Lipidic Liquid Crystalline Cubic Phases and Magnetocubosomes as Methotrexate Carriers
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Cubic Phases and Cubosomes
2.2.1. Dynamic Light Scattering (DLS) and Zeta Potential
2.2.2. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
2.3. Electrochemical Measurements
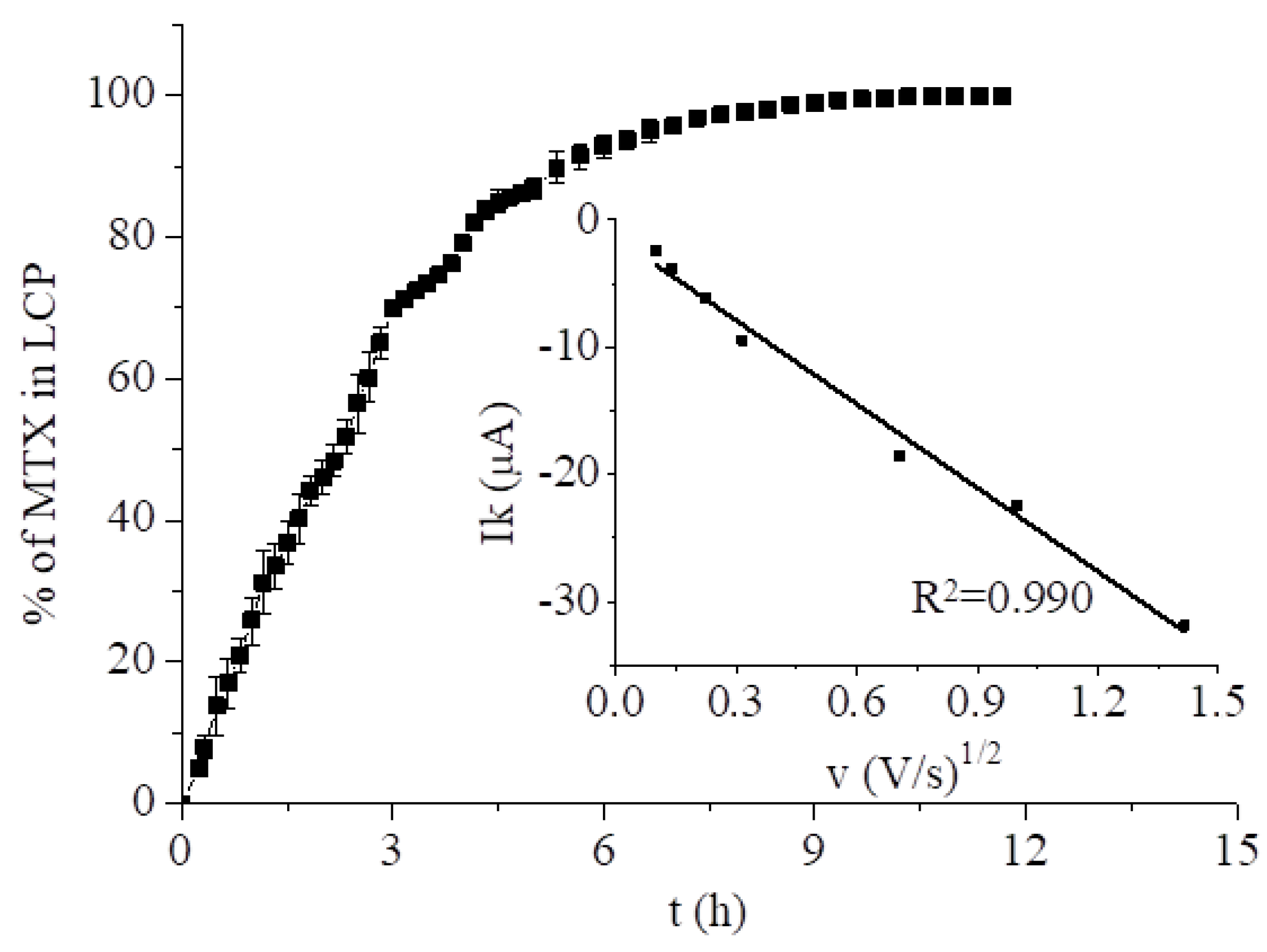
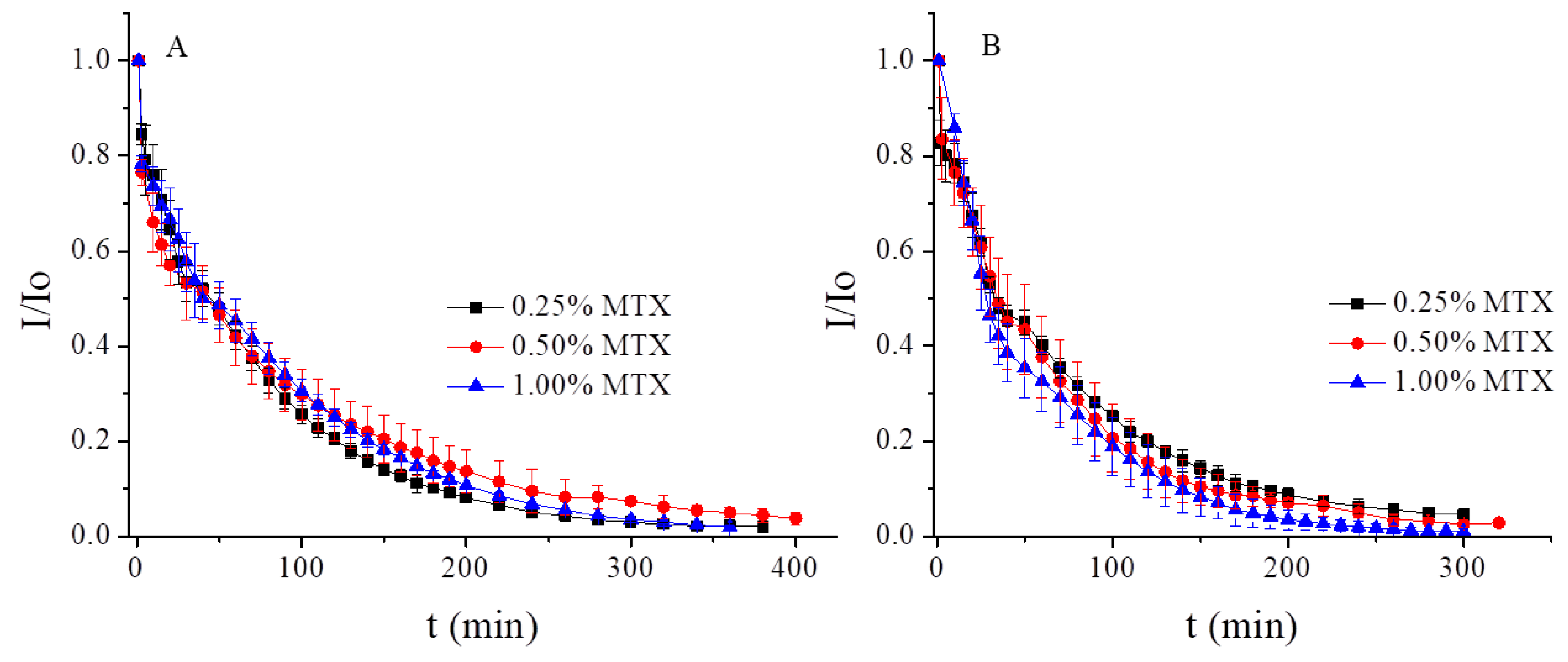
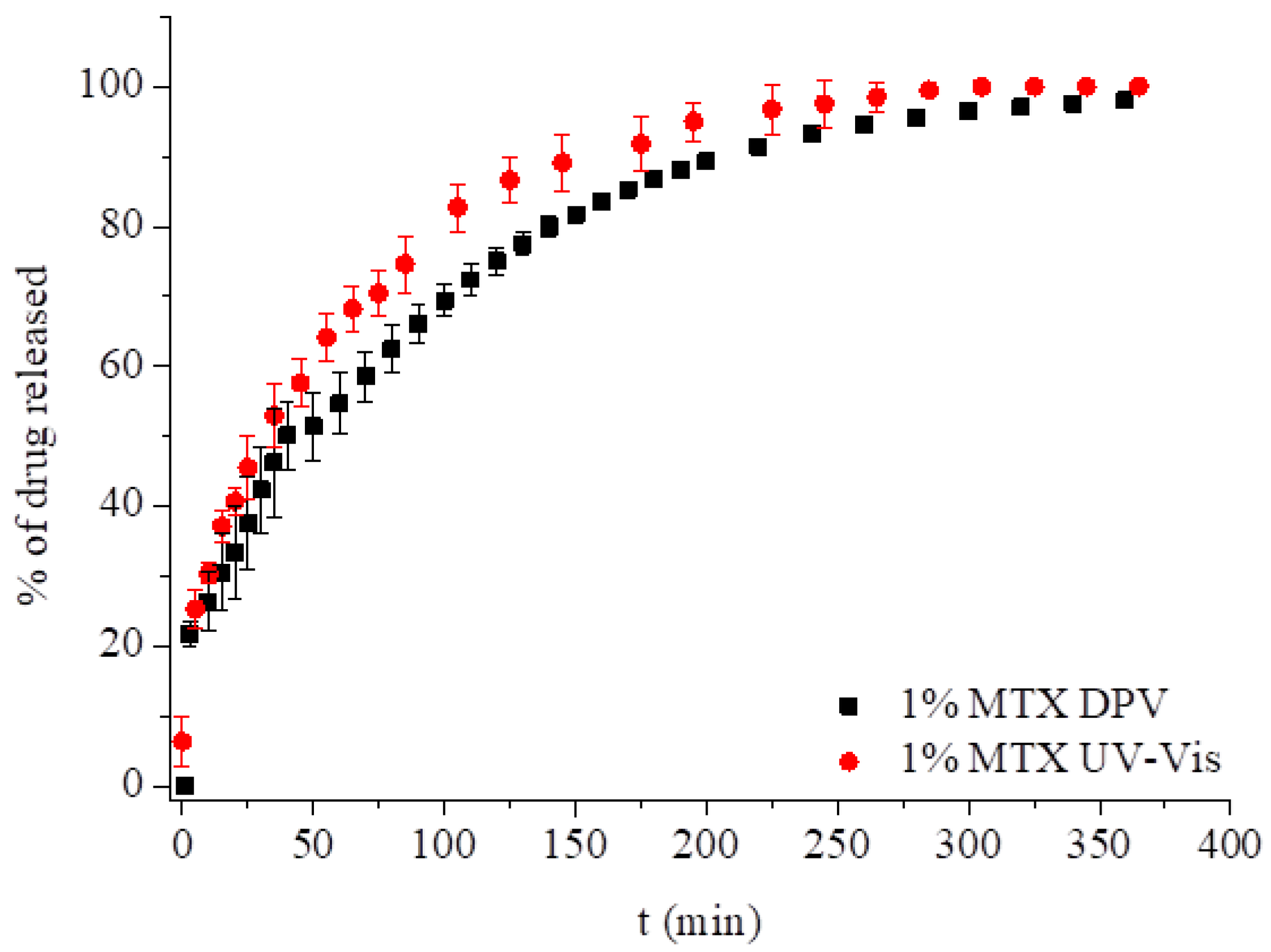
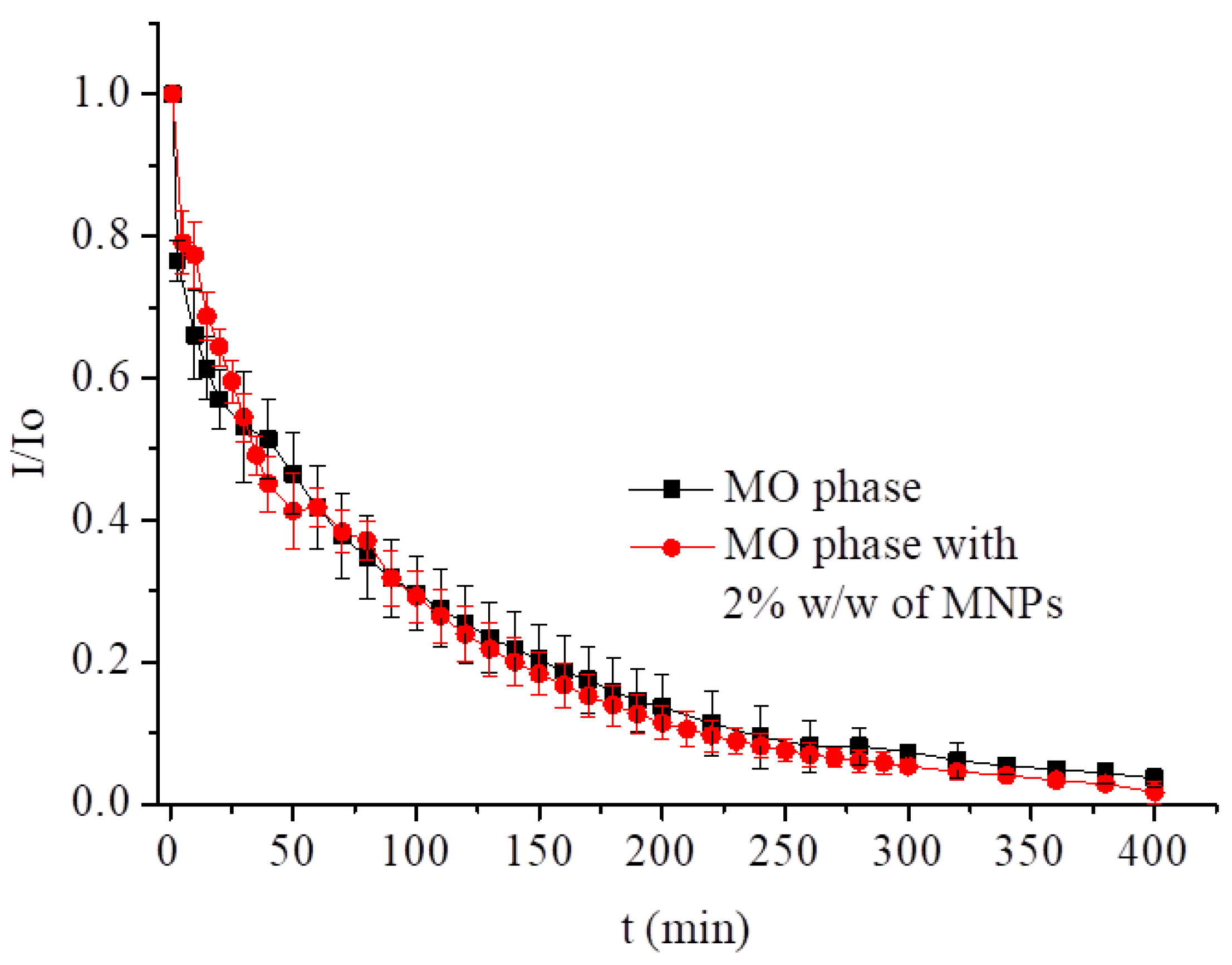
2.4. Modeling of the Kinetics of Drug Release
2.5. Magnetic Field Generator
2.6. Spectroscopic Measurements
3. Results and Discussion
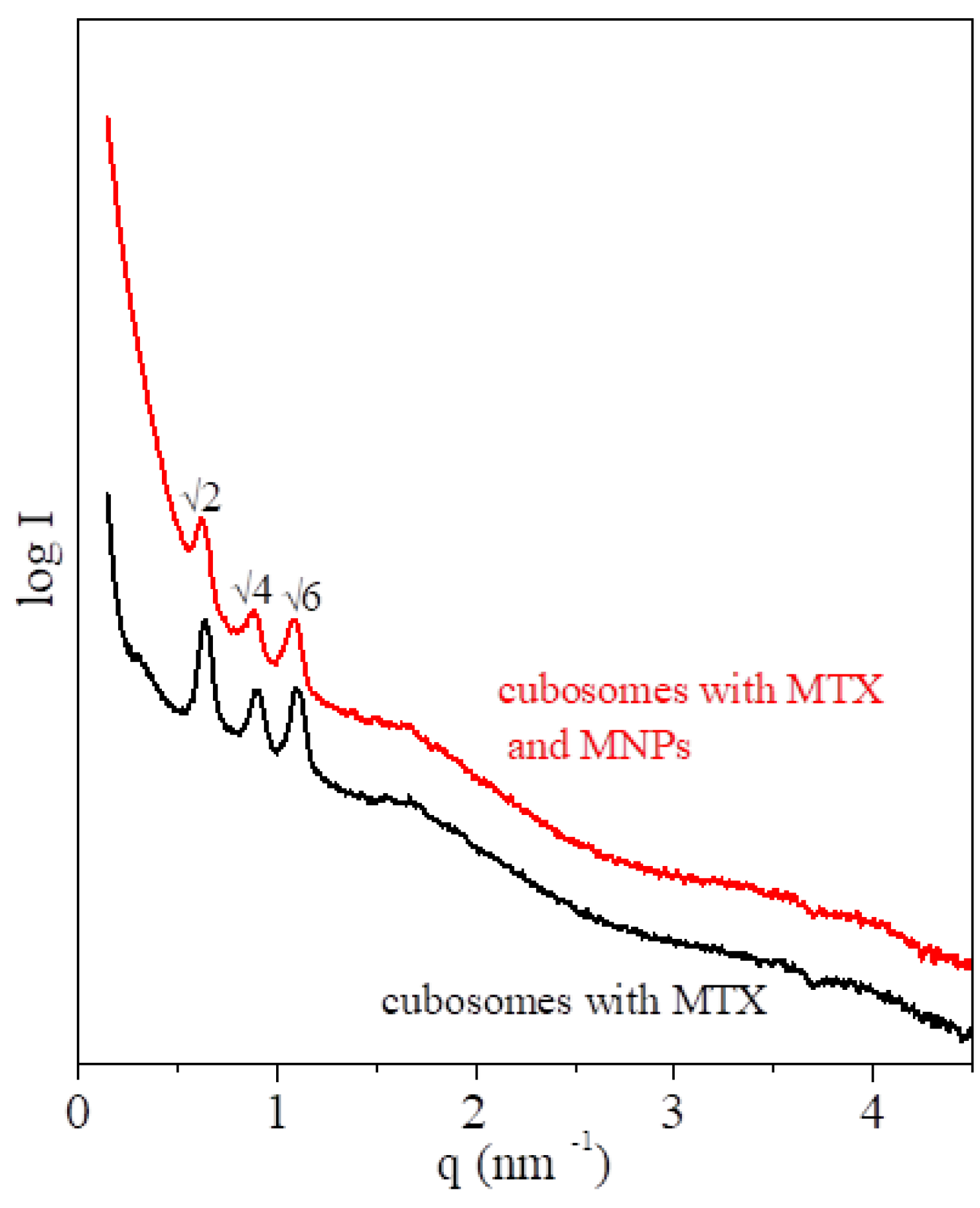
3.1. Structural Characterization of the MTX-Doped Cubic Phases
3.2. Electrochemical Measurements
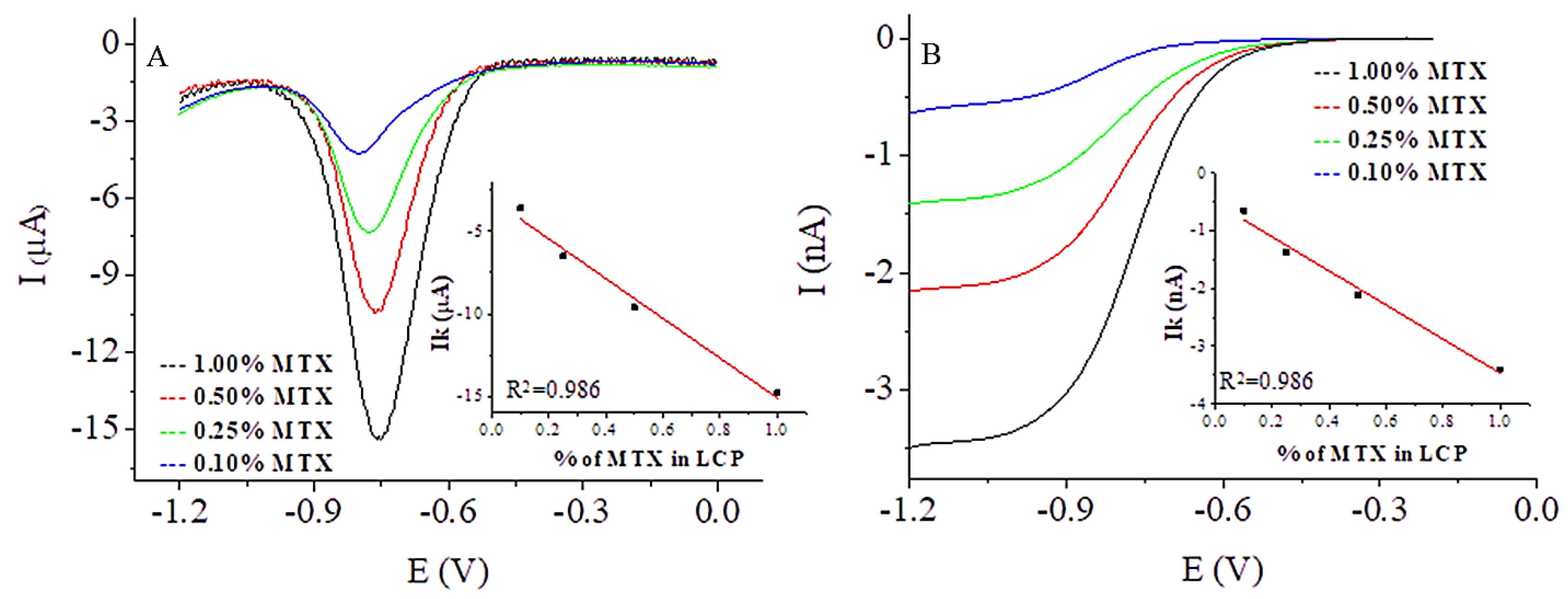
3.2.1. Methotrexate Incorporated in the Monoolein Cubic Phase
3.2.2. Behavior of MTX Incorporated into Hybrid LCP Systems
3.2.3. MTX Incorporated into Magnetocubosomes
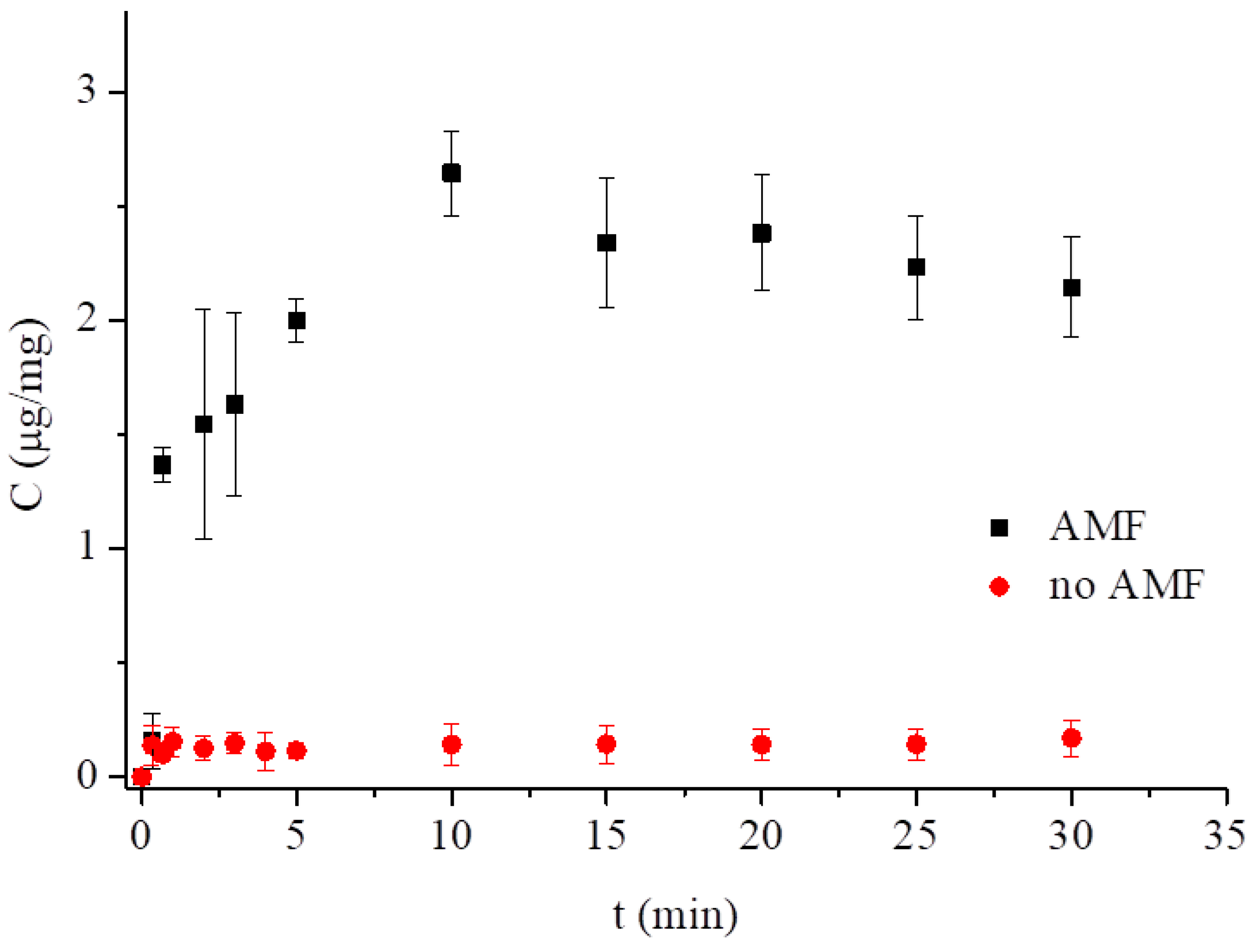
3.3. Low-Frequency Alternating Magnetic Field (AMF)-Stimulated Drug Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MTX | methotrexate |
| SAXS | small angle X-ray scattering |
| LCP | liquid crystalline phase |
| DDS | drug delivery system |
| AMF | alternating magnetic field |
| MO | monoolein |
| MNPs | magnetic nanoparticles |
| DLS | dynamic light scattering |
| Cryo-TEM | cryogenic transmission electron microscopy |
| DPV | differential pulse voltammetry |
| GC | glassy carbon |
| GCE | glassy carbon electrode |
| SWV | square-wave voltammetry |
| CV | cyclic voltammetry |
| EE | entrapment efficiency |
References
- Genestier, L.; Paillot, R.; Quemeneur, L.; Izeradjene, K.; Revillard, J.-P. Mechanisms of action of methotrexate. Immunopharmacology 2000, 47, 247–257. [Google Scholar] [CrossRef]
- Bleyer, W.A. The clinical pharmacology of Methotrexate. Cancer 1978, 41, 36–51. [Google Scholar] [CrossRef]
- Yoon, S.-A.; Choi, J.R.; Kim, J.-O.; Shin, J.-Y.; Zhang, X.H.; Kang, J.-H. Influence of Reduced Folate Carrier and Dihydrofolate Reductase Genes on Methotrexate-Induced Cytotoxicity. Cancer Res. Treat. 2010, 42, 163–171. [Google Scholar] [CrossRef]
- Kanarek, N.; Keys, H.R.; Cantor, J.R.; Lewis, C.A.; Chan, S.H.; Kunchok, T.; Abu-Remaileh, M.; Freinkman, E.; Schweitzer, L.D.; Sabatini, D.M. Histidine catabolism is a major determinant of methotrexate sensitivity. Nature 2018, 559, 632–655. [Google Scholar] [CrossRef] [PubMed]
- Rozensza, L.A.; Radnay, J. The effect of methotrexate on transformation and mitosis of normal human-blood lymphocytes in-vitro. Blood 1974, 43, 401–409. [Google Scholar]
- Bookbinder, S.A.; Espinoza, L.R.; Fenske, N.A.; Germain, B.F.; Vasey, F.B. Methotrexate therapy in the rheumatic disease. Clin. Exp. Rheumatol. 1984, 2, 185–193. [Google Scholar]
- Zeb, A.; Qureshi, O.S.; Kim, H.-S.; Cha, J.-H.; Kim, H.-S.; Kim, J.-K. Improved skin permeation of methotrexate via nanosized ultradeformable liposomes. Int. J. Nanomed. 2016, 11, 3813–3824. [Google Scholar] [CrossRef]
- Srinivas, P.S.; Babu, D.R.S. Formulation and evaluation of parenteral methotrexate nanoliposomes. Int. J. Pharm. Pharm. Sci. 2014, 6, 295–300. [Google Scholar]
- Rosenholm, J.M.; Peuhu, E.; Bate-Eya, L.T.; Eriksson, J.E.; Sahlgren, C.; Linden, M. Cancer-cell-specific induction of apoptosis using mesoporous silica nanoparticles as drug-delivery vectors. Small 2010, 6, 1234–1241. [Google Scholar] [CrossRef]
- de Oliveira Freitas, L.B.; Gonzalez Bravo, I.J.; de Almeida Macedo, W.A.; de Sousa, E.M.B. Mesoporous silica materials functionalized with folic acid: Preparation, characterization and release profile study with methotrexate. J. Sol-Gel Sci. Technol. 2016, 77, 186–204. [Google Scholar] [CrossRef]
- Kakkar, D.; Dumoga, S.; Kumar, R.; Chuttani, K.; Mishra, A.K. PEGylated solid lipid nanoparticles: Design, methotrexate loading and biological evaluation in animal models. Med. Chem. Commun. 2015, 6, 1452–1463. [Google Scholar] [CrossRef]
- Ferreira, M.; Silva, E.; Barreiros, L.; Segundo, M.A.; Costa Lima, S.A.; Reis, S. Methotrexate loaded lipid nanoparticles for topical management of skin-related diseases: Design, characterization and skin permeation potential. Int. J. Pharm. 2016, 512, 14–21. [Google Scholar] [CrossRef]
- Hashada, R.A.; Ishaka, R.A.H.; Geneidia, A.S.; Mansoura, S. Methotrexate loading in chitosan nanoparticles at a novel pH: Response surface modeling, optimization and characterization. Int. J. Biol. Macromol. 2016, 91, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Pretti, T.S.; Souza, M.A.; Santos, H.T.; Nascimento, A.C.G.G.; Santos, F.J.; Fraga, A.F.; Jafelicci, M.J.; Marques, R.F.C. Drug delivery nanotechnology applied to Methotrexate controlled release in human osteosarcona: In vitro test. Available online: http://www.metallum.com.br/7colaob/resumos/trabalhos_completos/08-020.docx (accessed on 15 April 2019).
- Guo, F.; Fan, Z.; Yang, J.; Li, Y.; Wang, Y.; Zhao, H.; Xie, L.; Hou, Z. A comparative evaluation of hydroxycamptothecin drug nanorods with and without methotrexate prodrug functionalization for drug delivery. Nanoscale Res. Lett. 2016, 11, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.D.F.; Pereira, L.G.R.; Barbosa, L.A.D.O.; Fialho, S.L.; Pereira, B.G.; Patricio, P.S.D.O.; Pinto, F.C.H.; Da Silva, G.R. Efficacy of methotrexate-loaded poly(e-caprolactone) implants in Ehrlich solid tumor-bearing mice. Drug Deliv. 2013, 2, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Hamishehkar, H.; Arsalani, N.; Entezami, A.A. A novel dual-responsive core-crosslinked magnetic-gold nanogel for triggered drug release. Mater. Sci. Eng. C 2016, 68, 436–444. [Google Scholar] [CrossRef]
- Lina, L.; Xua, W.; Lianga, H.; Hea, L.; Liua, S.; Lia, Y.; Lia, B.; Chena, Y. Construction of pH-sensitive lysozyme/pectin nanogel for tumor methotrexate delivery. Colloid. Surface. B 2015, 126, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, J.; Cao, F.; Lee, R.J.; Zhai, Z. Lyotropic liquid crystal systems in drug deliver. Drug Discov. Today 2010, 1032–1040. [Google Scholar] [CrossRef]
- Angelova, A.; Garamus, V.M.; Angelov, B. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemical and anti-tumor agents. Adv. Colloid Interf. Sci. 2017, 249, 331–345. [Google Scholar] [CrossRef]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The next generation of smart lipid nanoparticles? Angew. Chem. Int. Ed. 2018, 57, 2–23. [Google Scholar] [CrossRef]
- Kulkarni, C.V.; Wachter, W.; Iglesias-Salto, G.; Engelskirchenb, S.; Ahualliac, S. Monoolein: A magic lipid? Phys. Chem. Chem. Phys. 2011, 13, 3004–3021. [Google Scholar] [CrossRef]
- Nazaruk, E.; Szlȩzak, M.; Górecka, E.; Bilewicz, R.; Osornio, Y.M.; Uebelhart, P.; Landau, E.M. Design and Assembly of pH-Sensitive Lipidic Cubic Phase Matrices for Drug Release. Langmuir 2014, 30, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Negrini, R.; Sanchez-Ferrer, A.; Mezzenga, R. Influence of Electrostatic Interactions on the Release of Charged Molecules from Lipid Cubic Phases. Langmuir 2014, 30, 4280–4288. [Google Scholar] [CrossRef]
- Negrini, R.; Fong, W.-K.; Boyd, B.J.; Mezzenga, R. pH-responsive lyotropic liquid crystals and their potential therapeutic role in cancer treatment. Chem. Commun. 2015, 51, 6671–6674. [Google Scholar] [CrossRef] [PubMed]
- Negrini, R.; Mezzenga, R. pH-Responsive Lyotropic Liquid Crystals for Controlled Drug Delivery. Langmuir 2011, 27, 5296–5303. [Google Scholar] [CrossRef]
- Szlezak, M.; Nieciecka, D.; Joniec, A.; Pękała, M.; Gorecka, E.; Emo, M.; Stébé, M.J.; Krysiński, P.; Bilewicz, R. Monoolein cubic phase gels and cubosomes doped with magnetic nanoparticles - hybrid materials for controlled drug release. ACS Appl. Mater. Inter. 2017, 9, 2796–2805. [Google Scholar] [CrossRef]
- Fong, W.-K.; Negrini, R.; Vallooran, J.J.; Mezzenga, R.; Boyd, B.J. Responsive self-assembled nanostructured lipid systems for drug delivery and diagnostics. J. Colloid Interf. Sci. 2016, 484, 320–339. [Google Scholar] [CrossRef]
- Bonini, M.; Berti, D.; Baglioni, P. Nanostructures for magnetically triggered release of drugs and biomolecules. Curr. Opin. Colloid Interface Sci. 2013, 18, 459–467. [Google Scholar] [CrossRef]
- Vallooran, J.J.; Negrini, R.; Mezzenga, R. Controlling anisotropic drug diffusion in lipid-Fe3O4 nanoparticle hybrid mesophases by magnetic alignment. Langmuir 2013, 29, 999–1004. [Google Scholar] [CrossRef]
- Monnier, C.A.; Burnand, D.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Magnetoliposomes: Opportunities and challenges. Eur. J. Nanomed. 2014, 6, 201–215. [Google Scholar] [CrossRef]
- Joniec, A.; Sek, S.; Krysinski, P. Magnetoliposomes as Potential Carriers of Doxorubicin to Tumours. Chem.-Eur. J. 2016, 22, 17715–17724. [Google Scholar] [CrossRef]
- Montis, C.; Castroflorio, B.; Mendozza, M.; Salvatore, A.; Berti, D.; Baglioni, P. Magnetocubosomes for the delivery and controlled release of therapeutics. J. Colloid Interf. Sci. 2015, 449, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.K.; Kim, J.C. Cubic phase magnetic nanoparticles. Mol. Cryst. Liq. Cryst. 2015, 607, 123–134. [Google Scholar] [CrossRef]
- Wang, M.H.; Kim, J.-C. Magnetic field-responsive cubosomes containing magnetite and poly(N-isopropylacrylamide). J. Controlled Release 2013, 172, e139. [Google Scholar] [CrossRef]
- Ivkov, R.; DeNardo, S.J.; Daum, W.; Foreman, A.R.; Goldstein, R.; Nemkov, V.S.; DeNardo, G.L. Application of High Amplitude Alternating Magnetic Fields for Heat Induction of Nanoparticles Localized in Cancer. Clin. Cancer Res. 2005, 11, 7093–7103. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Hyde, S.T.; Andersson, S.; Ericsson, B.; Larsson, K. A Cubic Structure Consisting of a Lipid Bilayer Forming an Infinite Periodic Minimum Surface of the Gyroid Type in the Glycerolmonooleat-Water System. Z. Kristallogr. 1984, 168, 213–219. [Google Scholar] [CrossRef]
- Briggs, J.; Chung, H.; Caffrey, M. The Temperature-Composition Phase Diagram and Mesophase Structure Characterization of the Monoolein/Water System. J. Phys. 1996, 6, 723–751. [Google Scholar] [CrossRef]
- Qiu, H.; Caffrey, M. The Phase Diagram of the Monoolein/Water System: Metastability and Equilibrium Aspects. Biomaterials 2000, 21, 223–234. [Google Scholar] [CrossRef]
- Pontinha, A.D.R.; Jorge, S.M.A.; Diculescu, V.C.; Vivan, M.; Oliveira-Brett, A.M. Antineoplasic Drug Methotrexate Redox Mechanism Using a Glassy Carbon Electrode. Electroanalysis 2012, 24, 917–923. [Google Scholar] [CrossRef]
- Gao, L.; Wu, Y.; Liu, J.; Ye, B. Anodic voltammetric behaviors of methotrexate at a glassy carbon electrode and its determination in spiked human urine. J. Electroanal. Chem. 2007, 610, 131–136. [Google Scholar] [CrossRef]
- Acharya, D.P.; Moffat, B.A.; Polyzos, A.; Waddington, L.; Coia, G.; Wright, D.K.; Wang, H.X.; Egan, G.F.; Muir, B.W.; Hartley, P.G. Cubic mesophase nanoparticles doped with superparamagnetic iron oxide nanoparticles: A new class of MRI contrast agent. RSC Adv. 2012, 2, 6655–6662. [Google Scholar] [CrossRef]
- Nazaruk, E.; Miszta, P.; Filipek, S.; Landau, E.M.; Bilewicz, R. Lyotropic cubic phase gels for drug delivery: Tuning diffusion and sustained release from the mesophase. Langmuir 2015, 31, 12753–12761. [Google Scholar] [CrossRef] [PubMed]
- Mendozza, M.; Montis, C.; Caselli, L.; Wolf, M.; Baglionia, P.; Berti, D. On the thermotropic and magnetotropic phase behavior of lipid liquid crystals containing magnetic nanoparticles. Nanoscale 2018, 10, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Reimhult, E. Nanoparticle-triggered release from lipid membrane vesicles. New Biotechnol. 2015, 32, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Bixner, O.; Reimhult, E. Controlled megnetosomes: Embedding of magnetic nanoparticles into membranes of monodisperse lipid vesicles. J. Colloid Interf. Sci. 2016, 466, 62–71. [Google Scholar] [CrossRef]
- Shaghasemi, B.S.; Virk, M.M.; Reimhult, E. Optimization of Magneto-thermally Controlled Release Kinetics by Tuning of Magnetoliposome Composition and Structure. Sci. Rep. 2017, 7, 7474. [Google Scholar] [CrossRef]
- Fortin, J.-P.; Gazeau, F.; Wilhelm, C. Intracellular heating of living cells through Néel relaxation of magnetic nanoparticles. Eur. Biophys. J. 2008, 37, 223–228. [Google Scholar] [CrossRef]
- Leesajakul, W.; Nakano, M.; Taniguchi, A.; Handa, A. Interaction of cubosomes with plasma components resulting in the destabilization of cubosomes in plasma. Colloid. Surfaces B 2004, 34, 253–258. [Google Scholar] [CrossRef]
- Tran, N.; Bye, N.; Moffat, B.A.; Wright, D.K.; Cuddihy, A.; Hinton, T.M.; Hawley, A.M.; Reynolds, N.P.; Waddington, L.J.; Mulet, X.; et al. Dual-modality NIRF-MRI cubosomes and hexosomes: High throughput formulation and in vivo biodistribution. Mater. Sci. Eng. C 2017, 71, 584–593. [Google Scholar] [CrossRef]
- Falchi, A.M.; Rosa, A.; Atzeri, A.; Incani, A.; Lampis, S.; Meli, V.; Caltagirone, C.; Murgia, S. Effects of monoolein based cubosome formulations on lipid droplets and mitochondria of HeLa cells. Toxicol. Res. 2015, 4, 1025–1036. [Google Scholar] [CrossRef]
- Biffi, S.; Andolfi, L.; Caltagirone, C.; Garrovo, C.; Falchi, A.M.; Lippolis, V.; Lorenzon, A.; Maco, P.; Meli, V.; Monduzzi, M.; et al. Cubosomes for in vivo fluorescence lifetime imaging. Nanotechnology 2017, 28, 055102. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, E.; Majkowska, A.; Bilewicz, R. Lipidic Cubic-Phase Nanoparticles—Cubosomes for Efficient Drug Delivery to Cancer Cells. ChemPlusChem 2017, 82, 570–575. [Google Scholar] [CrossRef]
- Nazaruk, E.; Majkowska, A.; Godlewska, M.; Salamonczyk, M.; Gawel, D. Electrochemical and biological characterization of lyotropic liquid crystalline phases—Retardation of drug release from hexagonal mesophases. J. Electroanal. Chem. 2018, 813, 208–215. [Google Scholar] [CrossRef]
- Godlewska, M.; Majkowska-Pilip, A.; Stachurska, A.; Gawel, D.; Nazaruk, E. Voltammetric and biological studies of folate-targeted non-lamellar lipid mesophases. Electrochim. Acta 2019, 299, 1–11. [Google Scholar] [CrossRef]
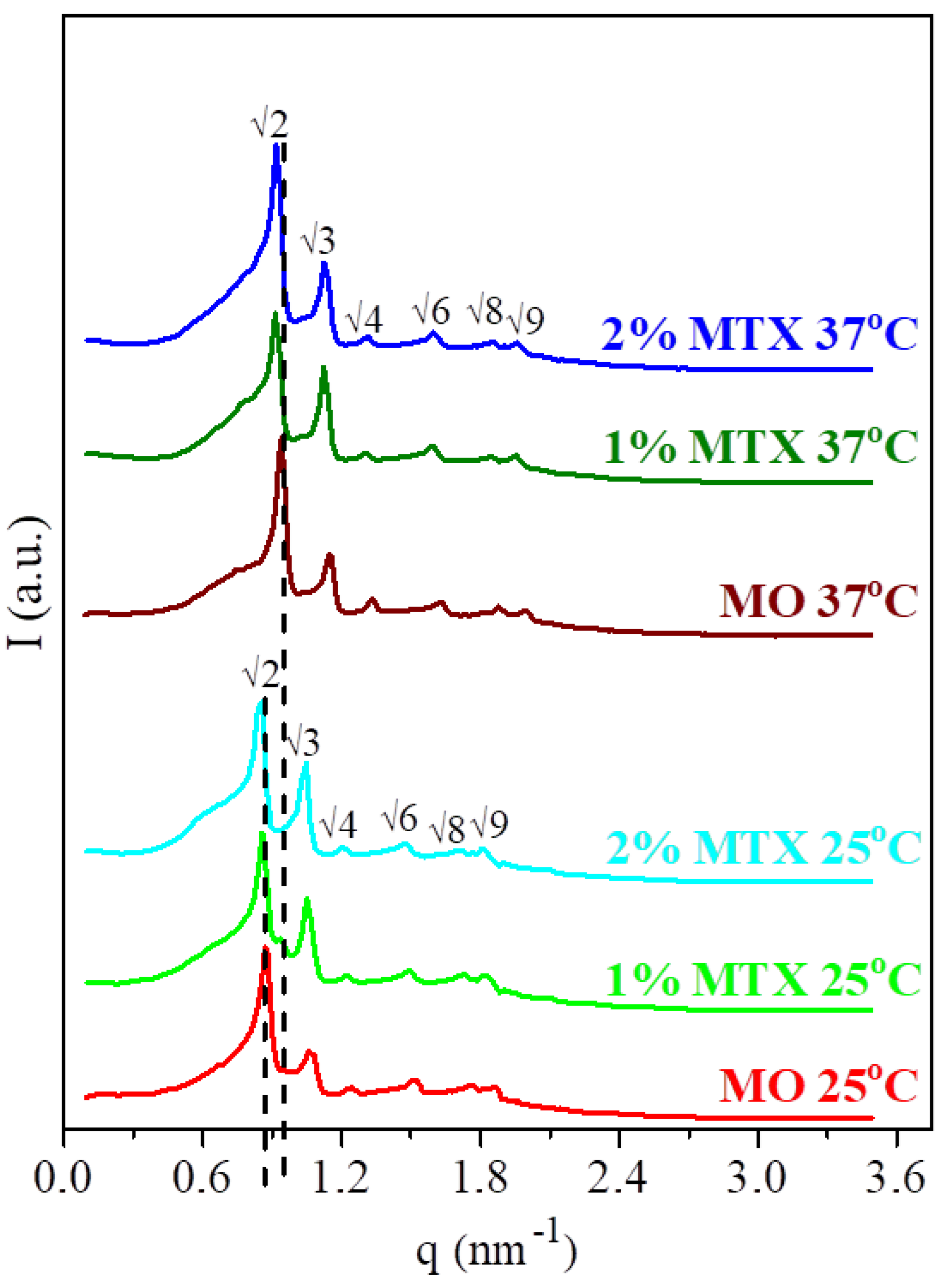
| T [°C] | Symmetry | a [nm] | l [nm] | dw [nm] | |
|---|---|---|---|---|---|
| MO/aq* 60/40% | 25 | Pn3̅m | 10.2 | 1.7 | 4.5 |
| 37 | Pn3̅m | 9.5 | 1.6 | 4.2 | |
| MO/MTX/aq* 59/1/40% | 25 | Pn3̅m | 10.3 | 1.8 | 4.5 |
| 37 | Pn3̅m | 9.8 | 1.7 | 4.3 | |
| MO/MTX/aq* 58/2/40% | 25 | Pn3̅m | 10.3 | 1.8 | 4.6 |
| 37 | Pn3̅m | 9.7 | 1.6 | 4.3 |
| Korsmeyer–Peppas | Higuchi | ||||
|---|---|---|---|---|---|
| % MTX | n | R2 | k [%/hn] | kH [%/h] | R2 |
| 1.0 at 25 °C | 0.44 ± 0.05 | 0.986 ± 0.004 | 56.08 ± 5.40 | 47.49 ± 1.49 | 0.977 ± 0.010 |
| 1.0 at 25 °C CUV-Vis | 0.44 ± 0.01 | 0.998 ± 0.001 | 61.20±1.92 | 58.78 ± 1.32 | 0.997±0.001 |
| 1.0 at 37 °C | 0.65 ± 0.19 | 0.921 ± 0.102 | 66.36 ± 6.32 | a | a |
| 0.5 at 25 °C | 0.41 ± 0.03 | 0.967 ± 0.006 | 62.34 ± 2.22 | 40.51 ± 9.49 | 0.943 ± 0.038 |
| 0.5 at 37 °C | 0.56 ± 0.11 | 0.950 ± 0.043 | 61.38 ± 8.76 | 66.22 ± 4.21 | 0.966 ± 0.020 |
| 0.5 and 2% MNPs at 25 °C | 0.47 ± 0.06 | 0.949 ± 0.014 | 60.60 ± 2.73 | 62.68 ± 5.85 | 0.945 ± 0.021 |
| 0.25 at 25 °C | 0.45 ± 0.08 | 0.975 ± 0.005 | 57.12 ± 3.80 | 53.97 ± 3.38 | 0.982 ± 0.008 |
| 0.25 at 37 °C | 0.57 ± 0.09 | 0.943 ± 0.014 | 63.51 ± 4.18 | 72.21 ± 4.11 | 0.962 ± 0.009 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mierzwa, M.; Cytryniak, A.; Krysiński, P.; Bilewicz, R. Lipidic Liquid Crystalline Cubic Phases and Magnetocubosomes as Methotrexate Carriers. Nanomaterials 2019, 9, 636. https://doi.org/10.3390/nano9040636
Mierzwa M, Cytryniak A, Krysiński P, Bilewicz R. Lipidic Liquid Crystalline Cubic Phases and Magnetocubosomes as Methotrexate Carriers. Nanomaterials. 2019; 9(4):636. https://doi.org/10.3390/nano9040636
Chicago/Turabian StyleMierzwa, Monika, Adrianna Cytryniak, Paweł Krysiński, and Renata Bilewicz. 2019. "Lipidic Liquid Crystalline Cubic Phases and Magnetocubosomes as Methotrexate Carriers" Nanomaterials 9, no. 4: 636. https://doi.org/10.3390/nano9040636
APA StyleMierzwa, M., Cytryniak, A., Krysiński, P., & Bilewicz, R. (2019). Lipidic Liquid Crystalline Cubic Phases and Magnetocubosomes as Methotrexate Carriers. Nanomaterials, 9(4), 636. https://doi.org/10.3390/nano9040636







