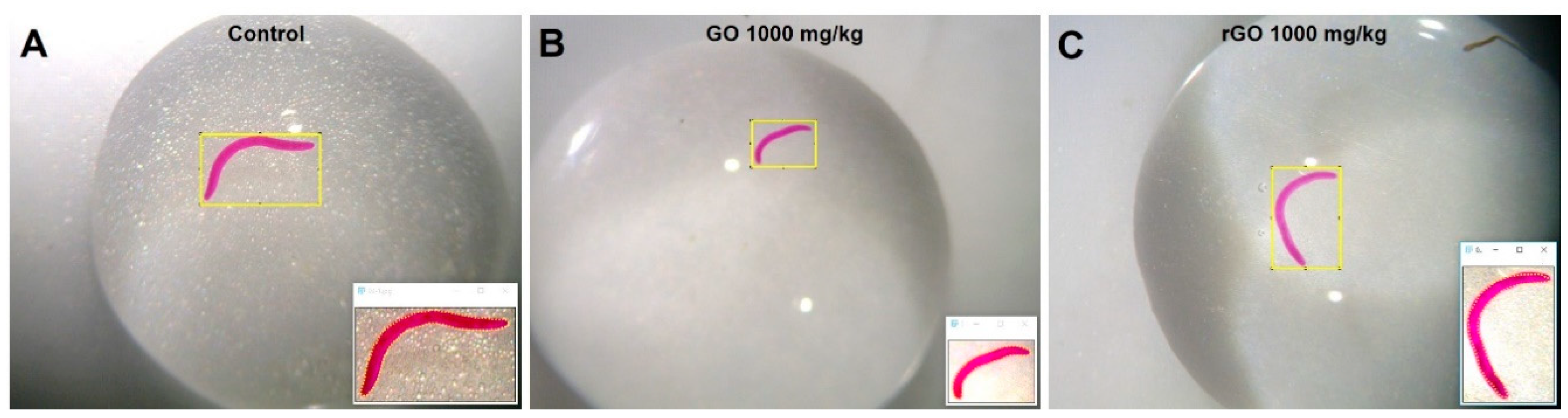
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ray, S.C. Application and uses of graphene oxide and reduced graphene oxide. In Applications of Graphene and Graphene-Oxide Based Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2015; pp. 39–55. ISBN 9780323375214. [Google Scholar]
- Qu, Y.; He, F.; Yu, C.; Liang, X.; Liang, D.; Ma, L.; Zhang, Q.; Lv, J.; Wu, J. Advances on graphene-based nanomaterials for biomedical applications. Mater. Sci. Eng. C 2018, 90, 764–780. [Google Scholar] [CrossRef] [PubMed]
- ReportLinker Global and China Graphene Industry Report, 2018–2023. Available online: https://www.reportlinker.com/p04539093/Global-and-China-Graphene-Industry-Report.html (accessed on 10 March 2019).
- Statista Market Volume of Graphene-Enhanced Composites Worlwide in 2017 and 2023, by Composite Type (in Tons). Available online: https://www.statista.com/statistics/960946/global-graphene-enhanced-composites-market-volume-by-type/ (accessed on 10 March 2019).
- Nurunnabi, M.; Parvez, K.; Nafiujjaman, M.; Revuri, V.; Khan, H.A.; Feng, X.; Lee, Y.K. Bioapplication of graphene oxide derivatives: Drug/gene delivery, imaging, polymeric modification, toxicology, therapeutics and challenges. RSC Adv. 2015, 5, 42141–42161. [Google Scholar] [CrossRef]
- Mendonça, M.C.P.; Soares, E.S.; De Jesus, M.B.; Ceragioli, H.J.; Batista, Â.G.; Nyúl-Tóth, Á.; Molnár, J.; Wilhelm, I.; Maróstica, M.R.; Krizbai, I.; et al. PEGylation of reduced graphene oxide induces toxicity in cells of the blood-brain barrier: An In Vitro and In Vivo study. Mol. Pharm. 2016, 13, 3913–3924. [Google Scholar] [CrossRef] [PubMed]
- Zare-Zardini, H.; Taheri-Kafrani, A.; Ordooei, M.; Amiri, A.; Karimi-Zarchi, M. Evaluation of toxicity of functionalized graphene oxide with ginsenoside Rh2, lysine and arginine on blood cancer cells (K562), red blood cells, blood coagulation and cardiovascular tissue: In Vitro and In Vivo studies. J. Taiwan Inst. Chem. Eng. 2018, 93, 70–78. [Google Scholar] [CrossRef]
- Castro-Ferreira, M.P.; Roelofs, D.; van Gestel, C.A.M.; Verweij, R.A.; Soares, A.M.V.M.; Amorim, M.J.B. Enchytraeus crypticus as model species in soil ecotoxicology. Chemosphere 2012, 87, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Soil Quality—Effects of Contaminants on Enchytraeidae (Enchytraeus sp.)—Determination of Effects on Reproduction; IOS: Geneva, Switzerland, 2014. [Google Scholar]
- OECD. Guidelines for the Testing of Chemicals. No. 220—Enchytraeid Reproduction Test; OECD: Paris, France, 2015. [Google Scholar]
- Bicho, R.C.; Santos, F.C.F.; Gonçalves, M.F.M.; Soares, A.M.V.M.; Amorim, M.J.B. Enchytraeid Reproduction TestPLUS: Hatching, growth and full life cycle test—An optional multi-endpoint test with Enchytraeus crypticus. Ecotoxicology 2015, 24, 1053–1063. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, J.; Wu, J.; Yin, Q.; Liang, H.; Chen, A.; Shao, L. Graphene oxide and reduced graphene oxide induced neural pheochromocytoma-derived PC12 cell lines apoptosis and cell cycle alterations via the ERK signaling pathways. Int. J. Nanomed. 2017, 12, 5511–5523. [Google Scholar] [CrossRef]
- Seifati, S.M.; Nasirizadeh, N.; Azimzadeh, M. Nano-biosensor based on reduced graphene oxide and gold nanoparticles, for detection of phenylketonuria-associated DNA mutation. IET Nanobiotechnol. 2018, 12, 417–422. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Series on the Safety of Manufactured Nanomaterials, No. 36: Guidance on Sample Preparation and Dosimetry for the Safety Testing of Manufactured Nanomaterials; OECD: Paris, France, 2012. [Google Scholar]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-Warthan, A.A.; Tremel, W. Graphene based metal and metal oxide nanocomposites: Synthesis, properties and their applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Das, S.; Singh, S.; Singh, V.; Joung, D.; Dowding, J.M.; Reid, D.; Anderson, J.; Zhai, L.; Khondaker, S.I.; Self, W.T.; et al. Oxygenated functional group density on graphene oxide: Its effect on cell toxicity. Part. Part. Syst. Charact. 2013, 30, 148–157. [Google Scholar] [CrossRef]
- Evariste, L.; Lagier, L.; Gonzalez, P.; Mottier, A.; Mouchet, F.; Cadarsi, S.; Lonchambon, P.; Daffe, G.; Chimowa, G.; Sarrieu, C.; et al. Thermal Reduction of Graphene Oxide Mitigates Its In Vivo Genotoxicity toward Xenopus Laevis Tadpoles. Nanomaterials 2019, 9, 584. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guiney, L.M.; Chang, C.H.; Mansukhani, N.D.; Ji, Z.; Wang, X.; Liao, Y.P.; Jiang, W.; Sun, B.; Hersam, M.C.; et al. Surface Oxidation of Graphene Oxide Determines Membrane Damage, Lipid Peroxidation, and Cytotoxicity in Macrophages in a Pulmonary Toxicity Model. ACS Nano 2018, 12, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Kim, Y.; Yang, J.; Roca, C.P.; Joo, S.W.; Choi, J. A systems toxicology approach reveals the Wnt-MAPK crosstalk pathway mediated reproductive failure in Caenorhabditis elegans exposed to graphene oxide (GO) but not to reduced graphene oxide (rGO). Nanotoxicology 2017, 11, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.J.B.; Pereira, C.; Soares, A.M.V.M.; Scott-Fordsmand, J.J. Does long term low impact stress cause population extinction? Environ. Pollut. 2017, 220, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, B.; Maria, V.L.; Römbke, J.; Amorim, M.J.B. Exposure of Folsomia candida (Willem 1902) to te fl ubenzuron over three generations—Increase of toxicity in the third generation. Appl. Soil Ecol. 2019, 134, 8–14. [Google Scholar] [CrossRef]
- Guimarães, B.; Maria, V.L.; Römbke, J.; Amorim, M.J.B. Multigenerational exposure of Folsomia candida to ivermectin—Using avoidance, survival, reproduction, size and cellular markers as endpoints. Geoderma 2019, 337, 273–279. [Google Scholar] [CrossRef]
- Bicho, R.C.; Ribeiro, T.; Rodrigues, N.P.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Effects of Ag nanomaterials (NM300K) and Ag salt (AgNO3) can be discriminated in a full life cycle long term test with Enchytraeus crypticus. J. Hazard. Mater. 2016, 318, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Bicho, R.C.; Santos, F.C.F.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Effects of copper oxide nanomaterials (CuONMs) are life stage dependent-full life cycle in Enchytraeus crypticus. Environ. Pollut. 2017, 224, 117–124. [Google Scholar] [CrossRef]
- Santos, F.C.F.; Gomes, S.I.L.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Hazard assessment of nickel nanoparticles in soil-The use of a full life cycle test with Enchytraeus crypticus. Environ. Toxicol. Chem. 2017, 36, 2934–2941. [Google Scholar] [CrossRef]
- Bicho, R.C.; Ribeiro, M.J.; Scott-Fordsmand, J.J.; Amorim, M. Tracing effects of NMs along their life cycle—Toxicity in soil (Enchytraeus crypticus). Poster presentation. In Proceedings of the SETAC Europe 25th Annual Meeting, Barcelona, Spain, 3–7 May 2015; p. 400. [Google Scholar]
- Kim, Y.; Jeong, J.; Yang, J.; Joo, S.W.; Hong, J.; Choi, J. Graphene oxide nano-bio interaction induces inhibition of spermatogenesis and disturbance of fatty acid metabolism in the nematode Caenorhabditis elegans. Toxicology 2018, 410, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska, A.M.; Olszyna, A.R. The ecotoxicity of graphene family materials: Current status, knowledge gaps and future needs. J. Nanopart. Res. 2015, 17, 40. [Google Scholar] [CrossRef]
- He, K.; Chen, G.; Zeng, G.; Peng, M.; Huang, Z.; Shi, J.; Huang, T. Stability, transport and ecosystem effects of graphene in water and soil environments. Nanoscale 2017, 9, 5370–5388. [Google Scholar] [CrossRef] [PubMed]
- An, W.; Zhang, Y.; Zhang, X.; Li, K.; Kang, Y.; Akhtar, S.; Sha, X.; Gao, L. Ocular toxicity of reduced graphene oxide or graphene oxide exposure in mouse eyes. Exp. Eye Res. 2018, 174, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zeng, G.; Tang, L.; Wang, J.; Wan, J.; Feng, H.; Song, B.; Huang, C.; Tang, X. Effect of exogenous carbonaceous materials on the bioavailability of organic pollutants and their ecological risks. Soil Biol. Biochem. 2018, 116, 70–81. [Google Scholar] [CrossRef]
- Ahmed, F.; Rodrigues, D.F. Investigation of acute effects of graphene oxide on wastewater microbial community: A case study. J. Hazard. Mater. 2013, 256, 33–39. [Google Scholar] [CrossRef]
- Esquivel-Gaon, M.; Nguyen, N.H.A.; Sgroi, M.F.; Pullini, D.; Gili, F.; Mangherini, D.; Pruna, A.I.; Rosicka, P.; Sevcu, A.; Castagnola, V. In Vitro and environmental toxicity of reduced graphene oxide as an additive in automotive lubricants. Nanoscale 2018, 10, 6539–6548. [Google Scholar] [CrossRef]
- De Marchi, L.; Pretti, C.; Gabriel, B.; Marques, P.A.A.P.; Freitas, R.; Neto, V. An overview of graphene materials: Properties, applications and toxicity on aquatic environments. Sci. Total Environ. 2018, 631, 1440–1456. [Google Scholar] [CrossRef]
- Jaworski, S.; Sawosz, E.; Kutwin, M.; Wierzbicki, M.; Hinzmann, M.; Grodzik, M.; Winnicka, A.; Lipińska, L.; Włodyga, K.; Chwalibog, A. In Vitro and In Vivo effects of graphene oxide and reduced graphene oxide on glioblastoma. Int. J. Nanomed. 2015, 10, 1585–1596. [Google Scholar]
- Contreras-Torres, F.F.; Rodríguez-Galván, A.; Guerrero-Beltrán, C.E.; Martínez-Lorán, E.; Vázquez-Garza, E.; Ornelas-Soto, N.; García-Rivas, G. Differential cytotoxicity and internalization of graphene family nanomaterials in myocardial cells. Mater. Sci. Eng. C 2017, 73, 633–642. [Google Scholar] [CrossRef]
- Guo, Z.; Xie, C.; Zhang, P.; Zhang, J.; Wang, G.; He, X.; Ma, Y.; Zhao, B.; Zhang, Z. Toxicity and transformation of graphene oxide and reduced graphene oxide in bacteria biofilm. Sci. Total Environ. 2017, 580, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
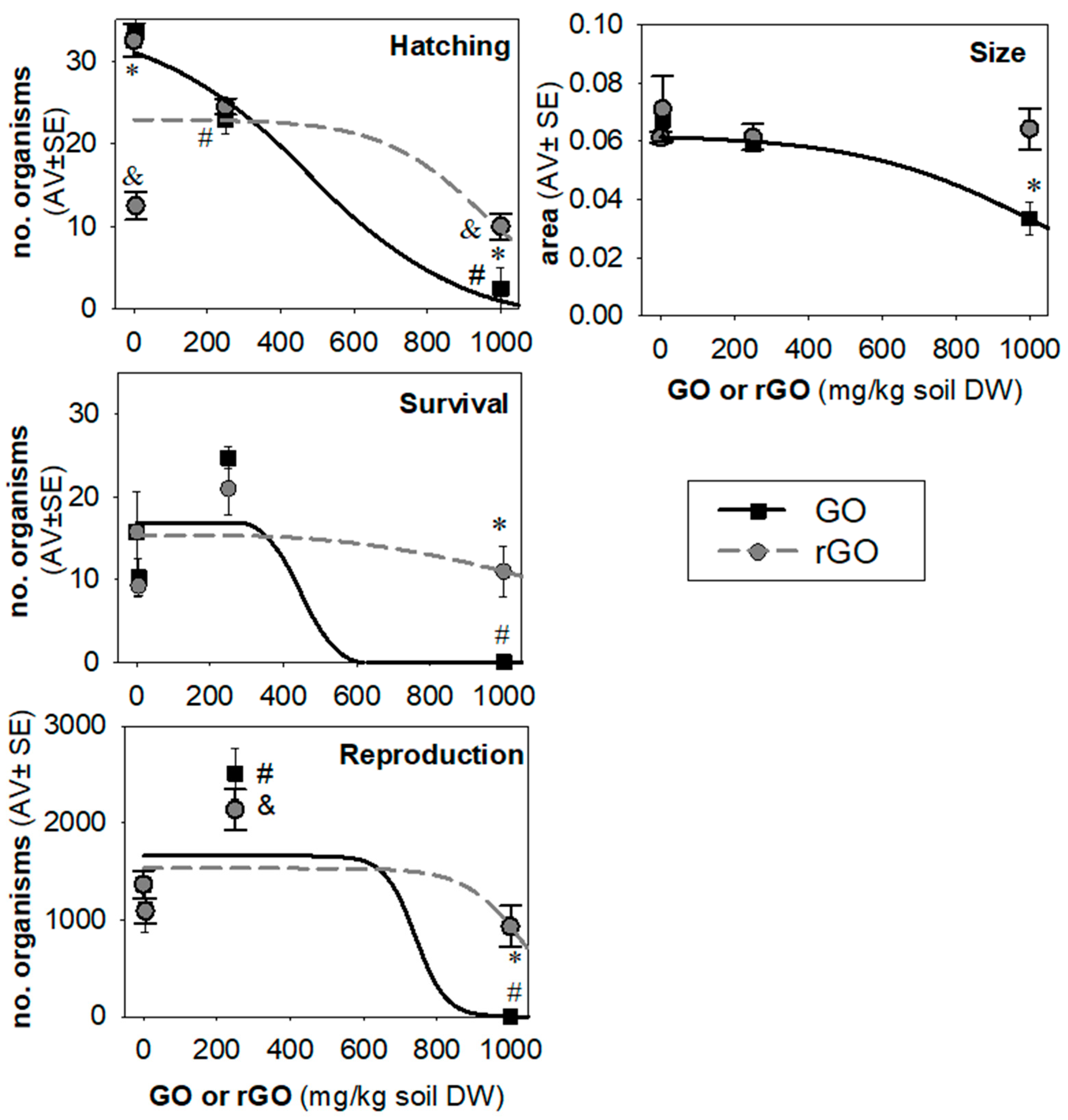
| Nanomaterial/Endpoint | EC10 (mg/kg) | EC20 (mg/kg) | EC50 (mg/kg) | EC80 (mg/kg) | Model and Parameters |
|---|---|---|---|---|---|
| Hatching | |||||
| GO | 129 (−36–294) | 202 (123–282) | 329 (197–460) | 455 (159–751) | Log 2 par S: 0.0009 Y0: 33; R2 = 0.9 |
| rGO | 753 (n.d.) | 834 (n.d.) | 973 (n.d.) | 1111 (n.d.) | Log 2 par S: 0.0008; Y0: 23.2; R2 = 0.3 |
| Size | |||||
| GO | 490 (82–898) | 685 (385–984) | 1017 (725–1310) | 1349 (858–1841) | Log 2 par S: 0.00092; Y0: 0.06; R2 = 0.2 |
| rGO | n.e. | n.e. | n.e. | n.e. | - |
| Reproduction | |||||
| GO | 650 (n.d.) | 683 (n.d.) | 740 (n.d.) | 798 (n.d.) | Log 2 par S: 0.006; Y0: 1658; R2 = 0.6 |
| rGO | 860 (n.d.) | 925 (n.d.) | 1034 (n.d.) | 1144 (n.d.) | Log 2 par S: 0.003; Y0: 1530; R2 = 0.2 |
| Survival | |||||
| GO | 353 (n.d.) | 384 (n.d.) | 447 (n.d.) | 492 (n.d.) | Thresh 2 par S: 0.002; Y0: 16.9; R2 = 0.5 |
| rGO | 696 (n.d.) | 881 (n.d.) | 1248 (n.d.) | 1512 (n.d.) | Thresh 2 par S: 0.00099; Y0: 15.3; R2 = 0.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendonça, M.C.P.; Rodrigues, N.P.; de Jesus, M.B.; Amorim, M.J.B. Graphene-Based Nanomaterials in Soil: Ecotoxicity Assessment Using Enchytraeus crypticus Reduced Full Life Cycle. Nanomaterials 2019, 9, 858. https://doi.org/10.3390/nano9060858
Mendonça MCP, Rodrigues NP, de Jesus MB, Amorim MJB. Graphene-Based Nanomaterials in Soil: Ecotoxicity Assessment Using Enchytraeus crypticus Reduced Full Life Cycle. Nanomaterials. 2019; 9(6):858. https://doi.org/10.3390/nano9060858
Chicago/Turabian StyleMendonça, Monique C. P., Natália P. Rodrigues, Marcelo B. de Jesus, and Mónica J. B. Amorim. 2019. "Graphene-Based Nanomaterials in Soil: Ecotoxicity Assessment Using Enchytraeus crypticus Reduced Full Life Cycle" Nanomaterials 9, no. 6: 858. https://doi.org/10.3390/nano9060858
APA StyleMendonça, M. C. P., Rodrigues, N. P., de Jesus, M. B., & Amorim, M. J. B. (2019). Graphene-Based Nanomaterials in Soil: Ecotoxicity Assessment Using Enchytraeus crypticus Reduced Full Life Cycle. Nanomaterials, 9(6), 858. https://doi.org/10.3390/nano9060858