Three-Dimensional Graphene Composite Containing Graphene-SiO2 Nanoballs and Its Potential Application in Stress Sensors
Abstract
1. Introduction
2. Materials and Methods
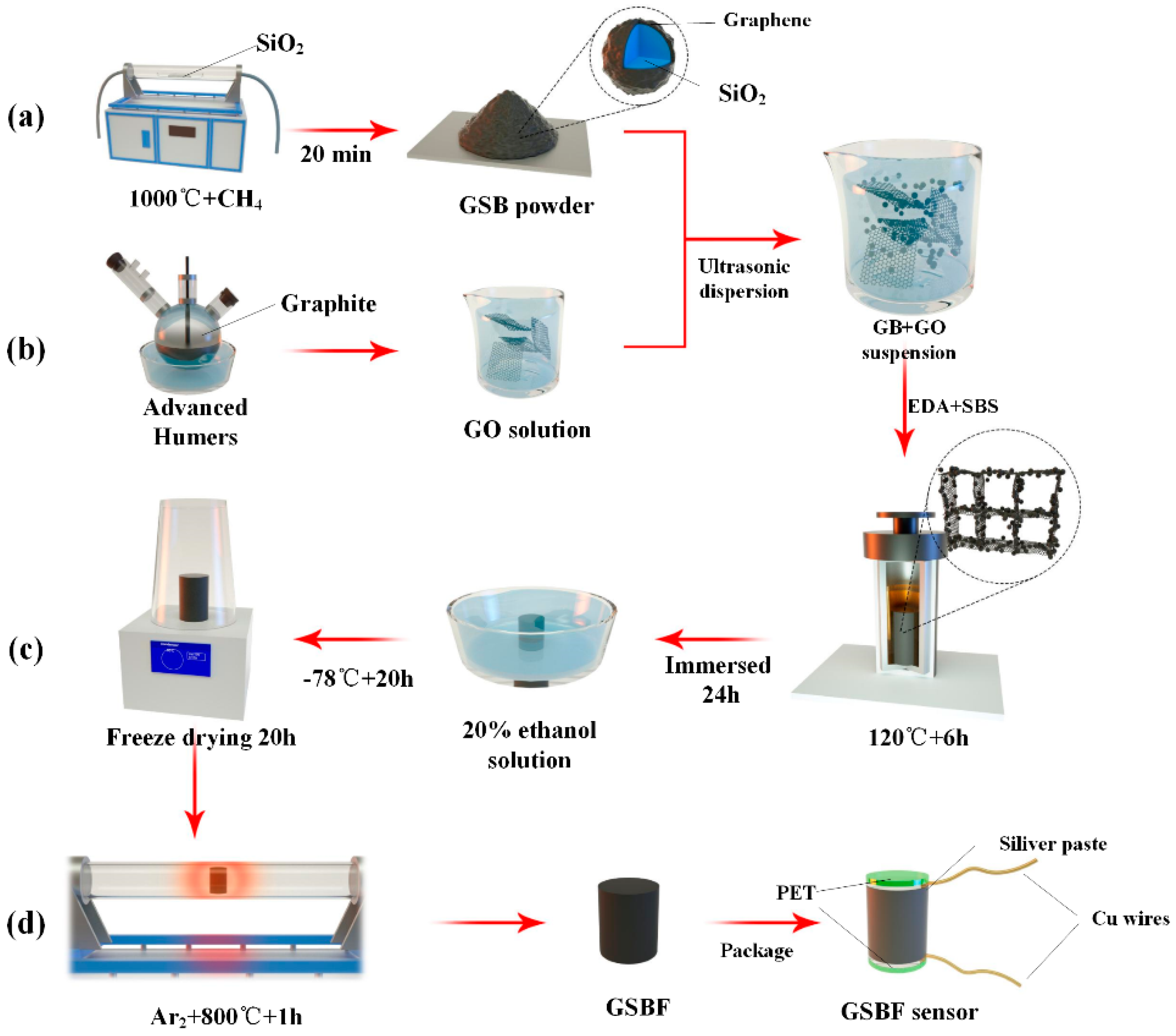
2.1. Synthesis of GSB
2.2. Preparation of GO
2.3. Preparation of GSBF Composite
2.4. Characterization
2.5. Mechanics and Electrical Measurement
3. Results and Discussion
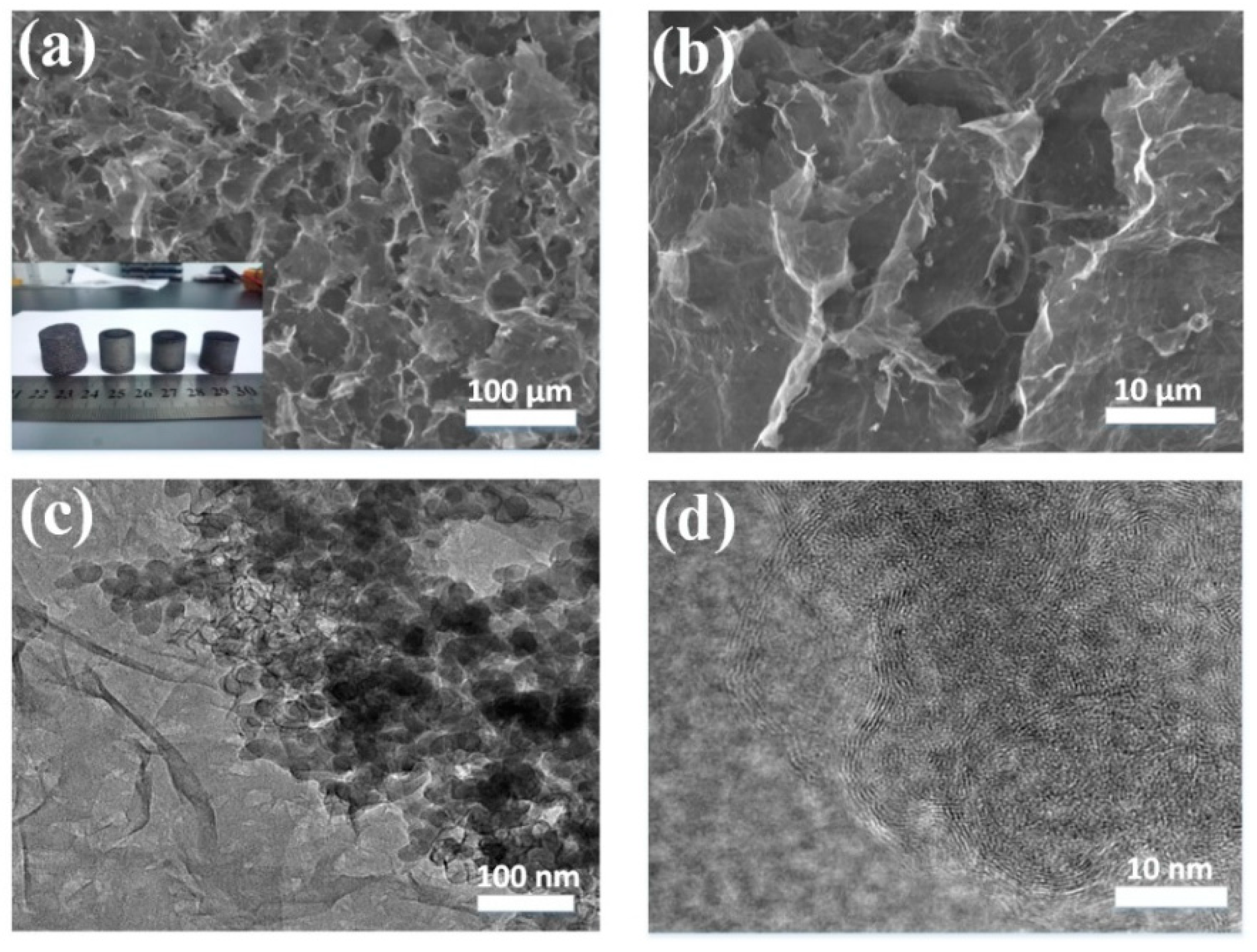
3.1. Microscopic Characterization
3.2. Structural Analysis
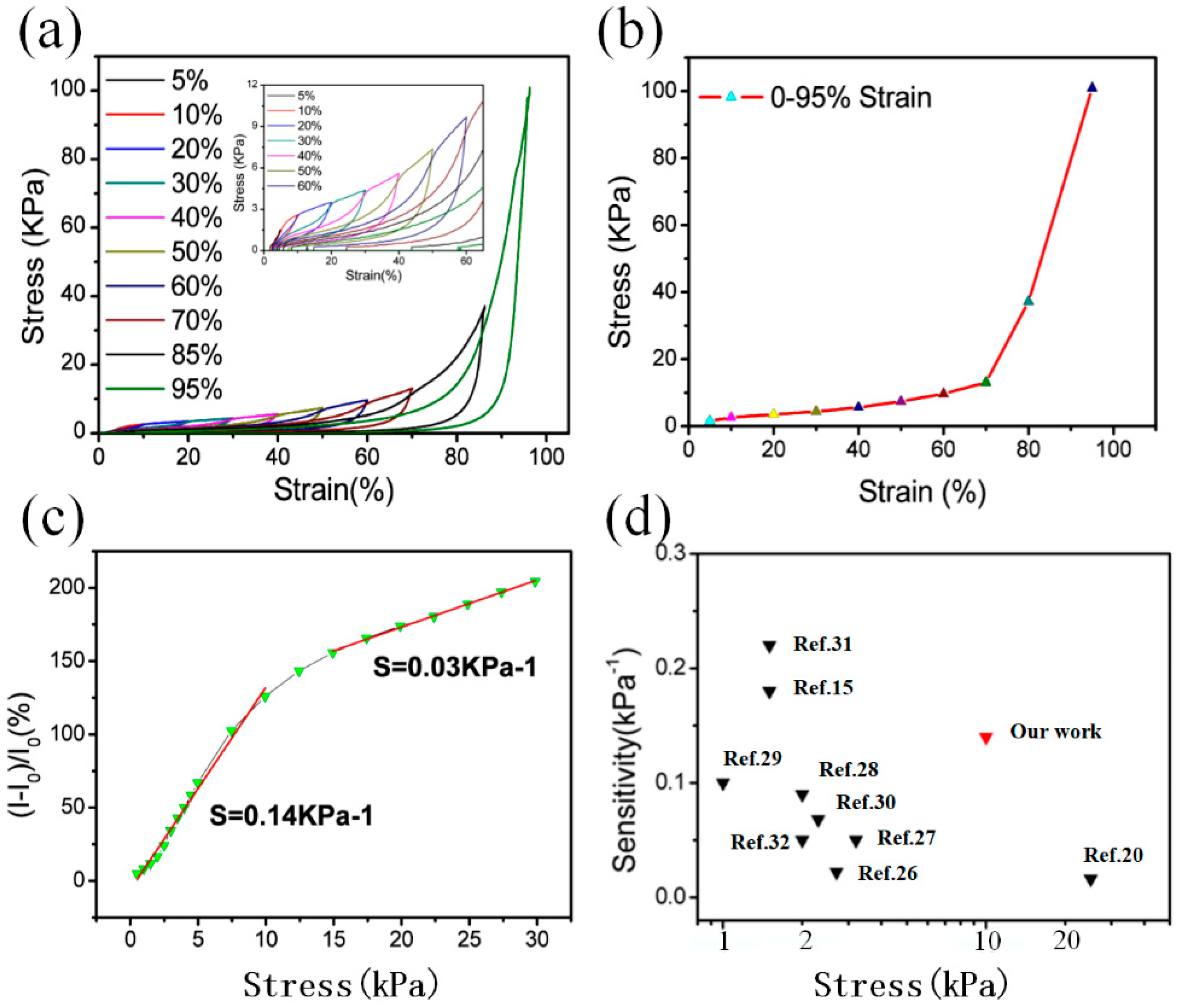
3.3. Stress-Strain Performance of GSBF
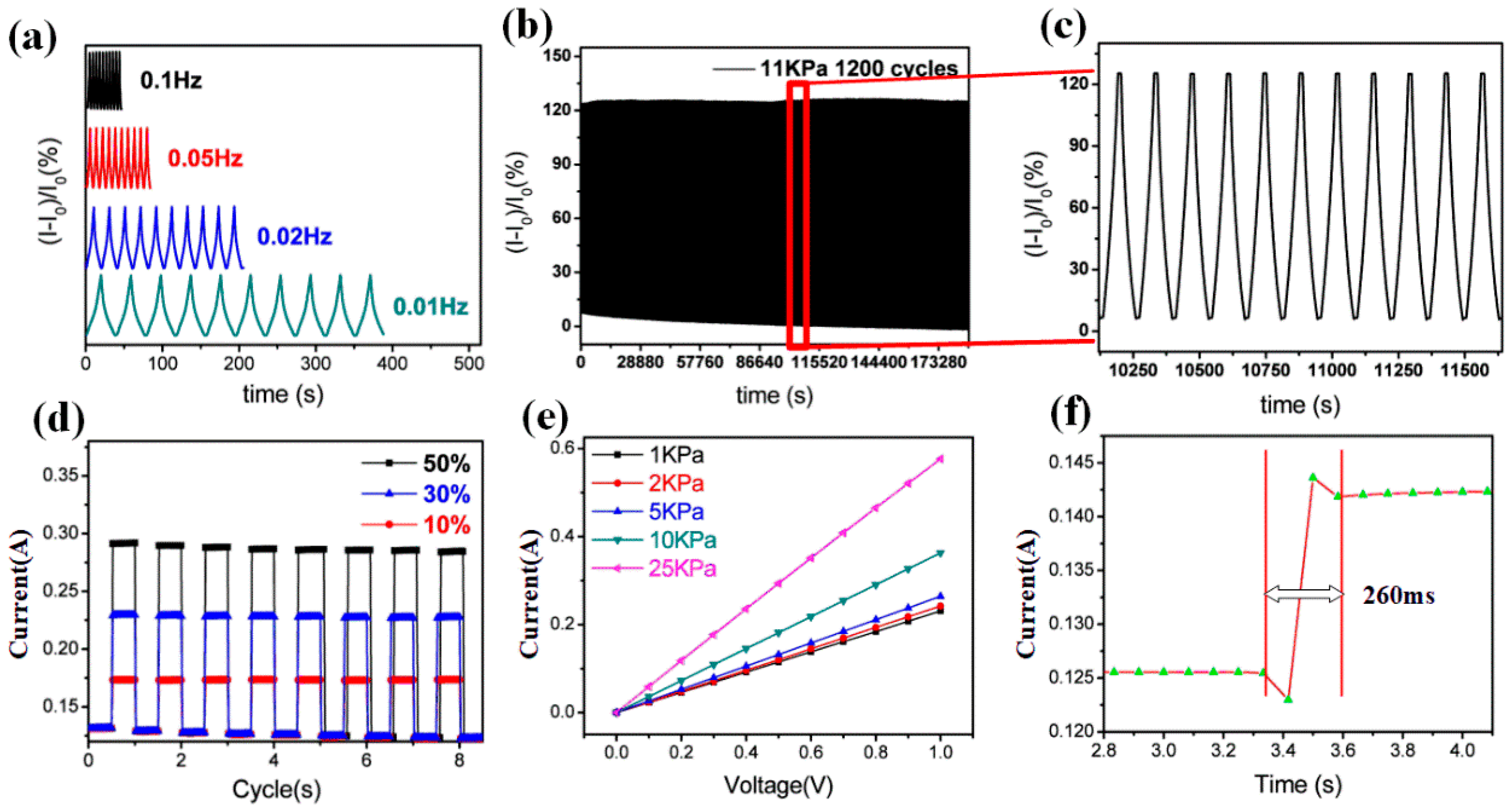
3.4. Stress Sensor Characterization of GSBF
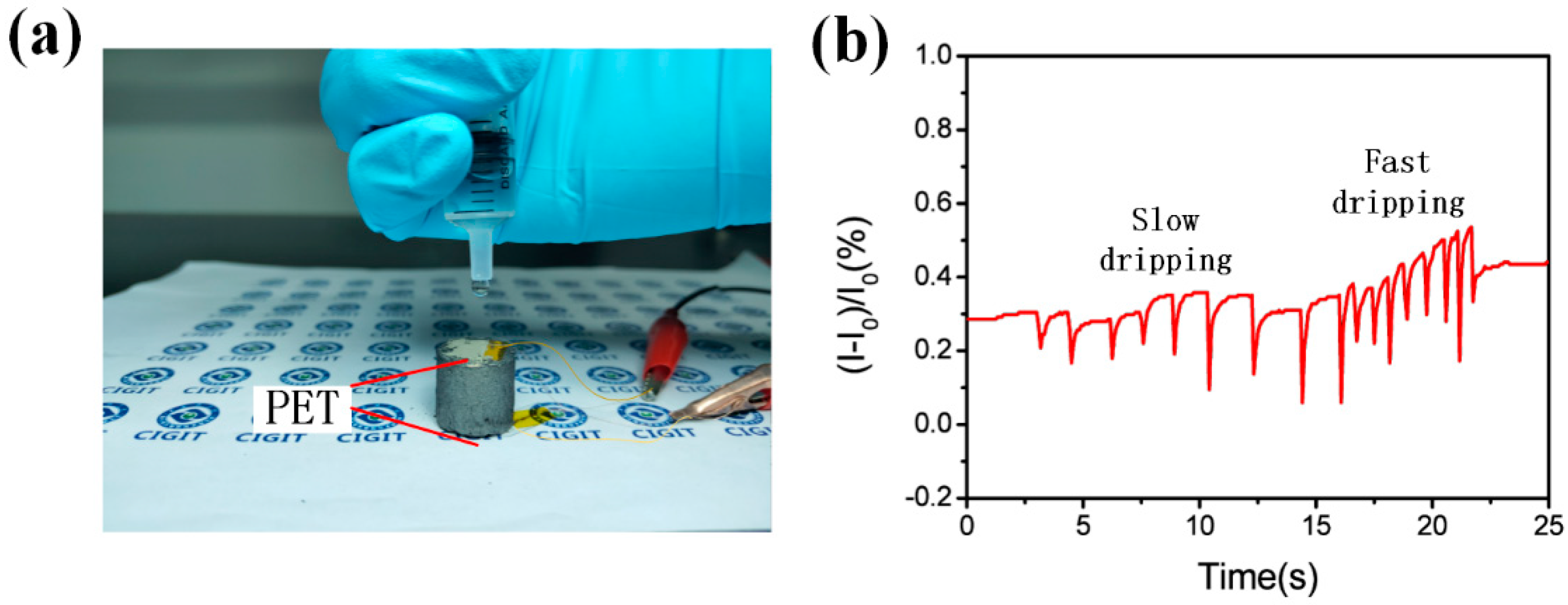
3.5. Water-Dripping Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Idowu, A.; Boesl, B.; Agarwal, A. 3D graphene foam-reinforced polymer composites—A review. Carbon 2018, 135, 52–71. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, J.; Chen, H.; Wang, R.; Li, D.; Qu, J.; Dai, L. Nitrogen-doped graphene foams as metal-free counter electrodes in high-performance dye-sensitized solar cells. Angew. Chem. Int. Ed. 2012, 51, 12124–12127. [Google Scholar] [CrossRef] [PubMed]
- Marsden, A.J.; Papageorgiou, D.G.; Vallés, C.; Liscio, A.; Palermo, V.; Bissett, M.A.; Young, R.J.; Kinloch, I.A. Electrical percolation in graphene–polymer composites. 2D Mater. 2018, 5. [Google Scholar] [CrossRef]
- Kemp, K.C.; Seema, H.; Saleh, M.; Le, N.H.; Mahesh, K.; Chandra, V.; Kim, K.S. Environmental applications using graphene composites: Water remediation and gas adsorption. Nanoscale 2013, 5, 3149–3171. [Google Scholar] [CrossRef] [PubMed]
- Tung, T.T.; Nine, M.J.; Krebsz, M.; Pasinszki, T.; Coghlan, C.J.; Tran, D.N.H.; Losic, D. Recent Advances in Sensing Applications of Graphene Assemblies and Their Composites. Adv. Funct. Mater. 2017, 27, 1702891. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, J.Z.; Chang, S.L.; Wu, Y.; Li, D. Biomimetic superelastic graphene-based cellular monoliths. Nat. Commun. 2012, 3, 1241. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xu, Z.; Gao, C. Multifunctional, ultra-flyweight, synergistically assembled carbon aerogels. Adv. Mater. 2013, 25, 2554–2560. [Google Scholar] [CrossRef] [PubMed]
- Son, I.H.; Park, J.H.; Kwon, S.; Choi, J.W.; Rummeli, M.H. Graphene Coating of Silicon Nanoparticles with CO2-Enhanced Chemical Vapor Deposition. Small 2016, 12, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Yavari, F.; Chen, Z.; Thomas, A.V.; Ren, W.; Cheng, H.M.; Koratkar, N. High sensitivity gas detection using a macroscopic three-dimensional graphene foam network. Sci. Rep. 2011, 1, 166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiao, F.; Guo, Y.; Wang, S.; Liu, Y. One-pot self-assembled three-dimensional TiO2-graphene hydrogel with improved adsorption capacities and photocatalytic and electrochemical activities. ACS Appl. Mater. Interfaces 2013, 5, 2227–2233. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Z.; Yi, H.; Wei, H.; Guo, Z.; Wang, X. One-step preparation of single-crystalline Fe2O3 particles/graphene composite hydrogels as high performance anode materials for supercapacitors. Nano Energy 2014, 7, 86–96. [Google Scholar] [CrossRef]
- Kuang, J.; Liu, L.; Gao, Y.; Zhou, D.; Chen, Z.; Han, B.; Zhang, Z. A hierarchically structured graphene foam and its potential as a large-scale strain-gauge sensor. Nanoscale 2013, 5, 12171–12177. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Zhang, P.; Xu, T.; Qu, L. Ultrasensitive Pressure Sensor Based on an Ultralight Sparkling Graphene Block. ACS Appl. Mater. Interfaces 2017, 9, 22885–22892. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Peng, Q.; Ding, Y.; Lin, Z.; Wang, C.; Li, Y.; Xu, F.; Li, J.; Yuan, Y.; He, X.; et al. Lightweight, Superelastic, and Mechanically Flexible Graphene/Polyimide Nanocomposite Foam for Strain Sensor Application. ACS Nano 2015, 9, 8933–8941. [Google Scholar] [CrossRef] [PubMed]
- Son, I.H.; Park, J.H.; Park, S.; Park, K.; Han, S.; Shin, J.; Doo, S.G.; Hwang, Y.; Chang, H.; Choi, J.W. Graphene balls for lithium rechargeable batteries with fast charging and high volumetric energy densities. Nat. Commun. 2017, 8, 1561. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, J.; Hmam, O.; Zidi, M.; Tavares, A.C.; Izquierdo, R.; Szkopek, T.; Siaj, M. One-Step In-Situ Growth of Core-Shell SiC@Graphene Nanoparticles/Graphene Hybrids by Chemical Vapor Deposition. Adv. Mater. Interfaces 2016, 3. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Z.; Xiao, J.; Chen, C.; Xiao, F.; Wang, S.; Liu, Y. Facile and green synthesis of palladium nanoparticles-graphene-carbon nanotube material with high catalytic activity. Sci. Rep. 2013, 3, 2527. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis ofGraphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhao, Z.; Wan, W.; Gogotsi, Y.; Qiu, J. Ultralight and highly compressible graphene aerogels. Adv. Mater. 2013, 25, 2219–2223. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Liu, L.; Zhang, L.; Shao, Q.; Zhou, W.; Chen, X.; Xie, S. A universal strategy to prepare functional porous graphene hybrid architectures. Adv. Mater. 2014, 26, 3681–3687. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Jing, Y.; Zeng, J.; Qi, Q.; Wang, C.; Goldfeld, D.; Xu, C.; Zheng, Y.; Sun, Y. Nanocables composed of anatase nanofibers wrapped in UV-light reduced graphene oxide and their enhancement of photoinduced electron transfer in photoanodes. J. Mater. Chem. 2011, 21. [Google Scholar] [CrossRef]
- Khan, F.A.; Ajmal, C.M.; Bae, S.; Seo, S.; Moon, H.; Baik, S. Silver Nanoflower Decorated Graphene Oxide Sponges for Highly Sensitive Variable Stiffness Stress Sensors. Small 2018, 14, e1800549. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Itagaki, N.; Im, H.J. Growth and Raman spectroscopy of thickness-controlled rotationally faulted multilayer graphene. Carbon 2019, 141, 76–82. [Google Scholar] [CrossRef]
- Zhang, J.H.; Sun, T.; Niu, A.; Tang, Y.M.; Deng, S.; Luo, W.; Xu, Q.; Wei, D.; Pei, D.S. Perturbation effect of reduced graphene oxide quantum dots (rGOQDs) on aryl hydrocarbon receptor (AhR) pathway in zebrafish. Biomaterials 2017, 133, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Lu, X.; Bao, W.; Chen, R. Three-Dimensional Interfacial Stress Sensor Based on Graphene Foam. IEEE Sens. J. 2018, 18, 7956–7963. [Google Scholar] [CrossRef]
- Tewari, A.; Gandla, S.; Bohm, S.; McNeill, C.R.; Gupta, D. Highly Exfoliated MWNT-rGO Ink-Wrapped Polyurethane Foam for Piezoresistive Pressure Sensor Applications. ACS Appl. Mater. Interfaces 2018, 10, 5185–5195. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Zhang, Y.; Shao, J.; Wang, W.; Si, W.; Huang, W.; Dong, X. Stretchable, Transparent, and Self-Patterned Hydrogel-Based Pressure Sensor for Human Motions Detection. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Da Silva, F.A.G.; de Araújo, C.M.S.; Alcaraz-Espinoza, J.J.; de Oliveira, H.P. Toward flexible and antibacterial piezoresistive porous devices for wound dressing and motion detectors. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1063–1072. [Google Scholar] [CrossRef]
- Yu, G.; Hu, J.; Tan, J.; Gao, Y.; Lu, Y.; Xuan, F. A wearable pressure sensor based on ultra-violet/ozone microstructured carbon nanotube/polydimethylsiloxane arrays for electronic skins. Nanotechnology 2018, 29, 115502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lv, L.; Cheng, Z.; Liang, Y.; Zhou, Q.; Zhao, Y.; Qu, L. Superelastic, Macroporous Polystyrene-Mediated Graphene Aerogels for Active Pressure Sensing. Chem. Asian J. 2016, 11, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Han, Y.; Zhang, X.; Zhou, Z.; Lu, C. Large-Area Compliant, Low-Cost, and Versatile Pressure-Sensing Platform Based on Microcrack-Designed Carbon Black@Polyurethane Sponge for Human-Machine Interfacing. Adv. Funct. Mater. 2016, 26, 6246–6256. [Google Scholar] [CrossRef]
- Huang, W.; Dai, K.; Zhai, Y.; Liu, H.; Zhan, P.; Gao, J.; Zheng, G.; Liu, C.; Shen, C. Flexible and Lightweight Pressure Sensor Based on Carbon Nanotube/Thermoplastic Polyurethane-Aligned Conductive Foam with Superior Compressibility and Stability. ACS Appl. Mater. Interfaces 2017, 9, 42266–42277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hao, M.; Xu, X.; Xiong, G.; Li, H.; Fisher, T.S. Flyweight 3D Graphene Scaffolds with Microinterface Barrier-Derived Tunable Thermal Insulation and Flame Retardancy. ACS Appl. Mater. Interfaces 2017, 9, 14232–14241. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-W.; Kim, B.; Li, J.; Meyyappan, M. Flexible, compressible, hydrophobic, floatable, and conductive carbon nanotube-polymer sponge. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]

| No. | Ratio (GO:GSB) | Recoverable Strain (%) | Sensitivity (kPa−1) | Sensing Range (kPa) | Young’s Modulus (kPa) | Electrical Conductivity (S/cm) |
|---|---|---|---|---|---|---|
| 1 | 10:0 | >95 | 0.4 | 0–2 | 48 | 0.1 |
| 2 | 10:1 | 95 | 0.14 | 0–10 | 112 | 0.184 |
| 3 | 10:2 | 90 | 0.12 | 0–8 | 119 | 0.186 |
| 4 | 10:3 | 80 | 0.08 | 0–5 | 98 | 0.188 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Sun, T.; Zhou, X.; Liu, X.; Li, X.; Zhou, K.; Dong, L.; Wei, D. Three-Dimensional Graphene Composite Containing Graphene-SiO2 Nanoballs and Its Potential Application in Stress Sensors. Nanomaterials 2019, 9, 438. https://doi.org/10.3390/nano9030438
Zhao B, Sun T, Zhou X, Liu X, Li X, Zhou K, Dong L, Wei D. Three-Dimensional Graphene Composite Containing Graphene-SiO2 Nanoballs and Its Potential Application in Stress Sensors. Nanomaterials. 2019; 9(3):438. https://doi.org/10.3390/nano9030438
Chicago/Turabian StyleZhao, Bowei, Tai Sun, Xi Zhou, Xiangzhi Liu, Xiaoxia Li, Kai Zhou, Lianhe Dong, and Dapeng Wei. 2019. "Three-Dimensional Graphene Composite Containing Graphene-SiO2 Nanoballs and Its Potential Application in Stress Sensors" Nanomaterials 9, no. 3: 438. https://doi.org/10.3390/nano9030438
APA StyleZhao, B., Sun, T., Zhou, X., Liu, X., Li, X., Zhou, K., Dong, L., & Wei, D. (2019). Three-Dimensional Graphene Composite Containing Graphene-SiO2 Nanoballs and Its Potential Application in Stress Sensors. Nanomaterials, 9(3), 438. https://doi.org/10.3390/nano9030438





