Development of Effective Lipase-Hybrid Nanoflowers Enriched with Carbon and Magnetic Nanomaterials for Biocatalytic Transformations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CaLB Nanoflowers
2.3. Characterization of CaLB Nanoflowers
2.4. Determination of Encapsulation Yield
2.5. Activity of CaLB Nanoflowers
2.6. Stability of CaLB Nanoflowers
2.7. Transesterification of Tyrosol Catalayzed by CaLB Nanoflowers
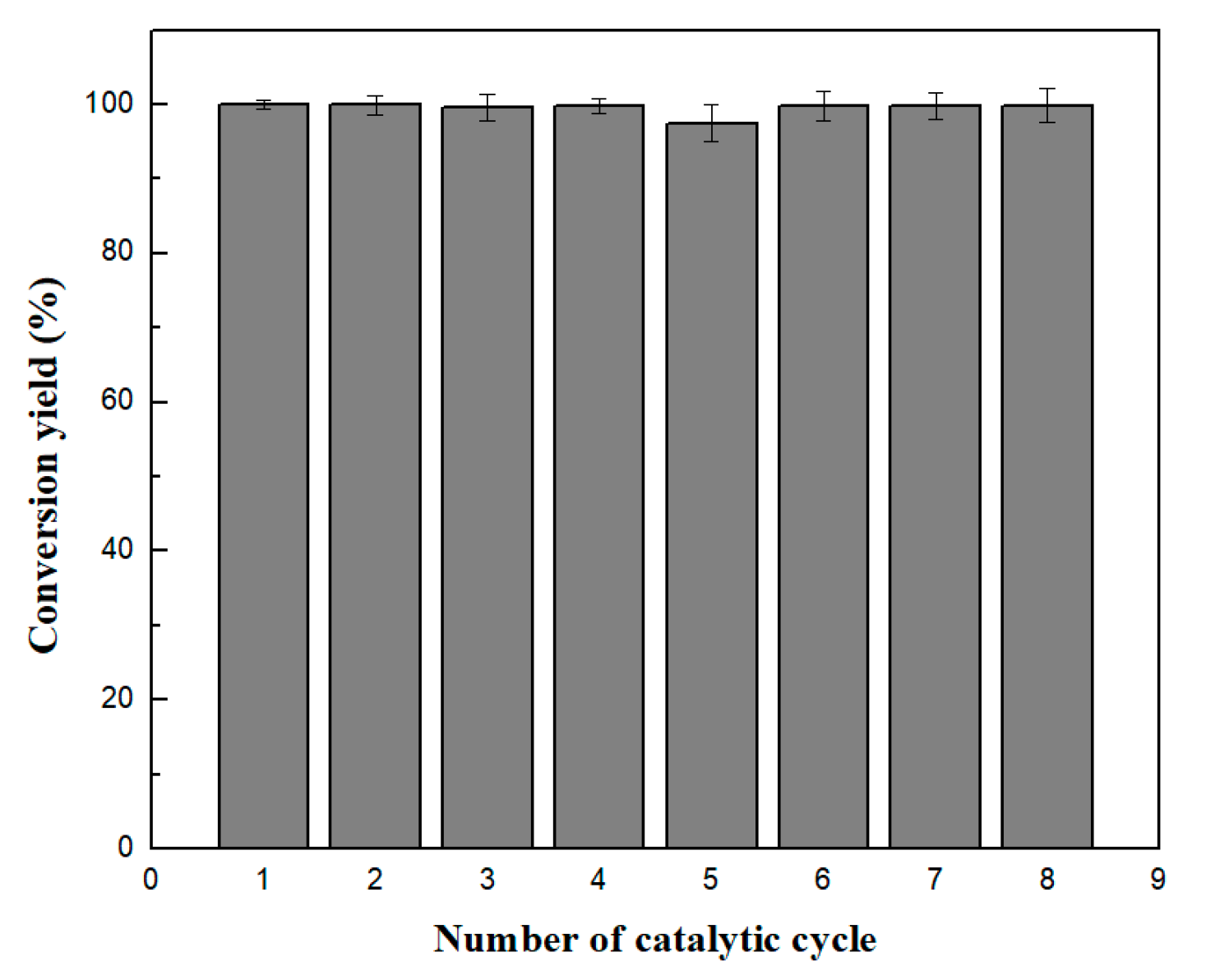
2.8. Reusability of CaLB Nanoflowers
3. Results and Discussion
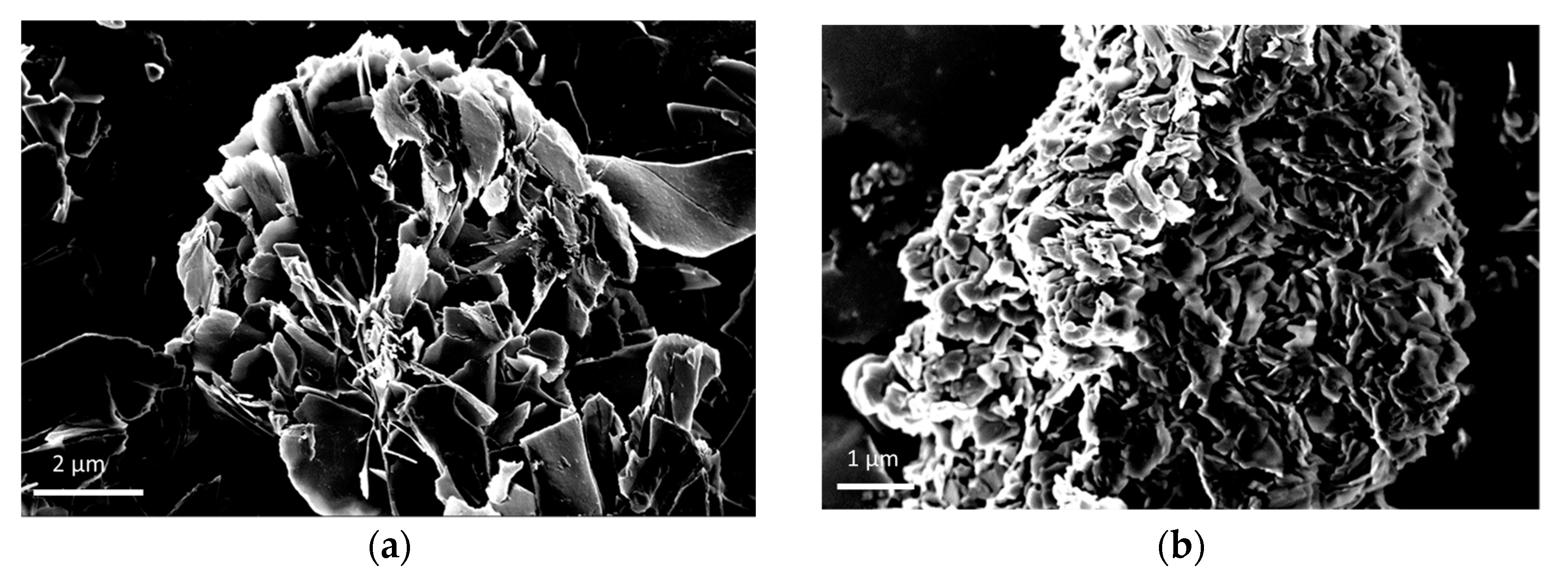
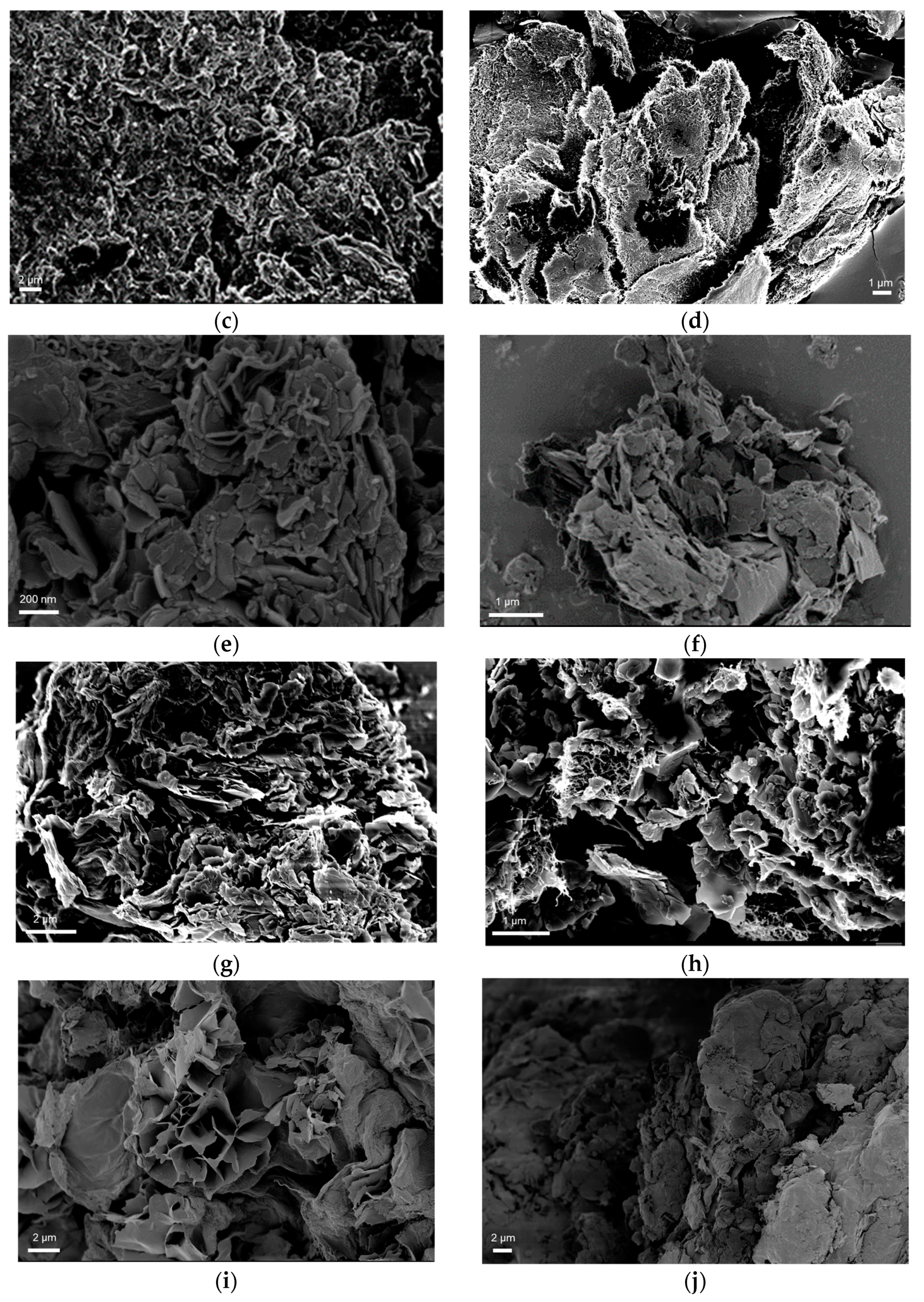
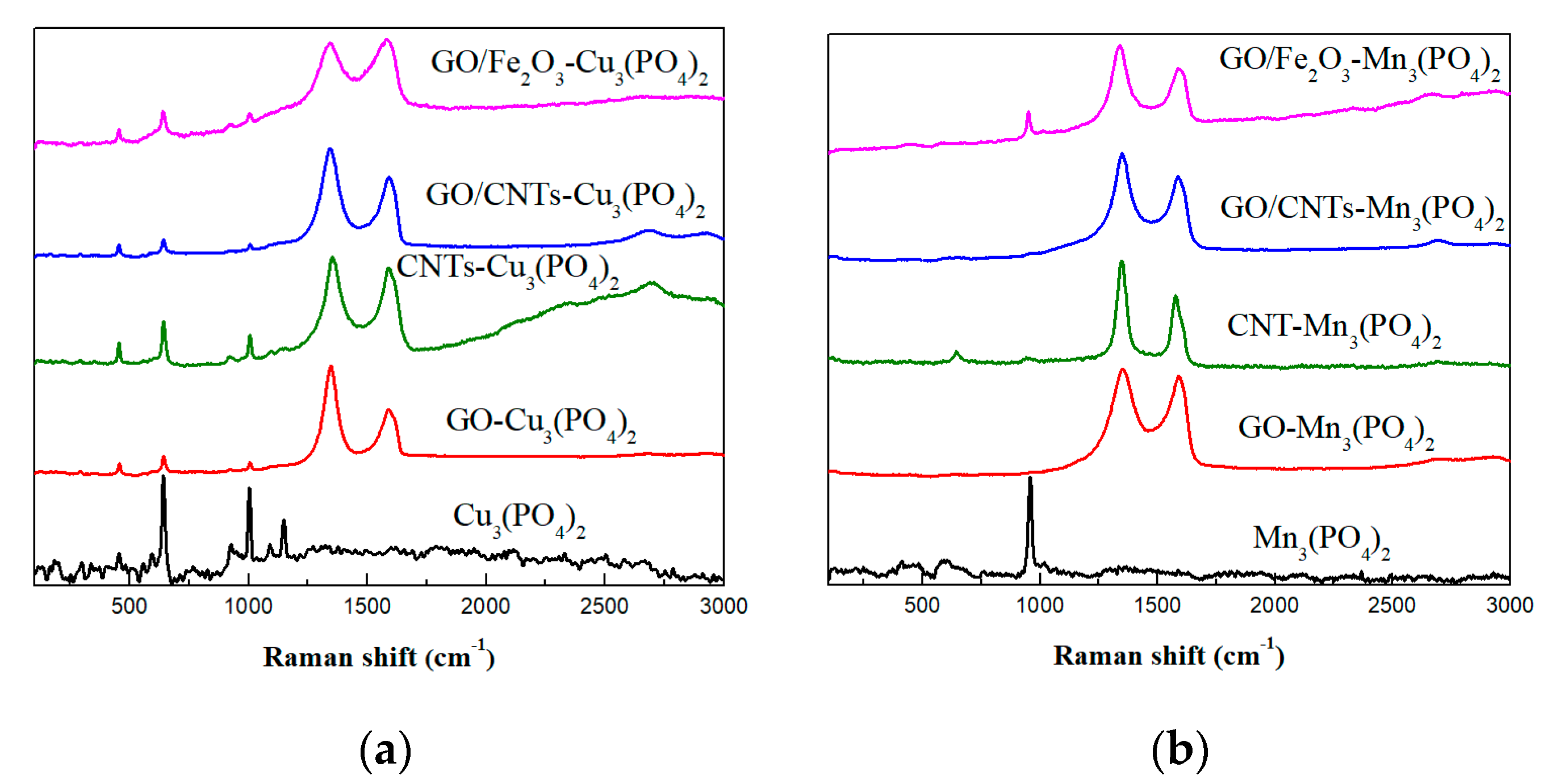
3.1. Morphological and Structural Characterization of CaLB Nanoflowers
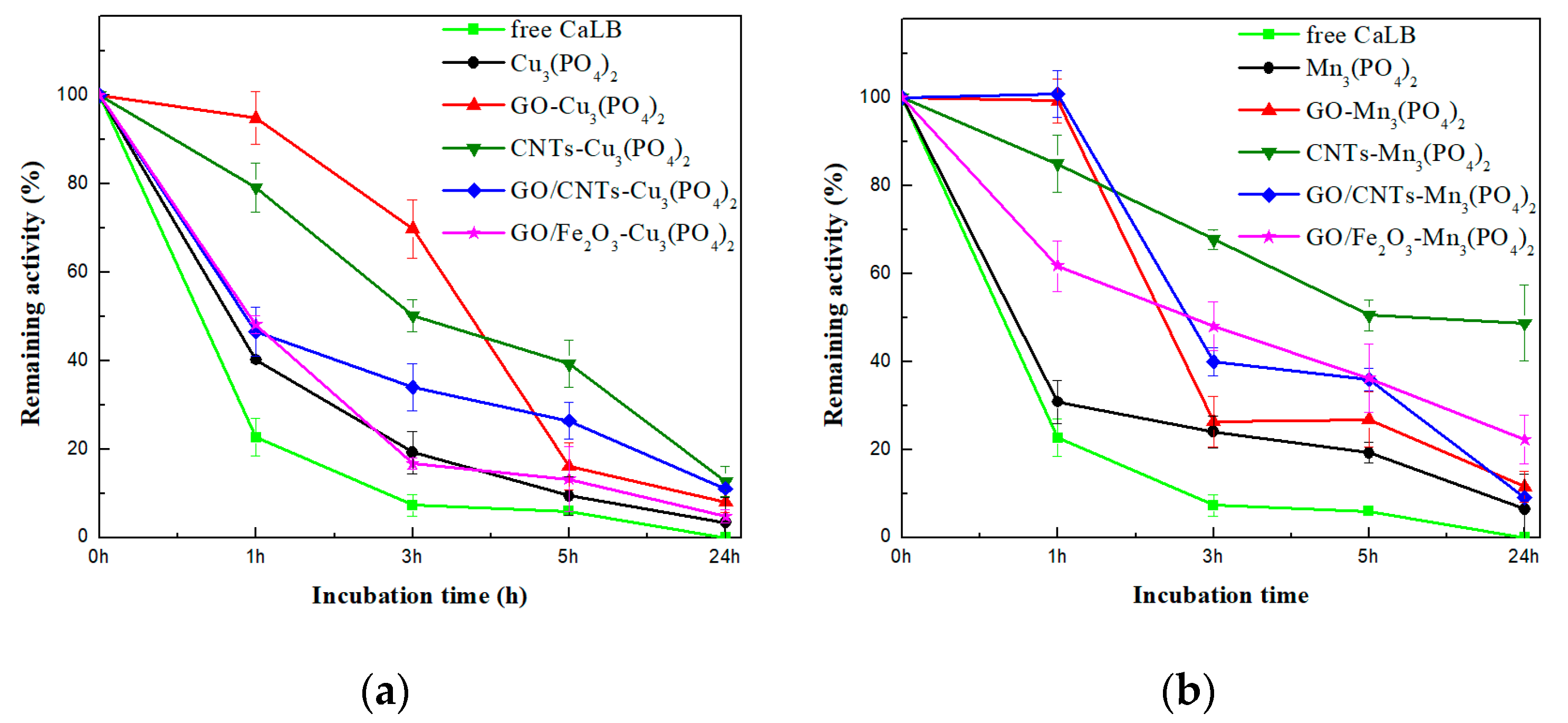
3.2. Biocatalytic Characterization of CaLB Nanoflowers
3.3. Transesterification of Tyrosol by CaLB Nanoflowers in Non-Aqueous Media
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Cipolatti, E.P.; Valério, A.; Henriques, R.O.; Moritz, D.E.; Ninow, J.L.; Freire, D.M.G.; Manoel, E.A.; Fernandez-Lafuente, R.; De Oliveira, D. Nanomaterials for biocatalyst immobilization – state of the art and future trends. RSC Adv. 2016, 6, 104675–104692. [Google Scholar] [CrossRef]
- Di Credico, B.; Redaelli, M.; Bellardita, M.; Calamante, M.; Cepek, C.; Cobani, E.; D’Arienzo, M.; Evangelisti, C.; Marelli, M.; Moret, M.; et al. Step-by-Step Growth of HKUST-1 on Functionalized TiO2 Surface: An Efficient Material for CO2 Capture and Solar Photoreduction. Catalysts 2018, 8, 353. [Google Scholar] [CrossRef]
- Di Credico, B.; Cobani, E.; Callone, E.; Conzatti, L.; Cristofori, D.; D’Arienzo, M.; Dirè, S.; Giannini, L.; Hanel, T.; Scotti, R.; et al. Size-controlled self-assembly of anisotropic sepiolite fibers in rubber nanocomposites. Appl. Clay Sci. 2018, 152, 51–64. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Patila, M.; Bornscheuer, U.T.; Gournis, D.; Stamatis, H. Graphene-based nanobiocatalytic systems: recent advances and future prospects. Trends Biotechnol. 2014, 32, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Kasture, P.; Gaud, R. Nanoflowers: the future trend of nanotechnology for multi-applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 413–422. [Google Scholar] [CrossRef]
- Cui, J.; Jia, S. Organic–inorganic hybrid nanoflowers: A novel host platform for immobilizing biomolecules. Co-ord. Chem. Rev. 2017, 352, 249–263. [Google Scholar] [CrossRef]
- Ge, J.; Lei, J.; Zare, R.N. Protein–inorganic hybrid nanoflowers. Nat. Nanotechnol. 2012, 7, 428–432. [Google Scholar] [CrossRef]
- Batule, B.S.; Park, K.S.; Gautam, S.; Cheon, H.J.; Kim, M.I.; Park, H.G. Intrinsic peroxidase-like activity of sonochemically synthesized protein copper nanoflowers and its application for the sensitive detection of glucose. Sens. Actuators B Chem. 2018, 283, 749–754. [Google Scholar] [CrossRef]
- Chen, X.; Xu, L.; Wang, A.; Li, H.; Wang, C.; Pei, X.; Zhang, P.; Wu, S.G. Efficient synthesis of the key chiral alcohol intermediate of Crizotinib using dual-enzyme@CaHPO4 hybrid nanoflowers assembled by mimetic biomineralization. J. Chem. Technol. Biotechnol. 2019, 94, 236–243. [Google Scholar] [CrossRef]
- Altinkaynak, C.; Tavlasoglu, S.; Kalin, R.; Sadeghian, N.; Ozdemir, H.; Ocsoy, I.; Özdemir, N. A hierarchical assembly of flower-like hybrid Turkish black radish peroxidase-Cu2+ nanobiocatalyst and its effective use in dye decolorization. Chemosphere 2017, 182, 122–128. [Google Scholar] [CrossRef]
- Li, P.; Zhang, B.; Fan, L.; Wang, H.; Tian, L.; Ali, N. Papain/Zn3(PO4)2 hybrid nanoflower: preparation, characterization and its enhanced catalytic activity as an immobilized enzyme. RSC Adv. 2016, 6, 46702–46710. [Google Scholar]
- López-Gallego, F.; Yate, L. Selective biomineralization of Co3 (PO4)2-sponges triggered by His-tagged proteins: efficient heterogeneous biocatalysts for redox processes. Chem. Commun. 2015, 51, 8753–8756. [Google Scholar] [CrossRef] [PubMed]
- Ocsoy, I.; Dogru, E.; Usta, S. A new generation of flowerlike horseradish peroxides as a nanobiocatalyst for superior enzymatic activity. Enzym. Microb. Technol. 2015, 75, 25–29. [Google Scholar] [CrossRef]
- Escobar, S.; Velasco-Lozano, S.; Lu, C.-H.; Lin, Y.-F.; Mesa, M.; Bernal, C.; López-Gallego, F. Understanding the functional properties of bio-inorganic nanoflowers as biocatalysts by deciphering the metal-binding sites of enzymes. J. Mater. Chem. B 2017, 5, 4478–4486. [Google Scholar] [CrossRef]
- Patel, S.K.; Otari, S.V.; Li, J.; Kim, D.R.; Kim, S.C.; Cho, B.-K.; Kalia, V.C.; Kang, Y.C.; Lee, J.-K. Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J. Hazard. Mater. 2018, 347, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.A.; Tzani, A.; Polydera, A.C.; Katapodis, P.; Voutsas, E.; Detsi, A.; Stamatis, H. Green biotransformations catalysed by enzyme-inorganic hybrid nanoflowers in environmentally friendly ionic solvents. Environ. Sci. Pollut. Res. 2018, 25, 26707–26714. [Google Scholar] [CrossRef]
- Zhu, L.; Gong, L.; Zhang, Y.; Wang, R.; Ge, J.; Liu, Z.; Zare, R.N. Rapid Detection of Phenol Using a Membrane Containing Laccase Nanoflowers. Chem. Asian J. 2013, 8, 2358–2360. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Xing, J.; Ge, Z. Preparation of laccase-loaded magnetic nanoflowers and their recycling for efficient degradation of bisphenol A. Sci. Total Environ. 2019, 651, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Huang, T.; Wang, Z.; Yang, H.; Geng, X.; Xu, G.; Samalo, M.; Sakinati, M.; Huo, D.; Hou, C. 3D graphene/copper oxide nano-flowers based acetylcholinesterase biosensor for sensitive detection of organophosphate pesticides. Sens. Actuators B Chem. 2019, 279, 95–101. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, J.; Liu, J.; Zhang, H.; Jiang, J.; Yu, R. A dual enzyme–inorganic hybrid nanoflower incorporated microfluidic paper-based analytic device (μPAD) biosensor for sensitive visualized detection of glucose. Nanoscale 2017, 9, 5658–5663. [Google Scholar] [CrossRef]
- Sharma, N.; Parhizkar, M.; Cong, W.; Mateti, S.; Kirkland, M.A.; Puri, M.; Sutti, A. Metal ion type significantly affects the morphology but not the activity of lipase–metal–phosphate nanoflowers. RSC Adv. 2017, 7, 25437–25443. [Google Scholar] [CrossRef]
- Zeng, J.; Xia, Y. Not just a pretty flower. Nat. Nanotechnol. 2012, 7, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Xiao, Y.; Wang, L.; Yin, Y.; Zheng, J.; Yang, H.; Chen, G. Facile synthesis of enzyme–inorganic hybrid nanoflowers and their application as an immobilized trypsin reactor for highly efficient protein digestion. RSC Adv. 2014, 4, 13888–13891. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, Y.; Liu, R.; Zhong, C.; Jia, S. Surfactant-activated lipase hybrid nanoflowers with enhanced enzymatic performance. Sci. Rep. 2016, 6, 27928. [Google Scholar] [CrossRef]
- Wang, X.; Shi, J.; Li, Z.; Zhang, S.; Wu, H.; Jiang, Z.; Yang, C.; Tian, C. Facile One-Pot Preparation of Chitosan/Calcium Pyrophosphate Hybrid Microflowers. ACS Appl. Mater. Interfaces 2014, 6, 14522–14532. [Google Scholar] [CrossRef]
- Li, H.; Hou, J.; Duan, L.; Ji, C.; Zhang, Y.; Chen, V. Graphene oxide-enzyme hybrid nanoflowers for efficient water soluble dye removal. J. Hazard. Mater. 2017, 338, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, J.; He, Y.; Abdulrazaq, M.A.; Yan, Y. Carbon nanotube-lipase hybrid nanoflowers with enhanced enzyme activity and enantioselectivity. J. Biotechnol. 2018, 281, 87–98. [Google Scholar] [CrossRef]
- Stergiou, D.V.; Diamanti, E.K.; Gournis, D.; Prodromidis, M.I.; Prodromidis, M. (Mamas) Comparative study of different types of graphenes as electrocatalysts for ascorbic acid. Electrochem. Commun. 2010, 12, 1307–1309. [Google Scholar] [CrossRef]
- Tsoufis, T.; Tomou, A.; Gournis, D.; Douvalis, A.P.; Panagiotopoulos, I.; Kooi, B.; Georgakilas, V.; Arfaoui, I.; Bakas, T. Novel Nanohybrids Derived from the Attachment of FePt Nanoparticles on Carbon Nanotubes. J. Nanosci. Nanotechnol. 2008, 8, 5942–5951. [Google Scholar] [CrossRef]
- Tzitzios, V.K.; Bakandritsos, A.; Georgakilas, V.; Basina, G.; Boukos, N.; Bourlinos, A.B.; Niarchos, D.; Petridis, D. Large-Scale Synthesis, Size Control, and Anisotropic Growth of &gamma-Fe2O3 Nanoparticles: Organosols and Hydrosols. J. Nanosci. Nanotechnol. 2007, 7, 2753–2757. [Google Scholar] [PubMed]
- Prestrelski, S.; Tedeschi, N.; Arakawa, T.; Carpenter, J. Dehydration-induced conformational transitions in proteins and their inhibition by stabilizers. Biophys. J. 1993, 65, 661–671. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, J.; Omenya, F.; O’Shea, M.; Chernova, N.A.; Zhang, R.; Wang, Q.; Quackenbush, N.F.; Piper, L.F.J.; Scanlon, D.; et al. Understanding the stability of MnPO4. J. Mater. Chem. A 2014, 2, 12827. [Google Scholar] [CrossRef]
- Kharbish, S.; Andráš, P.; Luptáková, J.; Milovská, S. Raman spectra of oriented and non-oriented Cu hydroxy-phosphate minerals: Libethenite, cornetite, pseudomalachite, reichenbachite and ludjibaite. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 2014, 130, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Sahana, M.B.; Vasu, S.; Sasikala, N.; Anandan, S.; Sepehri-Amin, H.; Sudakar, C.; Gopalan, R. Raman spectral signature of Mn-rich nanoscale phase segregations in carbon free LiFe1-xMnxPO4 prepared by hydrothermal technique. RSC Adv. 2014, 4, 64429–64437. [Google Scholar] [CrossRef]
- Tsirka, K.; Katsiki, A.; Chalmpes, N.; Gournis, D.; Paipetis, A.S. Mapping of Graphene Oxide and Single Layer Graphene Flakes—Defects Annealing and Healing. Front. Mater. 2018, 5, 1–11. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 32183. [Google Scholar] [CrossRef]
- Rong, J.; Zhang, T.; Qiu, F.; Zhu, Y. Preparation of Efficient, Stable, and Reusable Laccase–Cu3(PO4)2 Hybrid Microspheres Based on Copper Foil for Decoloration of Congo Red. ACS Sustain. Chem. Eng. 2017, 5, 4468–4477. [Google Scholar] [CrossRef]
- Lee, H.R.; Chung, M.; Kim, M.I.; Ha, S.H. Preparation of glutaraldehyde-treated lipase-inorganic hybrid nanoflowers and their catalytic performance as immobilized enzymes. Enzym. Microb. Technol. 2017, 105, 24–29. [Google Scholar] [CrossRef]
- Chatzikonstantinou, A.V.; Gkantzou, E.; Gournis, D.; Patila, M.; Stamatis, H. Stabilization of Laccase Through Immobilization on Functionalized GO-Derivatives. In Enzyme Nanoarchitectures: Enzymes Armored with Graphene, 1st ed.; Kumar, C.V., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 609, pp. 47–81. ISBN 9780128152409. [Google Scholar]
- Patila, M.; Diamanti, E.K.; Bergouni, D.; Polydera, A.C.; Gournis, D.; Stamatis, H. Preparation and biochemical characterisation of nanoconjugates of functionalized carbon nanotubes and cytochrome c. Nanomed. Res. J. 2018, 3, 10–18. [Google Scholar]
- Tzialla, A.A.; Pavlidis, I.V.; Felicissimo, M.P.; Rudolf, P.; Gournis, D.; Stamatis, H. Lipase immobilization on smectite nanoclays: Characterization and application to the epoxidation of α-pinene. Bioresour. Technol. 2010, 101, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Secundo, F.; Carrea, G. Mono- and disaccharides enhance the activity and enantioselectivity ofBurkholderia cepacia lipase in organic solvent but do not significantly affect its conformation. Biotechnol. Bioeng. 2005, 92, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Secundo, F.; Barletta, G.L.; Dumitriu, E. Carre Can an Inactivating Agent Increase Enzyme Activity in Organic Solvent? Effects of 18-Crown-6 on Lipase Activity, Enantioselectivity, and Conformation. Biotechnol. Bioeng. 2007, 97, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Fan, G.; Zhang, Y.; Xin, Y.; Zhang, L. Preparation and characterization of copper-Brevibacterium cholesterol oxidase hybrid nanoflowers. Int. J. Boil. Macromol. 2019, 126, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fei, X.; Tian, J.; Li, Y.; Zhi, H.; Wang, K.; Xu, L.; Wang, Y. Synthesis and continuous catalytic application of alkaline protease nanoflowers–PVA composite hydrogel. Catal. Commun. 2018, 116, 5–9. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Gao, J.; Kong, W.; Zhou, L.; He, Y.; Ma, L.; Wang, Y.; Yin, L.; Jiang, Y. Monodisperse core-shell magnetic organosilica nanoflowers with radial wrinkle for lipase immobilization. Chem. Eng. J. 2017, 309, 70–79. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, X.; Yang, J.; Han, H.; Li, Q.; Tang, J. Lipase-inorganic hybrid nanoflower constructed through biomimetic mineralization: A new support for biodiesel synthesis. J. Colloid Interface Sci. 2018, 514, 102–107. [Google Scholar] [CrossRef]
- Patila, M.; Pavlidis, I.V.; Kouloumpis, A.; Dimos, K.; Spyrou, K.; Katapodis, P.; Gournis, D.; Stamatis, H. Graphene oxide derivatives with variable alkyl chain length and terminal functional groups as supports for stabilization of cytochrome c. Int. J. Boil. Macromol. 2016, 84, 227–235. [Google Scholar] [CrossRef]
- Orfanakis, G.; Patila, M.; Catzikonstantinou, A.V.; Lyra, K.-M.; Kouloumpis, A.; Spyrou, K.; Katapodis, P.; Paipetis, A.; Rudolf, P.; Gournis, D.; et al. Hybrid Nanomaterials of Magnetic Iron Nanoparticles and Graphene Oxide as Matrices for the Immobilization of β-Glucosidase: Synthesis, Characterization, and Biocatalytic Properties. Front. Mater. 2018, 5, 1–11. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, M.; Hu, H.; Zhang, X. Chitosan beads immobilized manganese peroxidase catalytic potential for detoxification and decolorization of textile effluent. Int. J. Boil. Macromol. 2016, 89, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Farré, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 1–19. [Google Scholar] [CrossRef]
- Papadopoulou, A.A.; Katsoura, M.H.; Chatzikonstantinou, A.; Kyriakou, E.; Polydera, A.C.; Tzakos, A.G.; Stamatis, H. Enzymatic hybridization of α-lipoic acid with bioactive compounds in ionic solvents. Bioresour. Technol. 2013, 136, 41–48. [Google Scholar] [CrossRef]
- Aissa, I.; Sghair, R.M.; Bouaziz, M.; Laouini, D.; Sayadi, S.; Gargouri, Y. Synthesis of lipophilic tyrosyl esters derivatives and assessment of their antimicrobial and antileishmania activities. Lipids Heal. 2012, 11, 13. [Google Scholar] [CrossRef]
- Zhou, D.-Y.; Sun, Y.-X.; Shahidi, F. Preparation and antioxidant activity of tyrosol and hydroxytyrosol esters. J. Funct. Foods 2017, 37, 66–73. [Google Scholar] [CrossRef]
- Zaks, A.; Klibanov, A.M. The effect of water on enzyme action in organic media. J. Boil. Chem. 1988, 263, 8017–8021. [Google Scholar]
- Klibanov, A.M.; Klibanov, A.M.; Klibanov, A.M. Improving enzymes by using them in organic solvents. Nat. Cell Boil. 2001, 409, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kanwar, S.S. Organic Solvent Tolerant Lipases and Applications. Sci. World J. 2014, 2014, 1–15. [Google Scholar]
- Sheldon, R.A. Biocatalysis and Biomass Conversion in Alternative Reaction Media. Chem. A Eur. J. 2016, 22, 12984–12999. [Google Scholar] [CrossRef]
- Dyson, P.J.; Laurenczy, G. Determination of the Viscosity of the Ionic Liquids [bmim][PF6] and [bmim][TF2N] Under High CO2 Gas Pressure Using Sapphire NMR Tubes. Zeitschrift für Naturforschung B 2008, 63, 681–684. [Google Scholar] [CrossRef]
- Stefanovic, R.; Ludwig, M.; Webber, G.B.; Atkin, R.; Page, A.J. Nanostructure, Hydrogen Bonding and Rheology in Choline Chloride Deep Eutectic Solvents as a Function of the Hydrogen Bond Donor. Phys. Chem. Chem. Phys. 2017, 19, 3297–3306. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zou, X.; Lv, S.; Jin, Q.; Wang, X. Influence of ionic liquids on lipase activity and stability in alcoholysis reactions. RSC Adv. 2016, 6, 87703–87709. [Google Scholar] [CrossRef]
- Zeuner, B.; Ståhlberg, T.; Van Buu, O.N.; Kunov-Kruse, A.J.; Riisager, A.; Meyer, A.S. Dependency of the hydrogen bonding capacity of the solvent anion on the thermal stability of feruloyl esterases in ionic liquid systems. Green Chem. 2011, 13, 1550–1557. [Google Scholar] [CrossRef]
- Wang, S.; Meng, X.; Zhou, H.; Liu, Y.; Secundo, F.; Liu, Y. Enzyme Stability and Activity in Non-Aqueous Reaction Systems: A Mini Review. Catalysts 2016, 6, 32. [Google Scholar] [CrossRef]
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for Stabilization of Enzymes in Organic Solvents. ACS Catal. 2013, 3, 2823–2836. [Google Scholar] [CrossRef]

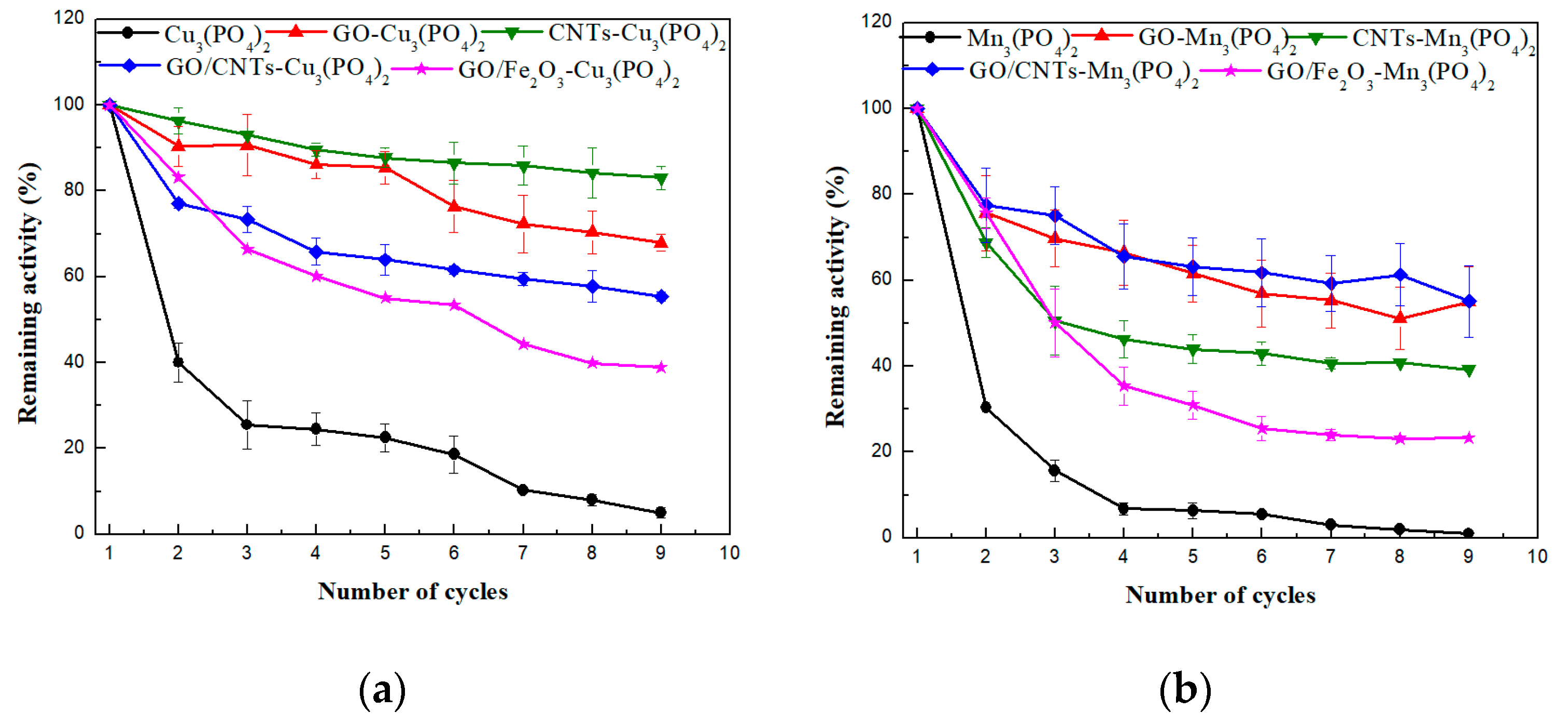
| Nanoflower | r | Nanoflower | r |
|---|---|---|---|
| Cu3(PO4)2 | 0.976 | Mn3(PO4)2 | 0.982 |
| GO-Cu3(PO4)2 | 0.991 | GO-Mn3(PO4)2 | 0.998 |
| CNTs-Cu3(PO4)2 | 0.899 | CNTs-Mn3(PO4)2 | 0.837 |
| GO/CNTs-Cu3(PO4)2 | 0.879 | GO/CNTs-Mn3(PO4)2 | 0.801 |
| GO/Fe2O3-Cu3(PO4)2 | 0.991 | GO/Fe2O3-Mn3(PO4)2 | 0.998 |
| Nanoflower | Encapsulation Yield (%) | Specific Activity (U g−1 Immobilized CaLB) |
|---|---|---|
| Cu3(PO4)2 | 57.6 ± 3.1 | 13.1 ± 0.5 |
| GO-Cu3(PO4)2 | 70.5 ± 1.7 | 174.4 ± 0.7 |
| CNTs-Cu3(PO4)2 | 57.5 ± 2.1 | 189.0 ± 3.9 |
| GO/CNTs-Cu3(PO4)2 | 61.6 ± 1.5 | 167.0 ± 1.7 |
| GO/Fe2O3-Cu3(PO4)2 | 59.0 ± 2.4 | 197.1 ± 2.5 |
| Mn3(PO4)2 | 49.0 ± 1.7 | 161.2 ± 2.6 |
| GO-Mn3(PO4)2 | 67.1 ± 3.6 | 284.7 ± 5.2 |
| CNTs-Mn3(PO4)2 | 57.6 ± 1.2 | 175.6 ± 4.0 |
| GO/CNTs-Mn3(PO4)2 | 65.9 ± 2.5 | 168.7 ± 1.0 |
| GO/Fe2O3-Mn3(PO4)2 | 60.9 ± 2.7 | 175.9 ± 1.9 |
| Reaction Medium | Conversion Yield (%) | |
|---|---|---|
| GO/Fe2O3-Cu3(PO4)2 CaLB-HNFs | GO/Fe2O3-Mn3(PO4)2 CaLB-HNFs | |
| n-Hexane | 99.6 ± 0.4 | 100.0 ± 0.3 |
| Acetonitrile | 80.3 ± 0.3 | 80.7 ± 0.8 |
| 2-Methyl-2-butanol | 30.2 ± 1.1 | 52.6 ± 1.2 |
| tert-Butyl-methylether | 98.9 ± 0.5 | 99.7 ± 0.6 |
| tert-Butanol | 23.2 ± 0.2 | 22.5 ± 0.4 |
| [BMIM][PF6] | 13.6 ± 1.6 | 20.0 ± 4.7 |
| ChCl:U | 33.2 ± 2.8 | 26.7 ± 4.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotiadou, R.; Patila, M.; Hammami, M.A.; Enotiadis, A.; Moschovas, D.; Tsirka, K.; Spyrou, K.; Giannelis, E.P.; Avgeropoulos, A.; Paipetis, A.; et al. Development of Effective Lipase-Hybrid Nanoflowers Enriched with Carbon and Magnetic Nanomaterials for Biocatalytic Transformations. Nanomaterials 2019, 9, 808. https://doi.org/10.3390/nano9060808
Fotiadou R, Patila M, Hammami MA, Enotiadis A, Moschovas D, Tsirka K, Spyrou K, Giannelis EP, Avgeropoulos A, Paipetis A, et al. Development of Effective Lipase-Hybrid Nanoflowers Enriched with Carbon and Magnetic Nanomaterials for Biocatalytic Transformations. Nanomaterials. 2019; 9(6):808. https://doi.org/10.3390/nano9060808
Chicago/Turabian StyleFotiadou, Renia, Michaela Patila, Mohamed Amen Hammami, Apostolos Enotiadis, Dimitrios Moschovas, Kyriaki Tsirka, Konstantinos Spyrou, Emmanuel P. Giannelis, Apostolos Avgeropoulos, Alkiviadis Paipetis, and et al. 2019. "Development of Effective Lipase-Hybrid Nanoflowers Enriched with Carbon and Magnetic Nanomaterials for Biocatalytic Transformations" Nanomaterials 9, no. 6: 808. https://doi.org/10.3390/nano9060808
APA StyleFotiadou, R., Patila, M., Hammami, M. A., Enotiadis, A., Moschovas, D., Tsirka, K., Spyrou, K., Giannelis, E. P., Avgeropoulos, A., Paipetis, A., Gournis, D., & Stamatis, H. (2019). Development of Effective Lipase-Hybrid Nanoflowers Enriched with Carbon and Magnetic Nanomaterials for Biocatalytic Transformations. Nanomaterials, 9(6), 808. https://doi.org/10.3390/nano9060808







_Stamatis.png)


