Gaseous Products Evolution Analyses for Catalytic Decomposition of AP by Graphene-Based Additives
Abstract
1. Introduction
2. Experimental Procedure
2.1. Sample Preparation
2.2. Experimental Techniques
3. Results and Discussion
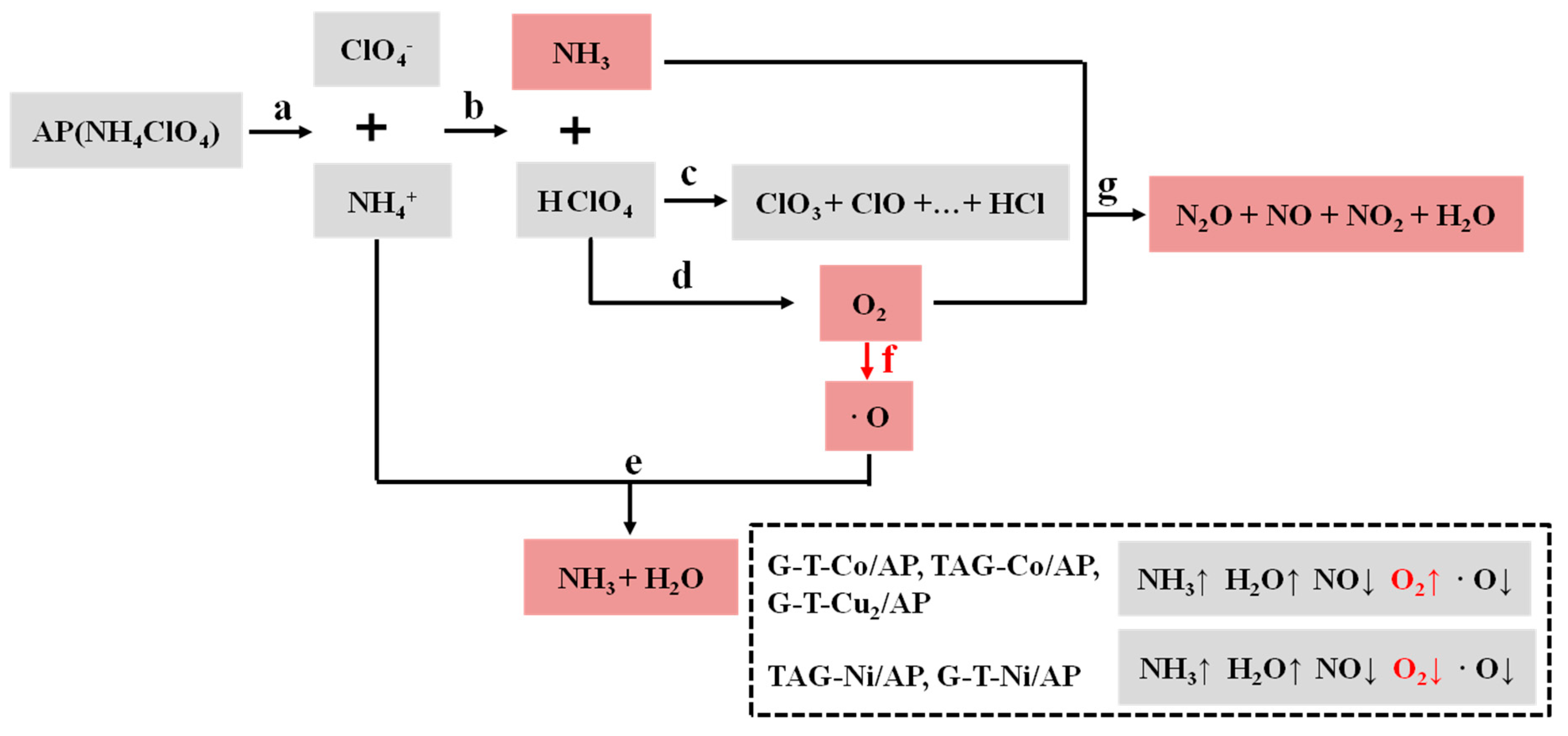
3.1. Possible Catalytic Decomposition Pathways of AP
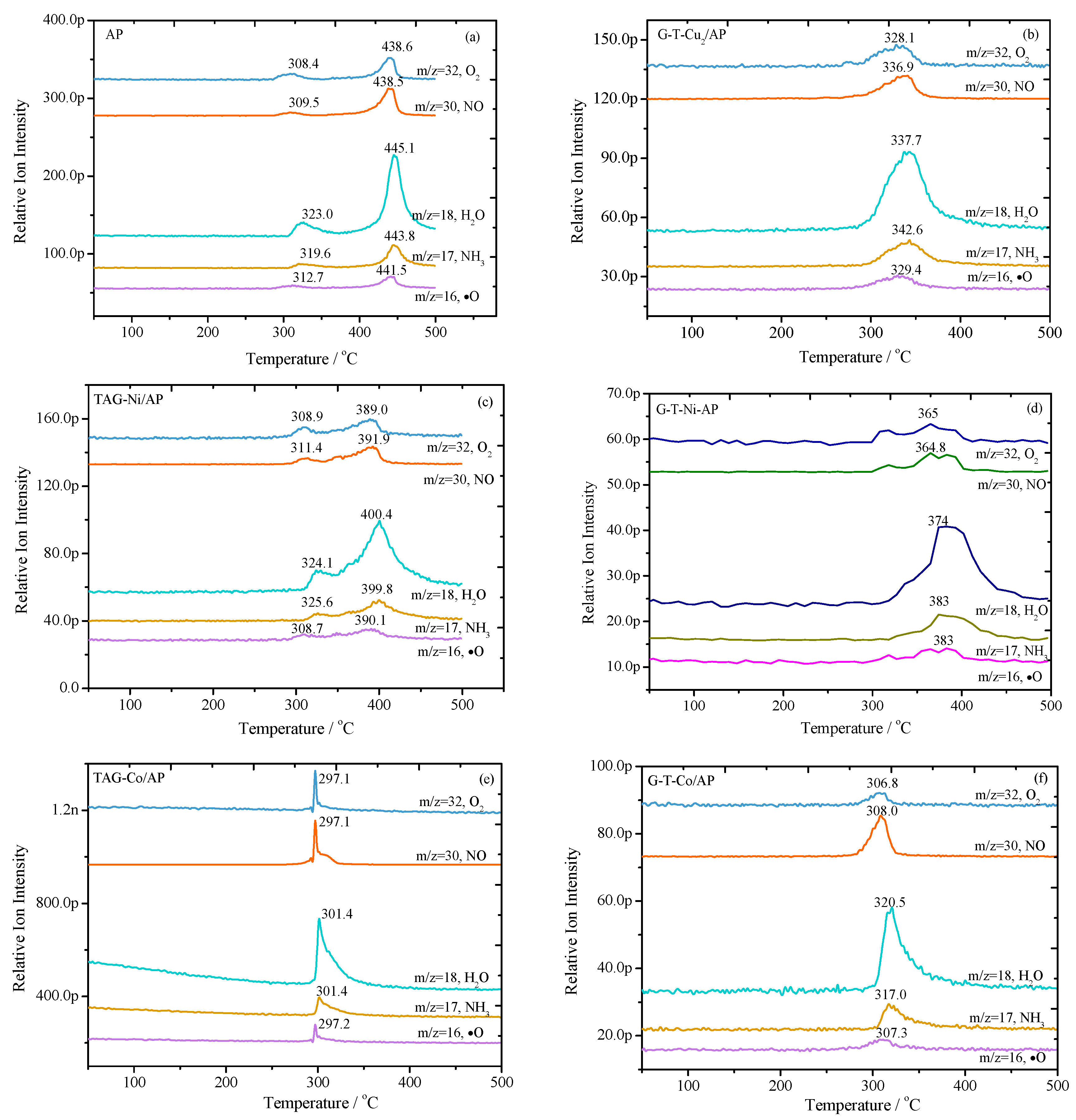
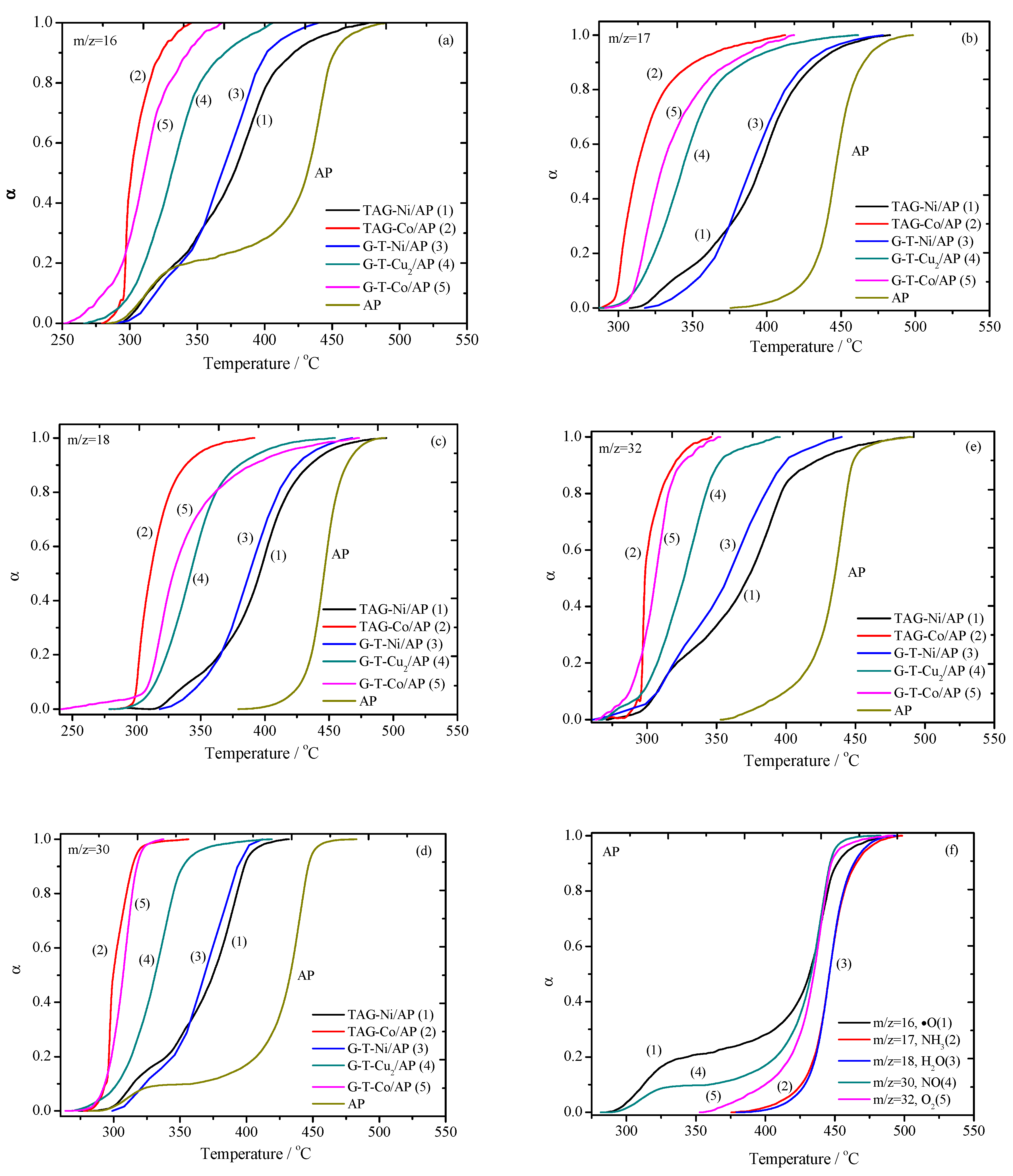
3.2. The Dependence of Gases’ Evolution Processes on Temperature for Catalytic Decomposition of AP
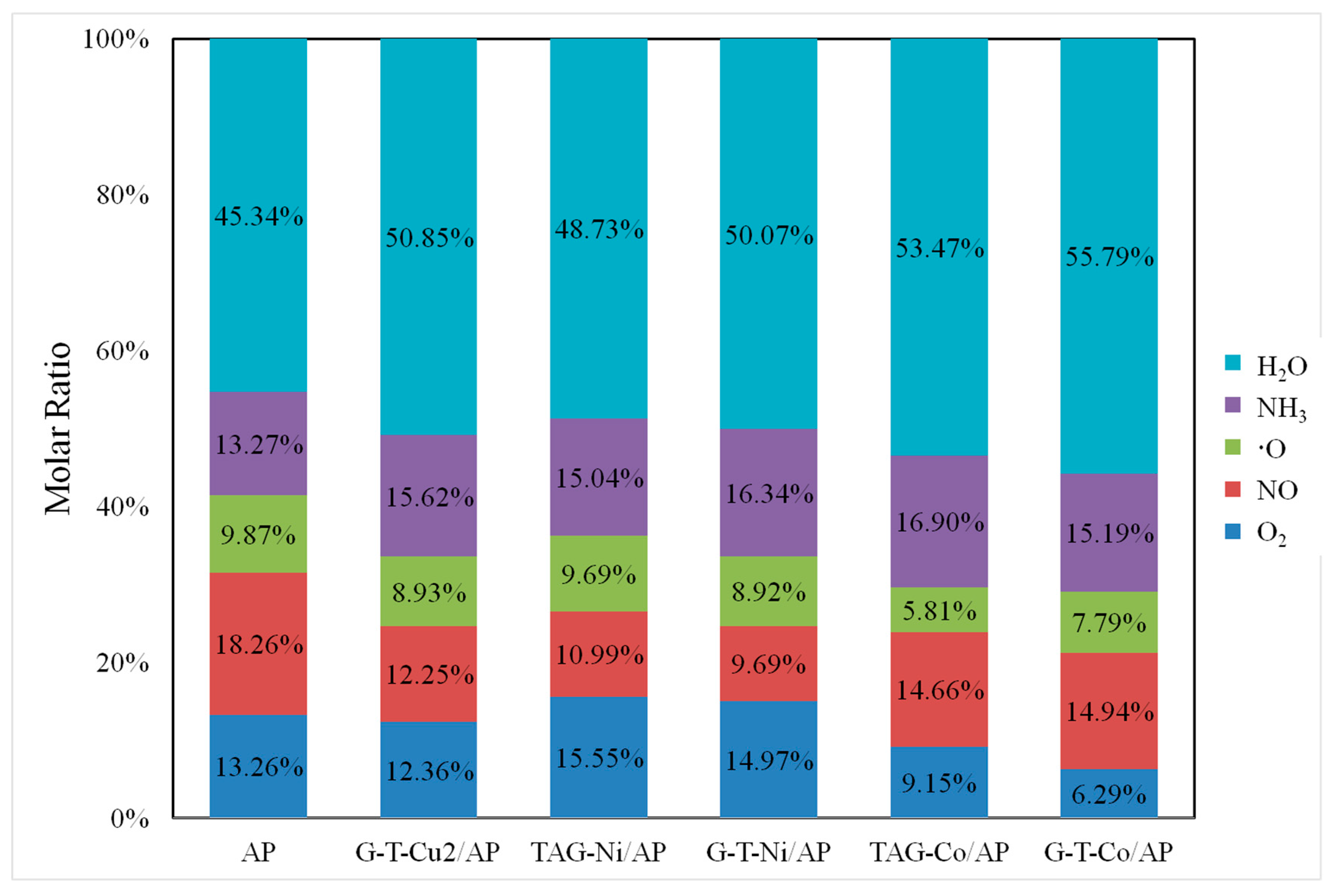
3.3. Quantitative Analyses of Gaseous Products’ Changes of AP in Presence of These Nanocatalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kappagantula, K.; Pantoya, M.L.; Hunt, E.M. Impact ignition of aluminum-teflon based energetic materials impregnated with nano-structured carbon additives. J. Appl. Phys. 2012, 112, 024902. [Google Scholar] [CrossRef]
- Krause, H.H. New Energetic Materials. In Energetic Materials: Particle Processing and Characterization; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; pp. 1–25. [Google Scholar]
- Singh, G.; Kapoor, I.P.S.; Mannan, S.M.; Kaur, J. Studies on energetic compounds: Part 8: Thermolysis of Salts of HNO3, and HClO4. J. Hazard. Mater. 2000, 79, 1–18. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Yang, X.; Lu, L.; Wang, X. Preparation of NiO nanoparticles and their catalytic activity in the thermal decomposition of ammonium perchlorate. Thermochim. Acta 2005, 437, 106–109. [Google Scholar]
- Zhang, W.; Li, P.; Xu, H.; Sun, R.; Qing, P.; Zhang, Y. Thermal decomposition of ammonium perchlorate in the presence of Al(OH)3·Cr(OH)3 nanoparticles. J. Hazard. Mater. 2014, 268, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, D.; Chen, Y.; Tan, L.; Kou, B.; Wan, F.; Jiang, W.; Li, F. Constructing sheet-on-sheet structured graphitic carbon nitride/reduced graphene oxide/layered MnO2 ternary nanocomposite with outstanding catalytic properties on thermal decomposition of ammonium perchlorate. Nanomaterials 2017, 7, 450. [Google Scholar] [CrossRef] [PubMed]
- Sergey, V.; Wight, C.A. Kinetics of Thermal Decomposition of Cubic Ammonium Perchlorate. Chem. Mater. 1999, 11, 3386–3393. [Google Scholar]
- Liu, T.; Wang, L.; Yang, P.; Hu, B. Preparation of nanometer CuFe2O4, by auto-combustion and its catalytic activity on the thermal decomposition of ammonium perchlorate. Mater. Lett. 2008, 62, 4056–4058. [Google Scholar] [CrossRef]
- Alizadeh-Gheshlaghi, E.; Shaabani, B.; Khodayari, A.; Azizian-Kalandaragh, Y.; Rahimi, R. Investigation of the catalytic activity of nano-sized CuO, Co3O4, and CuCo2O4, powders on thermal decomposition of ammonium perchlorate. Powder Technol. 2012, 217, 330–339. [Google Scholar] [CrossRef]
- Chen, L.; Li, L.; Li, G. Synthesis of CuO nanorods and their catalytic activity in the thermal decomposition of ammonium perchlorate. J. Alloys Compd. 2008, 464, 532–536. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Zhang, L. Selective preparation of nanorods and micro-octahedrons of Fe2O3, and their catalytic performances for thermal decomposition of ammonium perchlorate. Powder Technol. 2008, 185, 176–180. [Google Scholar] [CrossRef]
- Yuan, Y.; Wei, J.; Wang, Y.; Shen, P.; Li, F.; Li, P.; Zhao, F.; Gao, H. Hydrothermal preparation of Fe2O3/graphene nanocomposite and its enhanced catalytic activity on the thermal decomposition of ammonium perchlorate. Appl. Surf. Sci. 2014, 303, 354–359. [Google Scholar] [CrossRef]
- Memon, N.K.; Mcbain, A.W.; Son, S.F. Graphene Oxide/Ammonium Perchlorate Composite Material for Use in Solid Propellants. J. Propuls. Power 2016, 32, 682–686. [Google Scholar] [CrossRef]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and Thermal Reduction of Graphene Oxide: Reaction Mechanisms, Product Structures, and Reaction Design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Yan, Q.L.; Gozin, M.; Zhao, F.Q.; Cohen, A.; Pang, S.-P. Highly energetic compositions based on functionalized carbon nanomaterials. Nanoscale 2016, 8, 4799–4851. [Google Scholar] [CrossRef]
- He, W.; Guo, J.H.; Cao, C.K.; Liu, X.-K.; Lv, J.-Y.; Chen, S.-Y.; Liu, P.-J.; Yan, Q.-L. Catalytic Reactivity of Graphene Oxide Stabilized Transition Metal Complexes of Triaminoguanidine on Thermolysis of RDX. J. Phys. Chem. C 2018, 122, 14714–14724. [Google Scholar] [CrossRef]
- An, T.; He, W.; Chen, S.-W.; Zuo, B.-L.; Qi, X.F.; Zhao, F.Q.; Luo, Y.; Yan, Q.-L. Thermal Behavior and Thermolysis Mechanisms of AP under the Effects of GO-Doped Complexes of Triaminoguanidine. J. Phys. Chem. C 2018, 122, 14714–14724. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Nie, J.; Yu, L.; Zhong, Y.; Huang, C. Improve the catalytic activity of α-Fe2O3, particles in decomposition of ammonium perchlorate by coating amorphous carbon on their surface. J. Solid State Chem. 2011, 184, 387–390. [Google Scholar] [CrossRef]
- Jacobs, P.W.M.; Ng, W.L. Thermal decomposition of ammonium perchlorate single crystals. J. Solid State Chem. 1974, 4, 305. [Google Scholar] [CrossRef]
- Jacobs, P.W.M.; Pearson, G.S. The thermal decomposition of ammonium perchlorate (i) introduction experimental analysis of gaseous products and thermal decomposition experiments. Combust. Flame 1969, 13, 419. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N. A review on the use of nanometals as catalysts for the thermal decomposition of ammonium perchlorate. J. Saudi Chem. Soc. 2013, 17, 135–149. [Google Scholar] [CrossRef]
- Mallick, L.; Kumar, S.; Chowdhury, A. Thermal decomposition of ammonium perchlorate-A TGA-FTIR-MS study: Part I. Thermochim. Acta 2015, 610, 57–68. [Google Scholar] [CrossRef]
- Sanoop, A.P.; Rajeev, R.; George, B.K. Synthesis and characterization of a novel copper chromite catalyst for the thermal decomposition of ammonium perchlorate. Thermochim. Acta 2015, 606, 34–40. [Google Scholar] [CrossRef]
- Wang, X.B.; Li, J.Q.; Luo, Y.J. Preparation and Thermal Decomposition Behaviour of Ammonium Perchlorate/Graphene Aerogel Nanocomposites. Chin. J. Explos. Propellants 2012, 35, 76–80. [Google Scholar]
- Wang, X.; Li, J.; Luo, Y.; Huang, M. A Novel Ammonium Perchlorate/Graphene Aerogel Nanostructured Energetic Composite: Preparation and Thermal Decomposition. Sci. Adv. Mater. 2014, 6, 530–537. [Google Scholar] [CrossRef]
- Lan, Y.; Jin, M.; Luo, Y. Preparation and characterization of graphene aerogel/Fe2O3/ammonium perchlorate nanostructured energetic composite. J. Sol-Gel Sci. Technol. 2015, 74, 161–167. [Google Scholar] [CrossRef]
- Liu, H.; Jiao, Q.; Zhao, Y.; Li, H.; Sun, C.; Li, X.; Wu, H. Cu/Fe hydrotalcite derived mixed oxides as new catalyst for thermal decomposition of ammonium perchlorate. Mater. Lett. 2010, 64, 1698–1700. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Thermal decomposition of ammonium perchlorate. Thermochim. Acta 2006, 443, 1–36. [Google Scholar] [CrossRef]
- Rosser, W.A.; Inami, S.H.; Wise, H. Thermal decomposition of ammonium perchlorate. Combust. Flame 1968, 12, 427–435. [Google Scholar] [CrossRef]
| AP | GO | TAG | Metal | |
|---|---|---|---|---|
| G-T-Co/AP | 80% | 0.4% | 5.0% | 14.6% Cobalt |
| G-T-Cu2/AP | 80% | 0.4% | 5.7% | 13.9% Copper |
| G-T-Ni/AP | 80% | 0.5% | 6.6% | 12.9% Nickel |
| TAG-Co/AP | 80% | - | 6.3% | 13.7% Cobalt |
| TAG-Ni/AP | 80% | - | 7.1% | 12.9% Nickel |
| m/z = 16, ∙O | |||||
|---|---|---|---|---|---|
| To | Te | ∆T | n | nm | |
| TAG-Ni/AP | 289.9 | 478.0 | 188.1 | 4.541 × 10−10 | 9.69% |
| TAG-Co/AP | 279.4 | 346.7 | 67.3 | 5.803 × 10−10 | 5.81% |
| G-T-Ni/AP | 289 | 440 | 151 | 1.969 × 10−10 | 8.92% |
| G-T-Cu2/AP | 266.2 | 406.7 | 140.5 | 3.653 × 10−10 | 8.93% |
| G-T-Co/AP | 251.5 | 369.2 | 117.7 | 1.330 × 10−10 | 7.79% |
| AP | 282.7 | 491.2 | 208.5 | 5.734 × 10−10 | 9.87% |
| m/z = 17, NH3 | |||||
| TAG-Ni/AP | 307.8 | 483.1 | 175.3 | 7.052 × 10−10 | 15.04% |
| TAG-Co/AP | 287.6 | 412.5 | 124.9 | 16.89 × 10−10 | 16.90% |
| G-T-Ni/AP | 318 | 478 | 160 | 3.607 × 10−10 | 16.34% |
| G-T-Cu2/AP | 287.2 | 461.5 | 174.3 | 6.389 × 10−10 | 15.62% |
| G-T-Co/AP | 291.2 | 418.4 | 127.2 | 2.595 × 10−10 | 15.19% |
| AP | 375.6 | 498.4 | 122.8 | 7.706 × 10−10 | 13.27% |
| m/z = 18, H2O | |||||
| TAG-Ni/AP | 292.5 | 494.6 | 202.1 | 22.849 × 10−10 | 48.73% |
| TAG-Co/AP | 282.3 | 391.8 | 109.5 | 53.426 × 10−10 | 53.47% |
| G-T-Ni/AP | 318 | 468 | 150 | 11.052 × 10−10 | 50.07% |
| G-T-Cu2/AP | 278.7 | 454.5 | 175.8 | 20.80 × 10−10 | 50.85% |
| G-T-Co/AP | 241.3 | 473.3 | 232 | 9.531 × 10−10 | 55.79% |
| AP | 379.2 | 493.5 | 114.3 | 26.328 × 10−10 | 45.34% |
| m/z = 30, NO | |||||
| TAG-Ni/AP | 273.7 | 431.9 | 158.2 | 5.153 × 10−10 | 10.99% |
| TAG-Co/AP | 277.7 | 356.5 | 78.8 | 14.65 × 10−10 | 14.66% |
| G-T-Ni/AP | 299 | 412 | 113 | 2.138 × 10−10 | 9.69% |
| G-T-Cu2/AP | 265.3 | 419.0 | 153.7 | 5.010 × 10−10 | 12.25% |
| G-T-Co/AP | 263.7 | 337.6 | 73.9 | 2.552 × 10−10 | 14.94% |
| AP | 281.5 | 482.8 | 201.3 | 10.604 × 10−10 | 18.26% |
| m/z = 32, O2 | |||||
| TAG-Ni/AP | 270.8 | 488.9 | 218.1 | 7.291 × 10−10 | 15.55% |
| TAG-Co/AP | 266.4 | 346.3 | 79.9 | 9.146 × 10−10 | 9.15% |
| G-T-Ni/AP | 261 | 440 | 179 | 3.305 × 10−10 | 14.97% |
| G-T-Cu2/AP | 265.0 | 395.5 | 130.5 | 5.055 × 10−10 | 12.36% |
| G-T-Co/AP | 262.9 | 352.6 | 89.7 | 1.075 × 10−10 | 6.29% |
| AP | 352.8 | 491.4 | 138.6 | 7.701 × 10−10 | 13.26% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; An, T.; Gao, Y.; Lyu, J.-Y.; Tang, D.-Y.; Zhang, X.-X.; Zhao, F.; Yan, Q.-L. Gaseous Products Evolution Analyses for Catalytic Decomposition of AP by Graphene-Based Additives. Nanomaterials 2019, 9, 801. https://doi.org/10.3390/nano9050801
Chen S, An T, Gao Y, Lyu J-Y, Tang D-Y, Zhang X-X, Zhao F, Yan Q-L. Gaseous Products Evolution Analyses for Catalytic Decomposition of AP by Graphene-Based Additives. Nanomaterials. 2019; 9(5):801. https://doi.org/10.3390/nano9050801
Chicago/Turabian StyleChen, Shuwen, Ting An, Yi Gao, Jie-Yao Lyu, De-Yun Tang, Xue-Xue Zhang, Fengqi Zhao, and Qi-Long Yan. 2019. "Gaseous Products Evolution Analyses for Catalytic Decomposition of AP by Graphene-Based Additives" Nanomaterials 9, no. 5: 801. https://doi.org/10.3390/nano9050801
APA StyleChen, S., An, T., Gao, Y., Lyu, J.-Y., Tang, D.-Y., Zhang, X.-X., Zhao, F., & Yan, Q.-L. (2019). Gaseous Products Evolution Analyses for Catalytic Decomposition of AP by Graphene-Based Additives. Nanomaterials, 9(5), 801. https://doi.org/10.3390/nano9050801





