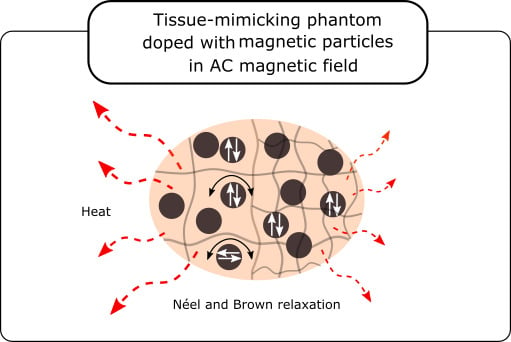
The Effect of Tissue-Mimicking Phantom Compressibility on Magnetic Hyperthermia
Abstract
1. Introduction
2. Materials and Methods
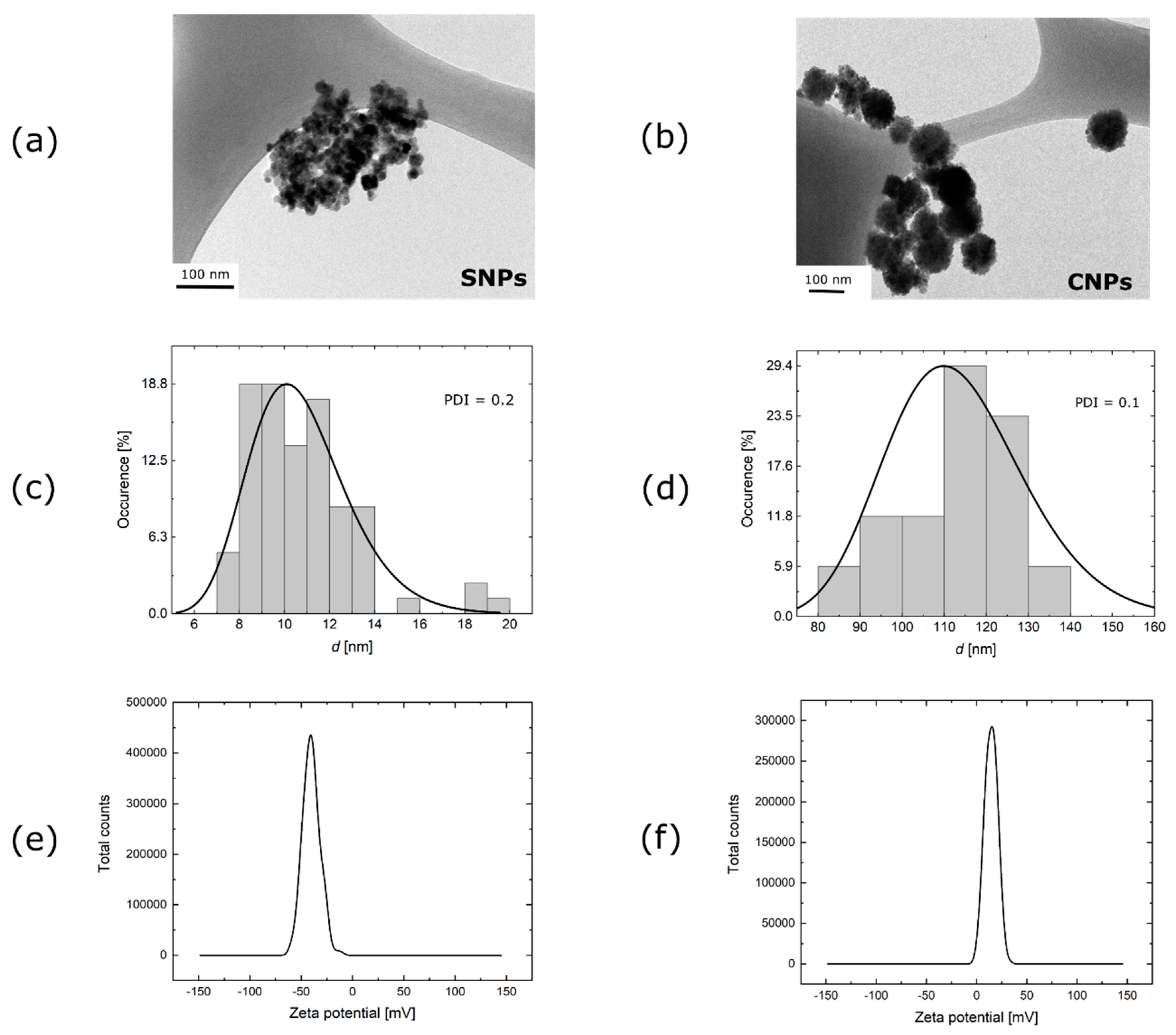
2.1. Magnetic Particles
2.2. Tissue-Mimicking Phantoms
2.3. Measurement Setup
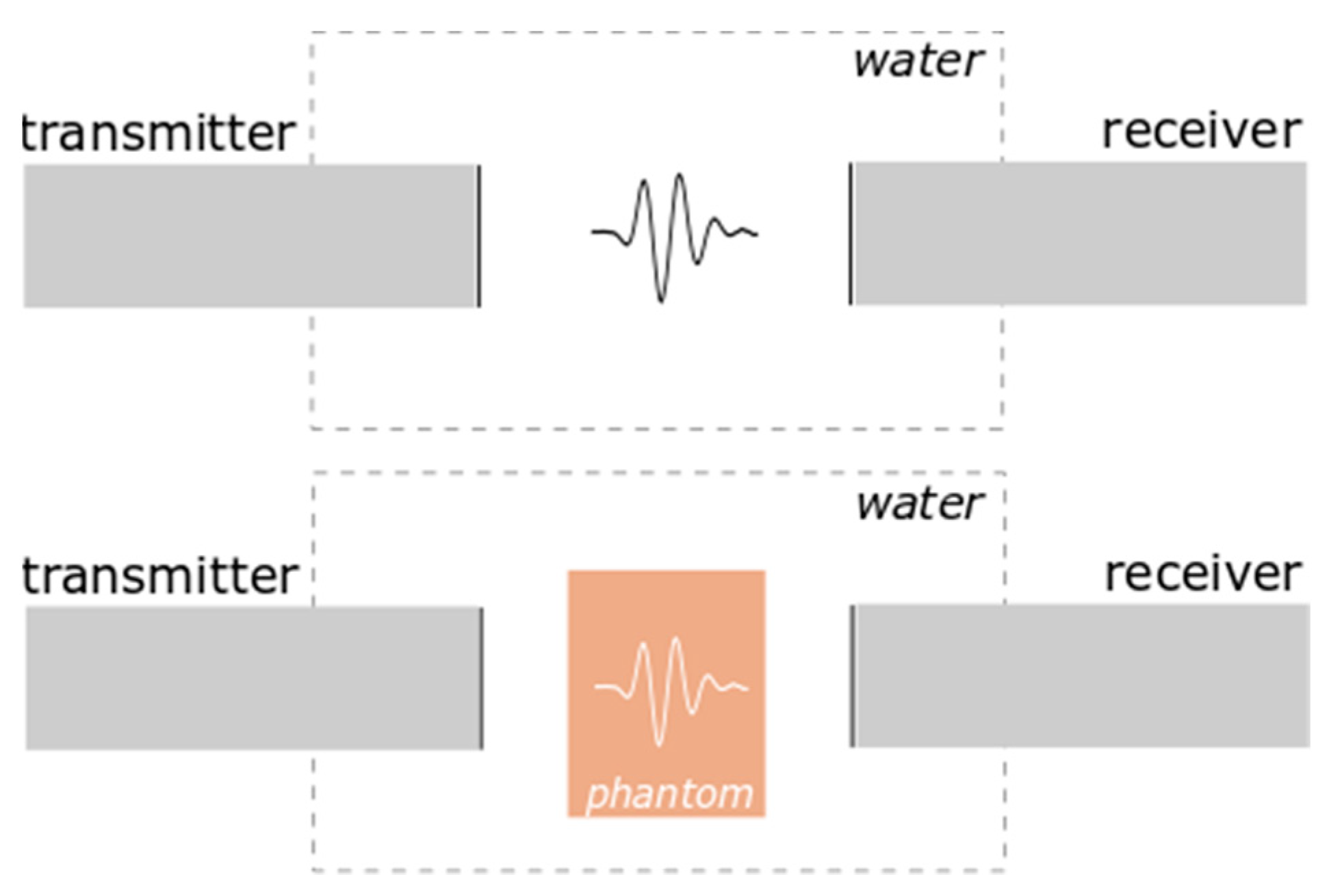
2.3.1. Ultrasonic Wave Velocity Measurements
2.3.2. Density Measurements
2.3.3. Hyperthermia Measurements
3. Results and Discussion
3.1. Elastic Properties
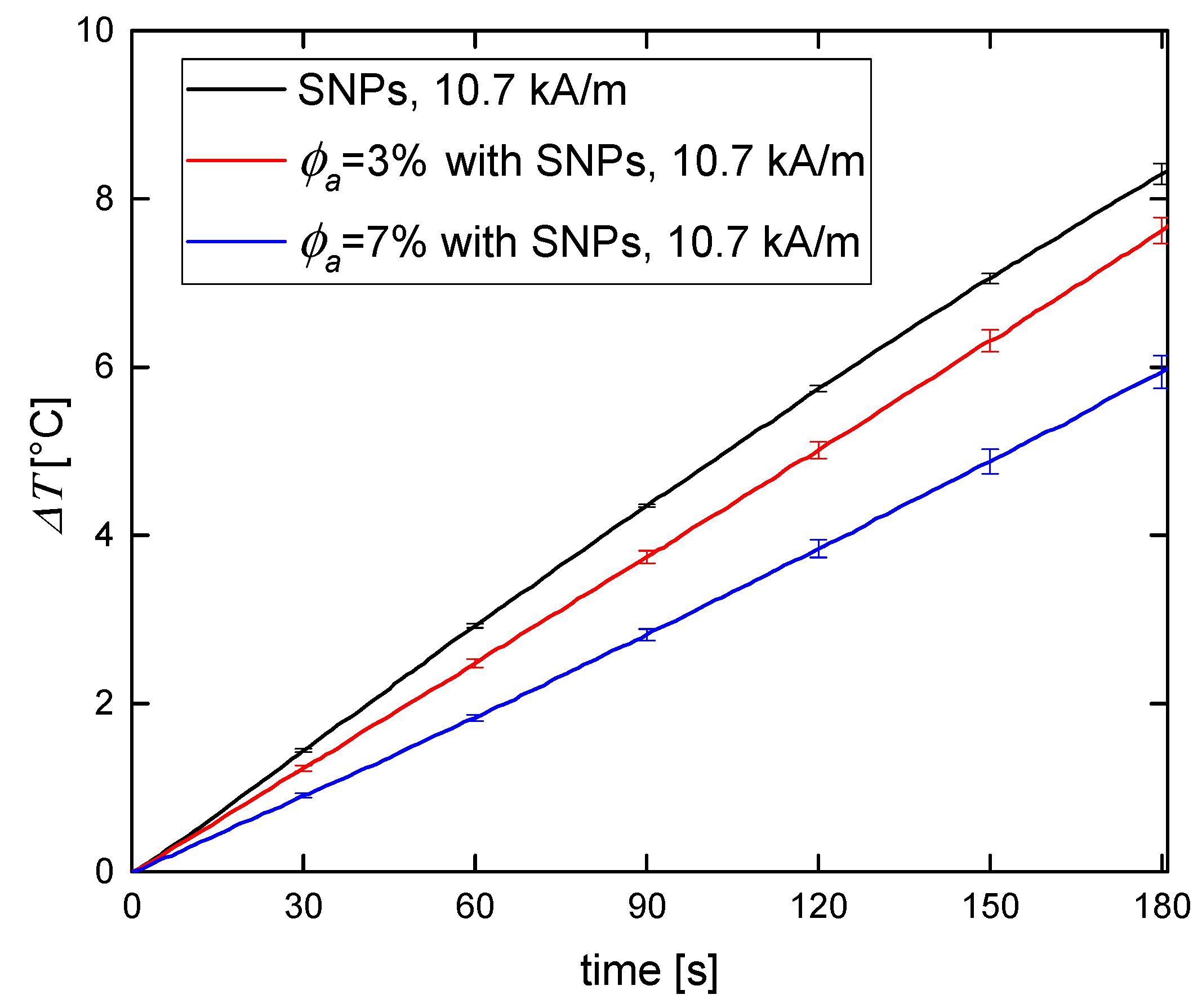
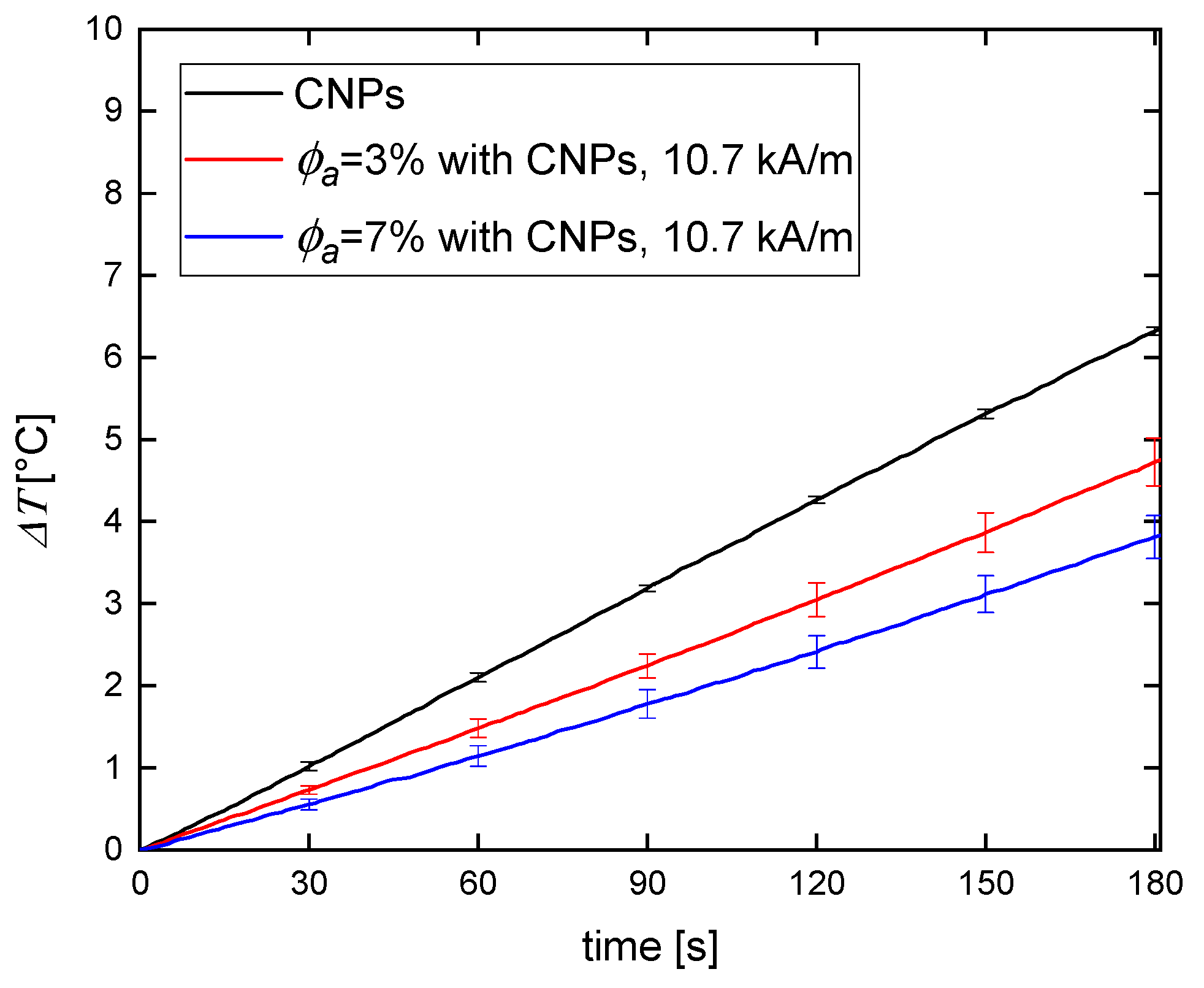
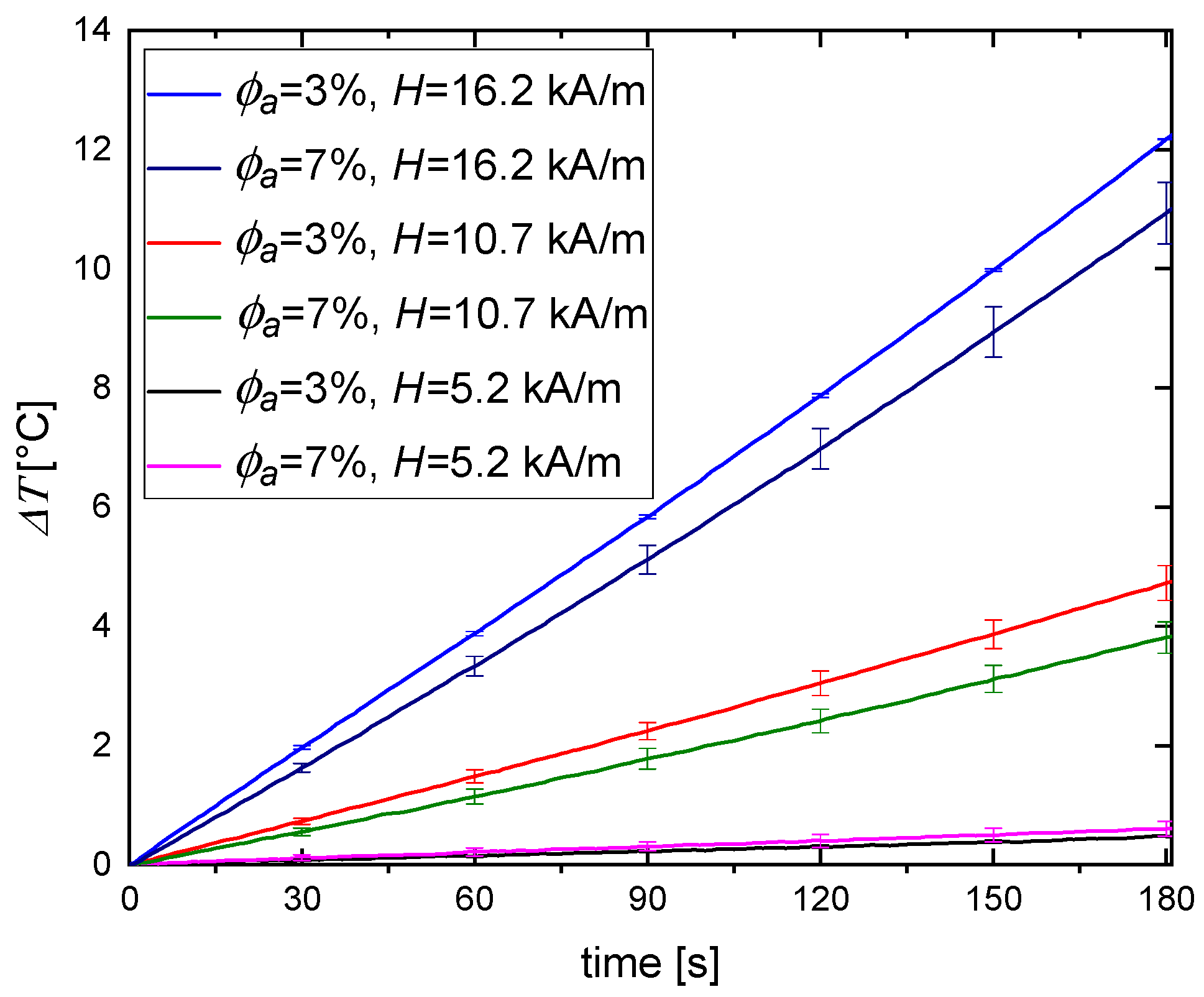
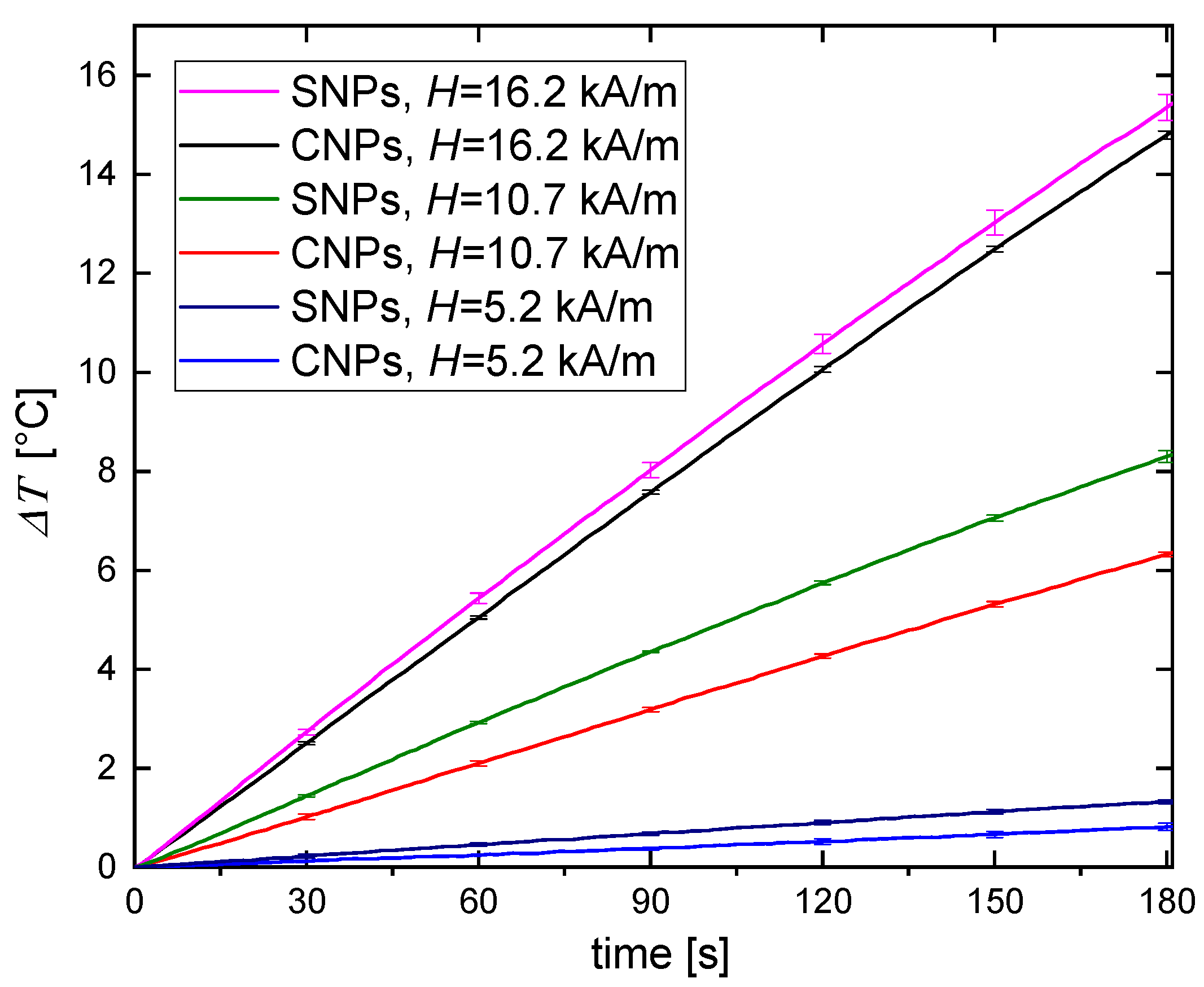
3.2. Magnetic Hyperthermia
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hergt, R.; Hiergeist, R.; Hilger, I.; Kaiser, W.; Lapatnikov, Y.; Margel, S.; Richter, U. Maghemite nanoparticles with very high AC-losses for application in RF-magnetic hyperthermia. J. Magn. Magn. Mater. 2004, 270, 345–357. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Gu, H.-C.; Wang, X.-M. Magnetite ferrofluid with high specific absorption rate for application in hyperthermia. J. Magn. Magn. Mater. 2007, 311, 228–233. [Google Scholar] [CrossRef]
- Iglesias, G.; Delgado, A.; Kujda, M.; Ramos-Tejada, M.; Ramos-Tejada, M.D.M. Magnetic hyperthermia with magnetite nanoparticles: Electrostatic and polymeric stabilization. Colloid Polym. Sci. 2016, 294, 1541–1550. [Google Scholar] [CrossRef]
- Darwish, M.S. Effect of carriers on heating efficiency of oleic acid-stabilized magnetite nanoparticles. J. Mol. Liq. 2017, 231, 80–85. [Google Scholar] [CrossRef]
- Natividad, E.; Andreu, I. Characterization of Magnetic Hyperthermia in Magnetic Nanoparticles. In Magnetic Characterization Techniques for Nanomaterials; Kumar, C.S.S.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 261–303. [Google Scholar]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. The Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef]
- Saraf, H.; Ramesh, K.; Lennon, A.; Merkle, A.; Roberts, J. Mechanical properties of soft human tissues under dynamic loading. J. Biomech. 2007, 40, 1960–1967. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef]
- Xue, S.-L.; Lin, S.-Z.; Li, B.; Feng, X.-Q. A nonlinear poroelastic theory of solid tumors with glycosaminoglycan swelling. J. Theor. Boil. 2017, 433, 49–56. [Google Scholar] [CrossRef]
- Plodinec, M.; Loparic, M.; Monnier, C.A.; Obermann, E.C.; Zanetti-Dällenbach, R.; Oertle, P.; Hyotyla, J.T.; Aebi, U.; Bentires-Alj, M.; Lim, R.Y.H.; et al. The nanomechanical signature of breast cancer. Nat. Nanotechnol. 2012, 7, 757–765. [Google Scholar] [CrossRef]
- Bazan, I.; Vazquez, M.; Ramos, A.; Vera, A.; Leija, L. A performance analysis of echographic ultrasonic techniques for non-invasive temperature estimation in hyperthermia range using phantoms with scatterers. Ultrasonics 2009, 49, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Miaskowski, A.; Sawicki, B. Magnetic Fluid Hyperthermia Modeling Based on Phantom Measurements and Realistic Breast Model. IEEE Trans. Biomed. Eng. 2013, 60, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhang, Y.; Gu, N. Estimation the tumor temperature in magnetic nanoparticle hyperthermia by infrared thermography: Phantom and numerical studies. J. Boil. 2018, 76, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kumar, A.; Singh, V.; Sethi, P.; Singh, M.; Pant, K. Development of tissue-equivalent phantoms for biomedical ultrasonic applications. Int. J. Biomed. Eng. Technol. 2008, 1, 273. [Google Scholar] [CrossRef]
- Narayanan, J.; Xiong, J.-Y.; Liu, X.Y. Determination of agarose gel pore size: Absorbance measurements vis a vis other techniques. J. Phys. Conf. Ser. 2006, 28, 83. [Google Scholar]
- Ernest, L.M.; Maritza, A.H.; Hairong, S.; Tomy, V.; Gary, R.F. Tissue-mimicking agar/gelatin materials for use in heterogeneous elastography phantoms. Phys. Med. Boil. 2005, 50, 5597. [Google Scholar]
- Manickam, K.; Machireddy, R.R.; Seshadri, S. Characterization of biomechanical properties of agar based tissue mimicking phantoms for ultrasound stiffness imaging techniques. J. Mech. Biomed. Mater. 2014, 35, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Mrówczyński, R.; Jedrzak, A.; Szutkowski, K.; Grześkowiak, B.F.; Coy, E.; Markiewicz, R.; Jesionowski, T.; Jurga, S. Cyclodextrin-Based Magnetic Nanoparticles for Cancer Therapy. Nanomaterials 2018, 8, 170. [Google Scholar] [CrossRef]
- Xuan, S.; Wang, Y.-X.J.; Yu, J.C.; Leung, K.C.-F. Tuning the Grain Size and Particle Size of Superparamagnetic Fe3O4Microparticles. Chem. Mater. 2009, 21, 5079–5087. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Celesti, C.; Triolo, C.; Patané, S.; Giofré, S.V.; Romeo, R.; Ziccarelli, I.; Mancuso, R.; Gabriele, B.; et al. A Smart Nanovector for Cancer Targeted Drug Delivery Based on Graphene Quantum Dots. Nanomaterials 2019, 9, 282. [Google Scholar] [CrossRef]
- Armisén, R. International Workshop on Gelidium; Juanes, J.A., Santelices, B., McLachlan, J.L., Eds.; Agar and agarose biotechnological applications; Springer: Dordrecht, The Netherlands, 1991; pp. 157–166. [Google Scholar]
- Zell, K.; Sperl, J.I.; Vogel, M.W.; Niessner, R.; Haisch, C. Acoustical properties of selected tissue phantom materials for ultrasound imaging. Phys. Med. Boil. 2007, 52, N475–N484. [Google Scholar] [CrossRef]
- Józefczak, A.; Kaczmarek, K.; Kubovčíková, M.; Rozynek, Z.; Hornowski, T. The effect of magnetic nanoparticles on the acoustic properties of tissue-mimicking agar-gel phantoms. J. Magn. Magn. Mater. 2017, 431, 172–175. [Google Scholar]
- Deatsch, A.E.; Evans, B.A. Heating efficiency in magnetic nanoparticle hyperthermia. J. Magn. Magn. Mater. 2014, 354, 163–172. [Google Scholar] [CrossRef]
- Avolio, M.; Guerrini, A.; Brero, F.; Innocenti, C.; Sangregorio, C.; Cobianchi, M.; Mariani, M.; Orsini, F.; Arosio, P.; Lascialfari, A. In-gel study of the effect of magnetic nanoparticles immobilization on their heating efficiency for application in Magnetic Fluid Hyperthermia. J. Magn. Magn. Mater. 2019, 471, 504–512. [Google Scholar] [CrossRef]
- Engelmann, U.M.; Seifert, J.; Mues, B.; Roitsch, S.; Ménager, C.; Schmidt, A.M.; Slabu, I. Heating efficiency of magnetic nanoparticles decreases with gradual immobilization in hydrogels. J. Magn. Magn. Mater. 2019, 471, 486–494. [Google Scholar] [CrossRef]
- Fu, R.; Yan, Y.; Roberts, C.; Liu, Z.; Chen, Y. The role of dipole interactions in hyperthermia heating colloidal clusters of densely-packed superparamagnetic nanoparticles. Sci. Rep. 2018, 8, 4704. [Google Scholar] [CrossRef] [PubMed]
- Sakellari, D.; Brintakis, K.; Kostopoulou, A.; Myrovali, E.; Simeonidis, K.; Lappas, A.; Angelakeris, M. Ferrimagnetic nanocrystal assemblies as versatile magnetic particle hyperthermia mediators. Mater. Sci. Eng. C 2016, 58, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, K.; Hornowski, T.; Antal, I.; Timko, M.; Józefczak, A. Magneto-ultrasonic heating with nanoparticles. J. Magn. Magn. Mater. 2019, 474, 400–405. [Google Scholar] [CrossRef]

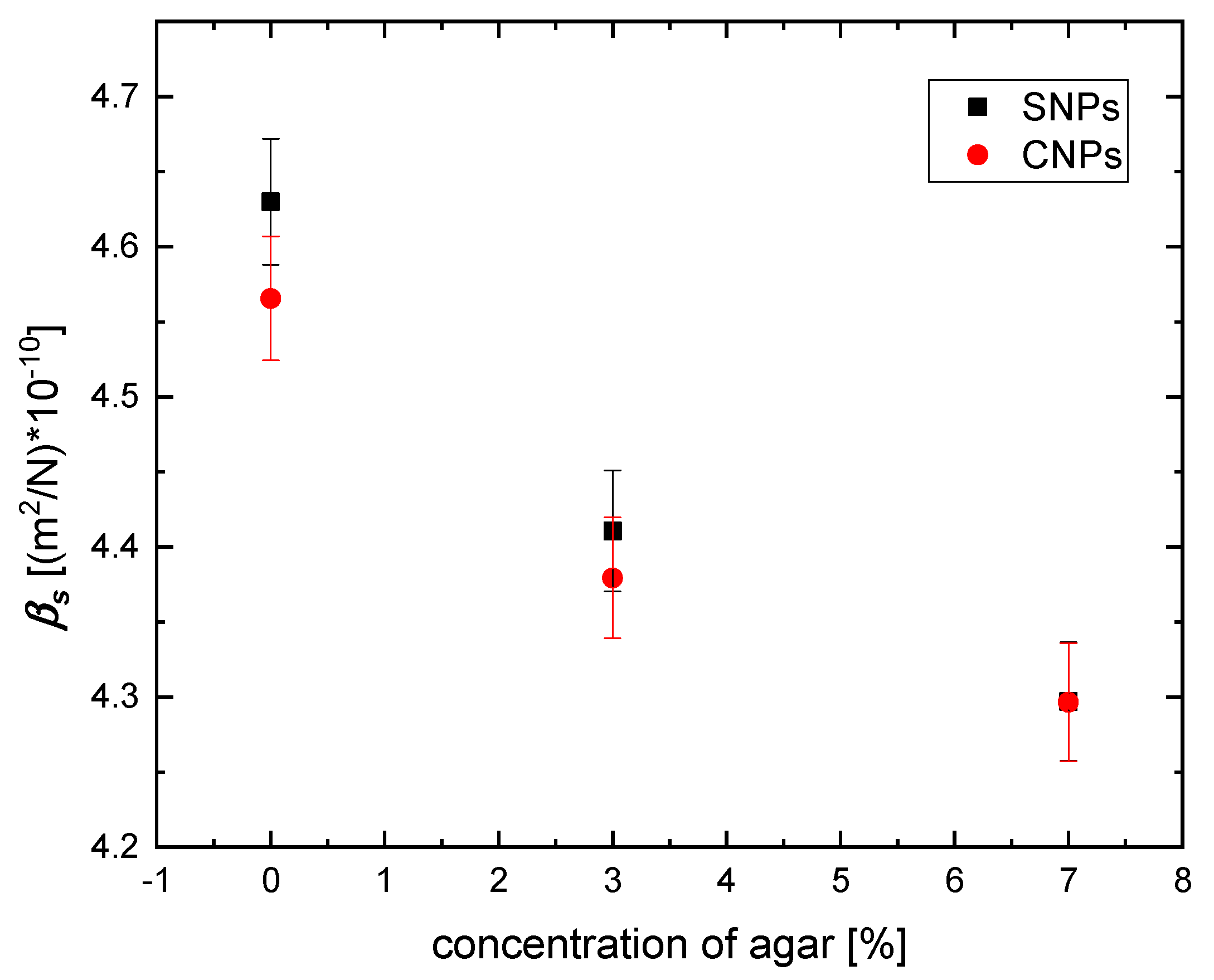
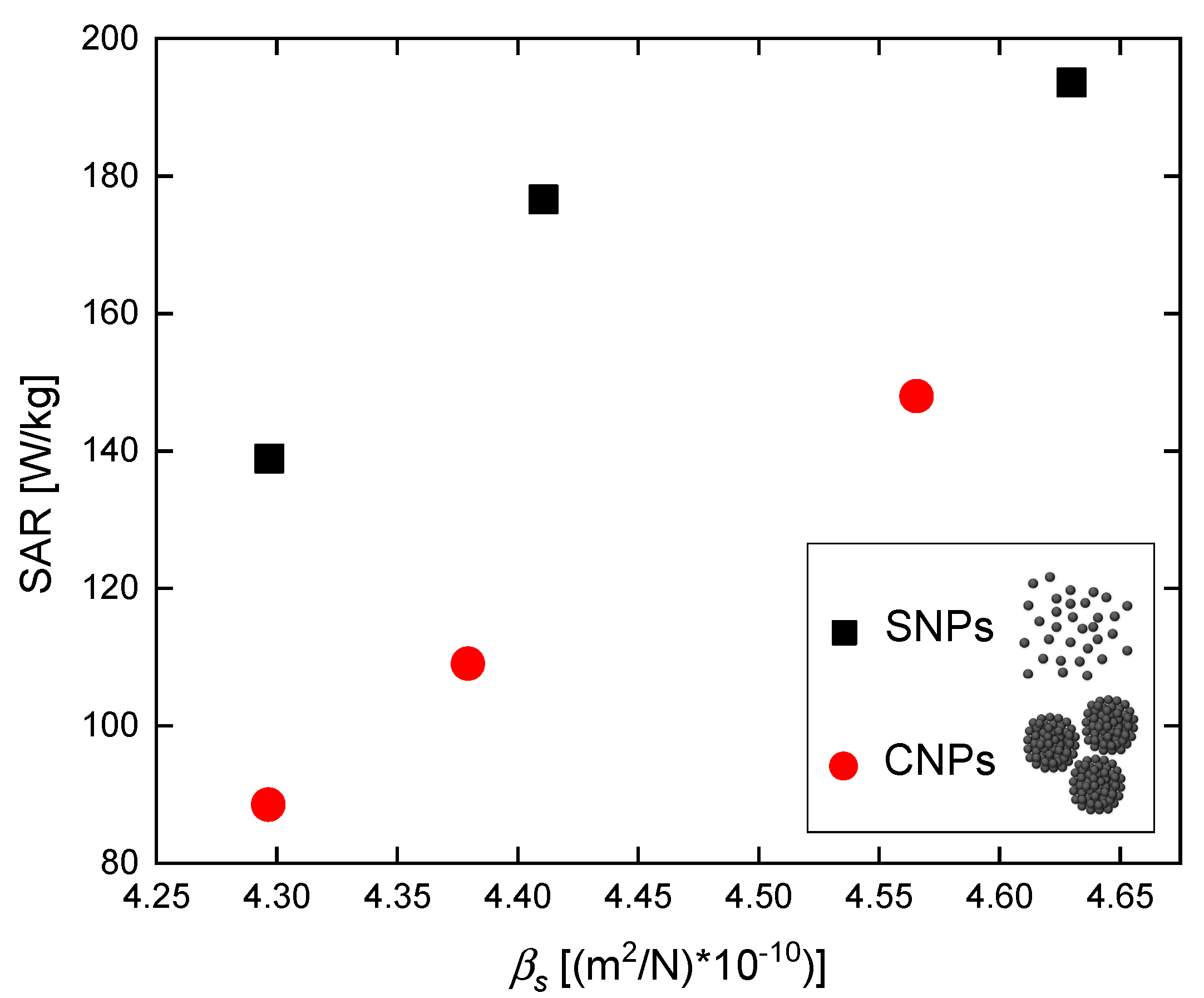
| Agar Concentration [%] | Type of Nanomaterials | Sound Velocity [m/s] | Density [kg/m3] | Bulk Modulus [GPa] | Compressibility βs [(m2/N)] |
|---|---|---|---|---|---|
| 0 | SNPs | 1479.29 | 987 | 2.160 | 4.630 × 10−10 |
| 3 | SNPs | 1498.30 | 1010 | 2.267 | 4.411 × 10−10 |
| 7 | SNPs | 1509.00 | 1022 | 2.327 | 4.297 × 10−10 |
| 0 | CNPs | 1483.68 | 995 | 2.190 | 4.566 × 10−10 |
| 3 | CNPs | 1503.60 | 1010 | 2.283 | 4.379 × 10−10 |
| 7 | CNPs | 1507.60 | 1024 | 2.328 | 4.297 × 10−10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek, K.; Mrówczyński, R.; Hornowski, T.; Bielas, R.; Józefczak, A. The Effect of Tissue-Mimicking Phantom Compressibility on Magnetic Hyperthermia. Nanomaterials 2019, 9, 803. https://doi.org/10.3390/nano9050803
Kaczmarek K, Mrówczyński R, Hornowski T, Bielas R, Józefczak A. The Effect of Tissue-Mimicking Phantom Compressibility on Magnetic Hyperthermia. Nanomaterials. 2019; 9(5):803. https://doi.org/10.3390/nano9050803
Chicago/Turabian StyleKaczmarek, Katarzyna, Radosław Mrówczyński, Tomasz Hornowski, Rafał Bielas, and Arkadiusz Józefczak. 2019. "The Effect of Tissue-Mimicking Phantom Compressibility on Magnetic Hyperthermia" Nanomaterials 9, no. 5: 803. https://doi.org/10.3390/nano9050803
APA StyleKaczmarek, K., Mrówczyński, R., Hornowski, T., Bielas, R., & Józefczak, A. (2019). The Effect of Tissue-Mimicking Phantom Compressibility on Magnetic Hyperthermia. Nanomaterials, 9(5), 803. https://doi.org/10.3390/nano9050803