Robust Synthesis of Size-Dispersal Triangular Silver Nanoprisms via Chemical Reduction Route and Their Cytotoxicity
Abstract
1. Introduction
2. Material and Methods.
2.1. Synthesis of Triangular Silver Nanoprisms (Ag-NPrs)
2.2. Characterization of Triangular Silver Nanoprisms (Ag-NPrs)
2.3. Silver Nanoprisms Silica Coating (Ag-NPrs@SiO2)
2.4. Cell Viability Assay
3. Results and Discussion
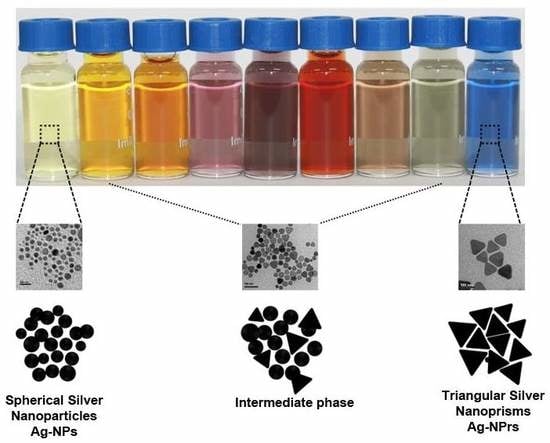
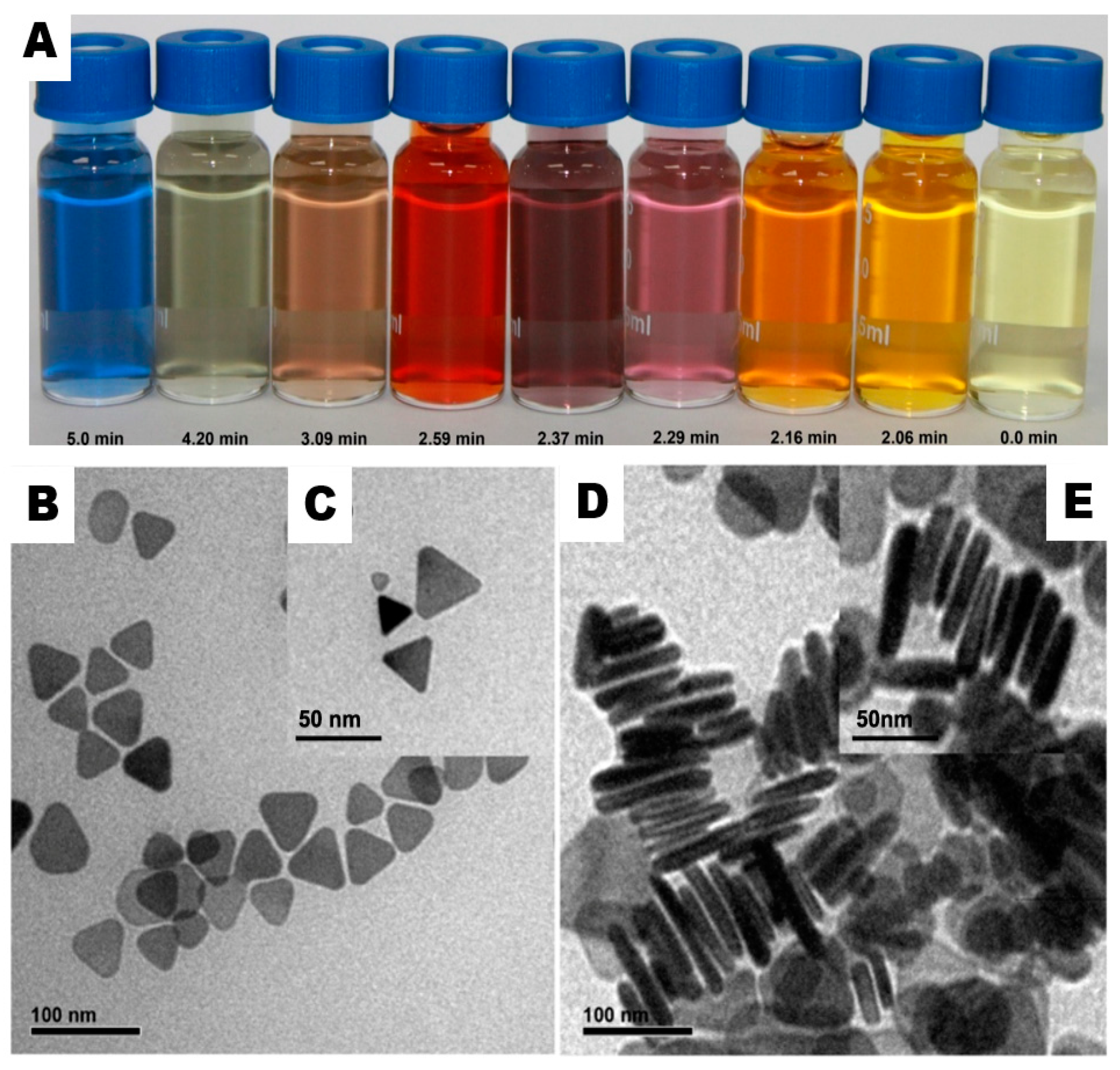
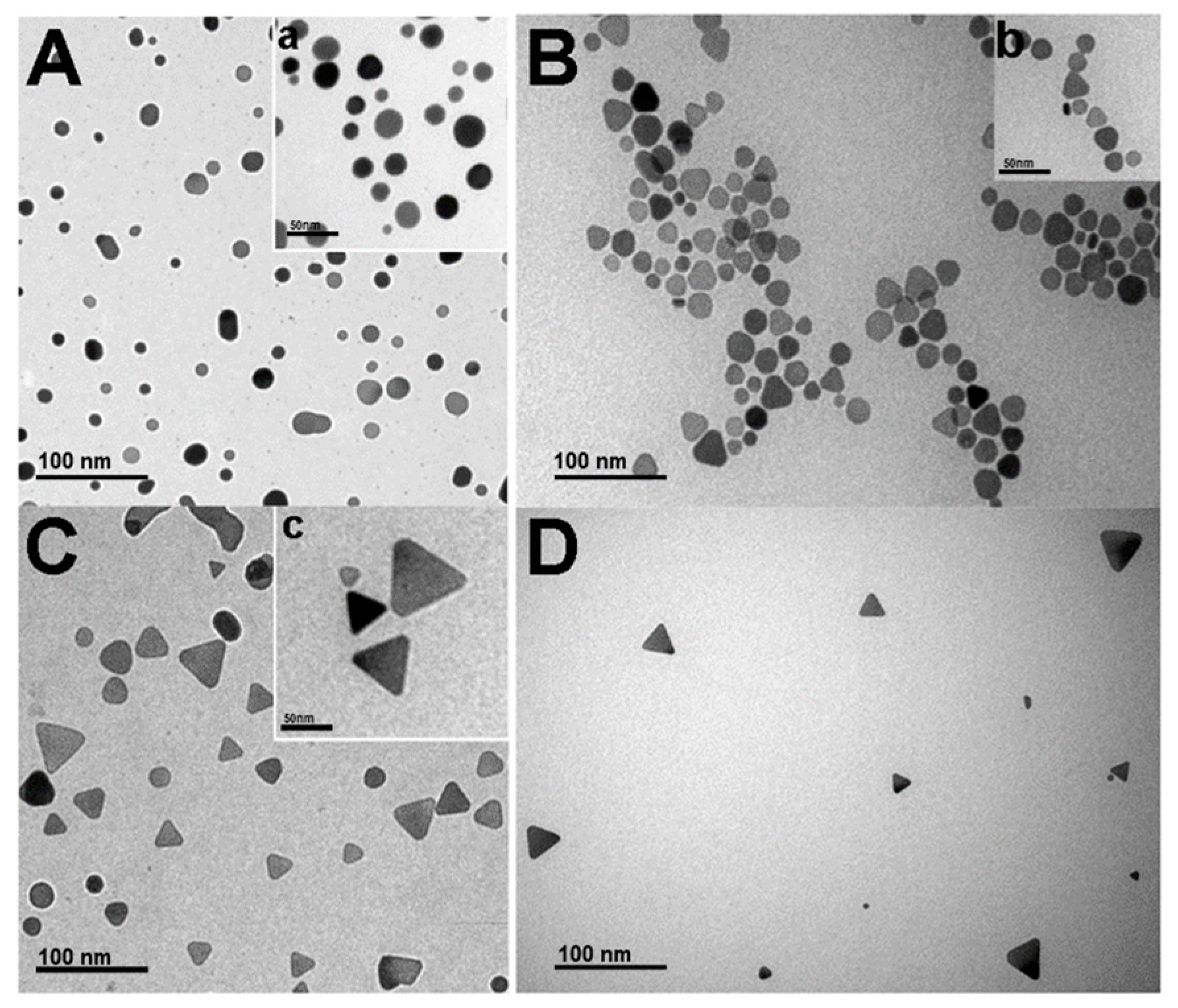
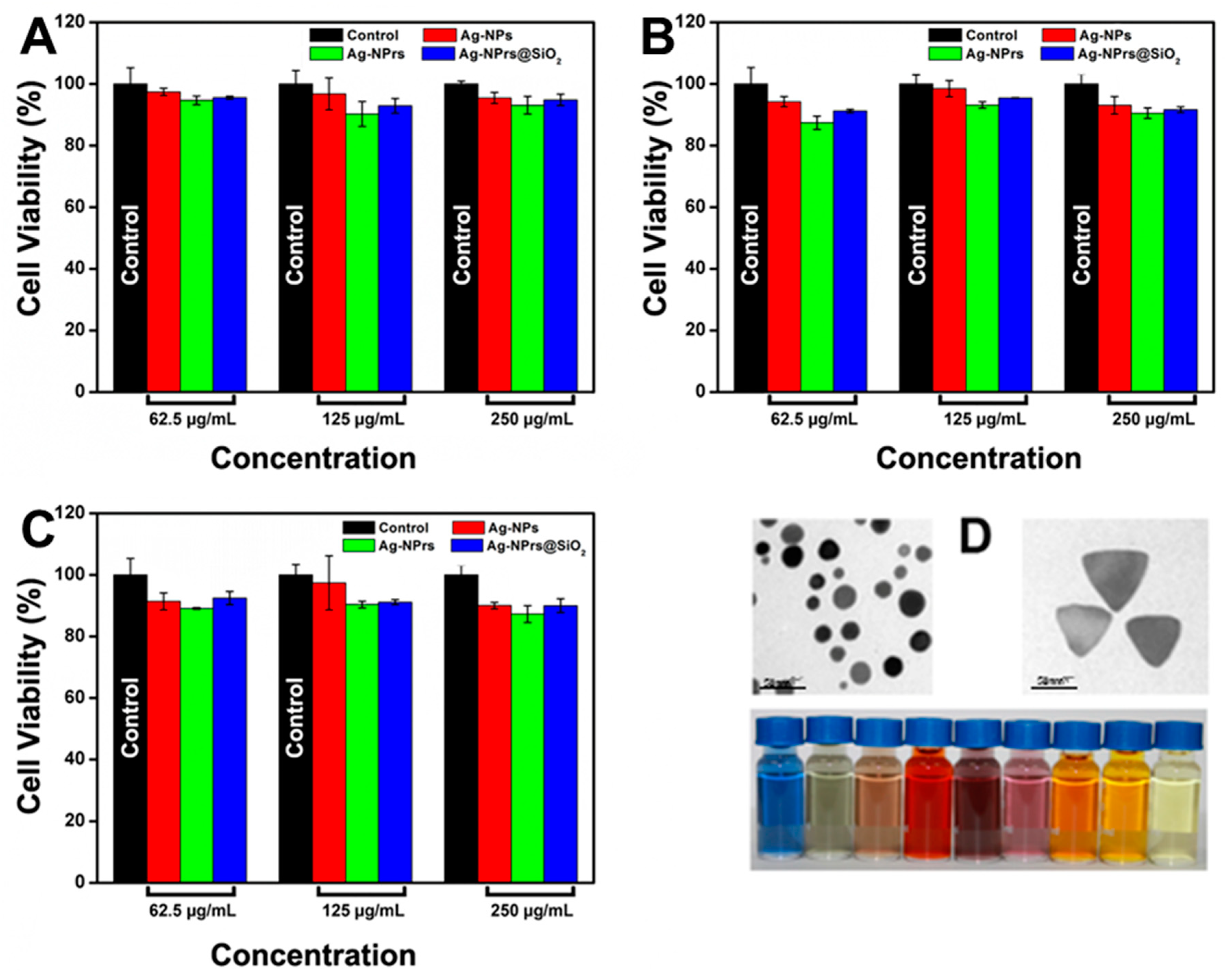
3.1. Preparation of Triangular Silver Nanoprisms (Ag-NPrs)
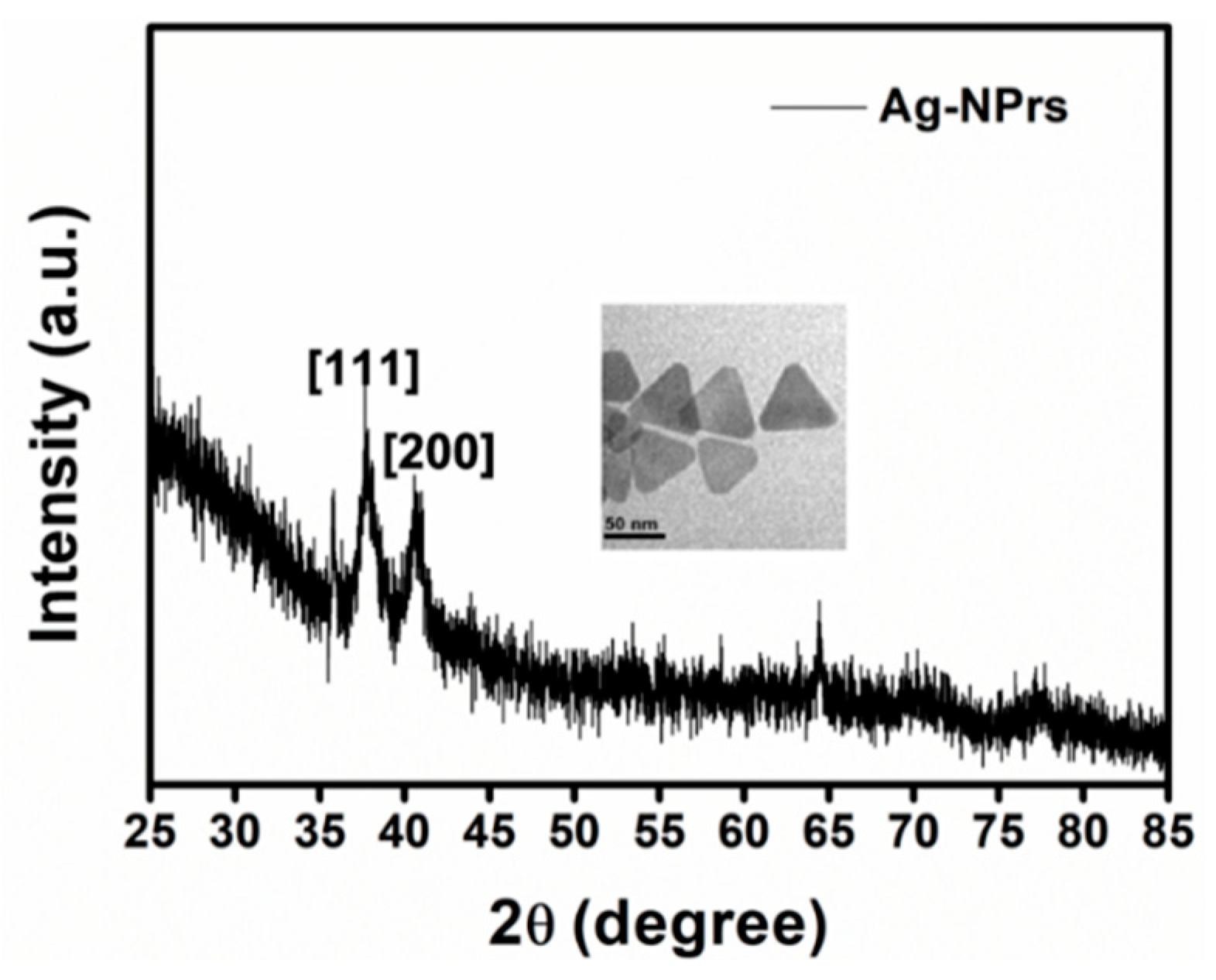
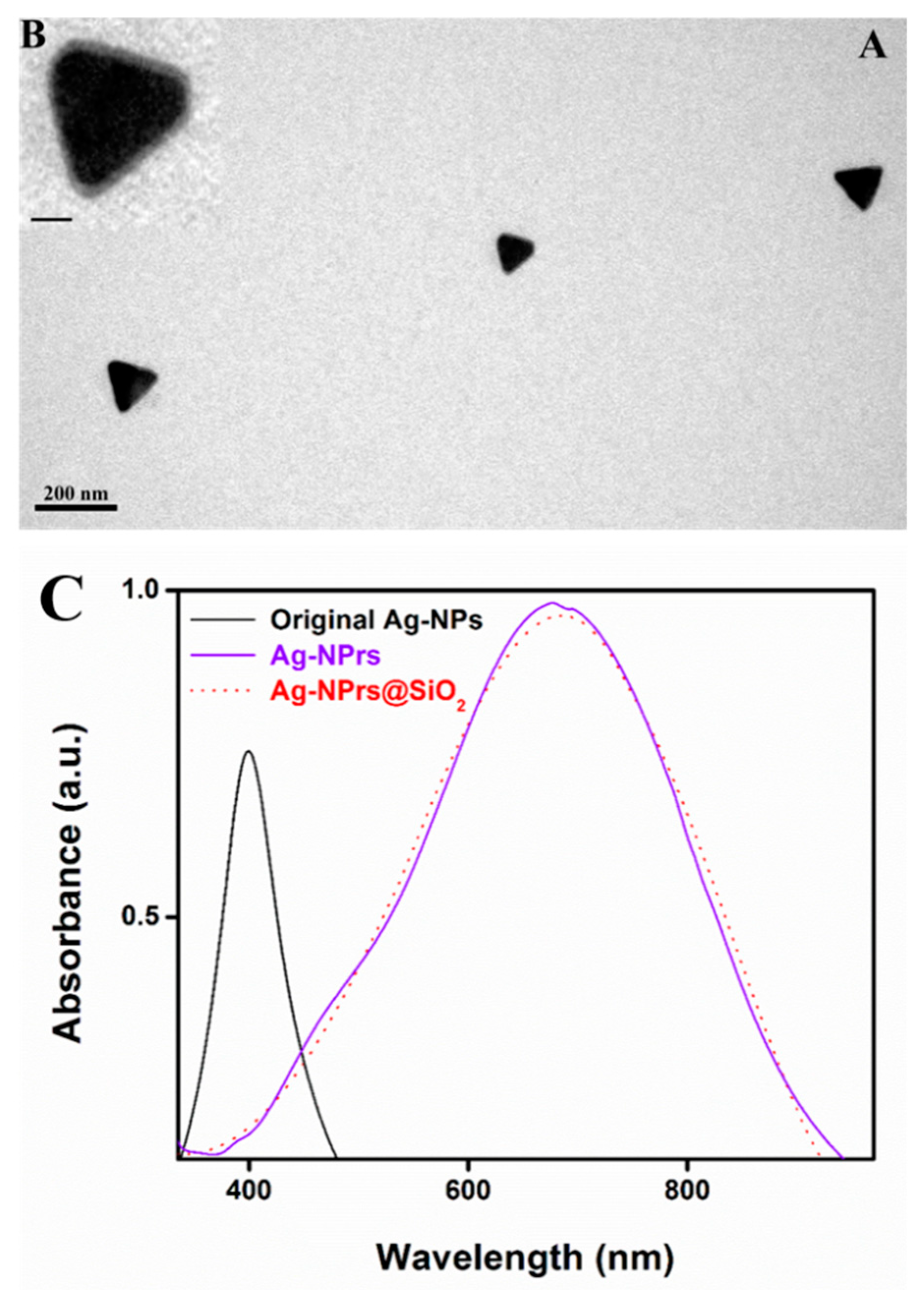
3.2. Characterization of Ag-NPrs
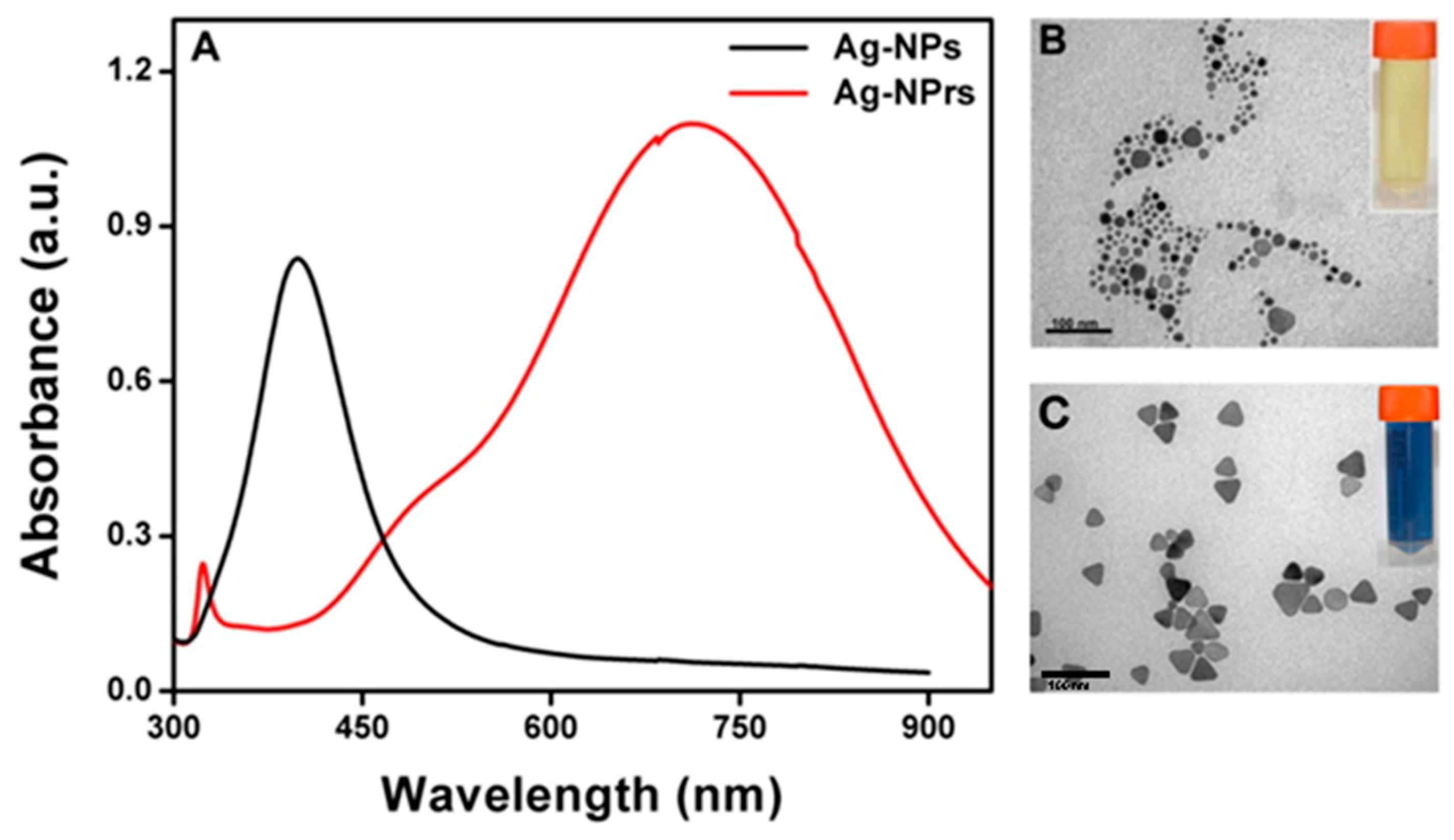
3.3. UV-Vis Measurement of the Ag-NPrs Formation
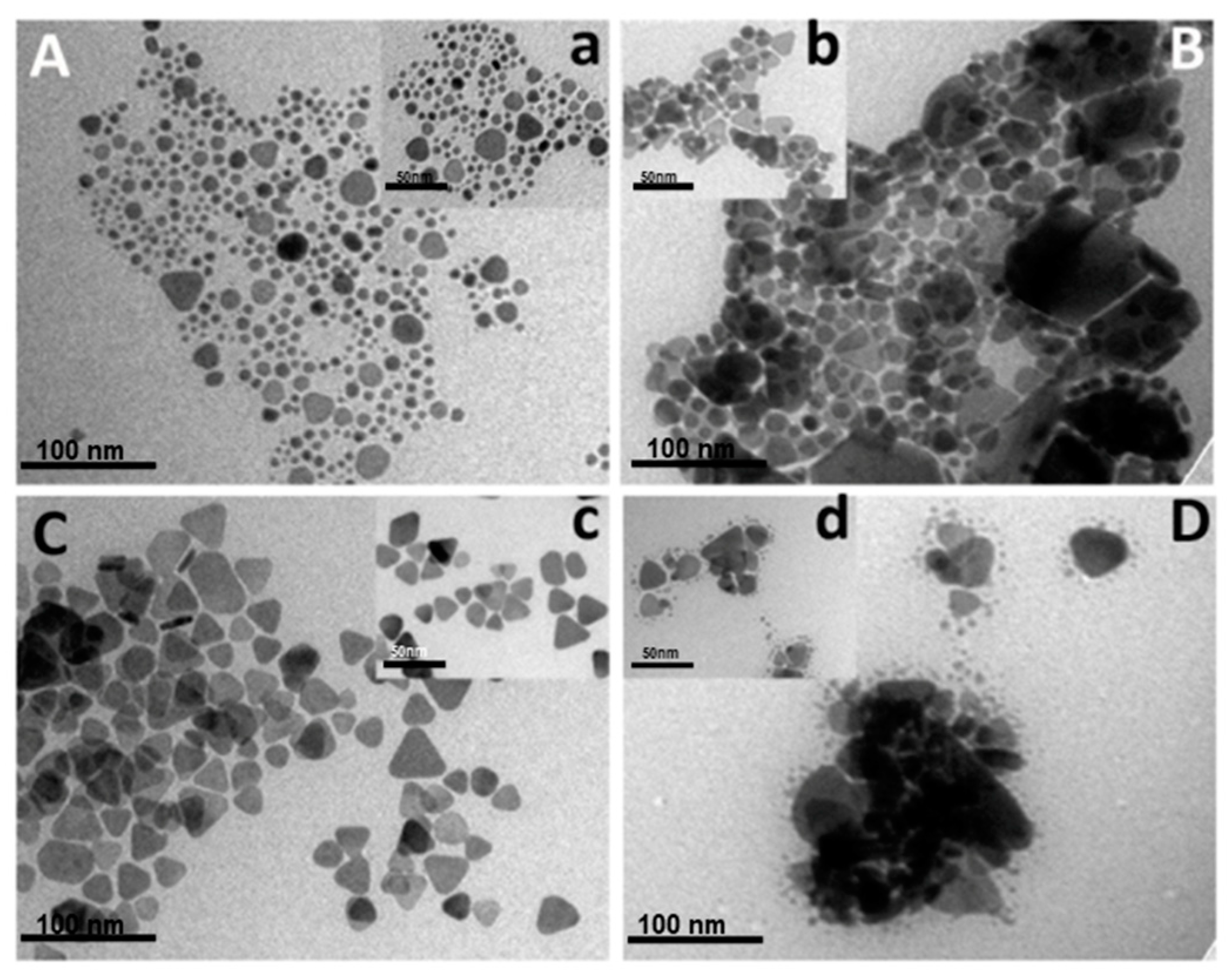
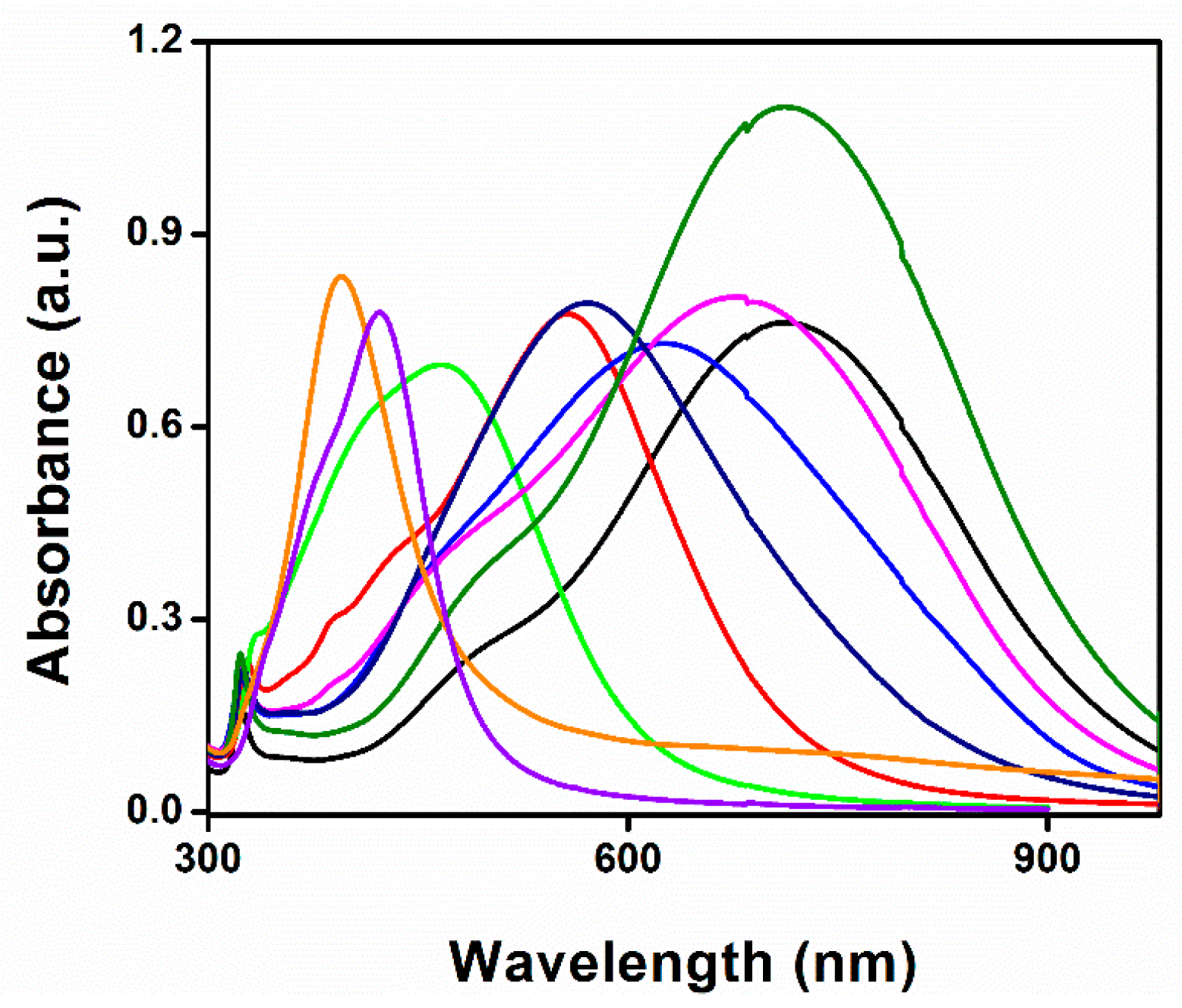
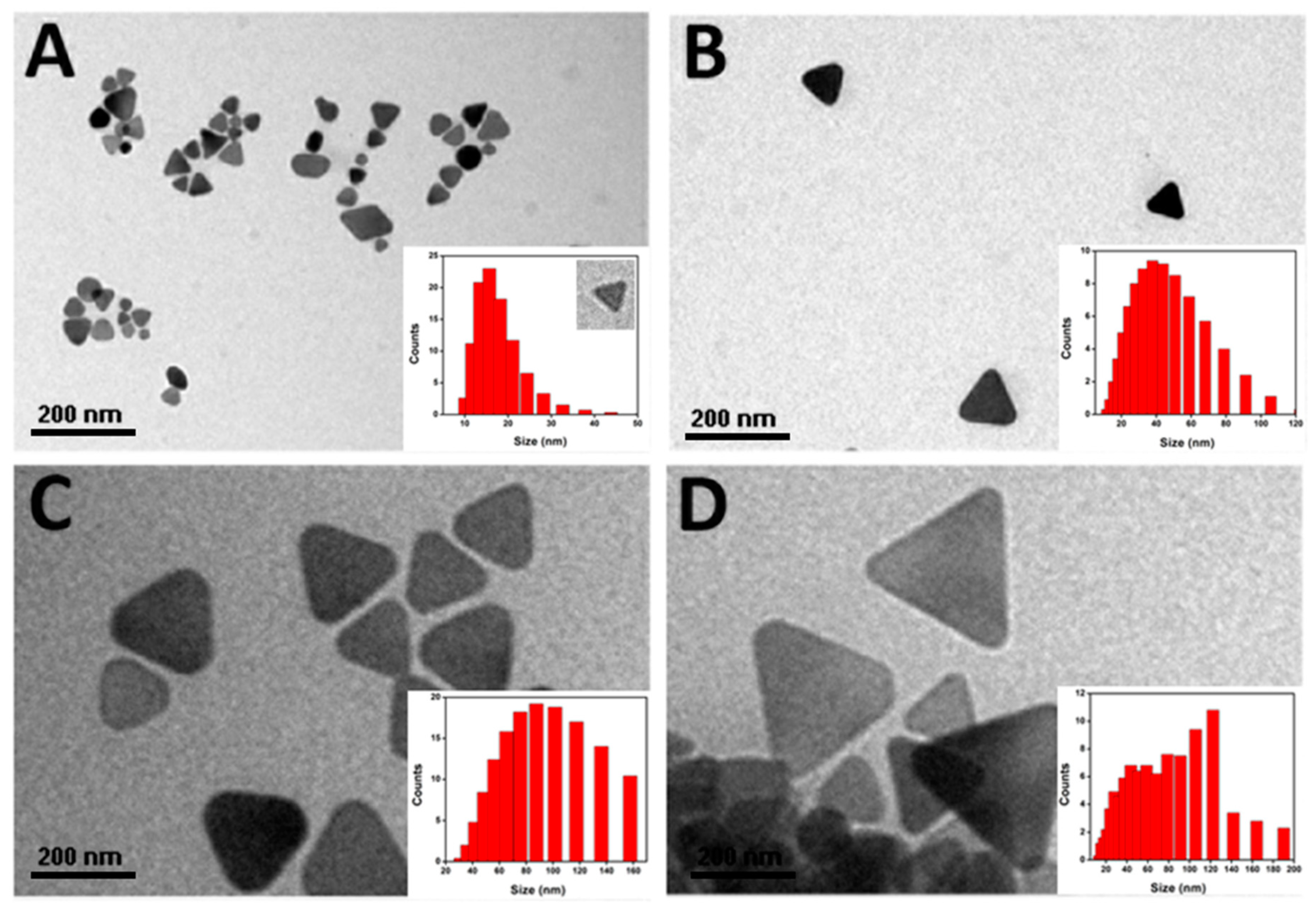
3.4. Size-Dispersal of Ag-NPrs
3.5. Silica Shell Formation and UV–Vis Spectra of Ag-NPrs, before and after Coating
3.6. Cytotoxicity of Ag-NPrs before and after Silica Coating
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- El-Sayed, M.A. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; McIlwrath, K.; Jackson, G.; Eichhorn, B. Enhanced CO tolerance for hydrogen activation in Au-Pt dendritic heteroaggregate nanostructures. J. Am. Chem. Soc. 2006, 128, 1780–1781. [Google Scholar] [CrossRef] [PubMed]
- Willets, K.A.; Van Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, B.; Angelomé, P.C.; Lechuga, L.M.; Liz-Marzán, L.M. LSPR-based nanobiosensors. Nano Today 2009, 4, 244–251. [Google Scholar] [CrossRef]
- Barnes, W.L.; Dereux, A.; Ebbesen, T.W. Surface plasmon subwavelength optics. Nature 2003, 424, 824. [Google Scholar] [CrossRef] [PubMed]
- Mirkin, C.A.; Letsinger, R.L.; Mucic, R.C.; Storhoff, J.J. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 1996, 382, 607. [Google Scholar] [CrossRef] [PubMed]
- Maillard, M.; Giorgio, S.; Pileni, M.P. Silver nanodisks. Adv. Mater. 2002, 14, 1084–1086. [Google Scholar] [CrossRef]
- Salzemann, C.; Urban, J.; Lisiecki, I.; Pileni, M.P. Characterization and growth process of copper nanodisks. Adv. Funct. Mater. 2005, 15, 1277–1284. [Google Scholar] [CrossRef]
- Zhang, J.; Langille, M.R.; Mirkin, C.A. Synthesis of silver nanorods by low energy excitation of spherical plasmonic seeds. Nano lett. 2011, 11, 2495–2498. [Google Scholar] [CrossRef]
- Pérez-Juste, J.; Liz-Marzán, L.M.; Carnie, S.; Chan, D.Y.; Mulvaney, P. Electric-field-directed growth of gold nanorods in aqueous surfactant solutions. Adv. Funct. Mater. 2004, 14, 571–579. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-dimensional nanostructures: synthesis, characterization, and applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Kim, F.; Connor, S.; Song, H.; Kuykendall, T.; Yang, P. Titelbild: Platonic Gold Nanocrystals. Angew. Chem. 2004, 116, 3699–3699. [Google Scholar]
- Millstone, J.E.; Park, S.; Shuford, K.L.; Qin, L.; Schatz, G.C.; Mirkin, C.A. Observation of a quadrupole plasmon mode for a colloidal solution of gold nanoprisms. J. Am. Chem. Soc. 2005, 127, 5312–5313. [Google Scholar] [CrossRef]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; You-Sheng, O.Y.; Chen, Y.B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biot. 2010, 85, 1115–1122. [Google Scholar] [CrossRef]
- Perez-Mayen, L.; Oliva, J.; Salas, P.; De la Rosa, E. Nanomolar detection of glucose using SERS substrates fabricated with albumin coated gold nanoparticles. Nanoscale 2016, 8, 11862–11869. [Google Scholar] [CrossRef]
- Millstone, J.E.; Hurst, S.J.; Métraux, G.S.; Cutler, J.I.; Mirkin, C.A. Colloidal gold and silver triangular nanoprisms. Small 2009, 5, 646–664. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, W.; Corredor, C.; Xu, S.; An, J.; Zhao, B.; Lombardi, J.R. Laser-induced growth of monodisperse silver nanoparticles with tunable surface plasmon resonance properties and a wavelength self-limiting effect. J. Phys. Chem. C 2007, 111, 14962–14967. [Google Scholar] [CrossRef]
- Xiong, Y.; Siekkinen, A.R.; Wang, J.; Yin, Y.; Kim, M.J.; Xia, Y. Synthesis of silver nanoplates at high yields by slowing down the polyol reduction of silver nitrate with polyacrylamide. J. Mater. Chem. 2007, 17, 2600–2602. [Google Scholar] [CrossRef]
- Xu, X.; Du, Z.; Wu, W.; Wang, Y.; Zhang, B.; Mao, X.; Jiang, L.; Yang, J.; Hou, S. Synthesis of triangular silver nanoprisms and spectroscopic analysis on the interaction with bovine serum albumin. Anal. Bioanal. Chem. 2017, 409, 5327–5336. [Google Scholar] [CrossRef]
- Sun, Y.; Mayers, B.; Xia, Y. Transformation of silver nanospheres into nanobelts and triangular nanoplates through a thermal process. Nano Lett. 2003, 3, 675–679. [Google Scholar] [CrossRef]
- Tsuji, M.; Gomi, S.; Maeda, Y.; Matsunaga, M.; Hikino, S.; Uto, K.; Tsuji, T.; Kawazumi, H. Rapid transformation from spherical nanoparticles, nanorods, cubes, or bipyramids to triangular prisms of silver with PVP, citrate, and H2O2. Langmuir 2012, 28, 8845–8861. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Métraux, G.S.; Millstone, J.E.; Mirkin, C.A. Mechanistic study of photomediated triangular silver nanoprism growth. J. Am. Chem. Soc. 2008, 130, 8337–8344. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Cao, Y.; Mirkin, C.A.; Kelly, K.L.; Schatz, G.C.; Zheng, J.G. Photoinduced conversion of silver nanospheres to nanoprisms. Science 2001, 294, 1901–1903. [Google Scholar] [CrossRef] [PubMed]
- Saade, J.; de Araújo, C.B. Synthesis of silver nanoprisms: a photochemical approach using light emission diodes. Mater. Chem. Phys. 2014, 148, 1184–1193. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Y.; Guo, S.; Goebl, J.; Yin, Y. Seeded growth of uniform Ag nanoplates with high aspect ratio and widely tunable surface plasmon bands. Nano Lett. 2010, 10, 5037–5042. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Xia, X.; Rycenga, M.; Henneghan, P.; Li, Q.; Xia, Y. Successive deposition of silver on silver nanoplates: lateral versus vertical growth. Angew. Chem. Int. Ed. 2011, 50, 244–249. [Google Scholar] [CrossRef]
- Métraux, G.S.; Mirkin, C.A. Rapid thermal synthesis of silver nanoprisms with chemically tailorable thickness. Adv. Mater. 2005, 17, 412–415. [Google Scholar] [CrossRef]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Ren, B.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef]
- Xu, H. Theoretical study of coated spherical metallic nanoparticles for single-molecule surface-enhanced spectroscopy. Appl. Phys. Lett. 2004, 85, 5980–5982. [Google Scholar] [CrossRef]
- Feng, J.J.; Gernert, U.; Sezer, M.; Kuhlmann, U.; Murgida, D.H.; David, C.; Richter, M.; Knorr, A.; Hildebrandt, P.; Weidinger, I.M. Novel Au-Ag hybrid device for electrochemical SE(R)R spectroscopy in a wide potential and spectral range. Nano Lett. 2008, 9, 298–303. [Google Scholar] [CrossRef]
- Xue, C.; Chen, X.; Hurst, S.J.; Mirkin, C.A. Self-Assembled Monolayer Mediated Silica Coating of Silver Triangular Nanoprisms. Adv. Mater. 2007, 19, 4071–4074. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotech. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Gong, P.; Li, H.; He, X.; Wang, K.; Hu, J.; Tan, W.; Zhang, S.; Yang, X. Preparation and antibacterial activity of Fe3O4@Ag nanoparticles. Nanotech 2007, 18, 285604. [Google Scholar] [CrossRef]
- Benn, T.M.; Westerhoff, P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Asz, J.; Asz, D.; Moushey, R.; Seigel, J.; Mallory, S.B.; Foglia, R.P. Treatment of toxic epidermal necrolysis in a pediatric patient with a nanocrystalline silver dressing. J. Pediatr. Surg. 2006, 41, e9–e12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vemula, P.K.; Ajayan, P.M.; John, G. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. 2008, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Braydich-Stolle, L.; Hussain, S.; Schlager, J.J.; Hofmann, M.C. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol. Sci. 2005, 88, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Senapati, D.; Wang, S.; Tovmachenko, O.; Singh, A.K.; Yu, H.; Ray, P.C. Effect of surface coating on the toxicity of silver nanomaterials on human skin keratinocytes. Chem. Phys. Lett. 2010, 487, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Mani, E. Mechanism and modeling of poly[vinylpyrrolidone] (PVP) facilitated synthesis of silver nanoplates. Phys. Chem. Chem. Phys. 2018, 20, 15507–15517. [Google Scholar] [CrossRef] [PubMed]
- Haber, J.; Sokolov, K. Synthesis of stable citrate-capped silver nanoprisms. Langmuir 2017, 33, 10525–10530. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, Y.N.; Kim, J.; Choi, W.M.; Park, S.H.; Kim, M.H. A systematic study of triangular silver nanoplates: one-pot green synthesis, chemical stability, and sensing application. Nanoscale 2017, 9, 11705–11712. [Google Scholar] [CrossRef]
- Popa, M.; Pradell, T.; Crespo, D.; Calderón-Moreno, J.M. Stable silver colloidal dispersions using short chain polyethylene glycol. Colloid Surf. A Physicochem. Eng. Asp. 2007, 303, 184–190. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Goebl, J.; Lu, Z.; Yin, Y. A systematic study of the synthesis of silver nanoplates: is citrate a “magic” reagent? J. Am. Chem. Soc. 2011, 133, 18931–18939. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Hallaj, T.; Salari, R. A highly sensitive plasmonic sensor for detection of selenium based on the shape transformation of silver nanoprisms. Sens. Actuat. B Chem. 2018, 273, 1307–1312. [Google Scholar] [CrossRef]
- Jin, R.; Cao, Y.C.; Hao, E.; Métraux, G.S.; Schatz, G.C.; Mirkin, C.A. Controlling anisotropic nanoparticle growth through plasmon excitation. Nature 2003, 425, 487. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Carroll, D.L. Silver nanoplates: size control in two dimensions and formation mechanisms. J. Phys. Chem. B 2004, 108, 5500–5506. [Google Scholar] [CrossRef]
- Foda, M.F.; Huang, L.; Shao, F.; Han, H.Y. Biocompatible and highly luminescent near-infrared CuInS2/ZnS quantum dots embedded silica beads for cancer cell imaging. ACS Appl. Mater. Interfaces 2014, 6, 2011–2017. [Google Scholar] [CrossRef]
- Carboni, M.; Carravetta, M.; Zhang, X.L.; Stulz, E. Efficient NIR light blockage with matrix embedded silver nanoprism thin films for energy saving window coating. J. Mater. Chem. C 2016, 4, 1584–1588. [Google Scholar] [CrossRef]
- Huang, L.; Luo, Z.; Han, H. Organosilane micellization for direct encapsulation of hydrophobic quantum dots into silica beads with highly preserved fluorescence. Chem. Commun. 2012, 48, 6145–6147. [Google Scholar] [CrossRef]

| No. | H2O | PVP (17.5 mM) | AgNO3 (0.01 M) | DSSH (0.1M) | TSCD (75 mM) | H2O2 | NaBH4 (100 mM) |
|---|---|---|---|---|---|---|---|
| 1 | 9.68 mL | 0.045 mL | 0.1 mL | 0.05 mL | 0.1 mL | 0.0245 mL | 0.1 mL |
| 2 | 9.68 mL | 0.045 mL | 0.1 mL | 0.1 mL | 0.05 mL | 0.0245 mL | 0.1 mL |
| 3 | 9.68 mL | 0.045 mL | 0.1 mL | 0.025 mL | 0.125 mL | 0.0245 mL | 0.1 mL |
| 4 | 9.68 mL | 0.045 mL | 0.1 mL | 0.125 mL | 0.025 mL | 0.0245 mL | 0.1 mL |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahlol, H.S.; Foda, M.F.; Ma, J.; Han, H. Robust Synthesis of Size-Dispersal Triangular Silver Nanoprisms via Chemical Reduction Route and Their Cytotoxicity. Nanomaterials 2019, 9, 674. https://doi.org/10.3390/nano9050674
Bahlol HS, Foda MF, Ma J, Han H. Robust Synthesis of Size-Dispersal Triangular Silver Nanoprisms via Chemical Reduction Route and Their Cytotoxicity. Nanomaterials. 2019; 9(5):674. https://doi.org/10.3390/nano9050674
Chicago/Turabian StyleBahlol, Hagar S., Mohamed F. Foda, Jing Ma, and Heyou Han. 2019. "Robust Synthesis of Size-Dispersal Triangular Silver Nanoprisms via Chemical Reduction Route and Their Cytotoxicity" Nanomaterials 9, no. 5: 674. https://doi.org/10.3390/nano9050674
APA StyleBahlol, H. S., Foda, M. F., Ma, J., & Han, H. (2019). Robust Synthesis of Size-Dispersal Triangular Silver Nanoprisms via Chemical Reduction Route and Their Cytotoxicity. Nanomaterials, 9(5), 674. https://doi.org/10.3390/nano9050674