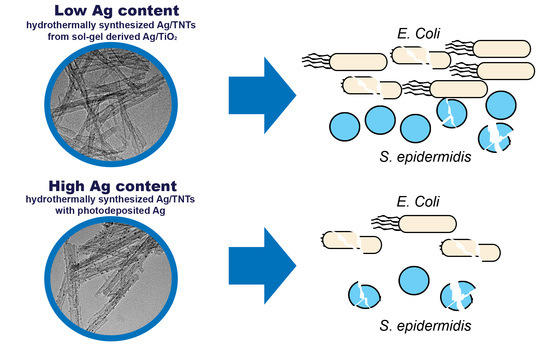
Influence of Preparation Procedure on Physicochemical and Antibacterial Properties of Titanate Nanotubes Modified with Silver
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TNTs
2.3. Preparation of Hybrid Ag/TNTs
2.3.1. Preparation of Ag/TNTs by Sol-Gel Combined with Hydrothermal Process
2.3.2. Preparation of Ag/TNTs by Photodeposition
2.3.3. Preparation of Ag/TNTs by NaBH4 Reduction
2.3.4. Preparation of Ag/TNTs by Electroless Reduction
2.4. Characterization Methods
2.5. Microbiological Study
2.5.1. Preparation of Culture Medium
2.5.2. Antibacterial Study of Nanomaterials
3. Results and Discussion
3.1. ICP Compositional Analysis
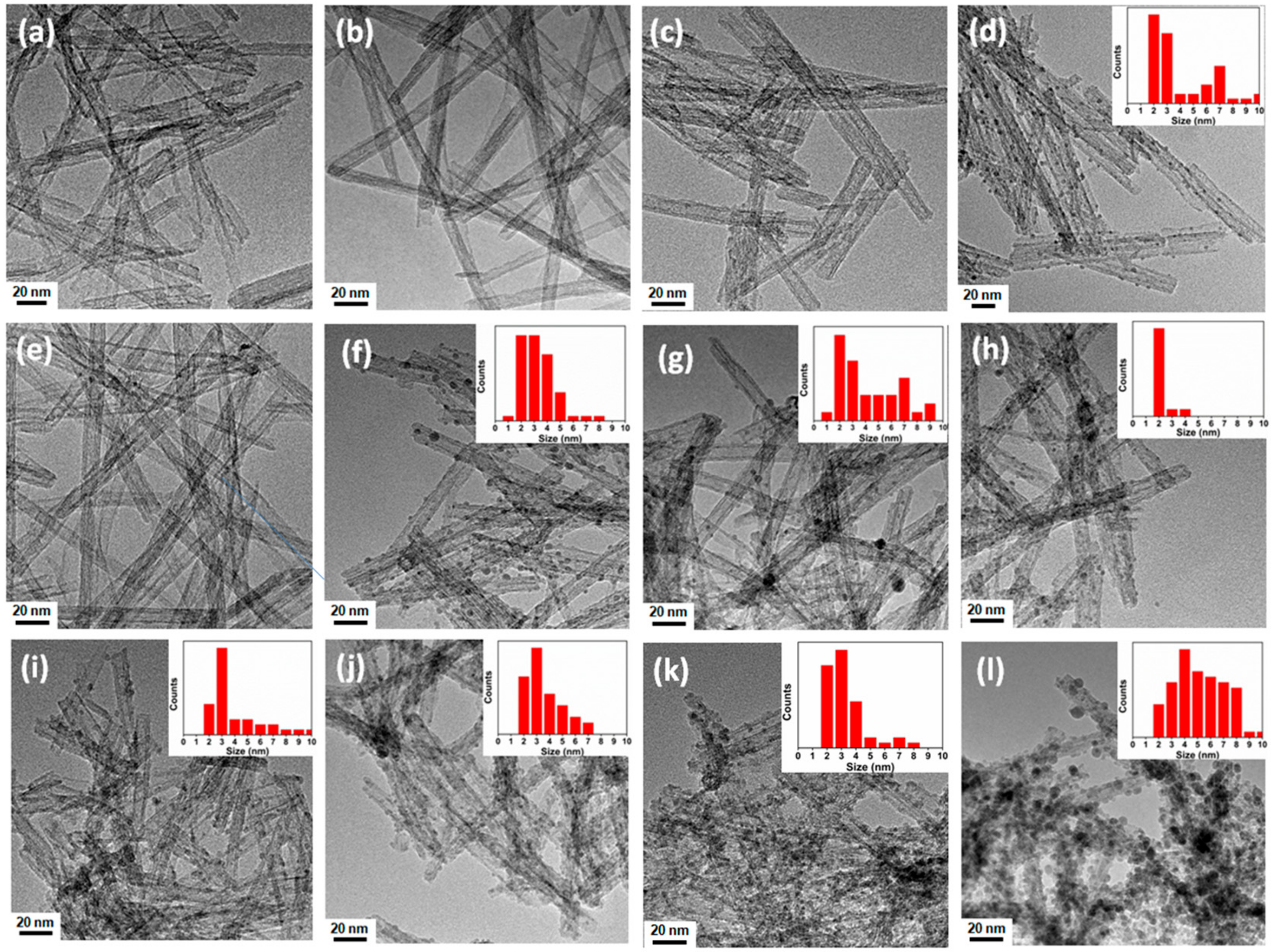
3.2. Morphological Analysis
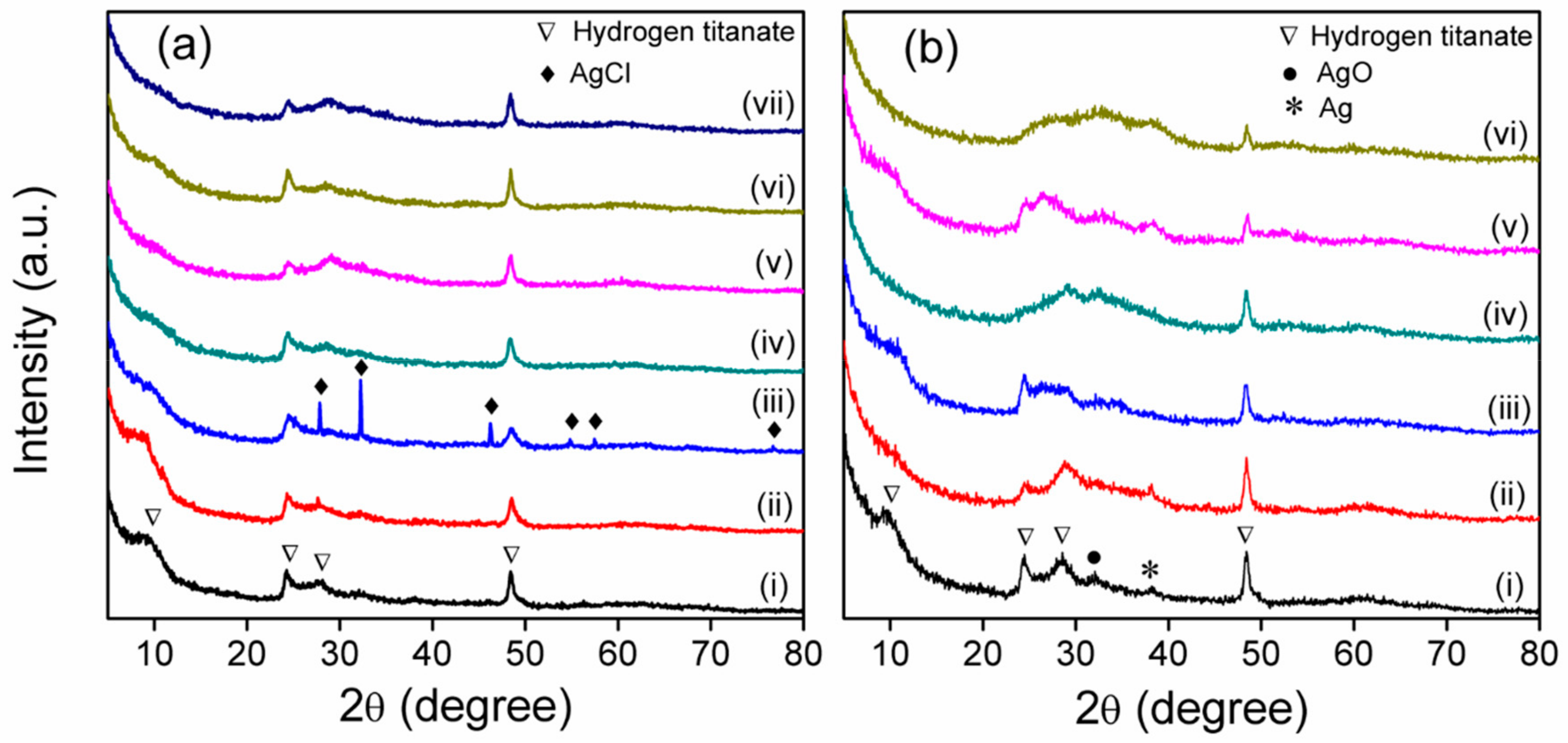
3.3. XRD Analysis
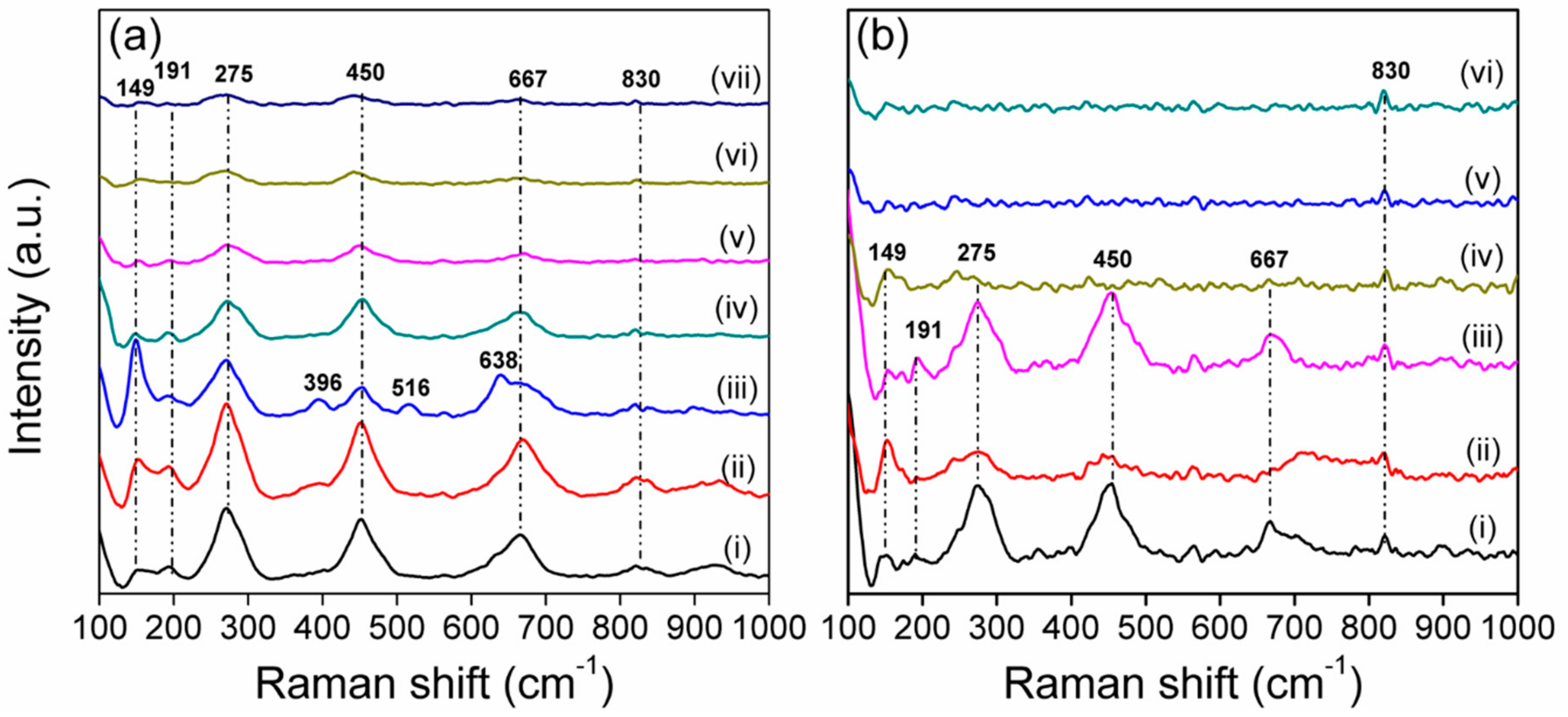
3.4. Raman Spectra Analysis
3.5. XPS Analysis
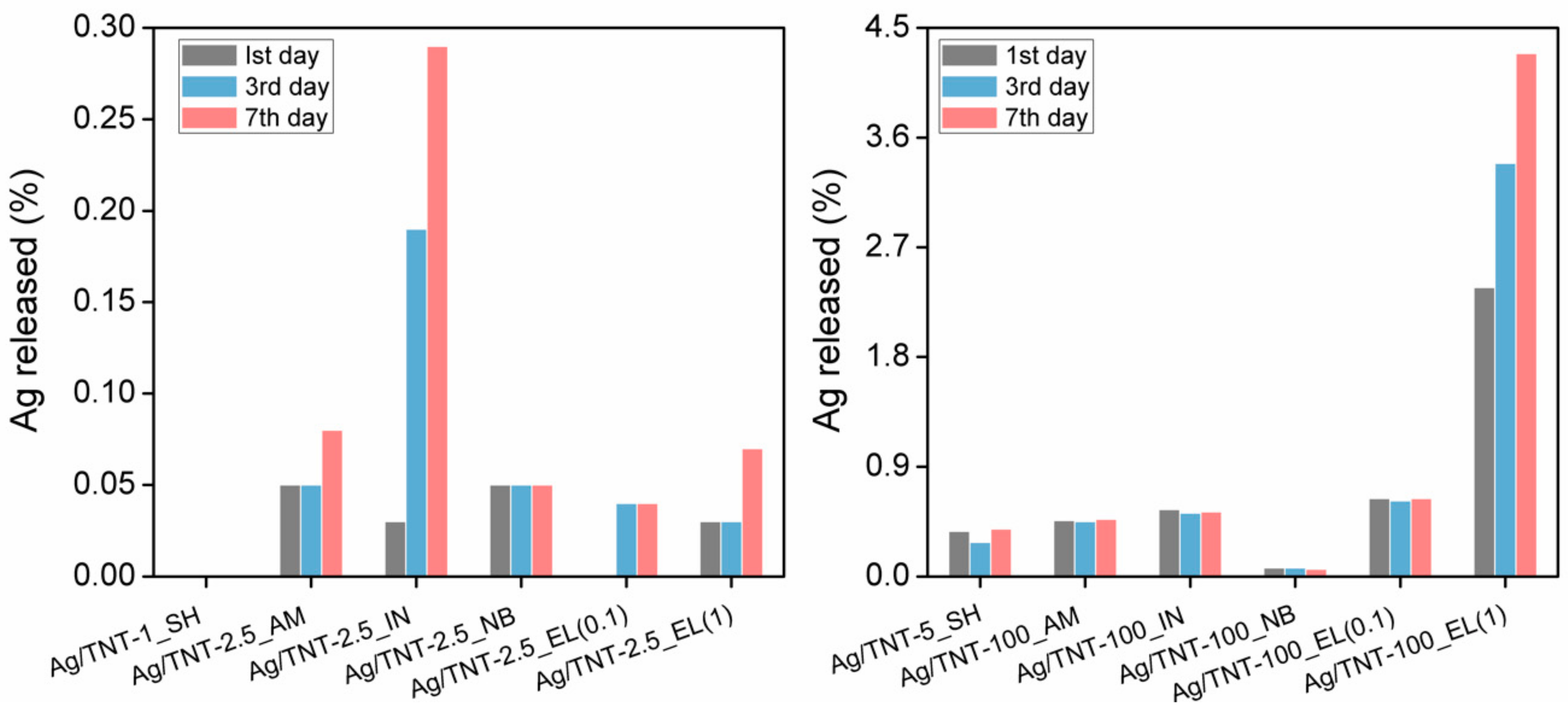
3.6. Ag+ Ion Release Measurement
3.7. Surface Charge Measurements
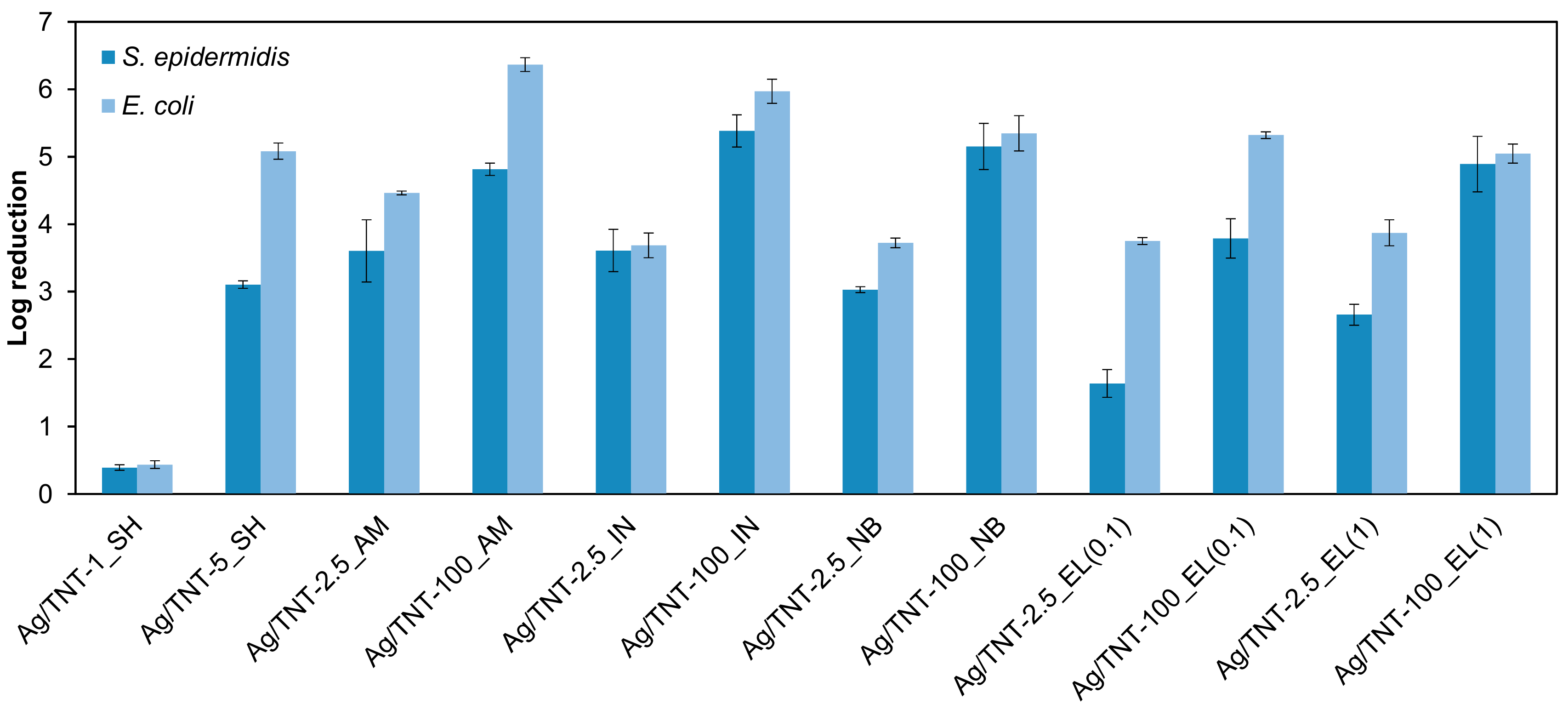
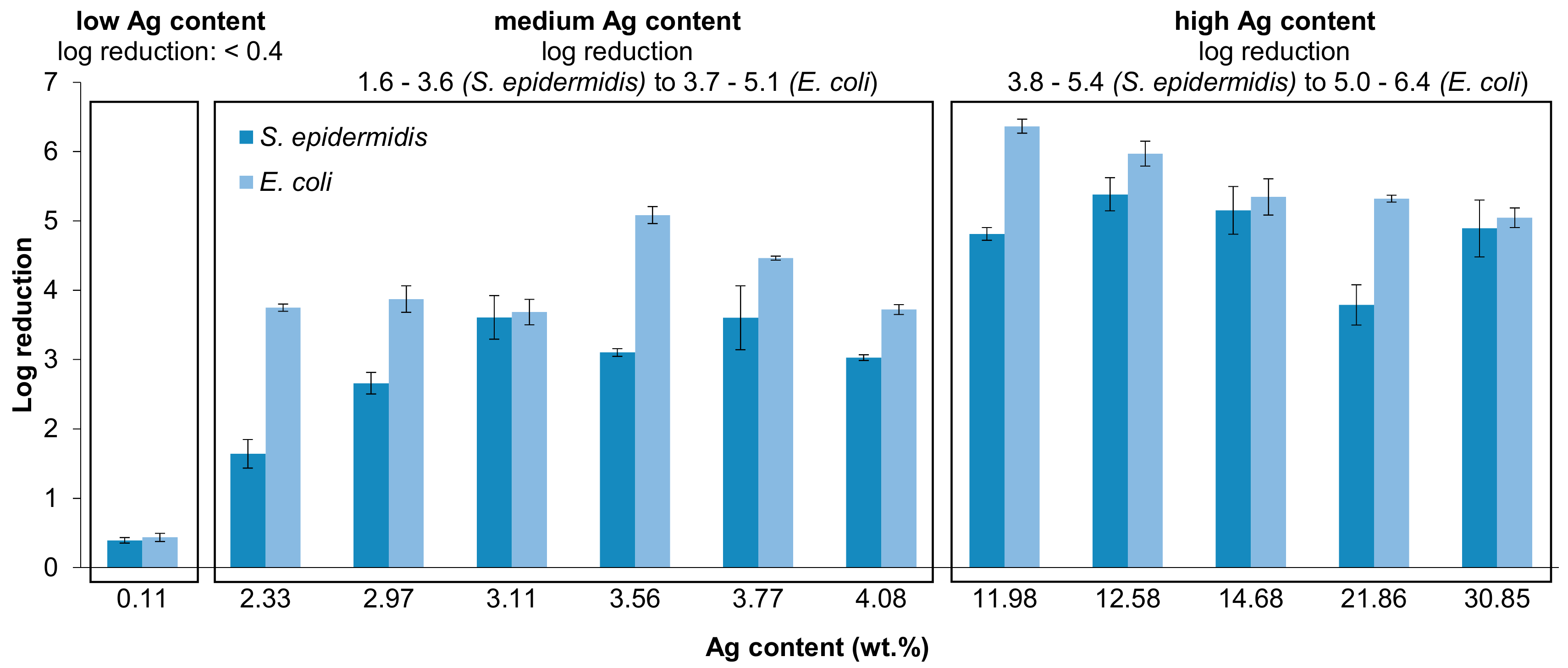
3.8. Antibacterial Properties of Ag/TNTs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, T.K.M.P.; Mandlimath, T.R.; Sangeetha, P.; Sakthivel, P.; Revathi, S.K.; Kumar, S.K.A.; Sahoo, S.K. Highly efficient performance of activated carbon impregnated with Ag, ZnO and Ag/ZnO nanoparticles as antimicrobial materials. RSC Adv. 2015, 5, 108034–108043. [Google Scholar] [CrossRef]
- Yuan, X.; Setyawati, M.I.; Leong, D.T.; Xie, J.P. Ultrasmall Ag+-rich nanoclusters as highly efficient nanoreservoirs for bacterial killing. Nano Res. 2014, 7, 301–307. [Google Scholar] [CrossRef]
- Biswas, P.; Bandyopadhyaya, R. Synergistic antibacterial activity of a combination of silver and copper nanoparticle impregnated activated carbon for water disinfection, Environmental Science. Nano 2017, 4, 2405–2417. [Google Scholar]
- Silver, S.; Phung, L.T.; Silver, G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J. Ind. Microbiol. Biotechnol. 2006, 33, 627–634. [Google Scholar] [CrossRef]
- Benn, T.M.; Westerhoff, P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Loher, S.; Schneider, O.D.; Maienfisch, T.; Bokorny, S.; Stark, W.J. Micro-organism-triggered release of silver nanoparticles from biodegradable oxide carriers allows preparation of self-sterilizing polymer surfaces. Small 2008, 4, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Weis, T.L.; Schurr, M.J.; Faith, N.G.; Czuprynski, C.J.; McAnulty, J.F.; Murphy, C.J.; Abbott, N.L. Surfaces modified with nanometer-thick silver-impregnated polymeric films that kill bacteria but support growth of mammalian cells. Biomaterials 2010, 31, 680–690. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Meng, F.; Chu, P.K. Biological actions of silver nanoparticles embedded in titanium controlled by micro-galvanic effects. Biomaterials 2011, 32, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Muñoz, R.; Borrego, B.; Juárez-Moreno, K.; García-García, M.; Morales, J.D.M.; Bogdanchikova, N.; Huerta-Saquero, A. Toxicity of silver nanoparticles in biological systems: Does the complexity of biological systems matter? Toxicol. Lett. 2017, 276, 11–20. [Google Scholar] [CrossRef]
- Bélteky, P.; Rónavári, A.; Igaz, N.; Szerencsés, B.; Tóth, I.Y.; Pfeiffer, I.; Kiricsi, M.; Kónya, Z. Silver nanoparticles: Aggregation behavior in biorelevant conditions and its impact on biological activity. Int. J. Nanomed. 2019, 14, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Moritz, M.; Geszke-Moritz, M. The newest achievements in synthesis, immobilization and practical applications of antibacterial nanoparticles. Chem. Eng. J. 2013, 228, 596–613. [Google Scholar] [CrossRef]
- Liu, S.X.; Qu, Z.P.; Han, X.W.; Sun, C.L. A mechanism for enhanced photocatalytic activity of silver-loaded titanium dioxide. Catal. Today 2004, 93, 877–884. [Google Scholar] [CrossRef]
- Li, M.; Noriega-Trevino, M.E.; Nino-Martinez, N.; Marambio-Jones, C.; Wang, J.; Damoiseaux, R.; Ruiz, F.; Hoek, E.M.V. Synergistic bactericidal activity Ag-TiO2 nanoparticles in both light and dark conditions. Environ. Sci. Technol. 2011, 45, 8989–8995. [Google Scholar] [CrossRef]
- Wei, L.; Wang, H.; Wang, Z.; Yu, M.; Chen, S. Preparation and long-term antibacterial activity of TiO2 nanotubes loaded with Ag nanoparticles and Ag ions. RSC Adv. 2015, 5, 74347–74352. [Google Scholar] [CrossRef]
- Yang, D.; Yang, N.; Ge, J. Controlled deposition of ultra-small Ag particles on TiO2 nanorods: Oxide/metal hetero-nanostructures with improved catalytic activity. CrystEngComm 2013, 15, 7230–7235. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Domínguez-Espíndola, R.B.; Casas-Flores, S.; Patrón-Soberano, O.A.; Camposeco-Solis, R.; Lee, S.-W. Antifungal nanocomposites inspired by titanate nanotubes for complete inactivation of Botrytis cinerea isolated from tomato infection. ACS Appl. Mater. Interfaces 2016, 8, 31625–31637. [Google Scholar] [CrossRef]
- Wang, H.; Wei, L.; Wang, Z.; Chen, S. Preparation, characterization and long-term antibacterial activity of Ag–poly (dopamine)–TiO2 nanotube composites. RSC Adv. 2016, 6, 14097–14104. [Google Scholar] [CrossRef]
- Martinez-Castanon, G.A.; Nino-Martinez, N.; Martinez-Gutierrez, F.; Martinez-Mendoza, J.R.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanoparticle Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346. [Google Scholar] [CrossRef]
- Kubacka, A.; Ferrer, M.; Martínez-Arias, A.; Fernández-García, M. Ag promotion of TiO2-anatase disinfection capability: Study of Escherichia coli inactivation. Appl. Catal. B Environ. 2008, 84, 87–93. [Google Scholar] [CrossRef]
- Es-Souni, M.; Fischer-Brandies, H.; Es-Souni, M. Versatile nanocomposite coatings with tunable cell adhesion and bactericidity. Adv. Funct. Mater. 2008, 18, 3179–3188. [Google Scholar] [CrossRef]
- Keleher, J.; Bashant, J.; Heldt, N.; Johnson, L.; Li, Y. Photo-catalytic preparation of silver-coated TiO2 particles for antibacterial applications. World J. Microbiol. Biotechnol. 2002, 18, 133–139. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Meyer, A.; Knijnenburg, J.T.N.; Panke, S.; Pratsinis, S.E. Quantifying the origin of released Ag+ ions from nanosilver. Langmuir 2012, 28, 15929–15936. [Google Scholar] [CrossRef]
- Rónavári, A.; Kovács, D.; Vágvölgyi, C.; Kónya, Z.; Kiricsi, M.; Pfeiffer, I. Ion exchange defines the biological activity of titanate nanotubes. J. Basic Microbiol. 2016, 56, 557–565. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.-M. Tunable optical properties of Ag–TiO2 nanorod composites based on interparticle plasmon coupling. Nano 2016, 11, 1650110. [Google Scholar] [CrossRef]
- Seery, M.K.; George, R.; Floris, P.; Pillai, S C. Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J. Photochem. Photobiol. A Chem. 2007, 189, 258–263. [Google Scholar] [CrossRef]
- Priya, R.; Baiju, K.V.; Shukla, S.; Biju, S.; Reddy, M.L.P.; Patil, K.; Warrier, K.G.K. Comparing ultraviolet and chemical reduction techniques for enhancing photocatalytic activity of silver oxide/silver deposited nanocrystalline anatase titania. J. Phys. Chem. C 2009, 113, 6243–6255. [Google Scholar] [CrossRef]
- Ye, J.; Cheng, H.; Li, H.; Yang, Y.; Zhang, S.; Rauf, A.; Zhao, Q.; Ning, G. Highly synergistic antimicrobial activity of spherical and flower-like hierarchical titanium dioxide/silver composites. J. Colloid Interface Sci. 2017, 504, 448–456. [Google Scholar] [CrossRef]
- Zhao, C.; Feng, B.; Li, Y.; Tan, J.; Lu, X.; Weng, J. Preparation and antibacterial activity of titanium nanotubes loaded with Ag nanoparticles in the dark and under the UV light. Appl. Surf. Sci. 2013, 280, 8–14. [Google Scholar] [CrossRef]
- Ma, R.; Sasaki, T.; Bando, Y. Layer-by-Layer Assembled multilayer films of titanate nanotubes, Ag-or Au-loaded nanotubes, and nanotubes/nanosheets with polycations. J. Am. Chem. Soc. 2004, 126, 10382–10388. [Google Scholar] [CrossRef]
- Cesano, F.; Bertarione, S.; Uddin, M.J.; Agostini, G.; Scarano, D.; Zecchina, A. Designing TiO2 based nanostructures by control of surface morphology of pure and silver loaded titanate nanotubes. J. Phys. Chem. C 2009, 114, 169–178. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Obregón-Alfaro, S.; Lozano-Sánchez, L.M.; Lee, S.-W. Rapid microwave-assisted synthesis of one-dimensional silver–H2Ti3O7 nanotubes. J. Mol. Catal. A Chem. 2012, 353, 163–170. [Google Scholar] [CrossRef]
- Yee, M.S.-L.; Khiew, P.S.; Lim, S.S.; Chiu, W.S.; Tan, Y.F.; Kok, Y.-Y.; Leong, C.-O. Enhanced marine antifouling performance of silver-titania nanotube composites from hydrothermal processing. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 520, 701–711. [Google Scholar] [CrossRef]
- Plodinec, M.; Gajović, A.; Jakša, G.; Žagar, K.; Čeh, M. High-temperature hydrogenation of pure and silver-decorated titanate nanotubes to increase their solar absorbance for photocatalytic applications. J. Alloys Compd. 2014, 591, 147–155. [Google Scholar] [CrossRef]
- Mozia, S.; Jose, M.; Sienkiewicz, P.; Szymanski, K.; Darowna, D.; Zgrzebnicki, M.; Markowska-Szczupak, A. Polyethersulfone ultrafiltration membranes modified with hybrid Ag/titanate nanotubes: Physicochemical characteristics, antimicrobial properties, and fouling resistance. Desalin. Water Treat 2018, 128, 106–118. [Google Scholar] [CrossRef]
- Miao, L.; Ina, Y.; Tanemura, S.; Jiang, T.; Tanemura, M.; Kaneko, K.; Toh, S.; Mori, Y. Fabrication and photochromic study of titanate nanotubes loaded with silver nanoparticles. Surf. Sci. 2007, 601, 2792–2799. [Google Scholar] [CrossRef]
- Chan, S.C.; Barteau, M.A. Preparation of highly uniform Ag/TiO2 and Au/TiO2 supported nanoparticle catalysts by photodeposition. Langmuir 2005, 21, 5588–5595. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Comparelli, R.; Fanizza, E.; Curri, M.L.; Agostiano, A.; Laub, D. Photocatalytic synthesis of silver nanoparticles stabilized by TiO2 nanorods: A semiconductor/metal nanocomposite in homogeneous nonpolar solution. J. Am. Chem. Soc. 2004, 126, 3868–3879. [Google Scholar] [CrossRef]
- Lai, Y.; Chen, Y.; Zhuang, H.; Lin, C. A facile method for synthesis of Ag/TiO2 nanostructures. Mater. Lett. 2008, 62, 3688–3690. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Lapkin, A.A.; Plucinski, P.K.; Torrente-Murciano, L.; Friedrich, J.M.; Walsh, F.C. Deposition of Pt, Pd, Ru and Au on the surfaces of titanate nanotubes. Top. Catal. 2006, 39, 151–160. [Google Scholar] [CrossRef]
- Kitano, M.; Wada, E.; Nakajima, K.; Hayashi, S.; Miyazaki, S.; Kobayashi, H.; Hara, M. Protonated titanate nanotubes with Lewis and Brønsted acidity: Relationship between nanotube structure and catalytic activity. Chem. Mater. 2013, 25, 385–393. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, W.; Du, G.H.; Peng, L. Trititanate nanotubes made via a single alkali treatment. Adv. Mater. 2002, 14, 1208–1211. [Google Scholar] [CrossRef]
- Yang, D.; Liu, H.; Liu, L.; Sarina, S.; Zheng, Z.; Zhu, H. Silver oxide nanocrystals anchored on titanate nanotubes and nanofibers: Promising candidates for entrapment of radioactive iodine anions. Nanoscale 2013, 5, 11011–11018. [Google Scholar] [CrossRef]
- Huang, J.; Ding, L.; Xi, Y.; Shi, L.; Su, G.; Gao, R.; Wang, W.; Dong, B.; Cao, L. Efficient silver modification of TiO2 nanotubes with enhanced photocatalytic activity. Solid State Sci. 2018, 80, 116–122. [Google Scholar] [CrossRef]
- Hodos, M.; Horváth, E.; Haspel, H.; Kukovecz, Á.; Kónya, Z.; Kiricsi, I. Photosensitization of ion-exchangeable titanate nanotubes by CdS nanoparticles. Chem. Phys. Lett. 2004, 399, 512–515. [Google Scholar] [CrossRef]
- Gajović, A.; Friščić, I.; Plodinec, M.; Iveković, D. High temperature Raman spectroscopy of titanate nanotubes. J. Mol. Struct. 2009, 924, 183–191. [Google Scholar] [CrossRef]
- Kukovecz, Á.; Kordás, K.; Kiss, J.; Kónya, Z. Atomic scale characterization and surface chemistry of metal modified titanate nanotubes and nanowires. Surf. Sci. Rep. 2016, 71, 473–546. [Google Scholar] [CrossRef]
- Qian, L.; Du, Z.-L.; Yang, S.-Y.; Jin, Z.-S. Raman study of titania nanotube by soft chemical process. J. Mol. Struct. 2005, 749, 103–107. [Google Scholar] [CrossRef]
- Mozia, S.; Borowiak-Paleń, E.; Przepiórski, J.; Grzmil, B.; Tsumura, T.; Toyoda, M.; Grzechulska-Damszel, J.; Morawski, A.W. Physico-chemical properties and possible photocatalytic applications of titanate nanotubes synthesized via hydrothermal method. J. Phys. Chem. Solids 2010, 71, 263–272. [Google Scholar] [CrossRef]
- Sallem, F.; Chassagnon, R.; Megriche, A.; El Maaoui, M.; Millot, N. Effect of mechanical stirring and temperature on dynamic hydrothermal synthesis of titanate nanotubes. J. Alloys Compd. 2017, 722, 785–796. [Google Scholar] [CrossRef]
- Ren, N.; Li, R.; Chen, L.; Wang, G.; Liu, D.; Wang, Y.; Zheng, L.; Tang, W.; Yu, X.; Jiang, H.; et al. In situ construction of a titanate–silver nanoparticle–titanate sandwich nanostructure on a metallic titanium surface for bacteriostatic and biocompatible implants. J. Mater. Chem. 2012, 22, 19151–19160. [Google Scholar] [CrossRef]
- Byeon, S.-H.; Lee, S.-O.; Kim, H. Structure and Raman spectra of layered titanium oxides. J. Solid State Chem. 1997, 130, 110–116. [Google Scholar] [CrossRef]
- Pótári, G.; Madarász, D.; Nagy, L.; László, B.; Sápi, A.; Oszkó, A.; Kukovecz, A.; Erdőhelyi, A.; Kónya, Z.; Kiss, J. Rh-Induced support transformation phenomena in titanate nanowire and nanotube catalysts. Langmuir 2013, 29, 3061–3072. [Google Scholar] [CrossRef]
- Bensebaa, F.; Zavaliche, F.; L‘Ecuyer, P.; Cochrane, R.W.; Veres, T. Microwave synthesis and characterization of Co–ferrite nanoparticles. J. Colloid Interface Sci. 2004, 277, 104–110. [Google Scholar] [CrossRef]
- Gilbert, J.B.; Rubner, M.F.; Cohen, R.E. Depth-profiling X-ray photoelectron spectroscopy (XPS) analysis of interlayer diffusion in polyelectrolyte multilayers. Proc. Natl. Acad. Sci. USA 2013, 110, 6651–6656. [Google Scholar] [CrossRef]
- Moulder, J.F. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corp.: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Amiri, O.; Salavati-Niasari, M.; Farangi, M.; Mazaheri, M.; Bagheri, S. Stable plasmonic-improved dye sensitized solar cells by silver nanoparticles between titanium dioxide layers. Electrochim. Acta 2015, 152, 101–107. [Google Scholar] [CrossRef]
- Hanaor, D.; Michelazzi, M.; Leonelli, C.; Sorrell, C.C. The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. J. Eur. Ceram. Soc. 2012, 32, 235–244. [Google Scholar] [CrossRef]
- Torres Sánchez, R.M.; Tavani, E.L. Temperature effects on the point of zero charge and isoelectric point of a red soil rich in kaolinite and iron minerals. J. Therm. Anal. 1994, 41, 1129–1139. [Google Scholar] [CrossRef]
- Kłodzińska, E.; Szumski, M.; Dziubakiewicz, E.; Hrynkiewicz, K.; Skwarek, E.; Janusz, W.; Buszewski, B. Effect of zeta potential value on bacterial behavior during electrophoretic separation. Electrophoresis 2010, 31, 1590–1596. [Google Scholar] [CrossRef]
- Nuryastuti, T.; van der Mei, H.C.; Busscher, H.J.; Kuijer, R.; Aman, A.T.; Krom, B.P. recA mediated spontaneous deletions of the icaADBC operon of clinical Staphylococcus epidermidis isolates: A new mechanism of phenotypic variations. Antonie van Leeuwenhoek 2008, 94, 317–328. [Google Scholar] [CrossRef][Green Version]
- Di Paola, A.; Garcı́a-López, E.; Marcı̀, G.; Martı́n, C.; Palmisano, L.; Rives, V.; Maria Venezia, A. Surface characterisation of metal ions loaded TiO2 photocatalysts: Structure—Activity relationship. Appl. Catal. B Environ. 2004, 48, 223–233. [Google Scholar] [CrossRef]
- Klitzke, S.; Metreveli, G.; Peters, A.; Schaumann, G.E.; Lang, F. The fate of silver nanoparticles in soil solution—Sorption of solutes and aggregation. Sci. Total Environ. 2015, 535, 54–60. [Google Scholar] [CrossRef]
- Baron, S. Medical Microbiology; University of Texas Medical Branch: Galveston, TX, USA, 1996. [Google Scholar]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef]
- Amato, E.; Diaz-Fernandez, Y.A.; Taglietti, A.; Pallavicini, P.; Pasotti, L.; Cucca, L.; Milanese, C.; Grisoli, P.; Dacarro, C.; Fernandez-Hechavarria, J.M.; et al. Synthesis, characterization and antibacterial activity against gram positive and gram negative bacteria of biomimetically coated silver nanoparticles. Langmuir 2011, 27, 9165–9173. [Google Scholar] [CrossRef]
- Hatchett, D.W.; White, H.S. Electrochemistry of sulfur adlayers on the low-index faces of silver. J. Phys. Chem. 1996, 100, 9854–9859. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G. Potent antibacterial activities of Ag/TiO2 nanocomposite powders synthesized by a one-pot sol-gel method. Environ. Sci. Technol. 2009, 43, 2905–2910. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Kakinen, A.; Titma, T.; Heinlaan, M.; et al. Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS ONE 2014, 9, 1–14. [Google Scholar] [CrossRef]
- Vidhu, V.K.; Philip, D. Biogenic synthesis of SnO2 nanoparticles: Evaluation of antibacterial and antioxidant activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 372–379. [Google Scholar] [CrossRef]
- Kumar Nair, K.; Kumar, P.; Kumar, V.; Harris, R.A.; Kroon, R.E.; Viljoen, B.; Shumbula, P.M.; Mlambo, M.; Swart, H.C. Synthesis and evaluation of optical and antimicrobial properties of Ag-SnO2 nanocomposites. Phys. B Condens. Matter. 2018, 535, 338–343. [Google Scholar] [CrossRef]
- Choi, O.; Deng, K.K.; Kim, N.J.; Ross, L.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticle, silver ions, and silver chloride colloids on microbial growth. Water. Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ahmed, A.I.; Mannaa, M.A. Structural, Photocatalytic, Biological and catalytic properties of SnO2/TiO2 nanoparticles. Ceram. Int. 2018, 44, 6201–6211. [Google Scholar] [CrossRef]
| No. | Method of Ag/TNTs Synthesis | Amount or Concentration of AgNO3 | Sample Name |
|---|---|---|---|
| 1 | Sol-gel and hydrothermal | 0.01 (Ag/Ti) | Ag/TNT-1_SH |
| 0.05 (Ag/Ti) | Ag/TNT-5_SH | ||
| 2 | Photodeposition (Ambient atmosphere) | 2.5 mM | Ag/TNT-2.5_AM |
| 100 mM | Ag/TNT-100_AM | ||
| 3 | Photodeposition (Inert atmosphere) | 2.5 mM | Ag/TNT-2.5_IN |
| 100 mM | Ag/TNT-100_IN | ||
| 4 | NaBH4 reduction | 2.5 mM | Ag/TNT-2.5_NB |
| 100 mM | Ag/TNT-100_NB | ||
| 5 | Electroless reduction (0.1 g SnCl2) | 2.5 mM | Ag/TNT-2.5_EL (0.1) |
| 100 mM | Ag/TNT-100_EL (0.1) | ||
| 6 | Electroless reduction (1 g SnCl2) | 2.5 mM | Ag/TNT-2.5_EL (1) |
| 100 mM | Ag/TNT-100_EL (1) |
| Sample Name | Ag (wt.%) | Sn (wt.%) | Sample Name | Ag (wt.%) | Sn (wt.%) |
|---|---|---|---|---|---|
| Ag/TNT-1_SH | 0.11 | - | Ag/TNT-2.5_NB | 4.08 | - |
| Ag/TNT-5_SH | 3.56 | - | Ag/TNT-100_NB | 14.68 | - |
| Ag/TNT-2.5_AM | 3.77 | - | Ag/TNT-2.5_EL (0.1) | 2.33 | 5.17 |
| Ag/TNT-100_AM | 11.98 | - | Ag/TNT-100_EL (0.1) | 21.86 | 4.67 |
| Ag/TNT-2.5_IN | 3.11 | - | Ag/TNT-2.5_EL (1) | 2.97 | 20.01 |
| Ag/TNT-100_IN | 12.58 | - | Ag/TNT-100_EL (1) | 30.85 | 14.38 |
| Element | Ti | O | C | Ag | Sn |
|---|---|---|---|---|---|
| Sample | at.% | ||||
| Ag/TNT-5_SH | 23 | 73 | 4 | traces | - |
| Ag/TNT-100_AM | 19 | 63 | 15 | 3 | - |
| Ag/TNT-100_IN | 19 | 63 | 16 | 2 | - |
| Ag/TNT-100_NB | 18 | 61 | 18 | 3 | - |
| Ag/TNT-100_EL (0.1) | 16 | 59 | 15 | 5 | 5 |
| Ag/TNT-100_EL (1) | 11 | 60 | 9 | 9 | 11 |
| Sample name | IEP | Sample name | IEP |
|---|---|---|---|
| TNTs | 3.13(0.09) | Ag/TNT-100_IN | 3.33(0.01) |
| TNTs (from sol-gel TiO2) | 3.73(0.06) | Ag/TNT-2.5_NB | 3.45(0.01) |
| Ag/TNT-1_SH | 3.89(0.04) | Ag/TNT-100_NB | 3.37(0.08) |
| Ag/TNT-5_SH | 3.68(0.01) | Ag/TNT-2.5_EL (0.1) | 3.39(0.01) |
| Ag/TNT-2.5_AM | 3.39(0.08) | Ag/TNT-100_EL (0.1) | 3.14(0.02) |
| Ag/TNT-100_AM | 3.29(0.04) | Ag/TNT-2.5_EL (1) | 3.69(0.03) |
| Ag/TNT-2.5_IN | 3.38(0.03) | Ag/TNT-100_EL (1) | 3.27(0.02) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jose, M.; Sienkiewicz, P.; Szymańska, K.; Darowna, D.; Moszyński, D.; Lendzion-Bieluń, Z.; Szymański, K.; Mozia, S. Influence of Preparation Procedure on Physicochemical and Antibacterial Properties of Titanate Nanotubes Modified with Silver. Nanomaterials 2019, 9, 795. https://doi.org/10.3390/nano9050795
Jose M, Sienkiewicz P, Szymańska K, Darowna D, Moszyński D, Lendzion-Bieluń Z, Szymański K, Mozia S. Influence of Preparation Procedure on Physicochemical and Antibacterial Properties of Titanate Nanotubes Modified with Silver. Nanomaterials. 2019; 9(5):795. https://doi.org/10.3390/nano9050795
Chicago/Turabian StyleJose, Manu, Paulina Sienkiewicz, Karolina Szymańska, Dominika Darowna, Dariusz Moszyński, Zofia Lendzion-Bieluń, Kacper Szymański, and Sylwia Mozia. 2019. "Influence of Preparation Procedure on Physicochemical and Antibacterial Properties of Titanate Nanotubes Modified with Silver" Nanomaterials 9, no. 5: 795. https://doi.org/10.3390/nano9050795
APA StyleJose, M., Sienkiewicz, P., Szymańska, K., Darowna, D., Moszyński, D., Lendzion-Bieluń, Z., Szymański, K., & Mozia, S. (2019). Influence of Preparation Procedure on Physicochemical and Antibacterial Properties of Titanate Nanotubes Modified with Silver. Nanomaterials, 9(5), 795. https://doi.org/10.3390/nano9050795