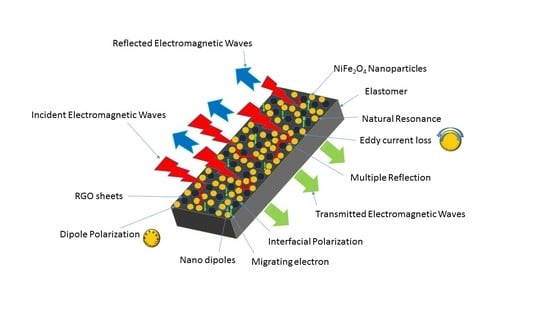
Polypropylene Nanocomposite Filled with Spinel Ferrite NiFe2O4 Nanoparticles and In-Situ Thermally-Reduced Graphene Oxide for Electromagnetic Interference Shielding Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NiFe2O4 Nanoparticles
2.3. Preparation of Graphene Oxide
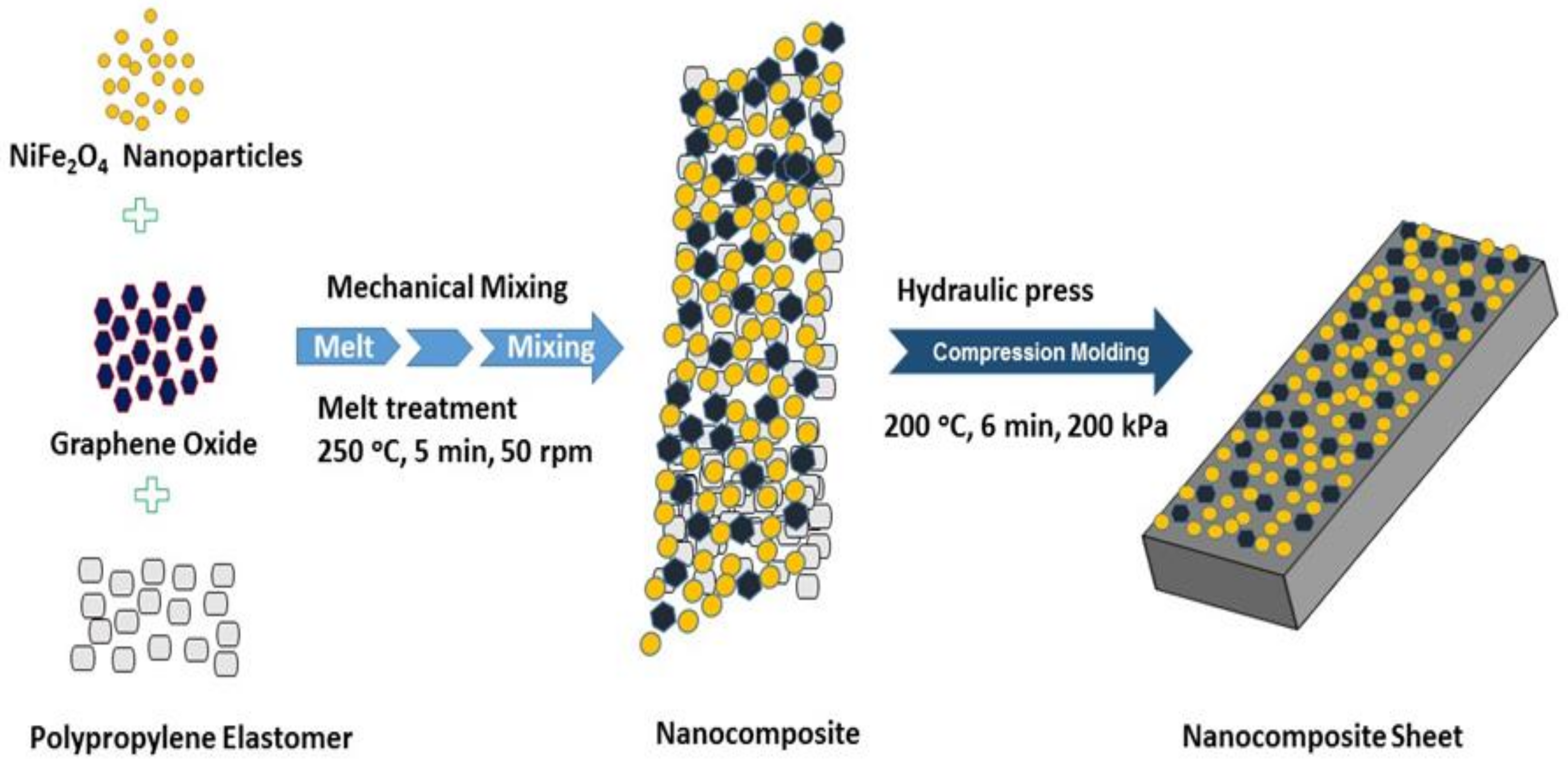
2.4. Formation of Polypropylene Elastomer Nanocomposites Filled with Spinel Ferrite NiFe2O4 Nanoparticles and In-Situ Thermally-Reduced Graphene Oxide (RGO)
2.5. Characterization
3. Results
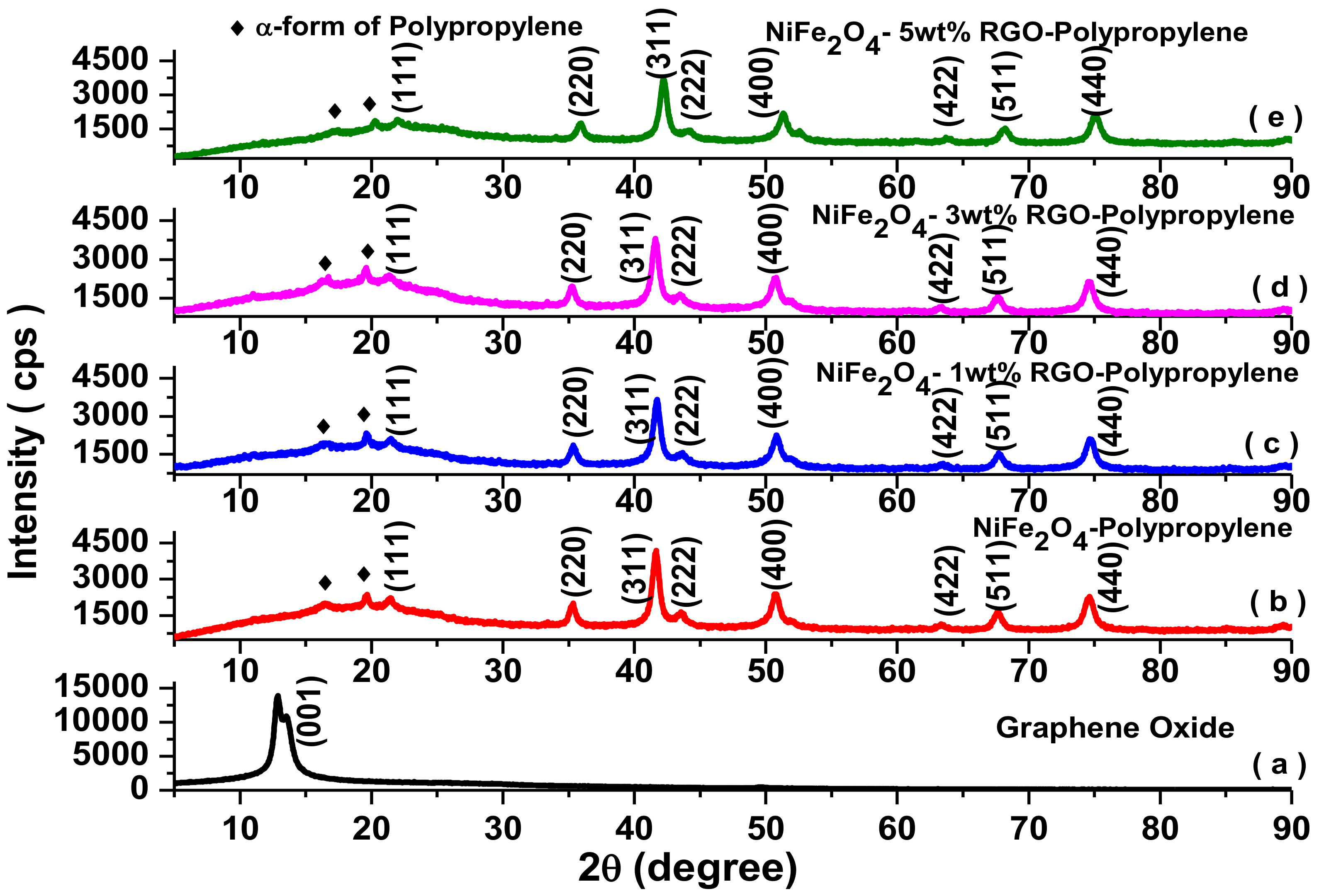
3.1. X-ray Diffraction Study
3.2. Field Emission Scanning Electron Microscopy (FE-SEM) and Transmission Eelectron Microscopy (TEM) Study
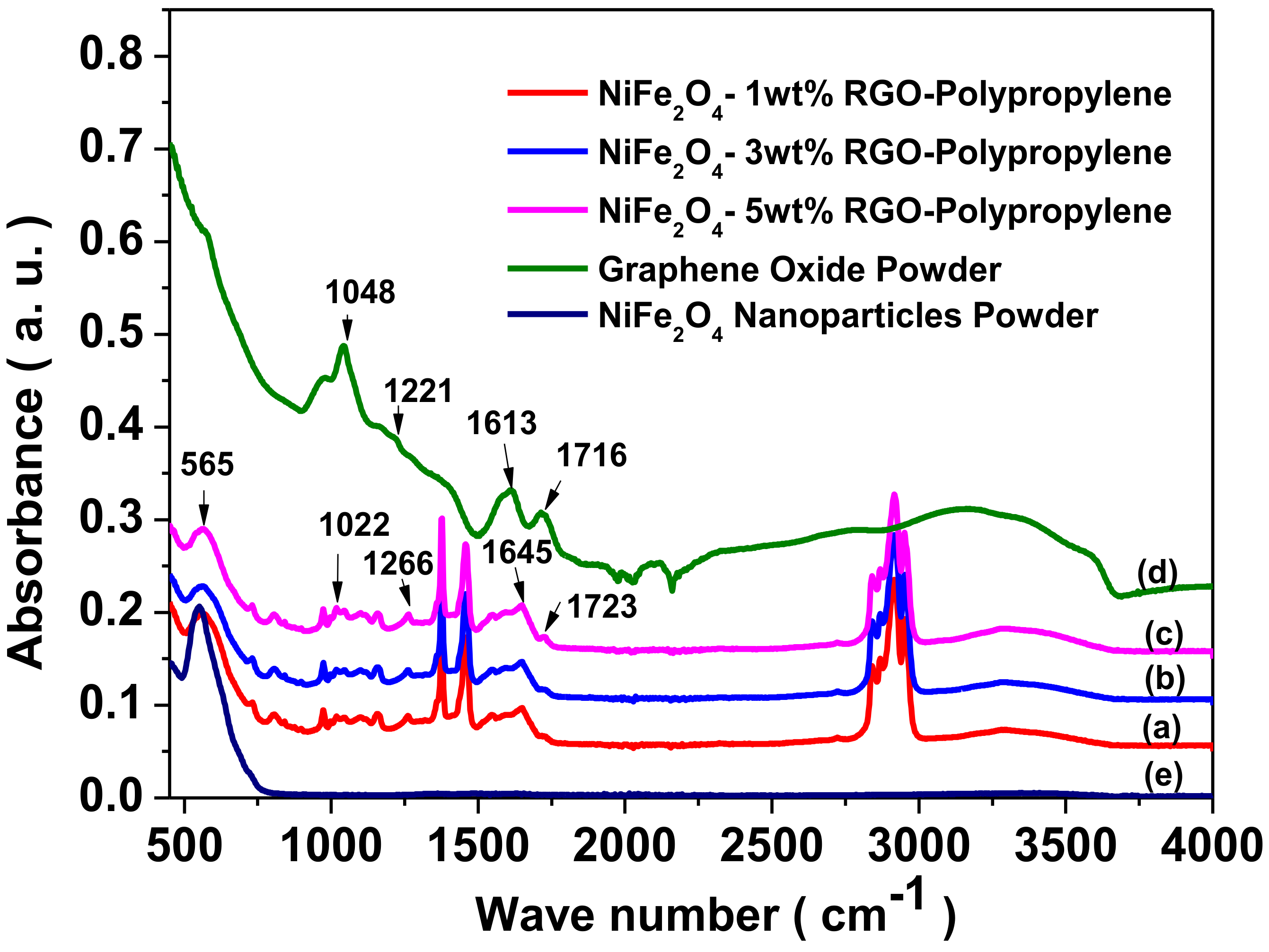
3.3. Fourier Transform Infrared Spectroscopy (FTIR) Study
3.4. Raman Spectra Study
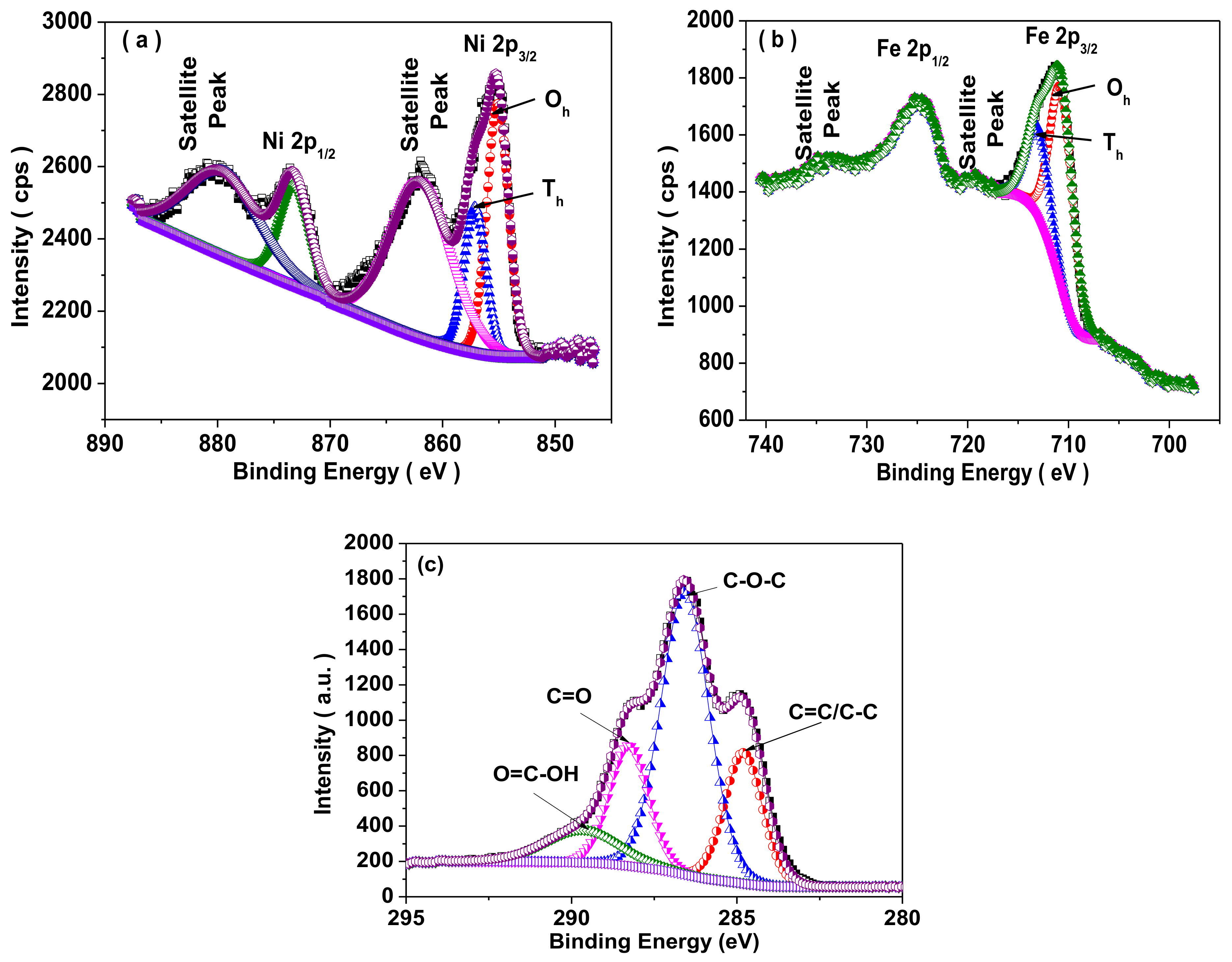
3.5. X-ray Photoelectron Spectroscopy (XPS) Study
3.6. Magnetic Property
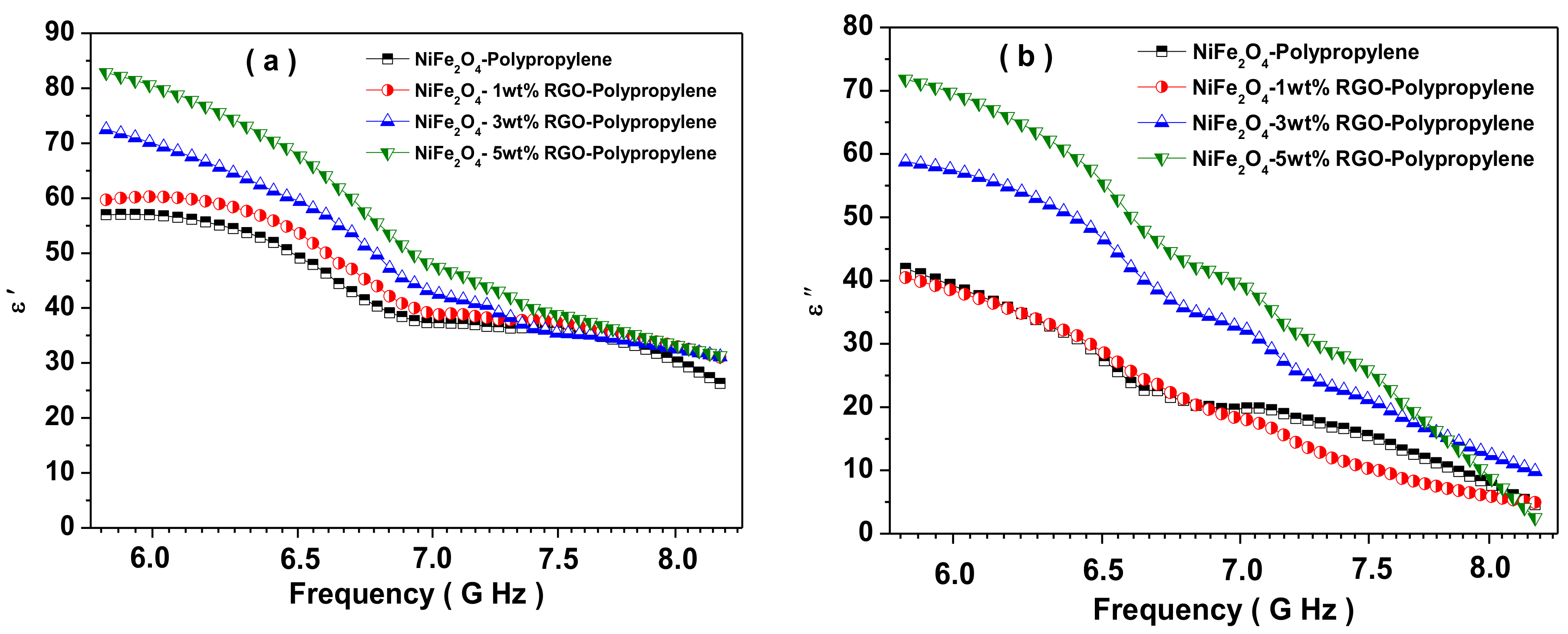
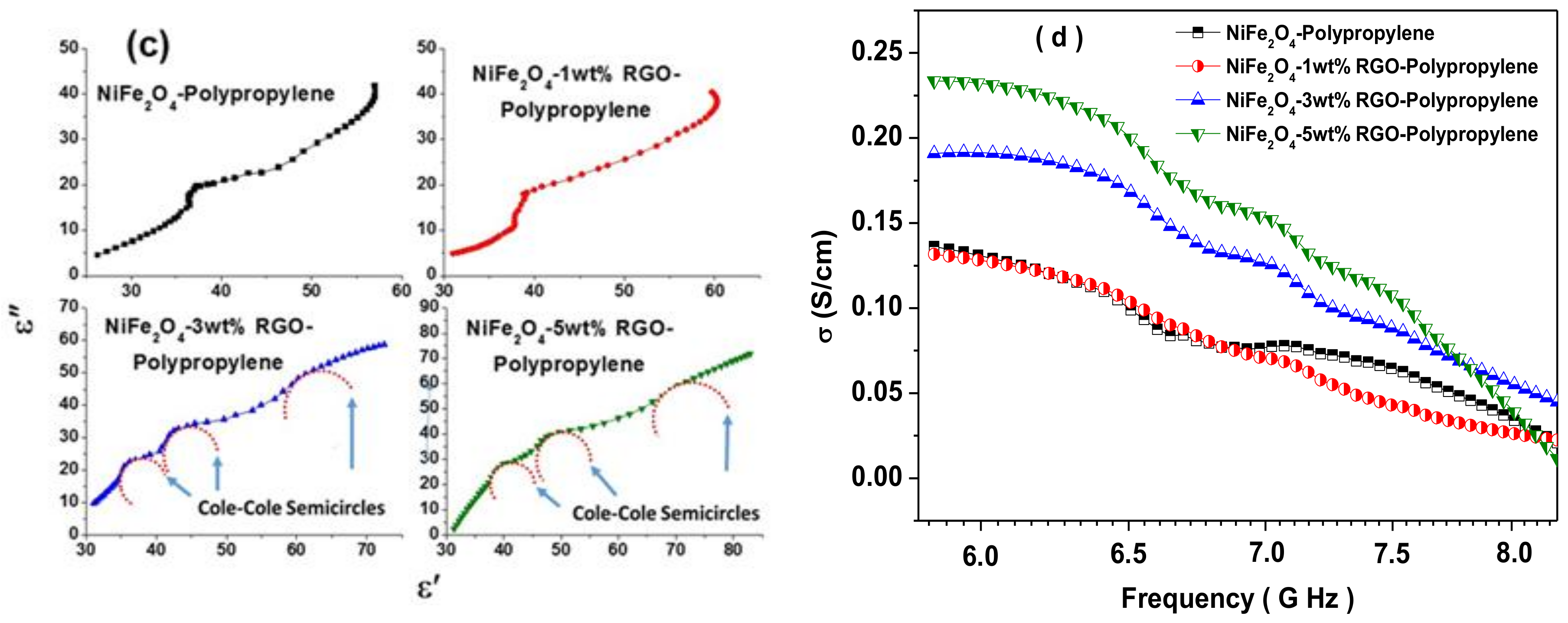
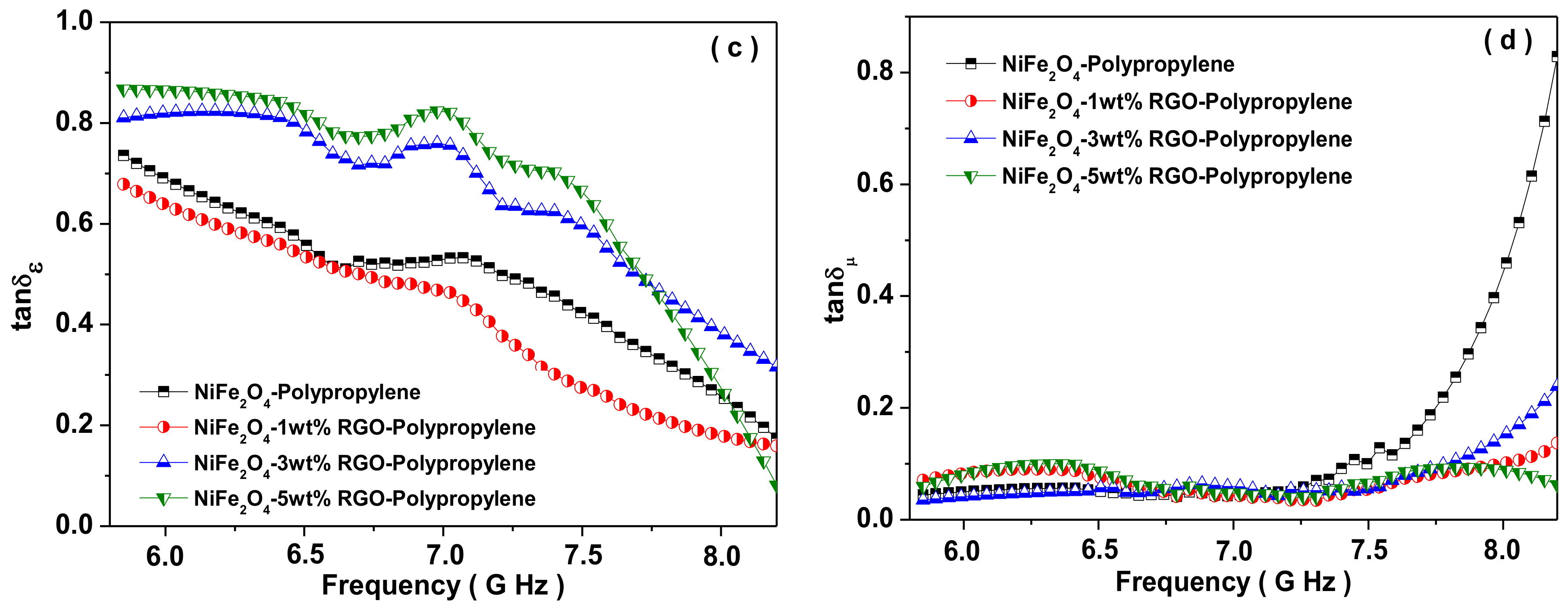
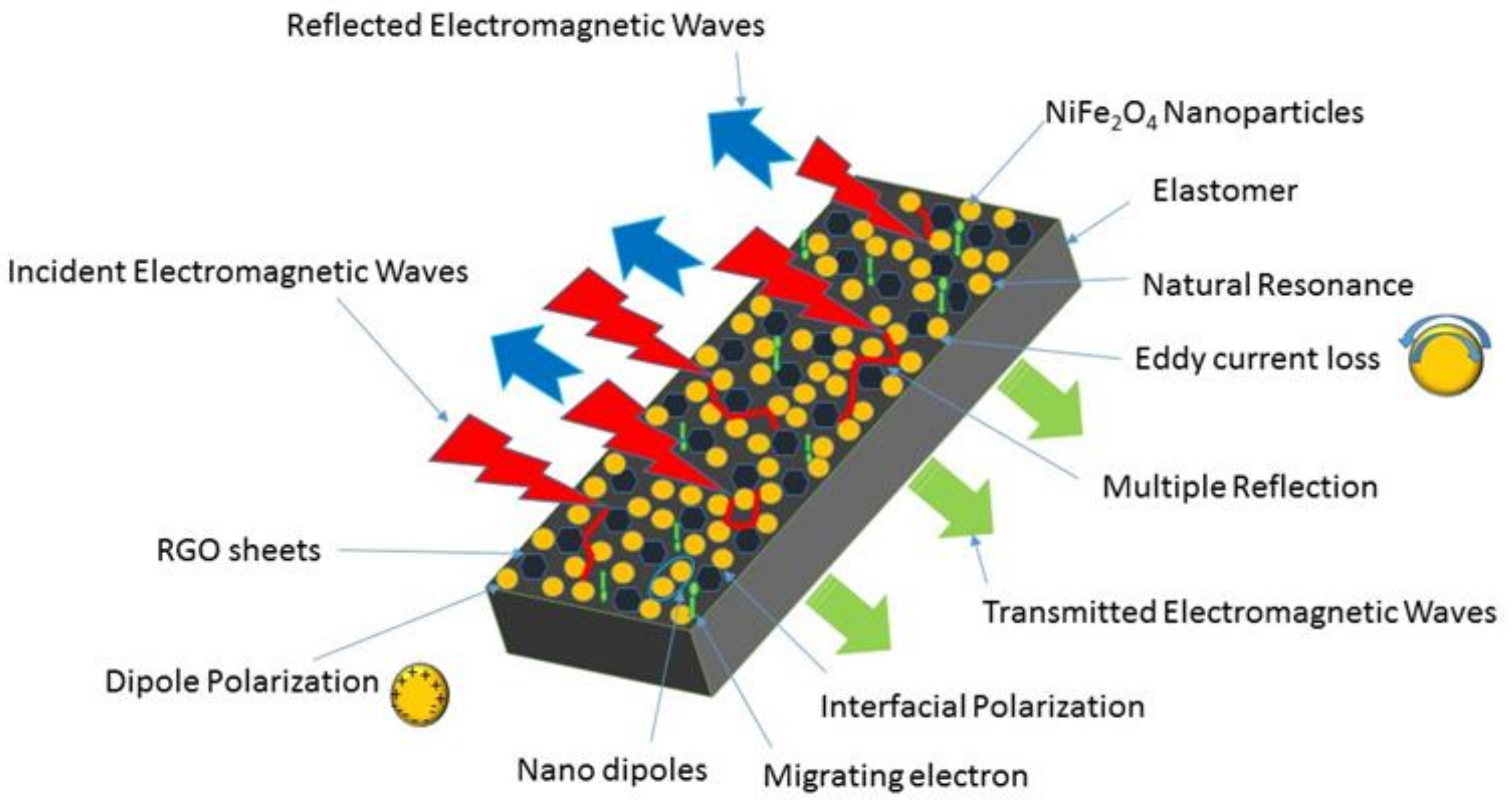
3.7. Electromagnetic Interference Shielding Effectiveness and Electromagnetic Parameters
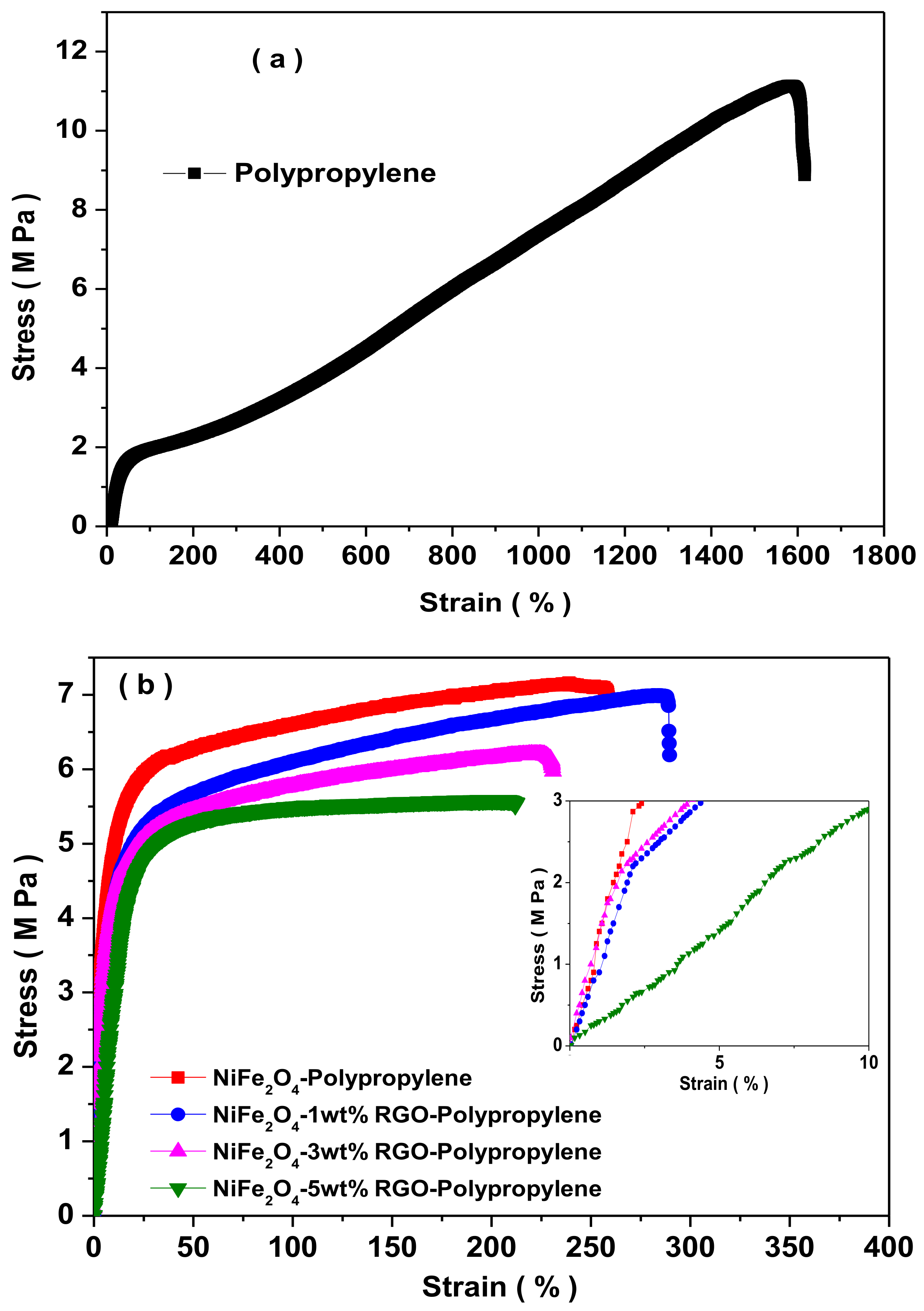
3.8. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Zhang, Z.; Dong, C.; Chen, G.; Wang, Y.; Guan, H. Carbon spheres@MnO2 coreshell nanocomposites with enhanced dielectric properties for electromagnetic shielding. Sci. Rep. 2017, 7, 15841. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lin, J.; Liu, Y.; Fu, H.; Ma, Y.; Jin, P.; Tan, J. Crackle template based metallic mesh with highly homogeneous light transmission for high performance transparent EMI shielding. Sci. Rep. 2016, 6, 25601. [Google Scholar] [CrossRef]
- Song, W.L.; Gong, C.; Li, H.; Cheng, X.D.; Chen, M.; Yuan, X.; Chen, H.; Yang, Y.; Fang, D. Graphene-Based Sandwich Structures for Frequency Selectable Electromagnetic Shielding. ACS Appl. Mater. Interfaces 2017, 9, 36119–36129. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Qi, X.; Cai, H.; Xie, R.; Long, L.; Bai, Z.; Jiang, Y.; Qin, S.; Zhong, W.; Du, Y. Preparation of porous Fe2O3 nanorods-reduced graphene oxide nanohybrids and their excellent microwave absorption properties. Sci. Rep. 2017, 7, 11213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rhee, K.Y.; Park, S.-J. Nanodiamond nanocluster-decorated graphene oxide/epoxy nanocomposites with enhanced mechanical behavior and thermal stability. Compos. Part B 2017, 114, 111–120. [Google Scholar] [CrossRef]
- Zhang, Y.; Rhee, K.Y.; Hui, D.; Park, S.-J. A critical review of nanodiamond based nanocomposites: Synthesis, properties and applications. Compos. Part B 2018, 143, 19–27. [Google Scholar] [CrossRef]
- Hsiao, S.T.; Ma, C.C.; Liao, W.H.; Wang, Y.S.; Li, S.M.; Huang, Y.C.; Yang, R.B.; Liang, W.F. Lightweight and Flexible Reduced Graphene Oxide/Water-Borne Polyurethane Composites with High Electrical Conductivity and Excellent Electromagnetic Interference Shielding Performance. ACS Appl. Mater. Interfaces 2014, 6, 10667–10678. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yang, E.; Cai, H.; Xie, R.; Bai, Z.; Jiang, Y.; Qin, S.; Zhong, W.; Du, Y. Water-assisted and controllable synthesis of core/shell/shell structured carbon-based nanohybrids, and their magnetic and microwave absorption properties. Sci. Rep. 2017, 7, 9851. [Google Scholar] [CrossRef] [PubMed]
- George, G.; Simon, S.M.; Prakashan, V.P.; Sajna, M.S.; Faisal, M.; Wilson, R.; Chandran, A.; Biju, P.R.; Joseph, C.; Unnikrishnan, N.V. Green and facile approach to prepare polypropylene/in situ reduced graphene oxide nanocomposites with excellent electromagnetic interference shielding properties. RSC Adv. 2018, 8, 30412. [Google Scholar] [CrossRef]
- Ameli, A.; Nofar, M.; Wang, S.; Park, C.B. Lightweight Polypropylene/Stainless-Steel Fiber Composite Foams with Low Percolation for Efficient Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2014, 6, 11091–11100. [Google Scholar] [CrossRef]
- Ameli, A.; Jung, P.U.; Park, C.B. Electrical properties and electromagnetic interference shielding effectiveness of polypropylene/carbon fiber composite foams. Carbon 2013, 60, 379–391. [Google Scholar] [CrossRef]
- Hong, M.S.; Choi, W.K.; An, K.H.; Kang, S.J.; Park, S.J.; Lee, Y.S.; Kim, B.J. Electromagnetic interference shielding behaviors of carbon fibers-reinforced polypropylene matrix composites: II. Effects of filler length control. J. Ind. Eng. Chem. 2014, 20, 3901–3904. [Google Scholar] [CrossRef]
- Biswas, S.; Panja, S.S.; Bose, S. Tailored distribution of nanoparticles in bi-phasic polymeric blends as emerging materials for suppressing electromagnetic radiation: Challenges and prospects. J. Mater. Chem. C 2018, 6, 3120–3142. [Google Scholar] [CrossRef]
- Pawar, S.P.; Gandi, M.; Bose, S. High performance electromagnetic wave absorbers derived from PC/SAN blends containing multiwall carbon nanotubes and Fe3O4 decorated onto graphene oxide sheets. RSC Adv. 2016, 6, 37633–37645. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.P.; Varshney, S.; Agrawal, N.; Sambyal, P.; Pandey, Y.; Singh, B.P.; Singh, V.N.; Gupta, B.K.; Dhawan, S.K. New insight into the shape-controlled synthesis and microwave shielding properties of iron oxide covered with reduced graphene oxide. RSC Adv. 2014, 4, 62413–62422. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Cao, M. Confinedly implanted NiFe2O4-rGO: Cluster tailoring and highly tunable electromagnetic properties for selective-frequency microwave absorption. Nano Res. 2018, 11, 1426–1436. [Google Scholar] [CrossRef]
- Liu, P.; Huang, Y.; Zhang, X. Cubic NiFe2O4 particles on graphene–polyaniline and their enhanced microwave absorption properties. Compos. Sci. Technol. 2015, 107, 54–60. [Google Scholar] [CrossRef]
- Ren, F.; Shi, Y.; Ren, P.; Si, X.; Wang, H. Cyanate Ester Resin Filled with Graphene Nanosheets and NiFe2O4-Reduced Graphene Oxide Nanohybrids for Efficient Electromagnetic Interference Shielding. Nano Brief Rep. Rev. 2017, 12, 750066. [Google Scholar] [CrossRef]
- He, J.-Z.; Wang, X.-X.; Zhang, Y.-L.; Cao, M.-S. Small magnetic nanoparticles decorating reduced graphene oxides to tune electromagnetic attenuation capacity. J. Mater. Chem. C 2016, 4, 7130–7140. [Google Scholar] [CrossRef]
- Sabet, M.; Jahangiri, H.; Ghashghaei, E. Synthesis of carbon nanotube, graphene, CoFe2O4, and NiFe2O4 polypyrrole nanocomposites and study their microwave absorption. J. Mater. Sci. Mater. Electron. 2018, 29, 10853–10863. [Google Scholar] [CrossRef]
- Bateer, B.; Zhang, J.; Zhang, H.; Zhang, X.; Wang, C.; Qi, H. Easily Dispersible NiFe2O4/RGO Composite for Microwave Absorption Properties in the X-Band. J. Electron. Mater. 2018, 47, 292–298. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Luo, C.; Wu, X.; Wang, Q.; Chen, W.; Li, J. Synthesis, characterization and enhanced electromagnetic properties of NiFe2O4@SiO2-decorated reduced graphene oxide nanosheets. Ceram. Int. 2016, 42, 17374–17381. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; Chen, X.; Wei, C. Conducting polymers-NiFe2O4 coated on reduced graphene oxide sheets as electromagnetic (EM) wave absorption materials. Synth. Met. 2016, 221, 291–298. [Google Scholar] [CrossRef]
- Liu, P.; Huang, Y.; Sun, X. NiFe2O4 clusters on the surface of reduced graphene oxide and their excellent microwave absorption properties. Mater. Lett. 2013, 112, 117–120. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuřitka, I.; Vilcakova, J.; Skoda, D.; Urbánek, P.; Machovsky, M.; Masař, M.; Kalina, L.; Havlica, J. Lightweight NiFe2O4-Reduced Graphene Oxide-Elastomer Nanocomposite Flexible Sheet for Electromagnetic Interference Shielding Application. Compos. Part B Eng. 2019, 166, 95–111. [Google Scholar] [CrossRef]
- Yadav, R.S.; Havlica, J.; Masilko, J.; Kalina, L.; Wasserbauer, J.; Hajdúchová, M.; Enev, V.; Kuřitka, I.; Kožáková, Z. Effects of annealing temperature variation on the evolution of structural and magnetic properties of NiFe2O4 nanoparticles synthesized by starch-assisted sol–gel auto-combustion method. J. Magn. Magn. Mater. 2015, 394, 439–447. [Google Scholar] [CrossRef]
- Nazim, S.; Kousar, T.; Shahid, M.; Khan, M.A.; Nasar, G.; Sher, M.; Warsi, M.F. New graphene-CoxZn1-xFe2O4 nano-heterostructures: Magnetically separable visible light photocatalytic materials. Ceram. Int. 2016, 42, 7647–7654. [Google Scholar] [CrossRef]
- Yang, H.; Yu, Z.; Wu, P.; Zou, H.; Liu, P. Electromagnetic interference shielding effectiveness of microcellular polyimide/in situ thermally reduced graphene oxide/carbon nanotubes nanocomposites. Appl. Surface Sci. 2018, 434, 318–325. [Google Scholar] [CrossRef]
- Bhawal, P.; Ganguly, S.; Das, T.K.; Mondal, S.; Choudhury, S.; Das, N.C. Superior electromagnetic interference shielding effectiveness and electro-mechanical properties of EMA-IRGO nanocomposites through the in-situ reduction of GO from melt blended EMA-GO composites. Compos. Part B 2018, 134, 46–60. [Google Scholar] [CrossRef]
- Bagotia, N.; Choudhary, V.; Sharma, D.K. Superior electrical, mechanical and electromagnetic interference shielding properties of polycarbonate/ethylene-methyl acrylate-in situ reduced graphene oxide nanocomposites. J. Mater. Sci. 2018, 53, 16047–16061. [Google Scholar] [CrossRef]
- Glover, A.J.; Cai, M.; Overdeep, K.R.; Kranbuehl, D.E.; Schniepp, H.C. In Situ Reduction of Graphene Oxide in Polymers. Macromolecules 2011, 44, 9821–9829. [Google Scholar] [CrossRef]
- Karakaş, Z.K.; Boncukçuoğlu, R.; Karakaş, İ.H. The effects of fuel type in synthesis of NiFe2O4 nanoparticles by microwave assisted combustion method. J. Phys. Conf. Ser. 2016, 707, 012046. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Liao, S.H.; Lin, Y.F.; Wang, C.A.; Pu, N.W.; Tsai, H.M.; Ma, C.C. Preparation and characterization of polypropylene-graft-thermally reduced graphite oxide with an improved compatibility with polypropylene-based nanocomposite. Nanoscale 2011, 3, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, X.; Zha, J.-W.; Zhao, J.; Dang, Z.-M.; Hu, G.-H. Dielectric properties of reduced graphene oxide/polypropylene composites with ultralow percolation threshold. Polymer 2013, 54, 1916–1922. [Google Scholar] [CrossRef]
- Xu, J.; Gai, S.; He, F.; Niu, N.; Gao, P.; Chen, Y.; Yang, P. Reduced graphene oxide/Ni1−xCoxAl-layered double hydroxide composites: Preparation and high supercapacitor performance. Dalton Trans. 2014, 43, 11667–11675. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Patil, R.; Rutman, D.; Kucknoor, A.S.; Wang, A.; Guo, Z. Ionic liquid assisted electrospinning of quantum dots/elastomer composite Nanofibers. Polymer 2011, 52, 1954–1962. [Google Scholar] [CrossRef]
- Jang, J.; Lee, D.K. Oxygen barrier properties of biaxially oriented polypropylene/polyvinyl alcohol blend films. Polymer 2004, 45, 1599–1607. [Google Scholar] [CrossRef]
- Zong, M.; Huang, Y.; Zhang, N.; Wu, H. Influence of (RGO)/(ferrite) ratios and graphene reduction degree on microwave absorption properties of graphene composites. J. Alloys Compd. 2015, 644, 491–501. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. A green approach for the reduction of graphene oxide by wild carrot root. Carbon 2012, 50, 914–921. [Google Scholar] [CrossRef]
- Anupama, M.K.; Srinatha, N.; Matteppanavar, S.; Angadi, B.; Sahoo, B.; Rudraswamy, B. Effect of Zn substitution on the structural and magnetic properties of nanocrystalline NiFe2O4 ferrites. Ceram. Int. 2018, 44, 4946–4954. [Google Scholar] [CrossRef]
- Chavan, A.R.; Kounsalye, J.S.; Chilwar, R.R.; Kale, S.B.; Jadhav, K.M. Cu2+ substituted NiFe2O4 thin films via spray pyrolysis technique and their high-frequency devices application. J. Alloys Compd. 2018, 769, 1132–1145. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Al-Hazmi, F.S.; Memesh, L.S.; Shokr, F.S.; Bronstein, L.M. Effect of mechanochemical synthesis on the structure, magnetic and optical behavior of Ni1-xZnxFe2O4 spinel ferrites. Ceram. Int. 2017, 43, 6192–6200. [Google Scholar] [CrossRef]
- Ding, Y.; Liao, Q.; Liu, S.; Guo, H.; Sun, Y.; Zhang, G.; Zhang, Y. Reduced Graphene Oxide Functionalized with Cobalt Ferrite Nanocomposites for Enhanced Efficient and Lightweight Electromagnetic Wave Absorption. Sci. Rep. 2016, 6, 32381. [Google Scholar] [CrossRef]
- Khurana, G.; Kumar, N.; Kotnala, R.K.; Nautiyal, T.; Katiyar, R.S. Temperature tuned defect induced magnetism in reduced graphene oxide. Nanoscale 2013, 5, 3346. [Google Scholar] [CrossRef]
- Ahmad, S.R.; Young, R.J.; Kinloch, I.A. Raman Spectra and Mechanical Properties of Graphene/Polypropylene Nanocomposites. Int. J. Chem. Eng. Appl. 2015, 6, 1–5. [Google Scholar]
- Nikolaeva, G.Y.; Sagitova, E.A.; Prokhorov, K.A.; Pashinin, P.P.; Nedorezova, P.M.; Klyamkina, A.N.; Guseva, M.A.; Gerasin, V.A. Using Raman spectroscopy to determine the structure of copolymers and polymer blends. J. Phys. Conf. Ser. 2017, 826, 012002. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuřitka, I.; Vilcakova, J.; Havlica, J.; Masilko, J.; Kalina, L.; Tkacz, J.; Enev, V.; Hajdúchová, M. Structural, magnetic, dielectric, and electrical properties of NiFe2O4 spinel ferrite nanoparticles prepared by honey-mediated sol-gel combustion. J. Phys. Chem. Solids 2017, 107, 150–161. [Google Scholar] [CrossRef]
- Aghavniana, T.; Moussy, J.-B.; Stanescu, D.; Belkhou, R.; Jedrecy, N.; Magnan, H.; Ohresser, P.; Arrio, M.-A.; Sainctavit, P.; Barbier, A. Determination of the cation site distribution of the spinel in multiferroic CoFe2O4/BaTiO3 layers by X-ray photoelectron spectroscopy. J. Electron Spectrosc. Relat. Phenom. 2015, 202, 16–21. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuřitka, I.; Vilcakova, J.; Havlica, J.; Kalina, L.; Urbánek, P.; Machovsky, M.; Skoda, D.; Masař, M.; Holek, M. Sonochemical synthesis of Gd3+ doped CoFe2O4 spinel ferrite nanoparticles and its physical properties. Ultrason. Sonochem. 2018, 40, 773–783. [Google Scholar] [CrossRef]
- Wang, D.W.; Du, A.; Taran, E.; Lu, G.Q.; Gentle, I.R. A water-dielectric capacitor using hydrated graphene oxide film. J. Mater. Chem. 2012, 22, 21085–21091. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, Y.; Gao, A.; Zhao, S.; Cui, J.; Zhang, G. Flexible Polydimethylsilane Nanocomposites Enhanced with a Three-Dimensional Graphene/Carbon Nanotube Bicontinuous Framework for High-Performance Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2018, 10, 26723–26732. [Google Scholar] [CrossRef] [PubMed]
- Manna, K.; Srivastava, S.K. Contrasting Role of Defect-Induced Carbon Nanotubes in Electromagnetic Interference Shielding. J. Phys. Chem. C 2018, 122, 19913–19920. [Google Scholar] [CrossRef]
- Cao, W.T.; Chen, F.F.; Zhu, Y.J.; Zhang, Y.G.; Jiang, Y.Y.; Ma, M.G.; Chen, F. Binary Strengthening and Toughening of MXene/Cellulose Nanofiber Composite Paper with Nacre-Inspired Structure and Superior Electromagnetic Interference Shielding Properties. ACS Nano 2018, 12, 4583–4593. [Google Scholar] [CrossRef]
- Biswas, S.; Arief, I.; Panja, S.S.; Bose, S. Electromagnetic screening in soft conducting composite-containing ferrites: The key role of size and shape anisotropy. Mater. Chem. Front. 2017, 1, 2574–2589. [Google Scholar] [CrossRef]
- Verma, M.; Singh, A.P.; Sambyal, P.; Singh, B.P.; Dhawan, S.K.; Choudhary, V. Barium ferrite decorated reduced graphene oxide nanocomposite for effective electromagnetic interference shielding. Phys. Chem. Chem. Phys. 2015, 17, 1610. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.P.; Stephen, S.; Bose, S.; Mittal, V. Tailored electrical conductivity, electromagnetic shielding and thermal transport in polymeric blends with graphene sheets decorated with nickel nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 14922–14930. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Choudhary, V.; Vijayan, N.; Kotnala, R.K. Improved Electromagnetic Interference Shielding Response of Poly(aniline)-Coated Fabrics Containing Dielectric and Magnetic Nanoparticles. J. Phys. Chem. C 2012, 116, 13403–13412. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Yip, M.; Tai, N. Electromagnetic interference shielding efficiency of polyaniline composites filled with graphene decorated with metallic nanoparticles. Compos. Sci. Technol. 2013, 80, 80–86. [Google Scholar] [CrossRef]
- Yang, Y.; Gupta, M.C. Novel Carbon Nanotube-Polystyrene Foam Composites for Electromagnetic Interference Shielding. Nano Lett. 2005, 5, 2131–2134. [Google Scholar] [CrossRef]
- Ramírez-Herrera, C.A.; Gonzalez, H.; de la Torre, F.; Benitez, L.; Cabañas-Moreno, J.G.; Lozano, K. Electrical Properties and Electromagnetic Interference Shielding Effectiveness of Interlayered Systems Composed by Carbon Nanotube Filled Carbon Nanofiber Mats and Polymer Composites. Nanomaterials 2019, 9, 238. [Google Scholar] [CrossRef]
- Mishra, M.; Singh, A.P.; Singh, B.P.; Singh, V.N.; Dhawan, S.K. Conducting ferrofluid: A high-performance microwave shielding material. J. Mater. Chem. A 2014, 2, 13159–13168. [Google Scholar] [CrossRef]
- Shao, Y.; Li, J.; Lu, W.; Xiao, J.Q.; Qiu, Y.; Chou, T.-W. Microbuckling-Enhanced Electromagnetic-Wave-Absorbing Capability of a Stretchable Fe3O4/Carbon Nanotube/Poly(dimethylsiloxane) Composite Film. ACS Appl. Nano Mater. 2018, 1, 2227–2236. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, A.; Ding, L.; Liu, Y.; Lu, H. A three-dimensional absorber hybrid with polar oxygen functional groups of MWNTs/graphene with enhanced microwave absorbing properties. Compos. Part B 2017, 108, 386–392. [Google Scholar] [CrossRef]
- Li, N.; Huang, G.W.; Li, Y.Q.; Xiao, H.M.; Feng, Q.P.; Hu, N.; Fu, S.Y. Enhanced Microwave Absorption Performance of Coated Carbon Nanotubes by Optimizing the Fe3O4 Nanocoating Structure. ACS Appl. Mater. Interfaces 2017, 9, 2973–2983. [Google Scholar] [CrossRef]
- Chen, C.Y.; Pu, N.W.; Liu, Y.M.; Chen, L.H.; Wu, C.H.; Cheng, T.Y.; Lin, M.H.; Ger, M.D.; Gong, Y.J.; Peng, Y.Y.; Grubb, P.M. Microwave absorption properties of holey graphene/silicone rubber composites. Compos. Part B 2018, 135, 119–128. [Google Scholar] [CrossRef]
- Reshi, H.A.; Singh, A.P.; Pillai, S.; Yadav, R.S.; Dhawan, S.K.; Shelke, V. Nanostructured La0.7Sr0.3MnO3 compounds for effective electromagnetic interference shielding in the X-band frequency range. J. Mater. Chem. C 2015, 3, 820–827. [Google Scholar] [CrossRef]
- Luo, J.; Zuo, Y.; Shen, P.; Yan, Z.; Zhang, K. Excellent microwave absorption properties by tuned electromagnetic parameters in polyaniline coated Ba0.9La0.1Fe11.9Ni0.1O19/reduced graphene oxide nanocomposites. RSC Adv. 2017, 7, 36433–36443. [Google Scholar] [CrossRef]
- Pan, H.; Xu, M.; Qi, Q.; Liu, X. Facile preparation and excellent microwave absorption properties of an RGO/Co0.33Ni0.67 lightweight absorber. RSC Adv. 2017, 7, 43831–43838. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Zhu, M.; Yu, D. Electromagnetic wave absorption polyimide fabric prepared by coating with core-shell NiFe2O4@ PANI nanoparticles. RSC Adv. 2017, 7, 42891–42899. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Zhu, P.-L.; Yu, S.-H.; Sun, R.; Wong, C.-P.; Liao, W.-H. Graphene paper for exceptional EMI shielding performance using large-sized graphene oxide sheets and doping strategy. Carbon 2017, 122, 74–81. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, Y.; Fu, Y. Flexible composite film of aligned polyaniline grown on the surface of magnetic barium titanate/polyvinylidene fluoride for exceptional microwave absorption performance. RSC Adv. 2017, 7, 36473–36481. [Google Scholar] [CrossRef]
- Bora, P.J.; Azeem, I.; Vinoy, K.J.; Ramamurthy, P.C.; Madras, G. Morphology controllable microwave absorption property of polyvinylbutyral (PVB)-MnO2 nanocomposites. Compos. Part B 2018, 132, 188–196. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, X.; Wang, S.; Wang, G.; Yin, P. Enhanced Microwave Absorption Material of Ternary Nanocomposites Based on MnFe2O4@SiO2. Polyaniline Polyvinylidene Fluoride RSC Adv. 2016, 6, 88104–88109. [Google Scholar] [CrossRef]
- Lv, H.; Ji, G.; Xiao, H.L.; Zhang, H.; Du, Y. A novel rod-like MnO2@Fe loading on graphene giving excellent electromagnetic absorption properties. J. Mater. Chem. C 2015, 3, 5056–5064. [Google Scholar] [CrossRef]
- Xu, W.; Wang, G.-S.; Yin, P.-G. Designed fabrication of reduced graphene oxides/Ni hybrids for effective electromagnetic absorption and shielding. Carbon 2018, 139, 759–767. [Google Scholar] [CrossRef]
- Quan, B.; Liang, X.; Ji, G.; Lv, J.; Dai, S.S.; Xu, G.; Du, Y. Laminated graphene oxide-supported high-efficiency microwave absorber fabricated by an in situ growth approach. Carbon 2018, 129, 310–320. [Google Scholar] [CrossRef]
- Crespo, M.; Mendez, N.; Gonzalez, M.; Baselga, J.; Pozuelo, J. Synergistic effect of magnetite nanoparticles and carbon nanofibres in electromagnetic absorbing composites. Carbon 2014, 74, 63–72. [Google Scholar] [CrossRef]
- Xu, F.; Chen, R.; Lin, Z.; Qin, Y.; Yuan, Y.; Li, Y.; Zhao, X.; Yang, M.; Sun, X.; Wang, S.; et al. Superflexible Interconnected Graphene Network Nanocomposites for High-Performance Electromagnetic Interference Shielding. ACS Omega 2018, 3, 3599–3607. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Aziz, S.A.; Abbas, Z.; Obaiys, S.J.; Khamis, A.M.; Hussain, I.R.; Zaid, M.H.M. Preparation of a Chemically Reduced Graphene Oxide Reinforced Epoxy Resin Polymer as a Composite for Electromagnetic Interference Shielding and Microwave-Absorbing Applications. Polymers 2018, 10, 1180. [Google Scholar] [CrossRef]
- Wen, B.; Cao, M.; Lu, M.; Cao, W.; Shi, H.; Liu, J.; Wang, X.; Jin, H.; Fang, X.; Wang, W.; et al. Reduced Graphene Oxides: Light-Weight and High-Efficiency Electromagnetic Interference Shielding at Elevated Temperatures. Adv. Mater. 2014, 26, 3484–3489. [Google Scholar] [CrossRef]




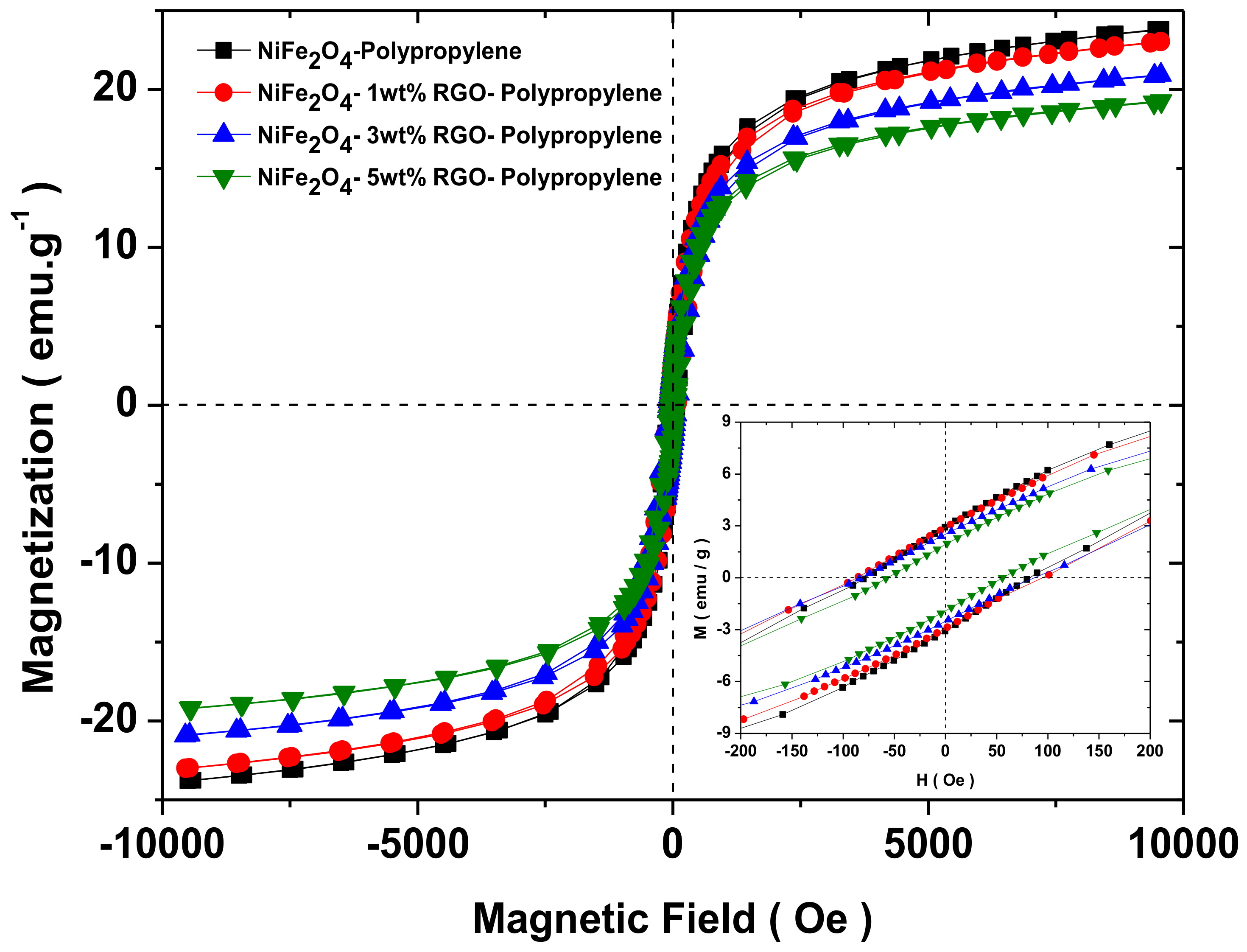



| Sample | Ms (emu/g) | Mr (emu/g) | Hc (Oe) |
|---|---|---|---|
| NiFe2O4-Polypropylene | 24.0 | 3.0 | 83.1 |
| NiFe2O4-1wt%RGO-Polypropylene | 23.2 | 2.8 | 94.0 |
| NiFe2O4-3wt%RGO-Polypropylene | 20.9 | 2.4 | 88.2 |
| NiFe2O4-5wt%RGO-Polypropylene | 19.3 | 1.9 | 57.5 |
| Sample | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|
| NiFe2O4-Polypropylene | 7.31 ± 0.12 | 30.01 ± 1.43 | 247.70 ± 30.91 |
| NiFe2O4-1wt%RGO-Polypropylene | 7.43 ± 0.38 | 26.28 ± 1.14 | 391.08 ± 34.46 |
| NiFe2O4-3wt%RGO-Polypropylene | 6.56 ± 0.32 | 25.29 ± 1.74 | 345.78 ± 42.43 |
| NiFe2O4-5wt%RGO-Polypropylene | 5.03 ± 0.23 | 22.34 ± 1.34 | 250.25 ± 40.65 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, R.S.; Kuřitka, I.; Vilčáková, J.; Machovský, M.; Škoda, D.; Urbánek, P.; Masař, M.; Gořalik, M.; Urbánek, M.; Kalina, L.; et al. Polypropylene Nanocomposite Filled with Spinel Ferrite NiFe2O4 Nanoparticles and In-Situ Thermally-Reduced Graphene Oxide for Electromagnetic Interference Shielding Application. Nanomaterials 2019, 9, 621. https://doi.org/10.3390/nano9040621
Yadav RS, Kuřitka I, Vilčáková J, Machovský M, Škoda D, Urbánek P, Masař M, Gořalik M, Urbánek M, Kalina L, et al. Polypropylene Nanocomposite Filled with Spinel Ferrite NiFe2O4 Nanoparticles and In-Situ Thermally-Reduced Graphene Oxide for Electromagnetic Interference Shielding Application. Nanomaterials. 2019; 9(4):621. https://doi.org/10.3390/nano9040621
Chicago/Turabian StyleYadav, Raghvendra Singh, Ivo Kuřitka, Jarmila Vilčáková, Michal Machovský, David Škoda, Pavel Urbánek, Milan Masař, Marek Gořalik, Michal Urbánek, Lukáš Kalina, and et al. 2019. "Polypropylene Nanocomposite Filled with Spinel Ferrite NiFe2O4 Nanoparticles and In-Situ Thermally-Reduced Graphene Oxide for Electromagnetic Interference Shielding Application" Nanomaterials 9, no. 4: 621. https://doi.org/10.3390/nano9040621
APA StyleYadav, R. S., Kuřitka, I., Vilčáková, J., Machovský, M., Škoda, D., Urbánek, P., Masař, M., Gořalik, M., Urbánek, M., Kalina, L., & Havlica, J. (2019). Polypropylene Nanocomposite Filled with Spinel Ferrite NiFe2O4 Nanoparticles and In-Situ Thermally-Reduced Graphene Oxide for Electromagnetic Interference Shielding Application. Nanomaterials, 9(4), 621. https://doi.org/10.3390/nano9040621