Cell In Vitro Testing with Soil Invertebrates—Challenges and Opportunities toward Modeling the Effect of Nanomaterials: A Surface-Modified CuO Case Study
Abstract
1. Introduction
2.1. Materials and Methods
2.1.1. Test Materials, Spiking, and Characterization
2.1.2. Cell and Coelomic Fluid Extraction
2.1.3. In Vitro Test Procedures and Flow Cytometry
2.1.4. Data Treatment
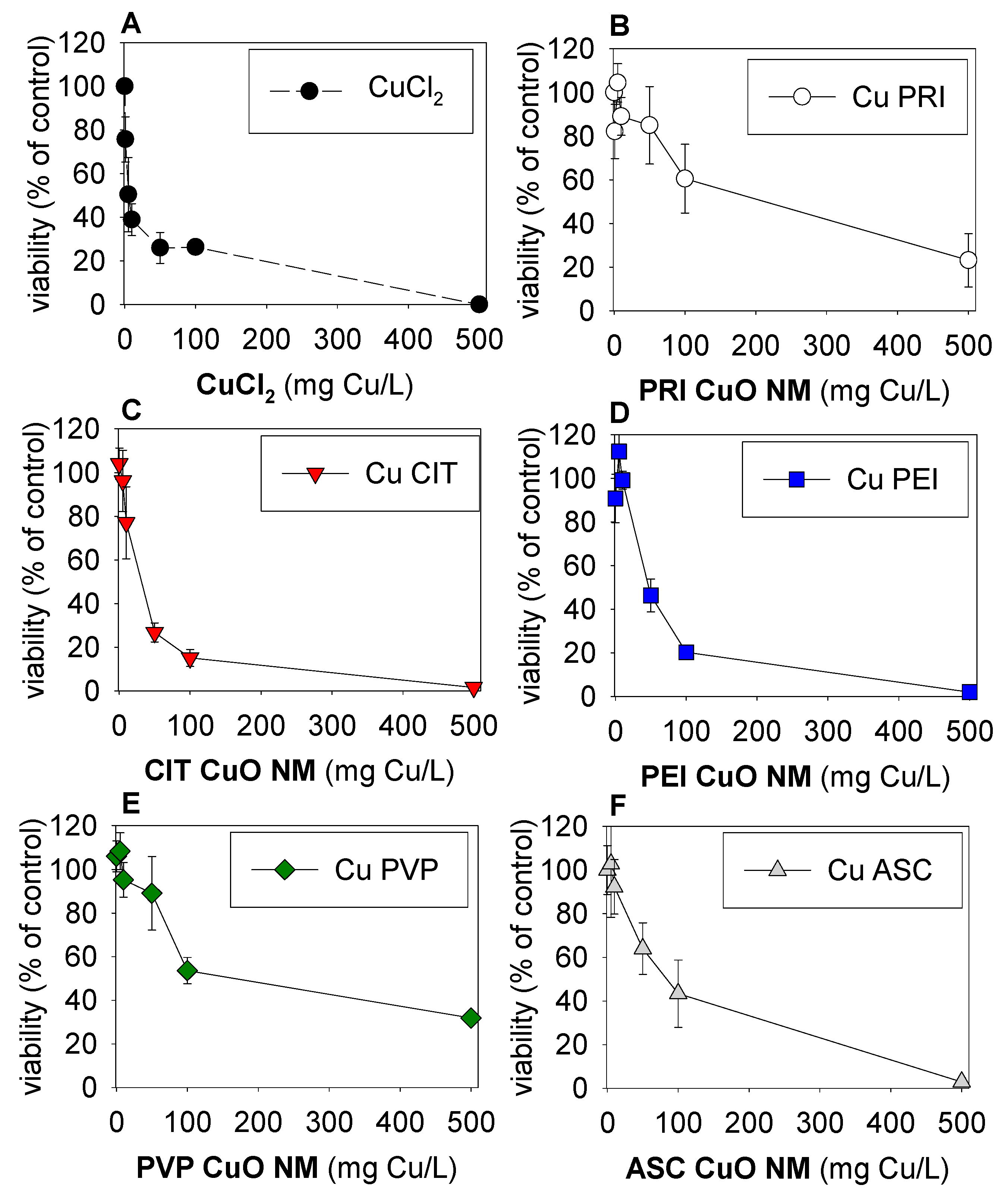
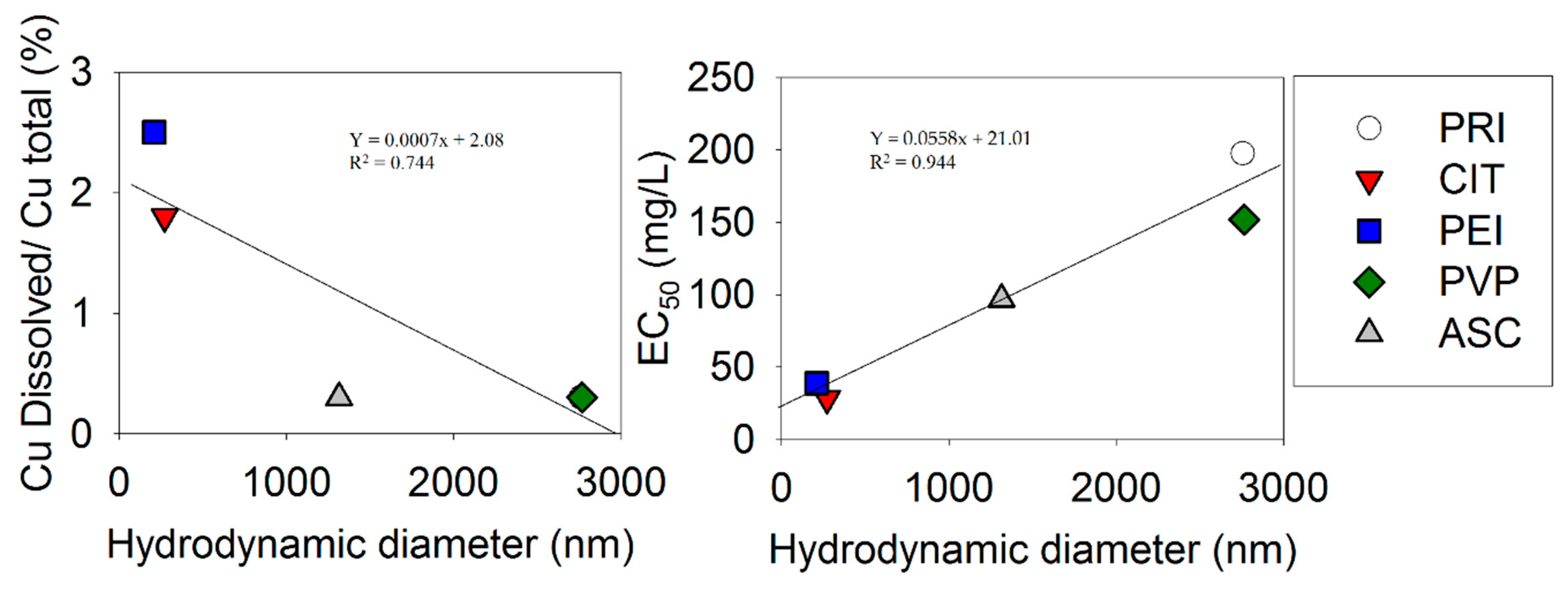
3. Results
4. Discussion
In Vitro Challenges and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Oomen, A.G.; Steinhäuser, K.G.; Bleeker, E.A.J.; Van Broekhuizen, F.; Sips, A.; Dekkers, S.; Wijnhoven, S.W.P.; Sayre, P.G. Risk assessment frameworks for nanomaterials: Scope, link to regulations, applicability, and outline for future directions in view of needed increase in efficiency. NanoImpact 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Scott-Fordsmand, J.J.; Peijnenburg, W.J.G.M.; Semenzin, E.; Nowack, B.; Hunt, N.; Hristozov, D.; Marcomini, A.; Irfan, M.A.; Jiménez, A.S.; Landsiedel, R.; et al. Environmental risk assessment strategy for nanomaterials. Int. J. Environ. Res. Public Health 2017, 14, 1251. [Google Scholar] [CrossRef] [PubMed]
- Kroll, A.; Pillukat, M.H.; Hahn, D.; Schnekenburger, J. Current in vitro methods in nanoparticle risk assessment: Limitations and challenges. Eur. J. Pharm. Biopharm. 2009, 72, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Tiede, K.; Hassellöv, M.; Breitbarth, E.; Chaudhry, Q.; Boxall, A.B.A. Considerations for environmental fate and ecotoxicity testing to support environmental risk assessments for engineered nanoparticles. J. Chromatogr. A 2009, 1216, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.S.; Autrup, H. Toxicity of silver nanoparticles-nanoparticle or silver ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.I.L.; Murphy, M.; Nielsen, M.T.; Kristiansen, S.M.; Amorim, M.J.B.; Scott-Fordsmand, J.J. Cu-nanoparticles ecotoxicity–Explored and explained? Chemosphere 2015, 139, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Alahdadi, I.; Behboudi, F. The effects of CuO and ZnO nanoparticles on survival, reproduction, absorption, overweight, and accumulation in Eisenia fetida earthworm tissues in two substrates. Int. J. Environ. Res. 2015, 9, 35–42. [Google Scholar]
- Niles, A.L.; Moravec, R.A.; Riss, T.L. In vitro viability and cytotoxicity testing and same-well multi-parametric combinations for high throughput screening. Curr. Chem. Genom. 2009, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Miclaus, T.; Scavenius, C.; Kwiatkowska, K.; Sobota, A.; Engelmann, P.; Scott-Fordsmand, J.J.; Enghild, J.J.; Sutherland, D.S. Species Differences Take Shape at Nanoparticles: Protein Corona Made of the Native Repertoire Assists Cellular Interaction. Environ. Sci. Technol. 2013, 47, 14367–14375. [Google Scholar] [CrossRef]
- Irizar, A.; Rivas, C.; García-Velasco, N.; De Cerio, F.G.; Etxebarria, J.; Marigómez, I.; Soto, M. Establishment of toxicity thresholds in subpopulations of coelomocytes (amoebocytes vs. eleocytes) of Eisenia fetida exposed in vitro to a variety of metals: Implications for biomarker measurements. Ecotoxicology 2015, 24, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.L.; Roch, P. Earthworm immunity: A model of immune competence: The 7th international symposium on earthworm ecology·Cardiff·Wales·2002. Pedobiologia 2003, 47, 676–688. [Google Scholar] [CrossRef]
- Lagadic, L.; Caquet, T. Invertebrates in testing of environmental chemicals: Are they alternatives? Environ. Health Perspect. 1998, 106, 593. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Engelmann, P.; Foldbjerg, R.; Szabó, M.; Somogyi, I.; Pollák, E.; Molnár, L.; Autrup, H.; Sutherland, D.S.; Scott-Fordsmand, J.; et al. Earthworms and humans in vitro: Characterizing evolutionarily conserved stress and immune responses to silver nanoparticles. Environ. Sci. Technol. 2012, 46, 4166–4173. [Google Scholar] [CrossRef] [PubMed]
- Bigorgne, E.; Foucaud, L.; Caillet, C.; Giambérini, L.; Nahmani, J.; Thomas, F.; Rodius, F. Cellular and molecular responses of E. fetida cœlomocytes exposed to TiO2 nanoparticles. J. Nanopart. Res. 2012, 14, 959. [Google Scholar] [CrossRef]
- Fugère, N.; Brousseau, P.; Krzystyniak, K.; Coderre, D.; Fournier, M. Heavy metal-specific inhibition of phagocytosis and different in vitro sensitivity of heterogeneous coelomocytes from Lumbricus terrestris (Oligochaeta). Toxicology 1996, 109, 157–166. [Google Scholar] [CrossRef]
- Maiorano, G.; Sabella, S.; Sorce, B.; Brunetti, V.; Malvindi, M.A.; Cingolani, R.; Pompa, P.P. Effects of cell culture media on the dynamic formation of protein-nanoparticle complexes and influence on the cellular response. ACS Nano 2010, 4, 7481–7491. [Google Scholar] [CrossRef] [PubMed]
- Tejamaya, M.; Römer, I.; Merrifield, R.C.; Lead, J.R. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef]
- Javed, R.; Ahmed, M.; ul Haq, I.; Nisa, S.; Zia, M. PVP and PEG doped CuO nanoparticles are more biologically active: Antibacterial, antioxidant, antidiabetic and cytotoxic perspective. Mater. Sci. Eng. C 2017, 79, 108–115. [Google Scholar] [CrossRef]
- Meyer, J.N.; Lord, C.A.; Yang, X.Y.; Turner, E.A.; Badireddy, A.R.; Marinakos, S.M.; Chilkoti, A.; Wiesner, M.R.; Auffan, M. Intracellular uptake and associated toxicity of silver nanoparticles in Caenorhabditis elegans. Aquat. Toxicol. 2010, 100, 140–150. [Google Scholar] [CrossRef]
- Su, Y.; He, Y.; Lu, H.; Sai, L.; Li, Q.; Li, W.; Wang, L.; Shen, P.; Huang, Q.; Fan, C. The cytotoxicity of cadmium based, aqueous phase—Synthesized, quantum dots and its modulation by surface coating. Biomaterials 2009, 30, 19–25. [Google Scholar] [CrossRef]
- Harush-Frenkel, O.; Debotton, N.; Benita, S.; Altschuler, Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem. Biophys. Res. Commun. 2007, 353, 26–32. [Google Scholar] [CrossRef]
- Yue, Z.-G.; Wei, W.; Lv, P.-P.; Yue, H.; Wang, L.-Y.; Su, Z.-G.; Ma, G.-H. Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Bastos, V.; Ferreira-de-Oliveira, J.M.P.; Carrola, J.; Daniel-da-Silva, A.L.; Duarte, I.F.; Santos, C.; Oliveira, H. Coating independent cytotoxicity of citrate- and PEG-coated silver nanoparticles on a human hepatoma cell line. J. Environ. Sci. 2017, 51, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Newton, B.; Lewis, E.; Fu, P.P.; Kafoury, R.; Ray, P.C.; Yu, H. Cytotoxicity of organic surface coating agents used for nanoparticles synthesis and stability. Toxicol. Vitr. 2015, 29, 762–768. [Google Scholar] [CrossRef]
- OECD. Guidelines for the Testing of Chemicals. no. 222. Earthworm Reproduction Test (Eisenia fetida/Eisenia andrei); OECD: Paris, France, 2016. [Google Scholar]
- Ortelli, S.; Costa, A.L.; Blosi, M.; Brunelli, A.; Badetti, E.; Bonetto, A.; Hristozov, D.; Marcomini, A. Colloidal characterization of CuO nanoparticles in biological and environmental media. Environ. Sci. Nano 2017, 4, 1264–1272. [Google Scholar] [CrossRef]
- Polcari, D.; Hernández-Castro, J.A.; Li, K.; Geissler, M.; Mauzeroll, J. Determination of the Relationship between Expression and Functional Activity of Multidrug Resistance-Associated Protein 1 using Scanning Electrochemical Microscopy. Anal. Chem. 2017, 89, 8988–8994. [Google Scholar] [CrossRef]
- Engelmann, P.; Hayashi, Y.; Bodó, K.; Ernszt, D.; Somogyi, I.; Steib, A.; Orbán, J.; Pollák, E.; Nyitrai, M.; Németh, P.; et al. Phenotypic and functional characterization of earthworm coelomocyte subsets: Linking light scatter-based cell typing and imaging of the sorted populations. Dev. Comp. Immunol. 2016, 65, 41–52. [Google Scholar] [CrossRef]
- Líbalová, H.; Costa, P.M.; Olsson, M.; Farcal, L.; Ortelli, S.; Blosi, M.; Topinka, J.; Costa, A.L.; Fadeel, B. Toxicity of surface-modified copper oxide nanoparticles in a mouse macrophage cell line: Interplay of particles, surface coating and particle dissolution. Chemosphere 2018, 196, 482–493. [Google Scholar] [CrossRef]
- Kwak, J.I.; Lee, W.-M.; Kim, S.W.; An, Y.-J. Interaction of citrate-coated silver nanoparticles with earthworm coelomic fluid and related cytotoxicity in Eisenia andrei. J. Appl. Toxicol. 2014, 34, 1145–1154. [Google Scholar] [CrossRef]
- Bastos, V.; Brown, D.; Johnston, H.; Daniel-da-Silva, A.L.; Duarte, I.F.; Santos, C.; Oliveira, H. Inflammatory responses of a human keratinocyte cell line to 10 nm citrate- and PEG-coated silver nanoparticles. J. Nanopart. Res. 2016, 18, 205. [Google Scholar] [CrossRef]
- Begum, A.N.; Aguilar, J.S.; Elias, L.; Hong, Y. Silver nanoparticles exhibit coating and dose-dependent neurotoxicity in glutamatergic neurons derived from human embryonic stem cells. Neurotoxicology 2016, 57, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ji, Z.; Chang, C.H.; Zhang, H.; Wang, M.; Liao, Y.P.; Lin, S.; Meng, H.; Li, R.; Sun, B.; et al. Use of coated silver nanoparticles to understand the relationship of particle dissolution and bioavailability to cell and lung toxicological potential. Small 2014, 10, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Tlotleng, N.; Vetten, M.A.; Keter, F.K.; Skepu, A.; Tshikhudo, R.; Gulumian, M. Cytotoxicity, intracellular localization and exocytosis of citrate capped and PEG functionalized gold nanoparticles in human hepatocyte and kidney cells. Cell Biol. Toxicol. 2016, 32, 305–321. [Google Scholar] [CrossRef]
- Kumar, G.; Degheidy, H.; Casey, B.J.; Goering, P.L. Flow cytometry evaluation of in vitro cellular necrosis and apoptosis induced by silver nanoparticles. Food Chem. Toxicol. 2015, 85, 45–51. [Google Scholar] [CrossRef] [PubMed]
| CuO | ζ-Potential (mV) | Hydrodynamic Diameter (nm) | Sedimentation Velocity (µm/s) | Cudissolved/CuOtotal Weight Ratio (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Milli-Q | PBS | DMEM | Milli-Q | PBS | DMEM | Milli-Q | PBS | DMEM | Milli-Q | PBS | DMEM | |
| PRI-PO43− | −9.1 ± 0.4 | −2.3 ± 2.1 | −8.2 ± 7.4 | 1093 ± 50 | 2756 ± 347 | 55 ± 6 | 0.12 | 0.43 | 0.04 | 0.2 (1.1) | <0.3 (0.1) | 67 (0.5) |
| CIT | −18.0 ± 0.3 | −3.4 ± 1.2 | −9.7 ± 0.6 | 368 ± 10 | 271 ± 43 | 37 ± 2 | 0.1 | 0.08 | 0.03 | 2 (0.5) | 1.8 (0.4) | 69 (1.0) |
| ASC | −17.4 ± 0.3 | −8.1 ± 0.1 | −9.2 ± 0.2 | 122 ± 1.4 | 1314 ± 525 | 73 ± 21 | 0.0 | 0.0 | 0.01 | 2 (0.5) | <0.3 (0.1) | 65 (0.4) |
| PEI | +28.3 ± 0.7 | +13.8 ± 0.1 | −10.1 ± 0.7 | 247 ± 14 | 209 ± 16 | 45 ± 14 | 0.05 | 0.03 | 0.1 | 2.8 (0.6) | 2.5 (0.6) | 67 (0.5) |
| PVP | −8.1 ± 2.3 | −0.9 ± 0.7 | −9.4 ± 0.8 | 797 ± 84 | 2765 ± 432 | 53 ± 25 | 0.06 | 0.2 | 0.03 | 0.2 (1.0) | <0.3 (0.1) | 66 (1.3) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, M.J.; Amorim, M.J.B.; Scott-Fordsmand, J.J. Cell In Vitro Testing with Soil Invertebrates—Challenges and Opportunities toward Modeling the Effect of Nanomaterials: A Surface-Modified CuO Case Study. Nanomaterials 2019, 9, 1087. https://doi.org/10.3390/nano9081087
Ribeiro MJ, Amorim MJB, Scott-Fordsmand JJ. Cell In Vitro Testing with Soil Invertebrates—Challenges and Opportunities toward Modeling the Effect of Nanomaterials: A Surface-Modified CuO Case Study. Nanomaterials. 2019; 9(8):1087. https://doi.org/10.3390/nano9081087
Chicago/Turabian StyleRibeiro, Maria J., Mónica J.B. Amorim, and Janeck J. Scott-Fordsmand. 2019. "Cell In Vitro Testing with Soil Invertebrates—Challenges and Opportunities toward Modeling the Effect of Nanomaterials: A Surface-Modified CuO Case Study" Nanomaterials 9, no. 8: 1087. https://doi.org/10.3390/nano9081087
APA StyleRibeiro, M. J., Amorim, M. J. B., & Scott-Fordsmand, J. J. (2019). Cell In Vitro Testing with Soil Invertebrates—Challenges and Opportunities toward Modeling the Effect of Nanomaterials: A Surface-Modified CuO Case Study. Nanomaterials, 9(8), 1087. https://doi.org/10.3390/nano9081087





