Effects of Different TiO2 Particle Sizes on the Microstructure and Optical Limiting Properties of TiO2/Reduced Graphene Oxide Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments and Measurements
2.3. Synthesis of Graphene Oxide (GO)
2.4. Synthesis of Tetrabutyl Titanate (TBT)-TiO2/rGO Nanocomposites
2.5. Synthesis of P25-TiO2/rGO Nanocomposites
3. Results and Discussion
3.1. Characterization of TiO2/rGO Nanocomposites
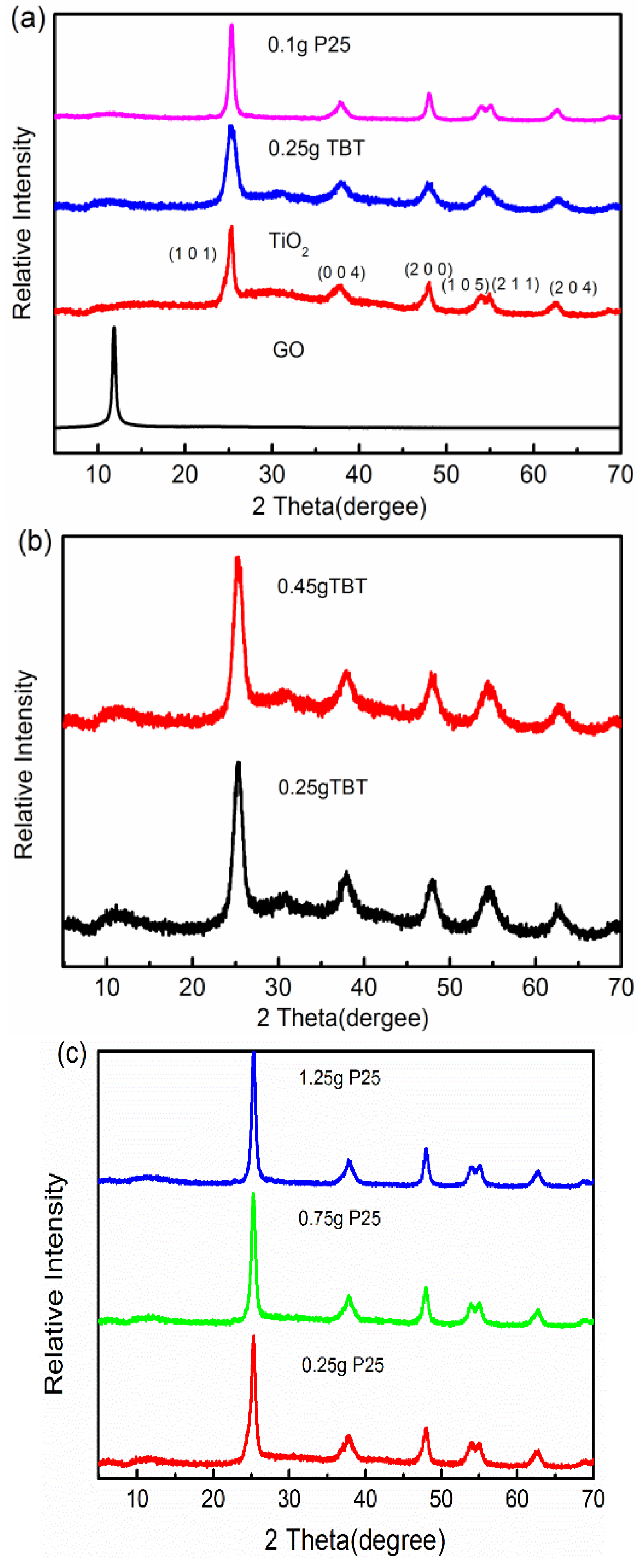
3.1.1. X-ray Powder Diffraction (XRD) Studies
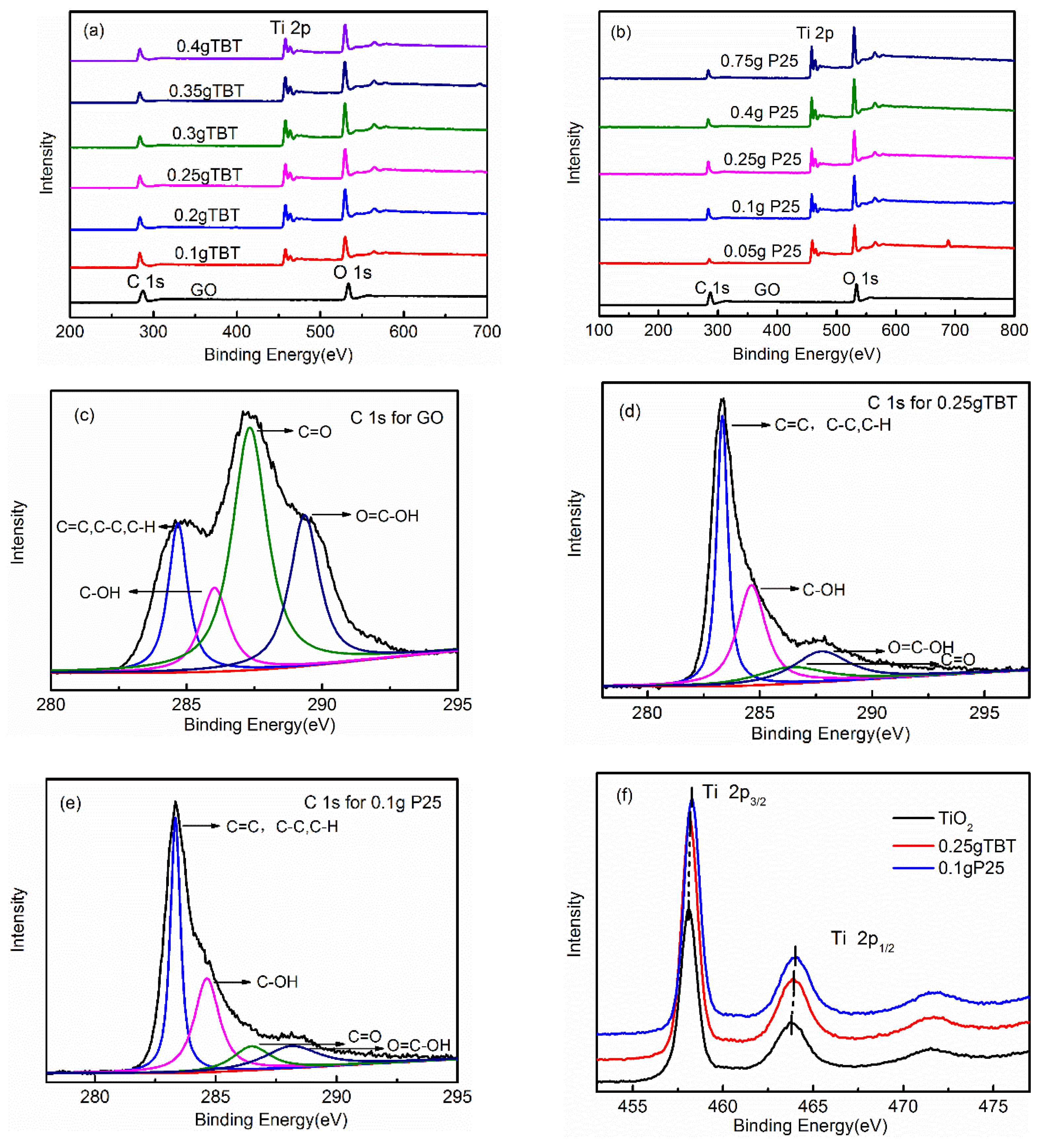
3.1.2. X-ray Photoelectron Spectroscopy (XPS) Spectra Analysis
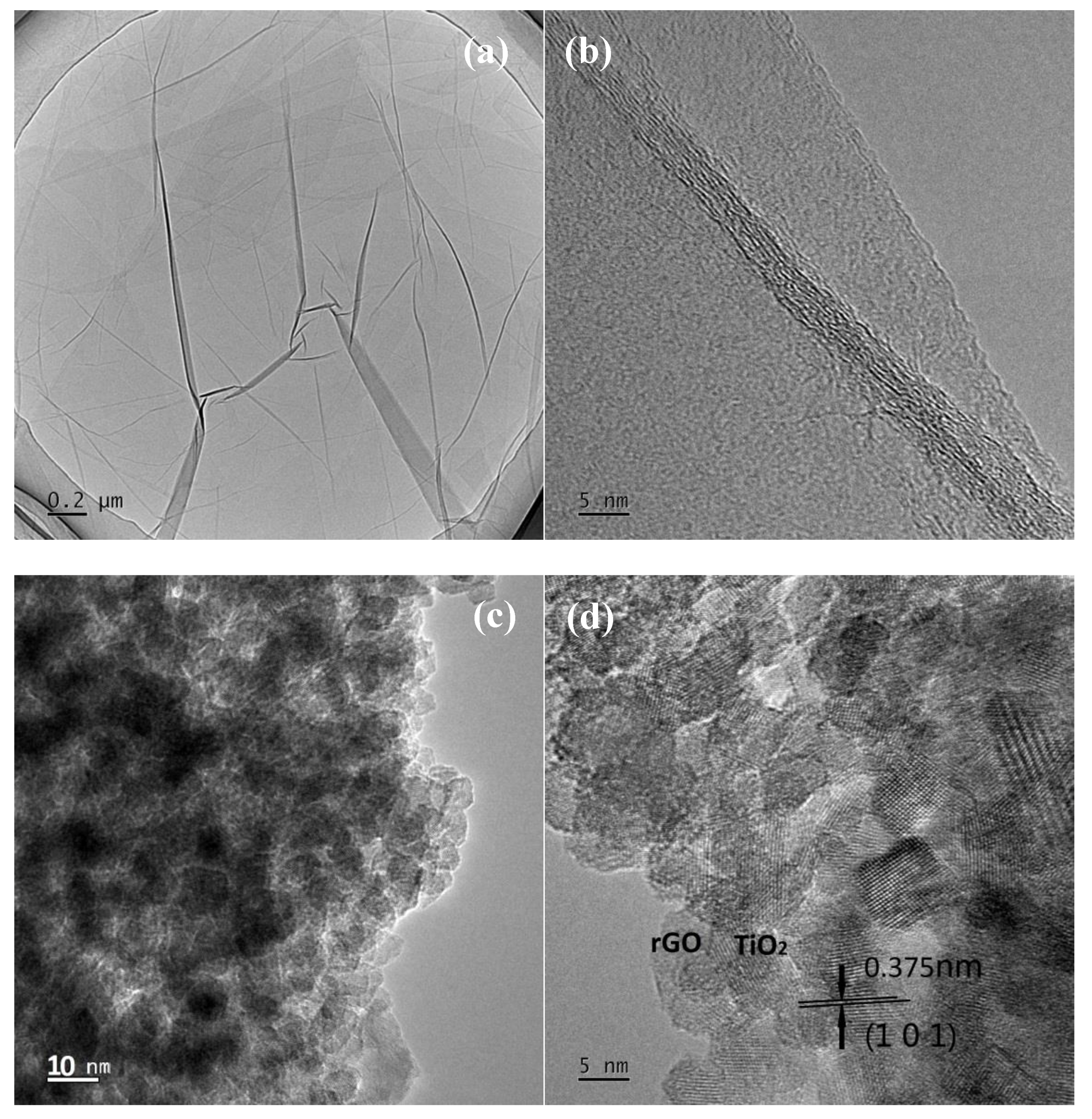
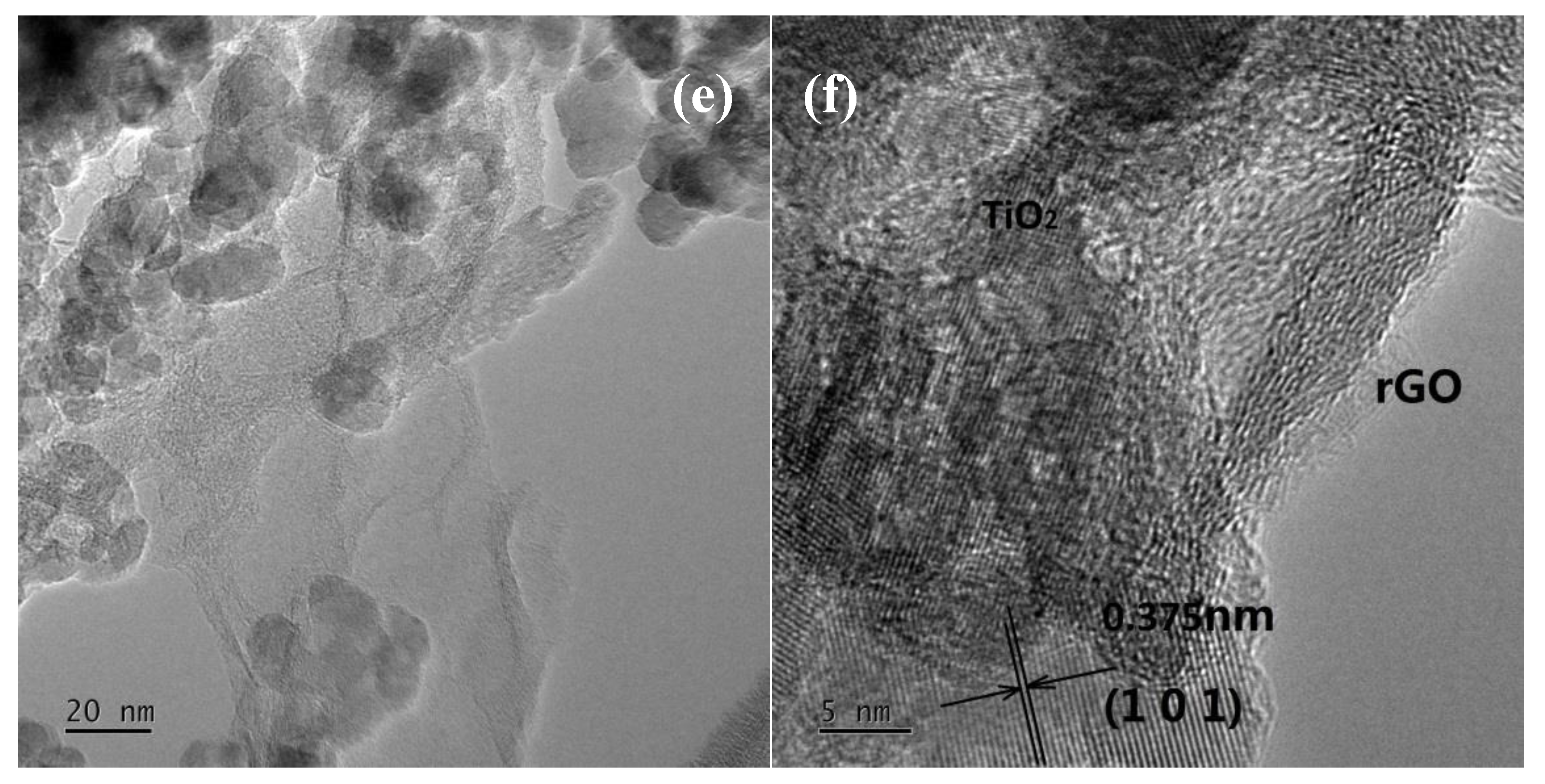
3.1.3. Transmission Electron Microscopy (TEM) Studies
3.1.4. Fourier-Transform Infrared (FTIR) Spectra Analysis
3.1.5. Raman Spectra Analysis
3.1.6. UV-Vis Absorption Spectra Analysis
3.2. Characterization of Nonlinear Optical (NLO) and Optical Limiting (OL) Performaces
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, C.R.; Xiao, J.; Yang, G.W. Giant nonlinear optical responses of carbyne. J. Mater. Chem. C 2016, 4, 4692–4698. [Google Scholar] [CrossRef]
- Dissanayake, D.; Cifuentes, M.P.; Humphrey, M.G. Optical limiting properties of (reduced) graphene oxide covalently functionalized by coordination complexes. Coord. Chem. Rev. 2018, 375, 489–513. [Google Scholar] [CrossRef]
- Muruganandi, G.; Saravanan, M.; Vinitha, G.; Raj, M.S.J.; Girisun, T.C.S. Barium borate nanorod decorated reduced graphene oxide for optical power limiting applications. Opt. Mater. 2018, 75, 612–618. [Google Scholar] [CrossRef]
- Saravanan, M.; Girisun, T.C.S.; Vinitha, G. Facile hydrothermal synthesis of CdFe2O4-reduced graphene oxide nanocomposites and their third-order nonlinear optical properties under CW excitation. J. Mol. Liq. 2018, 256, 519–526. [Google Scholar]
- Yu, Y.; Yan, L.; Yue, M.; Xu, H. Femtosecond laser-assisted synthesis of silver nanoparticles and reduced graphene oxide hybrid for optical limiting. R. Soc. Open Sci. 2018, 5, 171436. [Google Scholar] [CrossRef]
- Krivenkov, R.Y.; Mogileva, T.N.; Mikheev, K.G.; Okotrub, A.V.; Mikheev, G.M. Heat-Induced Dip of Optical Limiting Threshold in Carbon Nanotube Aqueous. J. Phys. Chem. C 2018, 122, 16339–16345. [Google Scholar] [CrossRef]
- Chen, K.; Su, W.; Wang, Y.; Ge, H.; Zhang, K.; Wang, Y.; Xie, X.; Gomes, V.G.; Sun, H.; Huang, L. Nanocomposites of carbon nanotubes and photon upconversion nanoparticles for enhanced optical limiting performance. J. Mater. Chem. C 2018, 6, 7311–7316. [Google Scholar] [CrossRef]
- Chantharasupawong, P.; Philip, R.; Endo, T.; Thomas, J. Enhanced optical limiting in nanosized mixed zinc ferrites. Appl. Phys. Lett. 2012, 100, 221108. [Google Scholar] [CrossRef]
- Philip, R.; Chantharasupawong, P.; Qian, H.; Jin, R.; Thomas, J. Evolution of Nonlinear Optical Properties: From Gold Atomic Clusters to Plasmonic Nanocrystals. Nano Lett. 2012, 12, 4661–4667. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, T.N.; Vaz, M.D.O.; Ramakrishnappa, T.; Teixeira, S.R.; Dupont, J. Ionic liquid assisted hydrothermal syntheses of Au doped TiO2 NPs for efficient visible-light photocatalytic hydrogen production from water, electrochemical detection and photochemical detoxification of hexavalent chromium (Cr6+). RSC Adv. 2017, 7, 43233–43244. [Google Scholar] [CrossRef]
- Zhao, M.; Peng, R.; Zheng, Q.; Wang, Q.; Chang, M.-J.; Liu, Y.; Song, Y.-L.; Zhang, H.-L. Broadband optical limiting response of a graphene-PbS nanohybrid. Nanoscale 2015, 7, 9268–9274. [Google Scholar] [CrossRef]
- Wu, J.-J.; Tao, Y.-R.; Fan, L.; Wu, Z.-Y.; Wu, X.-C.; Chun, Y. Visible light nonlinear absorption and optical limiting of ultrathin ZrSe3 nanoflakes. Nanotechnology 2016, 27, 465203. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, F.; Cao, Y.; Wang, C.; Wang, J.; Gu, Y. Nonlinear optical enhancement induced by synergistic effect of graphene nanosheets and CdS nanocrystals. Appl. Phys. Lett. 2016, 108, 252106. [Google Scholar] [CrossRef]
- Solati, E.; Dorranian, D. Nonlinear optical properties of the mixture of ZnO nanoparticles and graphene nanosheets. Appl. Phys. B-Lasers Opt. 2016, 122, 76. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene Oxide, Highly Reduced Graphene Oxide, and Graphene: Versatile Building Blocks for Carbon-Based Materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Lu, Y.H.; Chen, W.; Feng, Y.P.; He, P.M. Tuning the Electronic Structure of Graphene by an Organic Molecule. J. Phys. Chem. B 2009, 113, 2–5. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Zhang, X.; Xu, Y.; Chen, Y.; Tian, J. Nonlinear optical properties of graphene oxide in nanosecond and picosecond regimes. Appl. Phys. Lett. 2009, 94, 021902. [Google Scholar] [CrossRef]
- Zheng, X.; Feng, M.; Zhan, H. Giant optical limiting effect in Ormosil gel glasses doped with graphene oxide materials. J. Mater. Chem. C 2013, 1, 6759–6766. [Google Scholar] [CrossRef]
- Lim, G.-K.; Chen, Z.-L.; Clark, J.; Goh, R.G.S.; Ng, W.-H.; Tan, H.-W.; Friend, R.H.; Ho, P.K.H.; Chua, L.-L. Giant broadband nonlinear optical absorption response in dispersed graphene single sheets. Nat. Photonics 2011, 5, 554–560. [Google Scholar] [CrossRef]
- Kavitha, M.K.; John, H.; Gopinath, P.; Philip, R. Synthesis of reduced graphene oxide-ZnO hybrid with enhanced optical limiting properties. J. Mater. Chem. C 2013, 1, 3669–3676. [Google Scholar] [CrossRef]
- Eisenthal, K.B. Second harmonic spectroscopy of aqueous nano- and microparticle interfaces. Chem. Rev. 2006, 106, 1462–1477. [Google Scholar] [CrossRef]
- Chan, S.C.; Barteau, M.A. Preparation of highly uniform Ag/TiO2 and Au/TiO2 supported nanoparticle catalysts by photodeposition. Langmuir 2005, 21, 5588–5595. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.B.M.; Venkatramaiah, N.; Venkatesan, R.; Rao, D.N. Graphene oxide (GO)-Semiconductor (ZnO, TiO2) nanoparticles for broadband optical limiting. In Proceedings of the International Conference on Fibre Optics and Photonics 2012, Chennai, India, 9–12 December 2012. [Google Scholar]
- Ebrahimi, M.; Zakery, A.; Karimipour, M.; Molaei, M. Nonlinear optical properties and optical limiting measurements of graphene oxide—Ag@TiO2 compounds. Opt. Mater. 2016, 57, 146–152. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Pan, P.; Wang, M.-R.; Yang, Z.-C.; Liu, J.-L.; Wei, Z. Enhanced optical limiting of dispersible MWCNTs/TiO2 nanocomposite. Opt. Laser Technol. 2015, 67, 44–49. [Google Scholar] [CrossRef]
- Zhang, W.F.; He, Y.L.; Zhang, M.S.; Yin, Z.; Chen, Q. Raman scattering study on anatase TiO2 nanocrystals. J. Phys. D-Appl. Phys. 2000, 33, 912–916. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Enhanced photocatalytic H-2-production activity of graphene-modified titania nanosheets. Nanoscale 2011, 3, 3670–3678. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Pollak, F.H.; Tomkiewicz, M. Raman spectroscopy as a morphological probe for TiO2 aerogels. J. Phys. Chem. B 1997, 101, 2730–2734. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y.; Yu, W.; Huang, Z.; Fang, Y.; Long, L.; Song, Y.; Cifuentes, M.P.; Humphrey, M.G.; Zhang, L.; et al. TiO2-multi-walled carbon nanotube nanocomposites: Hydrothermal synthesis and temporally-dependent optical properties. RSC Adv. 2016, 6, 20120–20127. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-Graphene Composite as a High Performance Photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef]
- Wei, W.; He, T.; Teng, X.; Wu, S.; Ma, L.; Zhang, H.; Ma, J.; Yang, Y.; Chen, H.; Han, Y.; et al. Nanocomposites of Graphene Oxide and Upconversion Rare-Earth Nanocrystals with Superior Optical Limiting Performance. Small 2012, 8, 2271–2276. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Zhuang, Y.; Fu, X. New Insight for Enhanced Photocatalytic Activity of TiO2 by Doping Carbon Nanotubes: A Case Study on Degradation of Benzene and Methyl Orange. J. Phys. Chem. C 2010, 114, 2669–2676. [Google Scholar] [CrossRef]
- Jiang, G.; Lin, Z.; Chen, C.; Zhu, L.; Chang, Q.; Wang, N.; Wei, W.; Tang, H. TiO2 nanoparticles assembled on graphene oxide nanosheets with high photocatalytic activity for removal of pollutants. Carbon 2011, 49, 2693–2701. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Photocatalytic Reduction of Graphene Oxide Nanosheets on TiO2 Thin Film for Photoinactivation of Bacteria in Solar Light Irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, G.; Chen, Y.; Zeng, L.-J.; Zhu, C.-X.; Neoh, K.-G.; Wang, C.; Kang, E.-T. Conjugated Polymer-Grafted Reduced Graphene Oxide for Nonvolatile Rewritable Memory. Chem.-Eur. J. 2011, 17, 13646–13652. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Yumitori, S. Correlation of C-1s chemical state intensities with the O-1s intensity in the XPS analysis of anodically oxidized glass-like carbon samples. J. Mater. Sci. 2000, 35, 139–146. [Google Scholar] [CrossRef]
- Shah, M.S.A.S.; Park, A.R.; Zhang, K.; Park, J.H.; Yoo, P.J. Green Synthesis of Biphasic TiO2-Reduced Graphene Oxide Nanocomposites with Highly Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2012, 4, 3893–3901. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Bai, Y.; Cheng, Y.; Jin, W.; Xu, N. Preparation and characterization of visible-light-driven carbon-sulfur-codoped TiO2 photocatalysts. Ind. Eng. Chem. Res. 2006, 45, 4971–4976. [Google Scholar] [CrossRef]
- Liang, D.; Cui, C.; Hu, H.; Wang, Y.; Xu, S.; Ying, B.; Li, P.; Lu, B.; Shen, H. One-step hydrothermal synthesis of anatase TiO2/reduced graphene oxide nanocomposites with enhanced photocatalytic activity. J. Alloys Compd. 2014, 582, 236–240. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.-S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene oxide papers modified by divalent ions—Enhancing mechanical properties via chemical cross-linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef]
- Gao, Y.; Pu, X.; Zhang, D.; Ding, G.; Shao, X.; Ma, J. Combustion synthesis of graphene oxide-TiO2 hybrid materials for photodegradation of methyl orange. Carbon 2012, 50, 4093–4101. [Google Scholar] [CrossRef]
- Wang, A.; Yu, W.; Fang, Y.; Song, Y.; Jia, D.; Long, L.; Cifuentes, M.P.; Humphrey, M.G.; Zhang, C. Facile hydrothermal synthesis and optical limiting properties of TiO2-reduced graphene oxide nanocomposites. Carbon 2015, 89, 130–141. [Google Scholar] [CrossRef]
- Wu, H.; Fan, J.; Liu, E.; Hu, X.; Ma, Y.; Fan, X.; Li, Y.; Tang, C. Facile hydrothermal synthesis of TiO2 nanospindles-reduced graphene oxide composite with a enhanced photocatalytic activity. J. Alloys Compd. 2015, 623, 298–303. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic Effect of MoS2 and Graphene as Cocatalysts for Enhanced Photocatalytic H-2 Production Activity of TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef]
- Fresno, F.; Tudela, D.; Coronado, J.M.; Fernandez-Garcia, M.; Hungria, A.B.; Soria, J. Influence of Sn4+ on the structural and electronic properties of Ti1-xSnxO2 nanoparticles used as photocatalysts. Phys. Chem. Chem. Phys. 2006, 8, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, W.; Li, W. Construction of a graphene oxide-encapsulated Pt@TiO2 core/shell ternary composite nanostructure with enhanced optical limiting behavior. Carbon 2015, 93, 400–411. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Cui, X.; Jiang, Z. A green and facile synthesis of TiO2/graphene nanocomposites and their photocatalytic activity for hydrogen evolution. Int. J. Hydrogen Energy 2012, 37, 811–815. [Google Scholar] [CrossRef]
- Bersani, D.; Lottici, P.P.; Ding, X.Z. Phonon confinement effects in the Raman scattering by TiO2 nanocrystals. Appl. Phys. Lett. 1998, 72, 73–75. [Google Scholar] [CrossRef]
- Jawhari, T.; Roig, A.; Casado, J. Raman-spectroscopic characterization of some commercially available carbon-black materials. Carbon 1995, 33, 1561–1565. [Google Scholar] [CrossRef]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B. Efficient photochemical water splitting by a chemically modified n-TiO2 2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, S.; Kisch, H. Daylight photocatalysis by carbon-modified titanium dioxide. Angew. Chem. Int. Ed. 2003, 42, 4908–4911. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Ai, Z.; Jia, F.; Zhang, L.; Fan, X.; Zou, Z. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl. Catal. B-Environ. 2007, 69, 138–144. [Google Scholar] [CrossRef]
- Cronemeyer, D.C. Infrared absorption of reduced rutile tio2 single crystals. Phys. Rev. 1959, 113, 1222–1226. [Google Scholar] [CrossRef]
- Zheng, C.; Li, Y.; Huang, L.; Li, W.; Chen, W. Facile assembly of tetragonal Pt clusters on graphene oxide for enhanced nonlinear optical properties. Opt. Mater. 2015, 49, 152–157. [Google Scholar] [CrossRef]
- Vivien, L.; Anglaret, E.; Riehl, D.; Hache, F.; Bacou, F.; Andrieux, M.; Lafonta, F.; Journet, C.; Goze, C.; Brunet, M.; et al. Optical limiting properties of singlewall carbon nanotubes. Opt. Commun. 2000, 174, 271–275. [Google Scholar] [CrossRef]
- Sheikbahae, M.; Said, A.A.; Wei, T.H.; Hagan, D.J.; Vanstryland, E.W. sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 1990, 26, 760–769. [Google Scholar] [CrossRef]
- Zhang, D.; Gan, L.; Cao, Y.; Wang, Q.; Qi, L.; Guo, X. Understanding Charge Transfer at PbS-Decorated Graphene Surfaces toward a Tunable Photosensor. Adv. Mater. 2012, 24, 2715–2720. [Google Scholar] [CrossRef]
- Midya, A.; Mamidala, V.; Yang, J.-X.; Ang, P.K.L.; Chen, Z.-K.; Ji, W.; Loh, K.P. Synthesis and Superior Optical-Limiting Properties of Fluorene-Thiophene-Benzothiadazole Polymer-Functionalized Graphene Sheets. Small 2010, 6, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
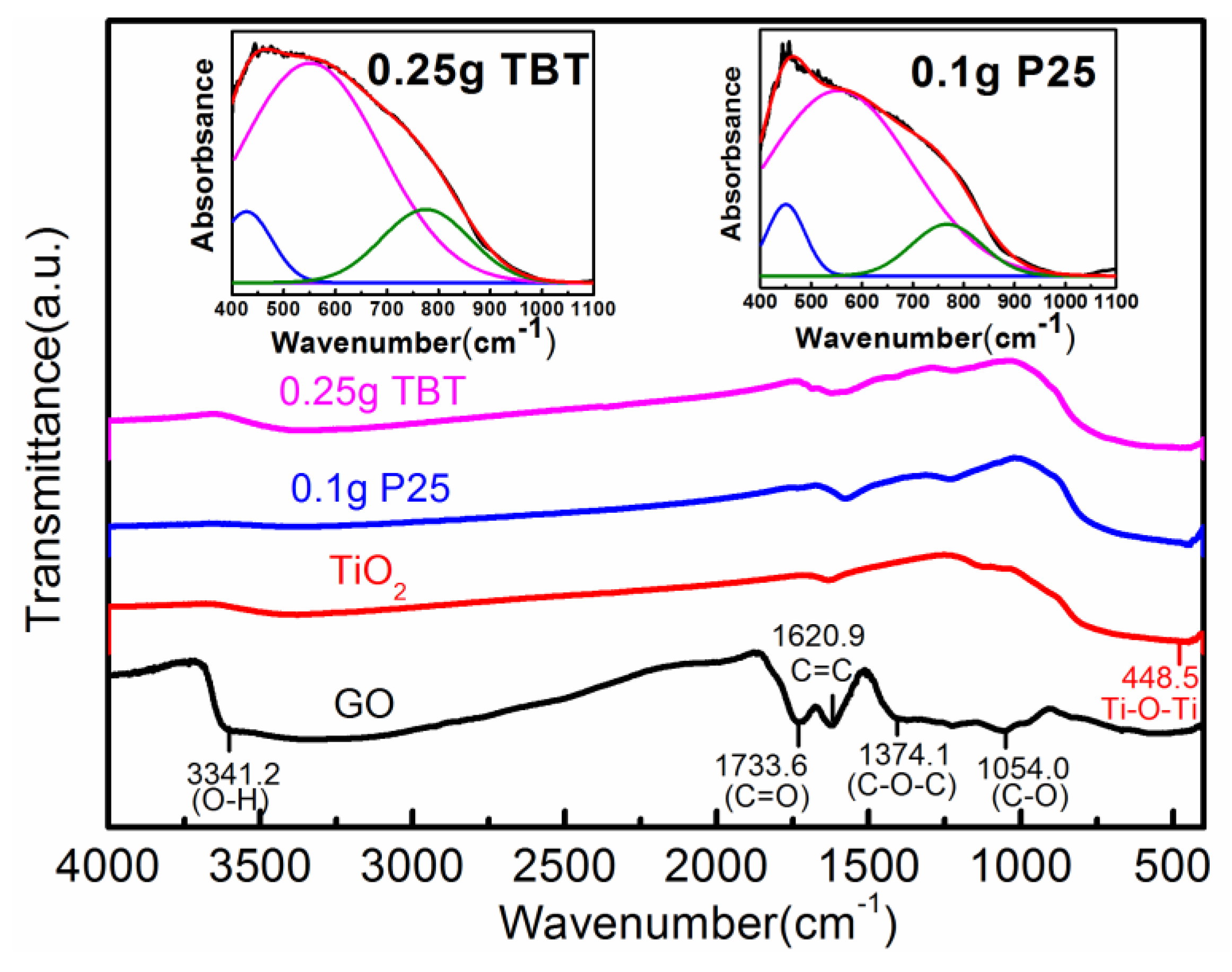
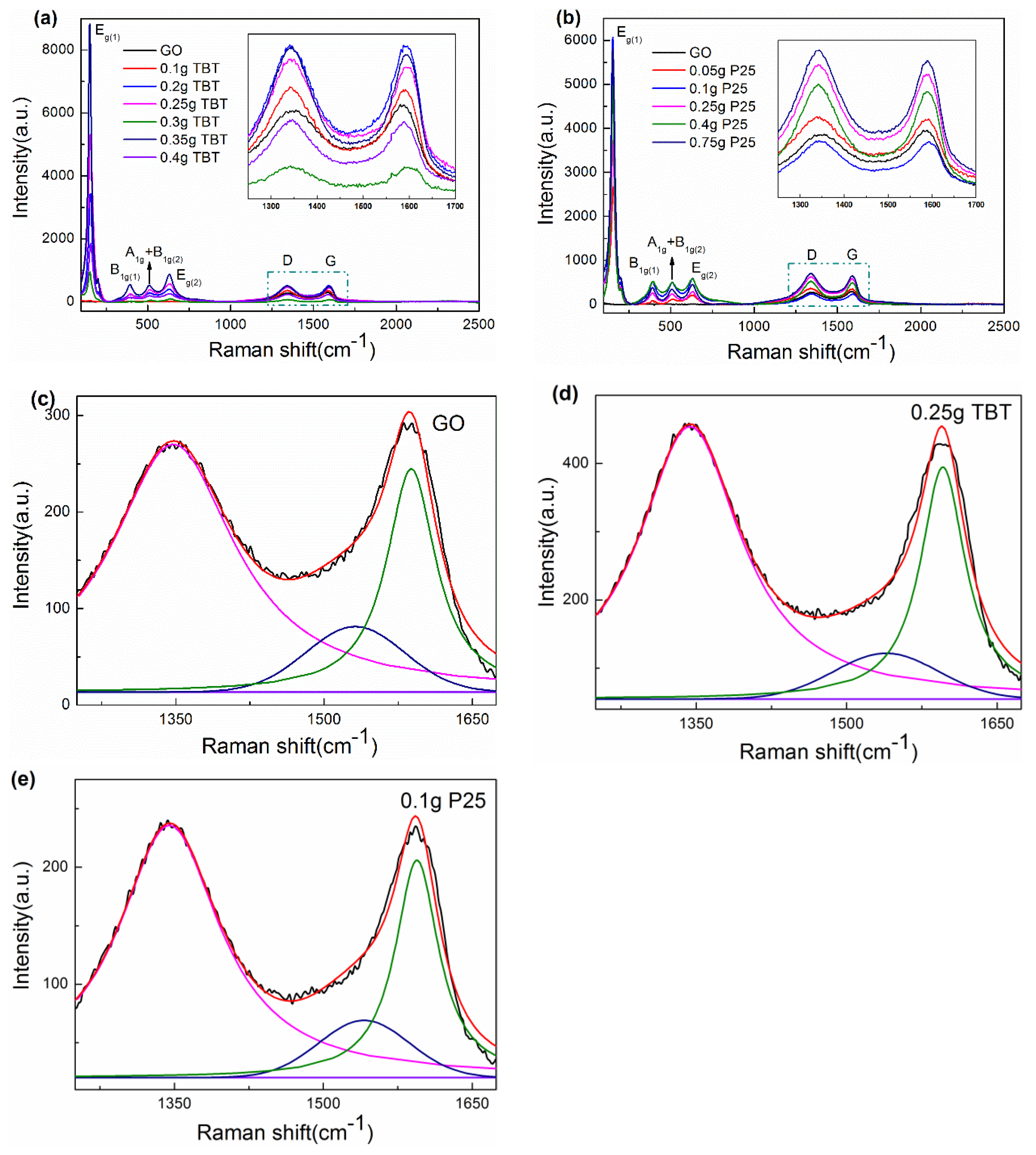
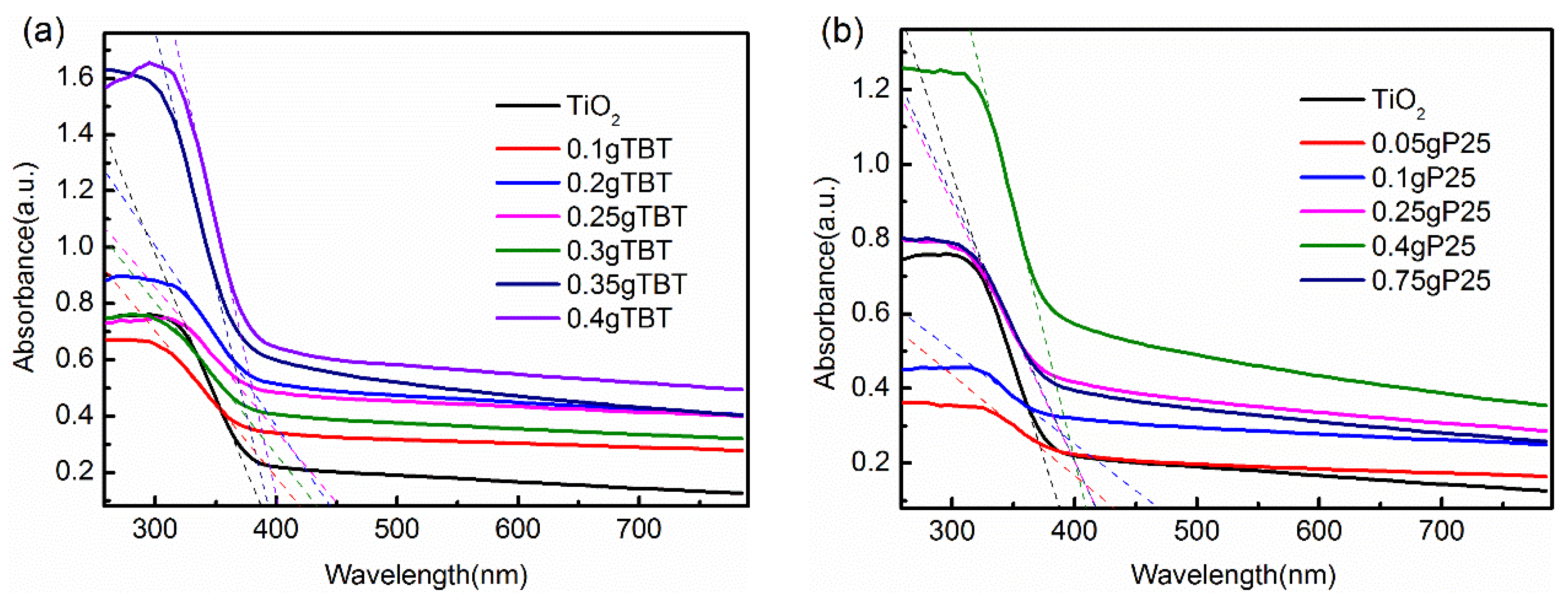


| Samples | Source Materials of TiO2 | Weight of Source Materials |
|---|---|---|
| 0.1 g TBT | TBT | 0.10 g |
| 0.2 g TBT | TBT | 0.20 g |
| 0.25 g TBT | TBT | 0.25 g |
| 0.3 g TBT | TBT | 0.30 g |
| 0.35 g TBT | TBT | 0.35 g |
| 0.4 g TBT | TBT | 0.40 g |
| Samples | Source Materials of TiO2 | Weight of Source Materials |
|---|---|---|
| 0.05 g P25 | P25 | 0.05 g |
| 0.1 g P25 | P25 | 0.10 g |
| 0.25 g P25 | P25 | 0.25 g |
| 0.4 g P25 | P25 | 0.40 g |
| 0.75 g P25 | P25 | 0.75 g |
| TBT Series Sample | P25 Series Samples | ||||
|---|---|---|---|---|---|
| Samples | λg (nm) | Eg (eV) | Samples | λg (nm) | Eg (eV) |
| 0.1 g TBT | 435 | 2.85 | TiO2 | 396 | 3.13 |
| 0.2 g TBT | 457 | 2.71 | 0.05 g P25 | 462 | 2.68 |
| 0.25 g TBT | 468 | 2.65 | 0.1 g P25 | 501 | 2.48 |
| 0.3 g TBT | 449 | 2.76 | 0.25 g P25 | 429 | 2.89 |
| 0.35 g TBT | 399 | 3.11 | 0.4 g P25 | 416 | 2.98 |
| 0.4 g TBT | 407 | 3.05 | 0.75 g P25 | 428 | 2.90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Zhao, L.; Zou, Y.; Song, L.; Dong, N.; Wang, J. Effects of Different TiO2 Particle Sizes on the Microstructure and Optical Limiting Properties of TiO2/Reduced Graphene Oxide Nanocomposites. Nanomaterials 2019, 9, 730. https://doi.org/10.3390/nano9050730
Ren Y, Zhao L, Zou Y, Song L, Dong N, Wang J. Effects of Different TiO2 Particle Sizes on the Microstructure and Optical Limiting Properties of TiO2/Reduced Graphene Oxide Nanocomposites. Nanomaterials. 2019; 9(5):730. https://doi.org/10.3390/nano9050730
Chicago/Turabian StyleRen, Yuyu, Lili Zhao, Yang Zou, Lixin Song, Ningning Dong, and Jun Wang. 2019. "Effects of Different TiO2 Particle Sizes on the Microstructure and Optical Limiting Properties of TiO2/Reduced Graphene Oxide Nanocomposites" Nanomaterials 9, no. 5: 730. https://doi.org/10.3390/nano9050730
APA StyleRen, Y., Zhao, L., Zou, Y., Song, L., Dong, N., & Wang, J. (2019). Effects of Different TiO2 Particle Sizes on the Microstructure and Optical Limiting Properties of TiO2/Reduced Graphene Oxide Nanocomposites. Nanomaterials, 9(5), 730. https://doi.org/10.3390/nano9050730





