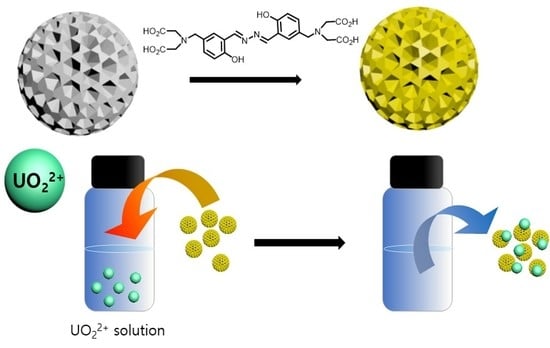
Spectroscopic Study of the Salicyladazine Derivative–UO22+ Complex and Its Immobilization to Mesoporous Silica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
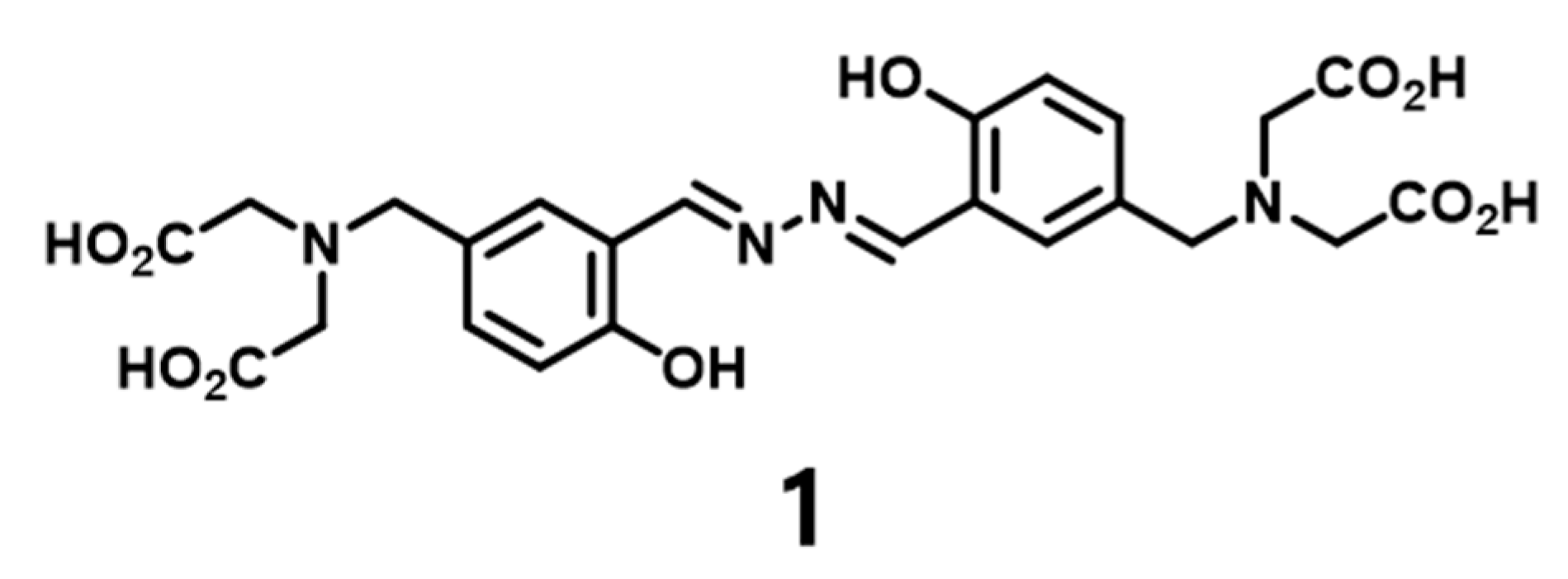
2.2. Synthesis of Compound 1
2.3. Preparation of MPS
2.4. Preparation of MPS-1
2.5. Photophysical Studies
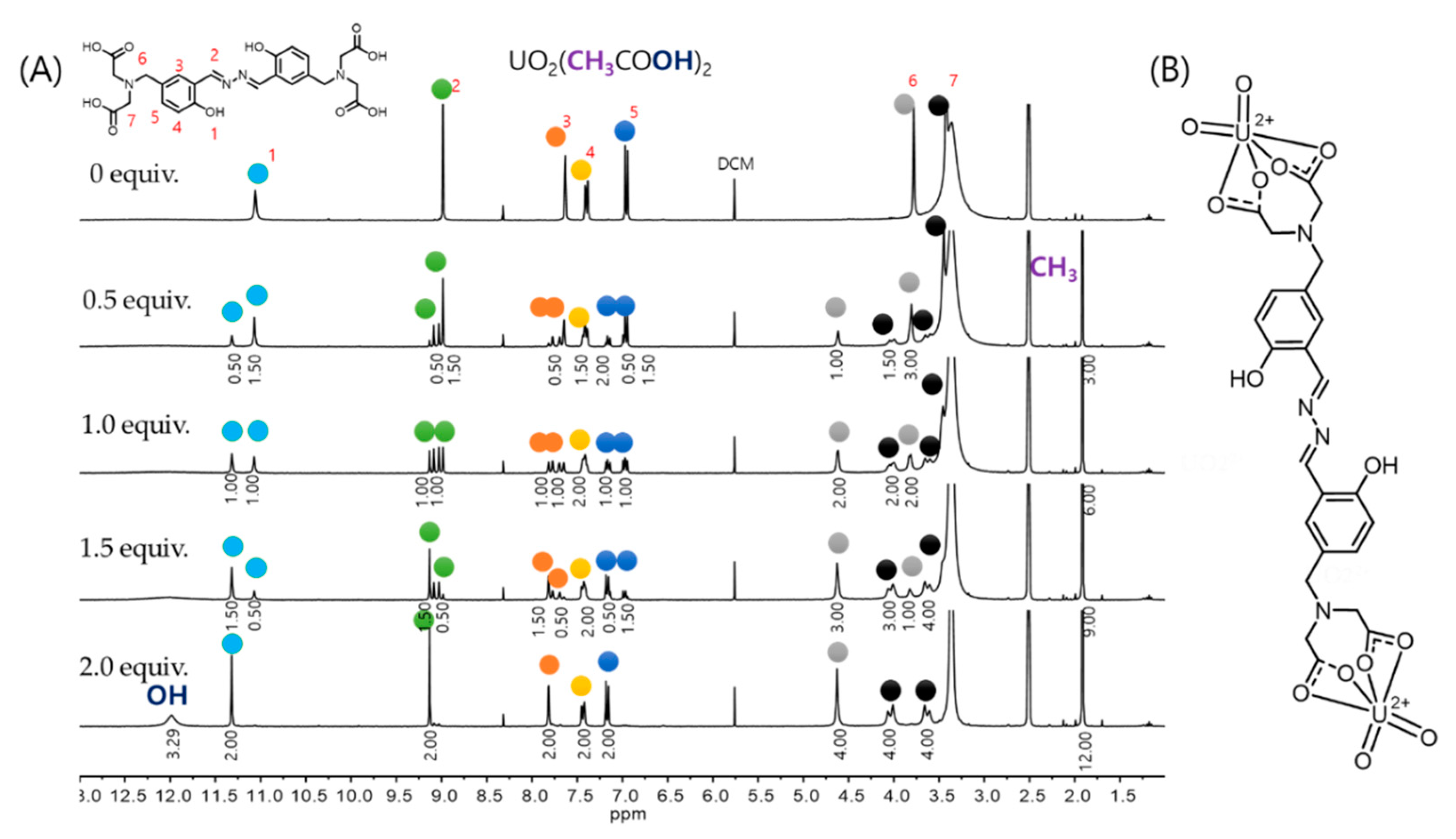
2.6. NMR Measurement
2.7. ICP-MS
3. Results and Discussion
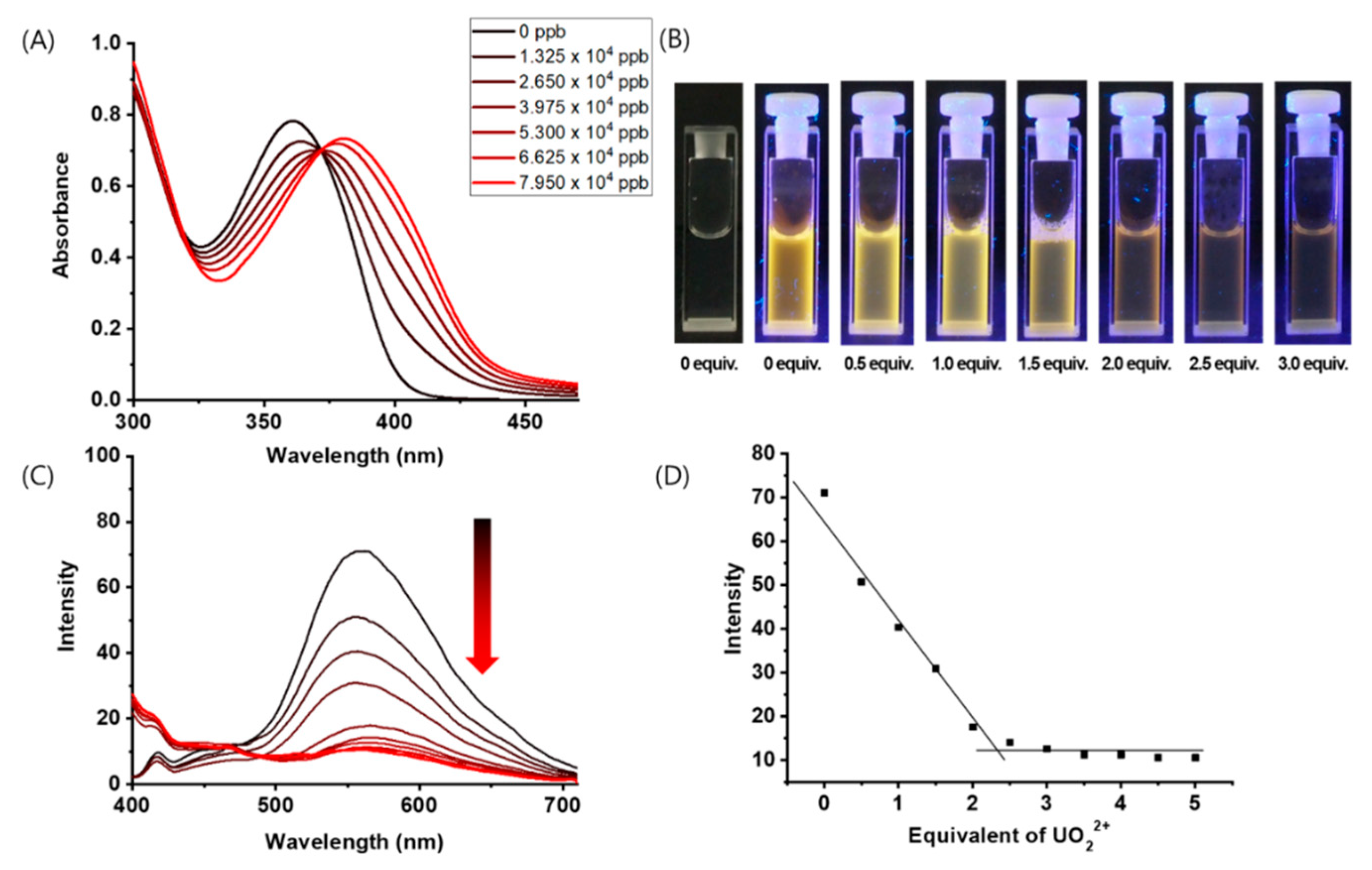
3.1. Spectroscopic Properties of Complex 1 with UO22+
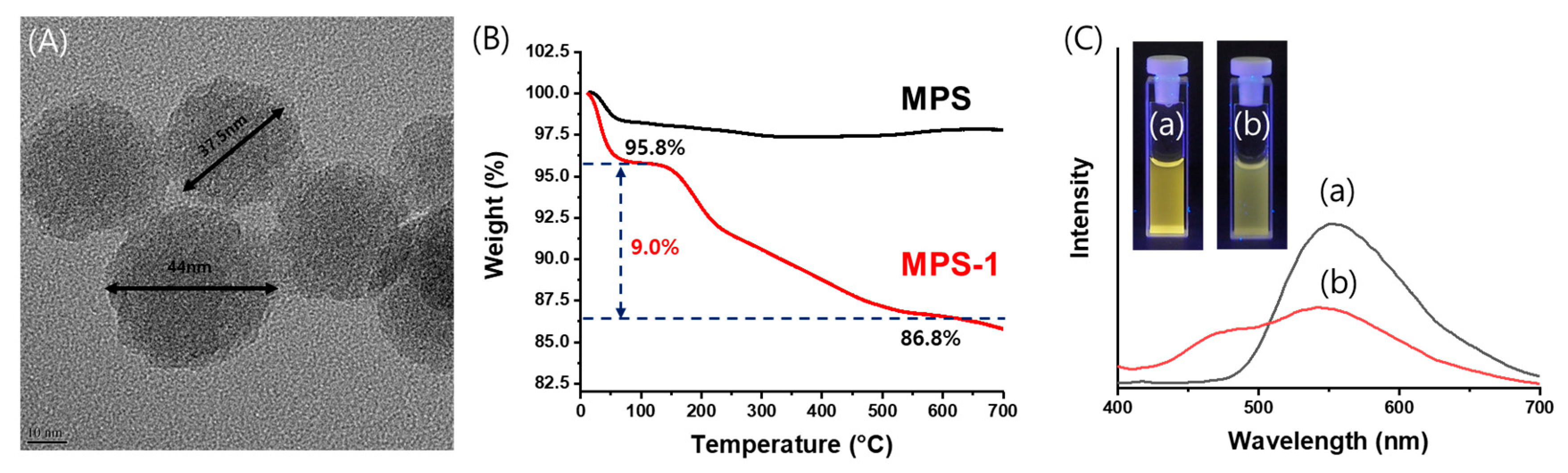
3.2. Immobilization of Compound 1 to Mesoporous Silica Nanoparticles
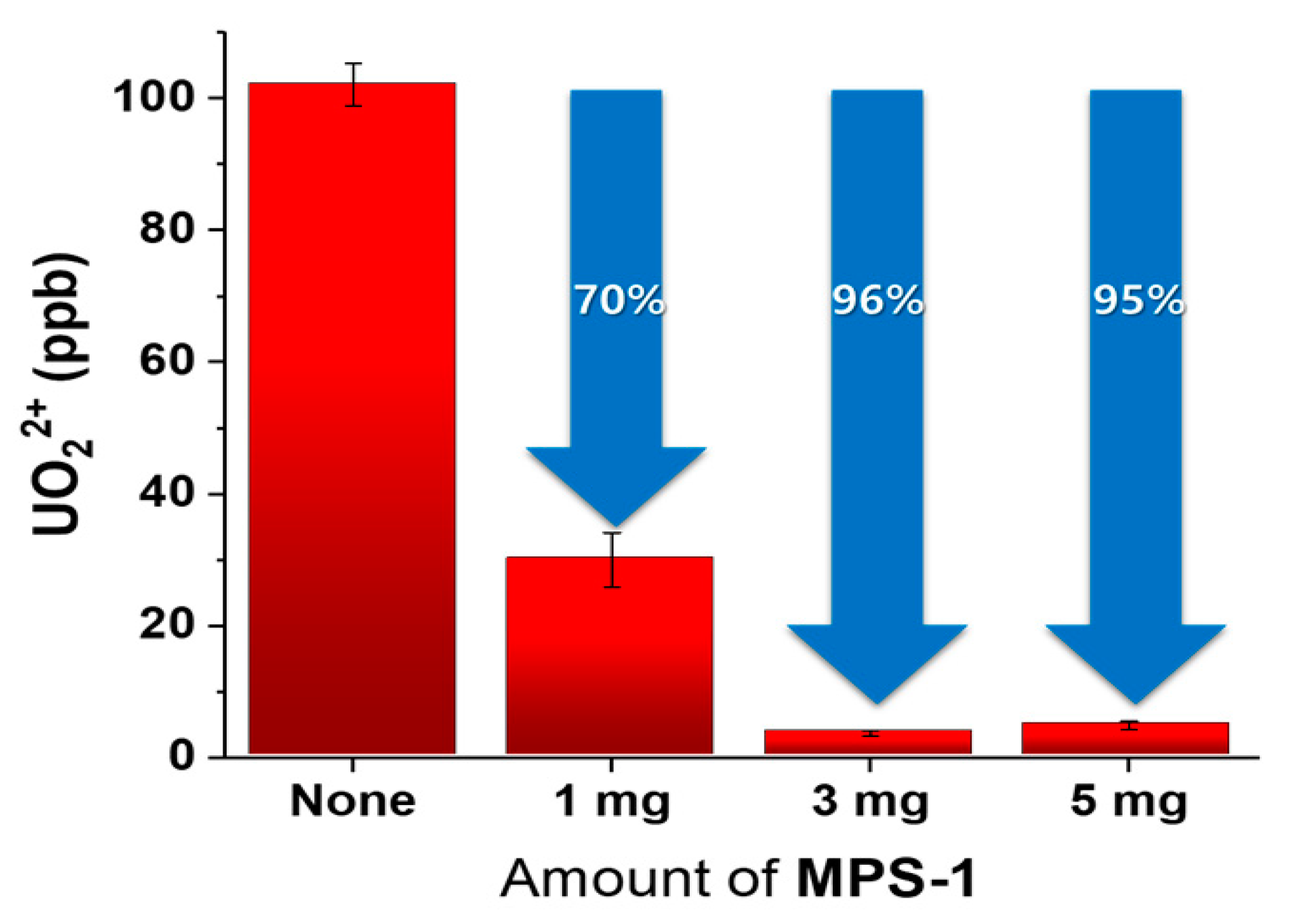
3.3. The Adsorption Capacity of MPS-1 for UO22+
3.4. Adsorption of UO22+ and Other Cations onto MPS-1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- O’Loughlin, E.J.; Kelly, S.D.; Cook, R.E.; Csencsits, R.; Kemner, K.M. Reduction of Uranium(VI) by Mixed Iron(II)/Iron(III) Hydroxide (Green Rust): Formation of UO2 Nanoparticles. Environ. Sci. Technol. 2003, 37, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.-L.; Sarma, D.; Qi, X.-H.; Du, K.-Z.; Huang, X.-Y.; Kanatzidis, M.G. Efficient Removal and Recovery of Uranium by a Layered Organic–Inorganic Hybrid Thiostannate. J. Am. Chem. Soc. 2016, 138, 12578–12585. [Google Scholar] [CrossRef] [PubMed]
- Asiabi, H.; Yamini, Y.; Shamsayei, M. Highly efficient capture and recovery of uranium by reusable layered double hydroxide intercalated with 2-mercaptoethanesulfonate. Chem. Eng. J. 2018, 337, 609–615. [Google Scholar] [CrossRef]
- Wu, P.; Hwang, K.; Lan, T.; Lu, Y. A DNAzyme-Gold Nanoparticle Probe for Uranyl Ion in Living Cells. J. Am. Chem. Soc. 2013, 135, 5254–5257. [Google Scholar] [CrossRef]
- Cao, X.-H.; Zhang, H.-Y.; Ma, R.-C.; Yang, Q.; Zhang, Z.-B.; Liu, Y.-H. Visual colorimetric detection of UO22+ using o-phosphorylethanolamine-functionalized gold nanoparticles. Sens. Actuators B 2015, 218, 67–72. [Google Scholar] [CrossRef]
- Elabd, A.A.; Attia, M.S. A new thin film optical sensor for assessment of UO22+ based on the fluorescence quenching of Trimetazidine doped in sol gel matrix. J. Lumin. 2015, 165, 179–184. [Google Scholar] [CrossRef]
- Xiao, S.J.; Zuo, J.; Zhu, Z.Q.; Ouyang, Y.Z.; Zhang, X.L.; Chen, H.W.; Zhang, L. Highly sensitive DNAzyme sensor for selective detection of trace uranium in ore and natural water samples. Sens. Actuators B 2015, 210, 656–660. [Google Scholar] [CrossRef]
- Elabd, A.A.; Attia, M.S. Spectrofluorimetric assessment of UO22+ by the quenching of the fluorescence intensity of Clopidogrel embedded in PMMA matrix. J. Lumin. 2016, 169, 313–318. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, H.; Hu, Q.; Wang, Y.; Hu, J.; Zhou, F.; Liu, P. “Turn-On” fluorescent chemosensor based on β-diketone for detecting Th4+ ions in Aqueous Solution and application in living cell imaging. Sens. Actuators B 2017, 253, 766–772. [Google Scholar] [CrossRef]
- Wen, J.; Huang, Z.; Hu, S.; Li, S.; Li, W.; Wang, X. Aggregation-induced emission active tetraphenylethene-based sensor for uranyl ion detection. J. Hazard. Mater. 2016, 318, 363–370. [Google Scholar] [CrossRef]
- Harvey, P.; Nonat, A.; Platas-Iglesias, C.; Natrajan, L.S.; Charbonniere, L.J. Sensing Uranyl(VI) Ions by Coordination and Energy Transfer to a Luminescent Europium(III) Complex. Angew. Chem. Int. Ed. 2018, 57, 9921–9924. [Google Scholar] [CrossRef]
- Lee, J.H.; Wang, Z.; Liu, J.; Lu, Y. Highly sensitive and selective colorimetric sensors for uranyl (UO22+): Development and comparison of labeled and label-free DNAzyme-gold nanoparticle systems. J. Am. Chem. Soc. 2008, 130, 14217–14226. [Google Scholar] [CrossRef]
- Sather, A.C.; Berryman, O.B.; Rebek, J. Selective recognition and extraction of the uranyl ion from aqueous solutions with a recyclable chelating resin. Chem. Sci. 2013, 4, 3601–3605. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, M.; Yan, Y.; Yu, H.; Yu, T.; Jiang, H.; Zhang, K.; Wang, S. A turn-on fluorescence probe for the selective and sensitive detection of fluoride ions. Anal. Bioanal. Chem. 2017, 409, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Li, Y.; Chen, X.; Li, S.; Ren, L.; Tang, B.Z. Aggregation-Induced Emission Probe for Light-Up and in Situ Detection of Calcium Ions at High Concentration. ACS Appl. Mater. Interfaces 2018, 10, 14410–14417. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, L.L.; Jiang, J.G.; Calin, N.; Lam, K.F.; Zhang, S.J.; Wu, H.H.; Wu, G.D.; Albela, B.; Bonneviot, L.; et al. Facile large-scale synthesis of monodisperse mesoporous silica nanospheres with tunable pore structure. J. Am. Chem. Soc. 2013, 135, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-T.; Han, J.; Gu, M.; Liu, J.; Li, Y.; Huang, Z.; Yu, H.-Z.; Hu, S.; Wang, X. Fluorescent recognition of uranyl ions by a phosphorylated cyclic peptide. Chem. Commun. 2015, 51, 11769–11772. [Google Scholar] [CrossRef] [PubMed]
- Starck, M.; Sisommay, N.; Laporte, F.A.; Oros, S.; Lebrun, C.; Delangle, P. Preorganized Peptide Scaffolds as Mimics of Phosphorylated Proteins Binding Sites with a High Affinity for Uranyl. Inorg. Chem. 2015, 54, 11557–11562. [Google Scholar] [CrossRef] [PubMed]
- Sessler, J.L.; Seidel, D.; Vivian, A.E.; Lynch, V.; Scott, B.L.; Keogh, D.W. Hexaphyrin(1.0.1.0.0.0): An Expanded Porphyrin Ligand for the Actinide Cations Uranyl (UO22+) and Neptunyl (NpO2+). Angew. Chem. Int. Ed. 2001, 40, 591–594. [Google Scholar] [CrossRef]
- Sessler, J.L.; Gorden, A.E.V.; Seidel, D.; Hannah, S.; Lynch, V.; Gordon, P.L.; Donohoe, R.J.; Drew Tait, C.; Webster Keogh, D. Characterization of the interactions between neptunyl and plutonyl cations and expanded porphyrins. Inorg. Chim. Acta 2002, 341, 54–70. [Google Scholar] [CrossRef]
- Anguera, G.; Brewster, J.T.; Moore, M.D.; Lee, J.; Vargas-Zúñiga, G.I.; Zafar, H.; Lynch, V.M.; Sessler, J.L. Naphthylbipyrrole-Containing Amethyrin Analogue: A New Ligand for the Uranyl (UO22+) Cation. Inorg. Chem. 2017, 56, 9409–9412. [Google Scholar] [CrossRef]
- Brewster, J.T.; He, Q.; Anguera, G.; Moore, M.D.; Ke, X.-S.; Lynch, V.M.; Sessler, J.L. Synthesis and characterization of a dipyriamethyrin–uranyl complex. Chem. Commun. 2017, 53, 4981–4984. [Google Scholar] [CrossRef] [PubMed]
- Marten-Ramos, P.; Costa, A.L.; Silva, M.R.; Pereira, L.C.J.; Pereira da Silva, P.S.; Seixas de Melo, J.S.; Martin-Gil, J. Luminescent properties of [UO2(TFA)2(DMSO)3], a promising material for sensing and monitoring the uranyl ion. Mater. Res. 2016, 19, 328–332. [Google Scholar] [CrossRef]
- Du, N.; Song, J.; Li, S.; Chi, Y.-X.; Bai, F.-Y.; Xing, Y.-H. A Highly Stable 3D Luminescent Indium-Polycarboxylic Framework for the Turn-off Detection of UO2(2+), Ru(3+), and Biomolecule Thiamines. ACS Appl. Mater. Interfaces 2016, 8, 28718–28726. [Google Scholar] [CrossRef] [PubMed]
- 2018 Drinking Water Standards and Advisory Tables. Available online: https://www.epa.gov/dwstandardsregulations/2018-drinking-water-standards-and-advisory-tables (accessed on 29 April 2019).
- Guidelines for Drinking-Water Quality, 4th ed. Available online: https://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/ (accessed on 29 April 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Park, J.; Lee, J.H.; Choi, M.Y.; Jung, J.H. Spectroscopic Study of the Salicyladazine Derivative–UO22+ Complex and Its Immobilization to Mesoporous Silica. Nanomaterials 2019, 9, 688. https://doi.org/10.3390/nano9050688
Park S, Park J, Lee JH, Choi MY, Jung JH. Spectroscopic Study of the Salicyladazine Derivative–UO22+ Complex and Its Immobilization to Mesoporous Silica. Nanomaterials. 2019; 9(5):688. https://doi.org/10.3390/nano9050688
Chicago/Turabian StylePark, Sujin, Jaehyeon Park, Ji Ha Lee, Myong Yong Choi, and Jong Hwa Jung. 2019. "Spectroscopic Study of the Salicyladazine Derivative–UO22+ Complex and Its Immobilization to Mesoporous Silica" Nanomaterials 9, no. 5: 688. https://doi.org/10.3390/nano9050688
APA StylePark, S., Park, J., Lee, J. H., Choi, M. Y., & Jung, J. H. (2019). Spectroscopic Study of the Salicyladazine Derivative–UO22+ Complex and Its Immobilization to Mesoporous Silica. Nanomaterials, 9(5), 688. https://doi.org/10.3390/nano9050688