Nanomaterials and Chemical Modifications for Enhanced Key Wood Properties: A Review
Abstract
:1. Introduction
2. Chemical Modification
- Filling the lumen with a chemical, usually resin: This decreases the sorption of water vapor but the sorption behavior over a longer period of time is not reduced.
- Bulking the cell wall and the cell lumen with a chemical: This reduces the swelling of wood and affects positively the long-term sorption behavior.
- Modifying treatments: The chemistry of the main cell wall components is changed, and covalent bonds are formed by converting the hydrophilic OH-groups into hydrophobic groups. This results in new wood products with enhanced properties.
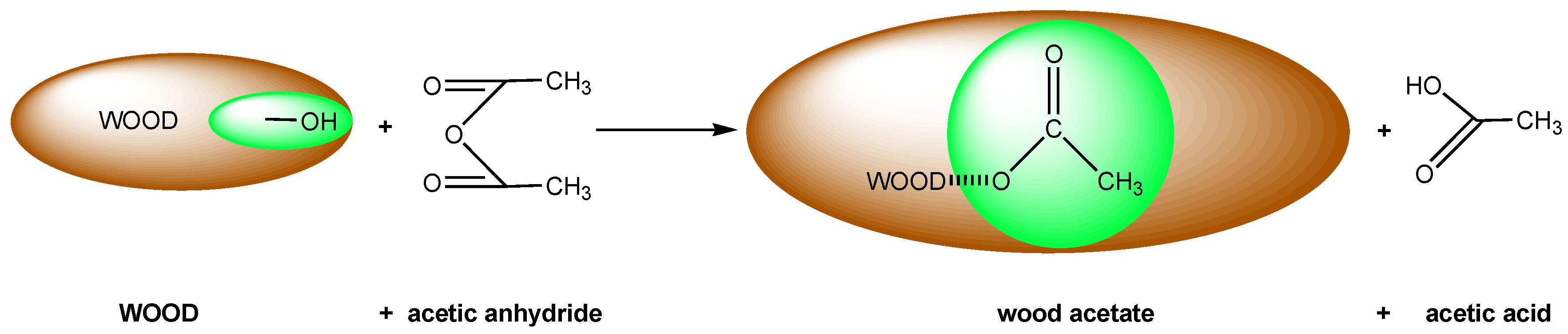
2.1. Acetylation
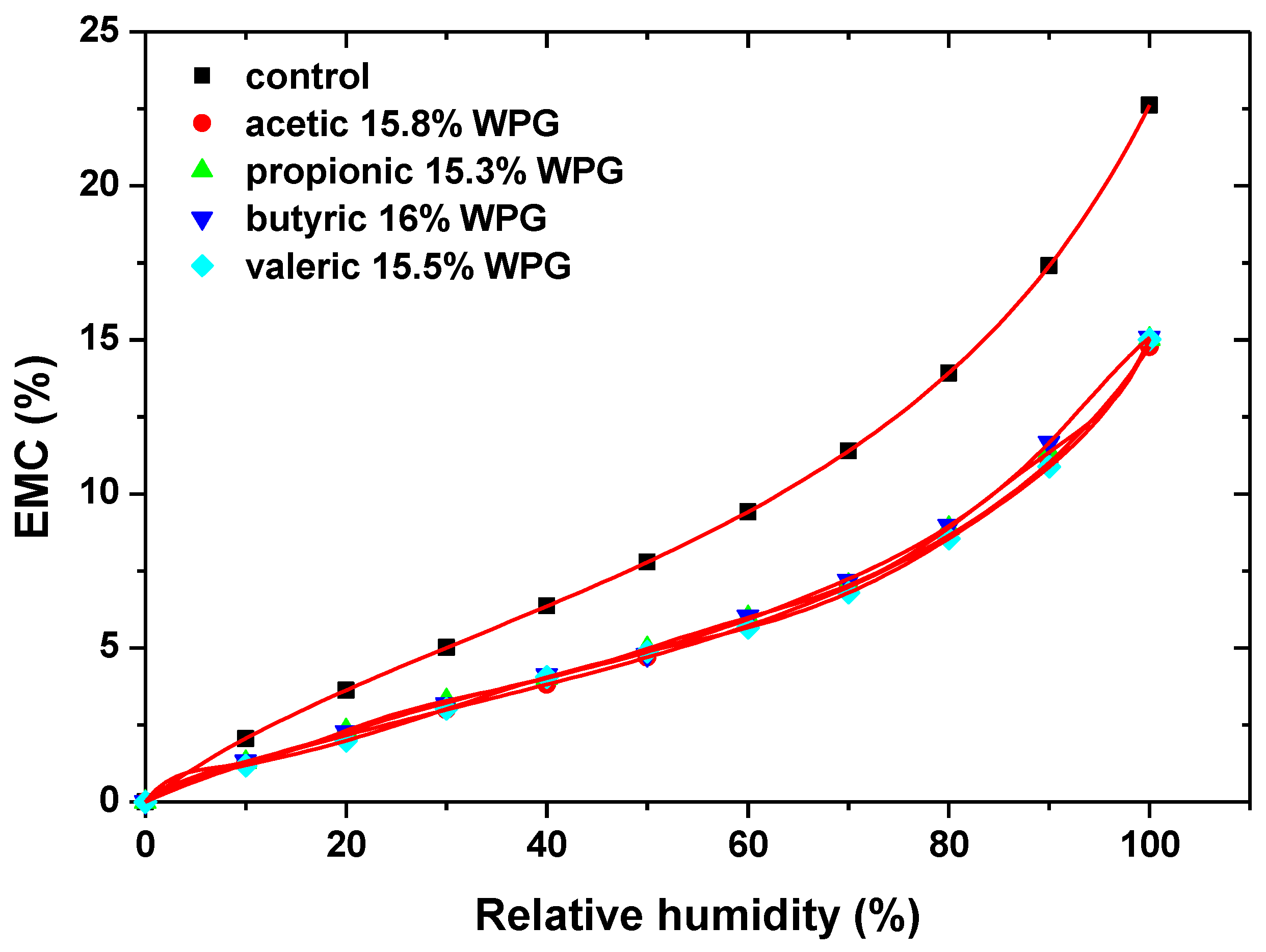
2.1.1. Water Vapor Sorption of Acetylated Wood
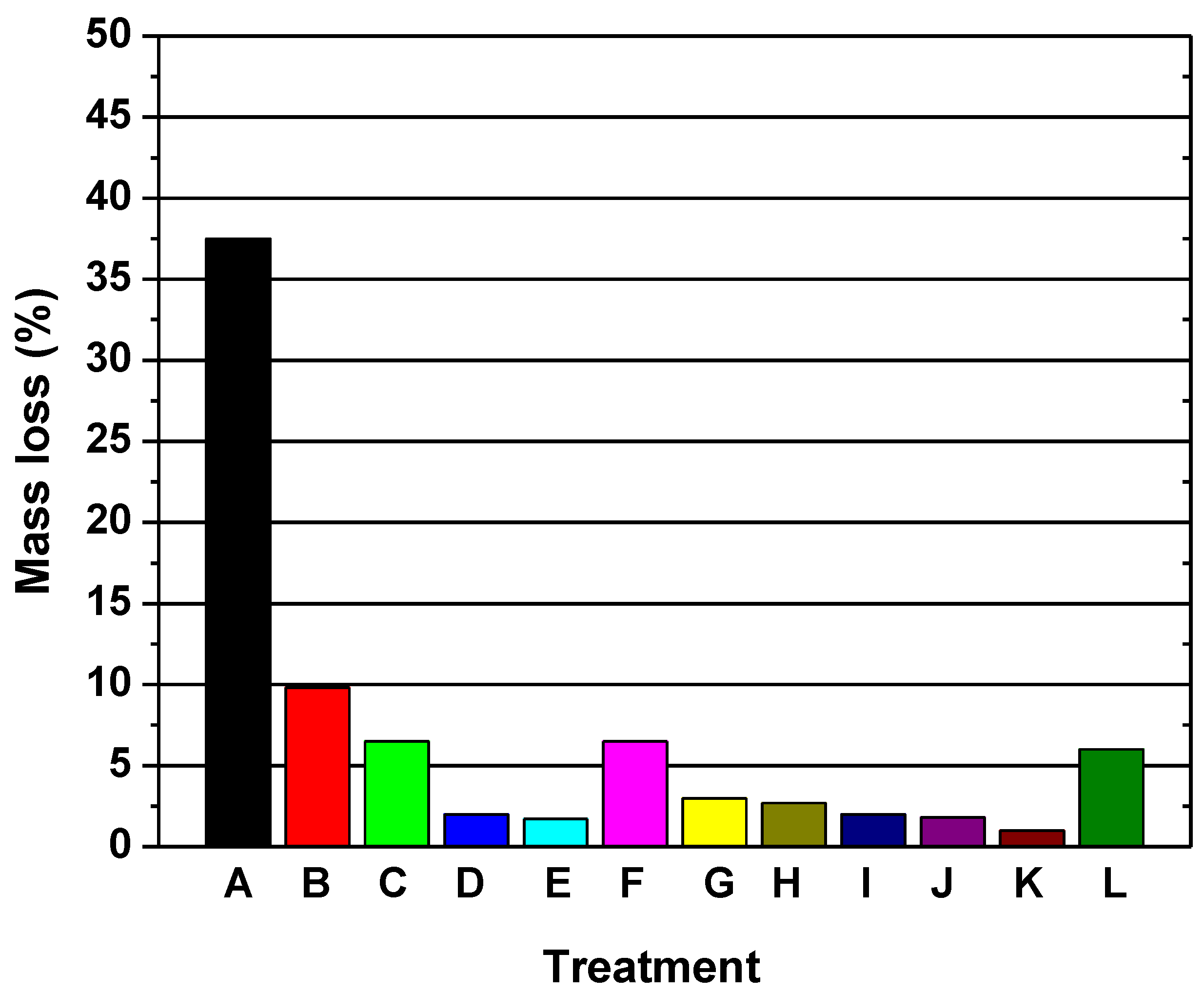
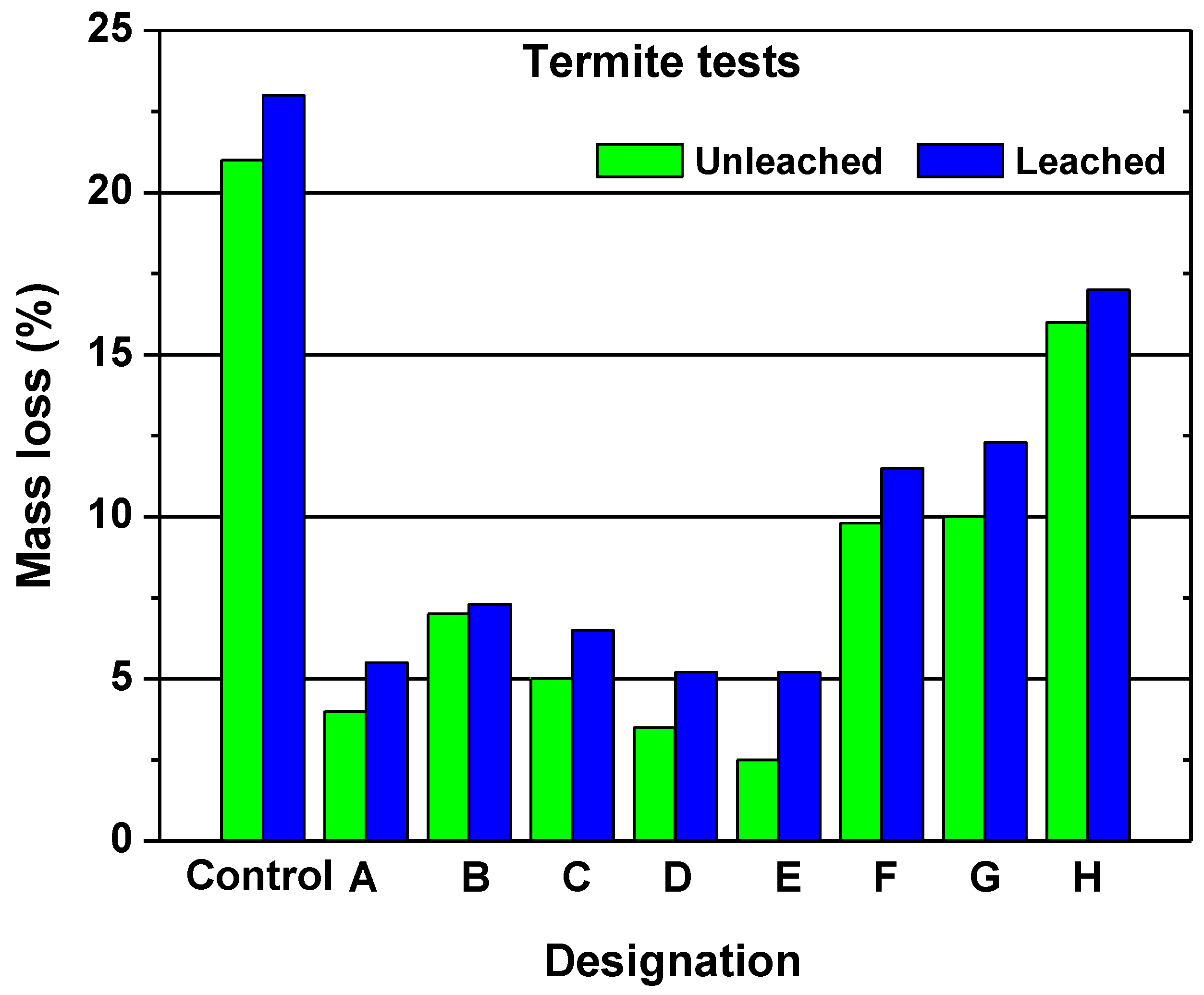
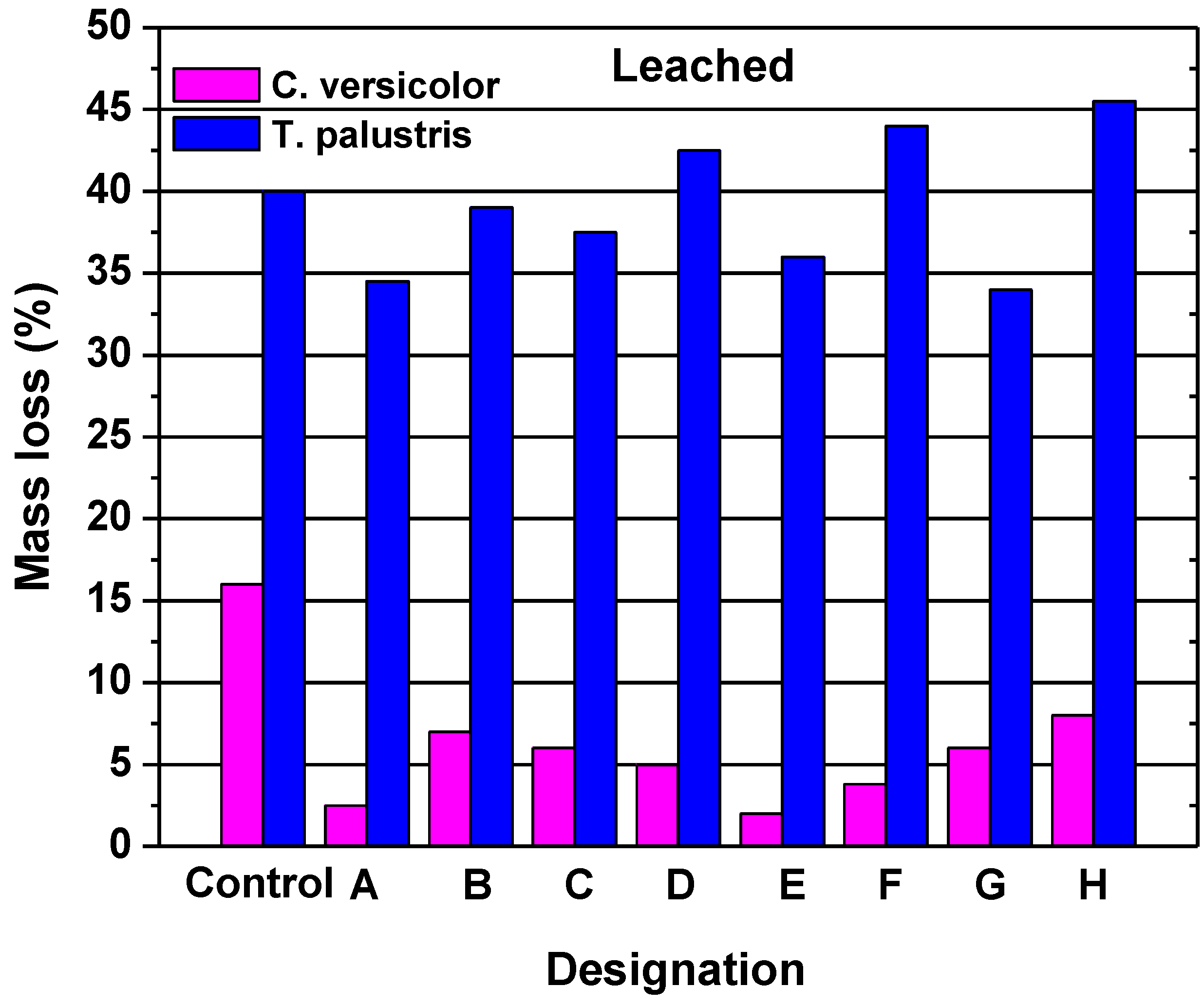
2.1.2. Durability of Acetylated Wood
2.2. Furfurylation
- Storage and mixing of chemicals: The treating solutions are mixed in a separate mixing tank where different chemicals (furfuryl alcohol, initiators/catalysts, buffering agents, surfactants, and water) are added. The mixed solution is pumped to one of the buffer tanks.
- Impregnation: the wooden material, i.e., treatable softwoods or hardwoods, is vacuum pressure impregnated with the treating solution by a full-cell process with a vacuum step, a pressure step, and a short post-vacuum step.
- Reaction and curing: An in situ polymerisation of the chemicals and grafting reactions with the polymeric components of the wood occur during this step. The curing chamber is heated with a direct injection of steam, and the temperature achieved depends on the product use. The chamber is operated as a closed system during the curing period, except for a ventilation period at the end. The ventilation gas is cooled, and the condensate is separated from the gas; thereafter, the condensate goes back to the condensate tank for reuse.
- Drying: The final drying of the modified wood material in a kiln dryer is essential to minimise emissions and to obtain a desirable final moisture content.
- Cleaning: the emissions during the process are managed by cleaning the ventilated gases.
- The biological durability of wood is up graded to “Class 1”. A decay test have showed that furfurylated wood of moderate loading has a comparable resistance with that of pine wood impregnated with copper chromium arsenate;
- The mechanical properties of wood, except for impact resistance, are enhanced when wood is treated with a furfuryl-alcohol polymer. Furfurylated wood is characterized by a greater hardness, elasticity, and rupture moduli as compared to untreated wood;
- Kebony wood, depending upon the loading, exhibits a strong dimensional stability and resistance to weathering; moreover, its water swelling and shrinking values are over 50% lower than untreated wood;
- Furfurylated wood is extremely resistant to marine borers at high levels (>50%) of weight percentage gain; and
- Studies regarding the ecotoxicology of furfurylated wood and leachates from furfurylated wood showed no significant ecotoxicity, while its combustion did not release any volatile organic compounds or polyaromatic hydrocarbons above the normal levels of wood combustion.
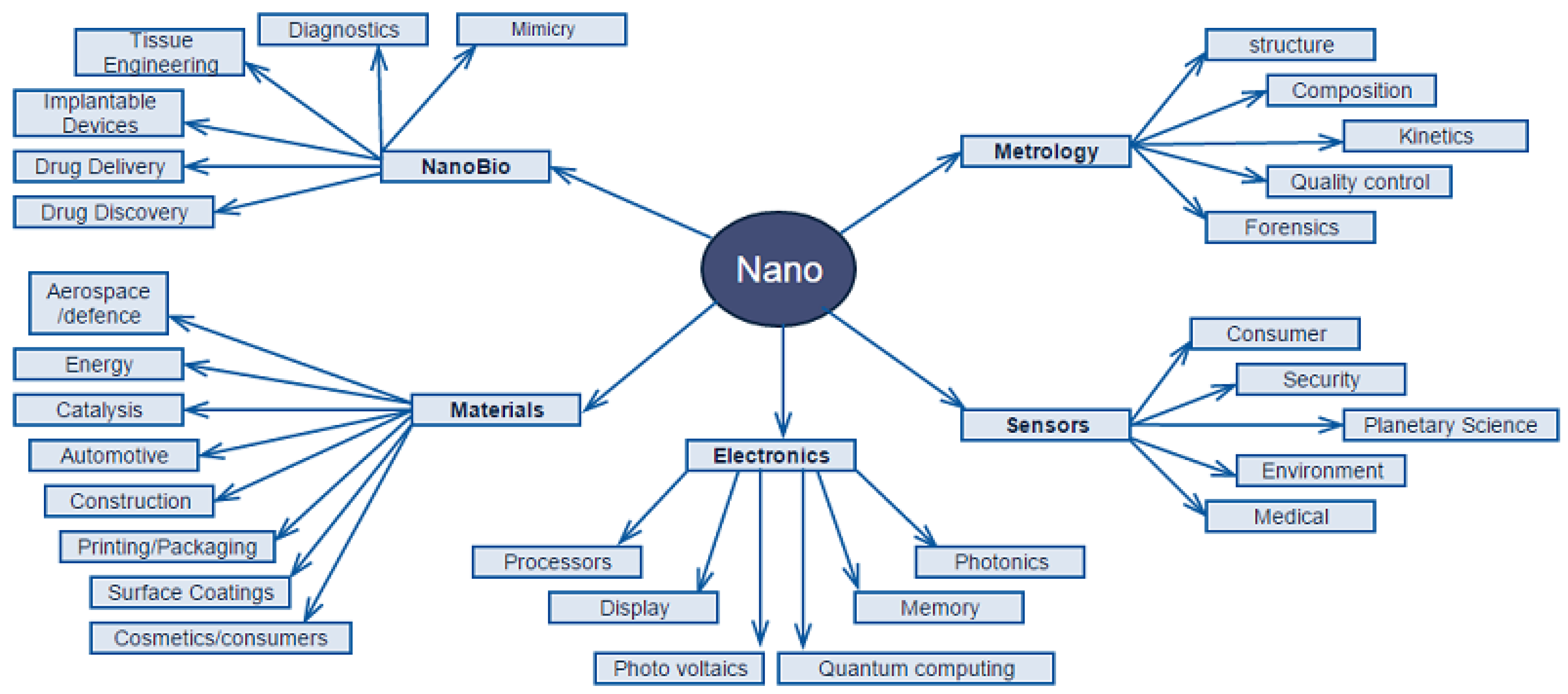
3. From Nanotechnology to Nanomaterials
Historical Points
- (i)
- 1857: The synthesis of colloidal gold particles by Michael Faraday.
- (ii)
- 1930s: Some novel catalysts in nanostructure form have been synthesized.
- (iii)
- 1940s: Precipitated and fumed silica nanoparticles were developed and manufactured for applications as substitutes for ultrafine carbon black for rubber reinforcements. The target was the USA and German markets.
- (iv)
- 1960s–1970s: Magnetic recording tapes were developed by metallic-like nanopowders.
- (v)
- 1976: The inert-gas evaporation for nanocrystal synthesis was mentioned for first time by Granqvist and Buhrman.
- (vi)
- Nowadays, the development of engineering nanophase/nanostructures or, generally, the development of nanotechnology is very rapid, and too many novel structural materials (either organic or inorganic) are investigated. Apart from that, we can also find possible ways to slightly or deeply modify/treat some useful parameters of nanomaterials (mechanical, optical, electronic functions, etc.).
4. Nanotechnology and Wood
4.1. Nanosized Metals
4.2. Polymeric Nanocarriers
4.3. Coating Treatments
5. Advantages of Nanomaterials in Wood Treatment
6. Conclusions
Funding
Conflicts of Interest
References
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites, 2nd ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Gerardin, P. New alternatives for wood preservation based on thermal and chemical modification of wood—A review. Ann. For. Sci. 2016, 73, 559–570. [Google Scholar] [CrossRef]
- Hill, C.A.S. Wood Modification—Chemical, Thermal and other Processes; John Wiley and Sons Ltd.: West Sussex, UK, 2006. [Google Scholar]
- Rowell, R.M. Acetylation of Wood—A review. Int. J. Lignocellul. Prod. 2014, 1, 1–27. [Google Scholar]
- Wegner, T.H.; Winandy, J.E.; Ritter, M.A. Nanotechnology opportunities in residential and non-residential construction. In Proceedings of the 2nd International Symposium on Nanotechnology in Construction, Bilbao, Spain, 13–16 November 2005; pp. 23–31. [Google Scholar]
- Wegner, T.H.; Jones, P. Advancing cellulose-based nanotechnology. Cellulose 2005, 13, 115–118. [Google Scholar] [CrossRef]
- Rowell, R.M. Chemical modification of wood. For. Prod. Abstr. 1983, 6, 366–382. [Google Scholar]
- Kumar, S. Chemical modification of wood. Wood Fiber Sci. 1996, 26, 270–280. [Google Scholar]
- Hon, D.N.S. Chemical Modification of Lignocellulosics; Marcel Dekker: New York, NY, USA, 2006. [Google Scholar]
- Papadopoulos, A.N. Chemical modification of solid wood and wood raw materials for composites production with linear chain carboxylic acid anhydrides: A brief Review. BioResources 2010, 5, 499–506. [Google Scholar]
- Bender, M.L. Mechanisms of catalysis of nucleophilic reactions of carboxylic acid derivatives. Chem. Revis. 1960, 60, 53–113. [Google Scholar] [CrossRef]
- Gold, V.; Jefferson, E.G. The hydrolysis of acetic anhydride. Part III. The catalytic efficiency of a series of tertiary amines. J. Chem. Soc. 1953, 1409–1415. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Kyzas, G.Z.; Mitropoulos, A.C. Lignocellulosic composites from acetylated sunflower stalks. Appl. Sci. 2019, 9, 646. [Google Scholar] [CrossRef]
- Fuschs, W. Zur kenntnis des genuimen lignis, I: De acetylierung des Finchtenholzes. Beriche Der Deutsehem Chemischem Gasellschaft 1928, 61, 948–951. [Google Scholar]
- Horn, O. Beriche Der Deutsehem. Chemischem Gasellschaft 1928, 61B, 2542. [Google Scholar]
- Stamm, A.J.; Tarkow, H. Dimensional stabilisation of wood. J. Colloid Chem. 1947, 51, 493–505. [Google Scholar] [CrossRef]
- Rowell, R.M. The Chemistry of Solid Wood; ACS: Washington, DC, USA, 1984. [Google Scholar]
- Beckers, E.P.J.; Militz, H. Acetylation of solid wood. Initial trials on lab and semi industrial scale. In Proceedings of the Second Pacific Rim Bio-based Composites Symposium, Vancouver, BC, Canada, 6–9 November 1994; pp. 125–135. [Google Scholar]
- Yasuda, R.; Minato, K.; Norimoto, M. Moisture adsorption thermodynamics of chemically modified wood. Holzforshung 1995, 49, 548–554. [Google Scholar] [CrossRef]
- Stamm, A.J. Wood and Cellulose Science; Ronald Press Co.: New York, NY, USA, 1964. [Google Scholar]
- Papadopoulos, A.N. Swelling, Cell Wall Porosity and Chemical Modification of Wood. Ph.D. Thesis, University of Wales Bangor, Bangor, UK, 2001. [Google Scholar]
- Goldstein, I.S.; Jeroski, E.B.; Lund, A.E.; Nielson, J.F.; Weaver, J.W. Acetylation of wood in lumber thickness. For. Prod. J. 1961, 11, 363–370. [Google Scholar]
- Beckers, E.P.J.; Militz, H.; Stevens, M. Acetylated Solid Wood. Laboratory Durability Test (Part II) and Field Tests; Document no. IRG/WP/95-40048; International Research Group on Wood Preservation: Stockholm, Sweden, 1995. [Google Scholar]
- Larsson-Brelid, P.; Simonson, R.; Bergman, O.; Nilsson, T. Resistance of acetylated wood to biological degradation. Holz als Roh- und Werkst 2000, 58, 331–337. [Google Scholar] [CrossRef]
- Suttie, E.D.; Hill, C.A.S.; Jones, D.; Orsler, R.J. Chemically modified solid wood. I. Resistance to fungal attack. Maerialst und Organisms 1999, 32, 159–182. [Google Scholar]
- Papadopoulos, A.N.; Hill, C.A.S. The biological effectiveness of wood modified with linear chain carboxylic acid anhydrides against Coniophora puteana. Holz als Roh- und Werkstoff 2002, 60, 329–332. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Militz, H.; Pfeffer, A. The biological behaviours of pine wood modified with linear chain carboxylic acid anhydrides against soft rot decay. Int. Biodegradation Biodeterior. 2010, 64, 409–412. [Google Scholar] [CrossRef]
- Kartal, S.N.; Yoshimura, T.; Imamura, Y. Decay and termite resistance of boron treated and chemically modified wood by in situ co-polymerization of allyl glycidyl ether (AGE) with methyl methacrylate (MMA). Int. Biodegradation Biodeterior. 2004, 53, 111–117. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Duquesnoy, P.; Cragg, S.M.; Pitman, A.J. The resistance of wood modified with linear chain carboxylic acid anhydrides to attack by the marine wood borer Limnoria quadripunctata Hothius. Int. Biodegradation Biodeterior. 2008, 61, 199–202. [Google Scholar] [CrossRef]
- Borges, L.M.S.; Cragg, S.M.; Williams, J.R. Comparing the Resistance of a Number of Lesser Known Species of Tropical Hardwoods to the Marine Borer Limnoria Using a Short-Term Laboratory Assay; Document no. IRG/WP 03-10500; The International Research Group on Wood Preservation: Stockholm, Sweden, 2003. [Google Scholar]
- Papadopoulos, A.N.; Avtzis, D.; Avtzis, N. The biological effectiveness of wood modified with linear chain carboxylic acid anhydrides against the subterranean termites Reticulitermes flavipes. Holz als Roh- und Werkstoff 2003, 66, 249–252. [Google Scholar] [CrossRef]
- Stamm, A.J. Dimensional stabilization of wood with furfuryl alcohol. Wood Technol. Chem. Aspects 1977, 43, 141–149. [Google Scholar]
- Westin, M. Development and Evaluation of New Alternative Wood Preservation Treatments; Final Report to the Swedish Council for Forestry and Agricultural Research, Stockholm University: Stochholm, Sweden, 1996; pp. 1–25. [Google Scholar]
- Mantanis, G.I. Chemical modification of wood by acetylation or furfurylation: A review of the present scaled-up technologies. BioResources 2017, 12, 4478–4489. [Google Scholar] [CrossRef]
- Filgueira, D.; Moldes, D.; Fuentealba, C.; Garcia, D.E. Condensed tannins from pine bark: A novel wood surface modifier assisted by laccase. Ind. Crops Prod. 2017, 103, 185–194. [Google Scholar] [CrossRef]
- Fernández-Costas, C.; Palanti, S.; Carpentier, J.; Sanroman, M.A.; Moldes, D. A sustainable treatment for wood preservation: Enzymatic grafting of wood extractives. ACS Sustain. Chem. Eng. 2017, 5, 7557–7567. [Google Scholar] [CrossRef]
- Fernández-Costas, C.; Gouveia, S.; Sanromán, M.A.; Moldes, D. Structural characterization of Kraft lignins from different spent cooking liquors by 1D and 2D Nuclear Magnetic Resonance spectroscopy. Biomass Bioenergy 2014, 63, 156–166. [Google Scholar] [CrossRef]
- Fernández-Costas, C.; Palanti, S.; Sanromán, M.A.; Moldes, D. Enzymatic grafting of kraft lignin as a wood bio-protection strategy. Part 1: Factors affecting the process. Holzforschung 2017, 71, 681–687. [Google Scholar] [CrossRef]
- Fernández-Costas, C.; Palanti, S.; Sanromán, M.A.; Moldes, D. Enzymatic grafting of karft lignin as a wood bioprotection strategy. Part 2: Effectiveness against wood destroying basidiomycetes. Effect of cooper entrapment. Holzforschung 2017, 71, 689–695. [Google Scholar]
- Boehm, H.P.; Setton, R.; Stumpp, E. Nomenclature and terminology of graphite intercalation compounds. Carbon 1986, 24, 241–245. [Google Scholar] [CrossRef]
- Borm, P.J.; Robbins, D.; Haubold, S.; Kuhlbusch, T.; Fissan, H.; Donaldson, K.; Schins, R.; Stone, V.; Kreyling, W.; Lademann, J.; et al. The potential risks of nanomaterials: A review carried out for ECETOC. Part. Fibre Toxicol. 2006, 3, 11. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N.; Seredych, M.; Bandosz, T.J.; Deliyanni, E.A. Removal of dorzolamide from biomedical wastewaters with adsorption onto graphite oxide/poly(acrylic acid) grafted chitosan nanocomposite. Bioresour. Technol. 2014, 152, 399–406. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Travlou, N.A.; Deliyanni, E.A. The role of chitosan as nanofiller of graphite oxide for the removal of toxic mercury ions. Colloids Surfaces B Biointerfaces 2014, 113, 467–476. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bomis, G.; Kosheleva, R.I.; Efthimiadou, E.K.; Favvas, E.P.; Kostoglou, M.; Mitropoulos, A.C. Nanobubbles effect on heavy metal ions adsorption by activated carbon. Chem. Eng. J. 2019, 356, 91–97. [Google Scholar] [CrossRef]
- Roco, M. Nanotechnology’s Future. Scientific American, 1 August 2006. [Google Scholar]
- De Filpo, G.; Nicoletta, F.P.; Chidichimo, G. Flexible nano-photoelectrochromic film. Chem. Mater. 2006, 18, 4662–4666. [Google Scholar] [CrossRef]
- De Filpo, G.; Mormile, S.; Nicoletta, F.P.; Chidichimo, G. Fast, self-supplied, all solid photoelectrochromic film. J. Power Sources 2010, 195, 4365–4369. [Google Scholar] [CrossRef]
- Zikeli, F.; BVinciguerra, V.; D’Annibale, A.; Capitani, D.; Romagnoli, M.; Mugnozza, G. Preparation of lignin nanoparticles from wood waste for wood surface treatment. Nanomaterials 2019, 9, 281. [Google Scholar] [CrossRef]
- Xu, Q.; Ji, Y.; Sun, Q.; Fu, Y.; Xu, Y.; Jin, L. Fabrication of cellulose nanocrystal/chitosan hydrogel for controlled drug release. Nanomaterials 2019, 9, 253. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ekielski, A. The self-assembly of lignin and its application in nanoparticle synthesis: A short review. Nanomaterials 2019, 9, 243. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Schimdt, O. Nanotachnology in wood based composite panels. Int. J. Bioinorg. Hybrid Nanomater. 2014, 3, 65–73. [Google Scholar]
- Goffredo, G.B.; Accoroni, S.; Totti, T.; Romagnoli, T.; Valentini, L.; Munafò, P. Titanium dioxide based nanotreatments to inhibit microalgal fouling on building stone surfaces. Build. Environ. 2017, 112, 209–222. [Google Scholar] [CrossRef]
- Gupta, A.; Kim, B.S. Shape memory polyurethane biocomposites based on toughened polycaprolactone promoted by nano-chitosan. Nanomaterials 2019, 9, 225. [Google Scholar] [CrossRef]
- Yang, W.; Jiao, L.; Liu, W.; Dai, H. Manufacture of highly transparent and hazy cellulose nanofibril films via coating TEMPO-oxidized wood fibers. Nanomaterials 2019, 9, 107. [Google Scholar] [CrossRef]
- Wang, H.; Yao, Q.; Wang, C.; Ma, Z.; Sun, Q.; Fan, B.; Jin, C.; Chen, Y. Hydrothermal synthesis of nanooctahedra MnFe2O4 onto the wood surface with soft magnetism, fire resistance and electromagnetic wave absorptions. Nanomaterials 2017, 7, 118. [Google Scholar] [CrossRef] [PubMed]
- De Filpo, G.; Palermo, A.M.; Rachiele, F.; Nicoletta, F.P. Preventing fungal growth in wood by titanium dioxide nanoparticles. Int. Biodeterioration Biodegradation 2014, 85, 217–222. [Google Scholar] [CrossRef]
- Wegner, T.H.; Jones, P. Nanotechnology for the forest products industry. Wood Fiber Sci. 2005, 37, 549–551. [Google Scholar]
- Xu, C.; Stromme, M. Sustainable porous carbon materials derived from wood-based biopolymers for CO2 capture. Nanomaterials 2019, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Darweesh, A.A.; Bauman, S.J.; Debu, D.T.; Herzog, J.B. The Role of rayleigh-wood anomalies and surface plasmons in optical enhancement for nano-gratings. Nanomaterials 2018, 8, 809. [Google Scholar] [CrossRef]
- Lou, Z.; Zhang, Y.; Zhou, M.; Han, H.; Cai, J.; Yang, L.; Yuan, C.; Li, Y. Synthesis of magnetic wood fiber board and corresponding multi-layer magnetic composite board, with electromagnetic wave absorbing properties. Nanomaterials 2018, 8, 441. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Papadopoulos, A.N. A review of methods used to determine the size of the cell wall microvoids of wood. J. Inst. Wood Sci. 2001, 15, 337–345. [Google Scholar]
- Teng, T.; Arip, M.; Sudesh, K.; Lee, H. Conventional technology and nanotechnology in wood preservation: A review. BioResources 2018, 13, 9220–9252. [Google Scholar] [CrossRef]
- Polleti Papi, M.A.; Caetano, F.R.; Berganini, M.F.; Marcolino-Junior, L.H. Facile synthesis of a silver nanoparicles/polypyrrole nanocomposite for non-enzymatic glucose determination. Mater. Sci. Eng. 2017, C75, 88–94. [Google Scholar] [CrossRef]
- Gupta, A.; Srivastana, R. Zinc oxide nanoleaves: A scalable disperser assisted sonochemical approach for synthesis and an antibacterial application. Ultrason. Sonochem. 2018, 41, 47–58. [Google Scholar] [CrossRef]
- Moya, R.; Zuniga, A.; Berrocal, A.; Vega, J. Effect of silver nanoparticles synthesized with NPsAg-ethylene glycol on brown decay and white decay fungi of nine tropical woods. J. Nanosci. Nanotechnol. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Royas, O.J.; Lucia, L.A. Green modification of surface characteristics of cellulose materials at the molecular or nano scale: A review. BioResources. 2015, 10, 6095–6206. [Google Scholar] [CrossRef]
- Civardi, C.; Schwarze, F.; Wick, P. Micronized copper wood protection: An efficiency and potential health and risk assessment for copper based nanoparticles. Environ. Pollut. 2015, 200, 20–32. [Google Scholar] [CrossRef]
- Nikolic, M.; Lawther, J.M.; Sanadi, A.R. Use of nanofilers in wood coating: A scientific review. J. Coat. Technol. Res. 2015, 12, 445–461. [Google Scholar] [CrossRef]
- Matsunaga, H.; Kiguchi, M.; Evans, P. Microdistribution of copper carbonate and iron oxide nanoparticles in treated wood. J. Nanoparticle Res. 2009, 11, 1087–1098. [Google Scholar] [CrossRef]
- Xue, W.; Kennepohl, P.; Ruddick, W. Chemistry of copper preservative treated wood. In Proceedings of the 45th International Group on Wood Protection, St. George, UT, USA, 11–15 May 2014; pp. 1–8. [Google Scholar]
- Civardi, C.; Kaiser, J.; Hirsch, C.; Mucchino, C.; Wichser, A.; Wick, P.; Schwarze, F. Release of copper amended particles from micronized copper pressure treated wood during mechanical abrasion. J. Nanotechnol. 2016, 14, 1–10. [Google Scholar] [CrossRef]
- Akhtari, M.; Ganjipour, M. Effect of nano-silver and nano-copper and nano zinc oxide on Paulownia wood exposed to white rot fungus. Agric. Sci. Dev. 2013, 2, 116–119. [Google Scholar]
- Akhtari, M.; Nicholas, D. Evaluation of particulate zinc and copper as wood preservatives for termite control. Eur. J. Wood Wood Prod. 2013, 71, 395–396. [Google Scholar] [CrossRef]
- Jafari, A.; Omidvar, A.; Rasouli, D. The effect of nano copper oxide on physical properties and leaching resistance of wood polystyrene polymer. J. Wood For. Sci. Technol. 2018, 25, 9–25. [Google Scholar]
- Mantanis, G.; Papadopoulos, A.N. The sorption of water vapour of wood treated with a nanotechnology compound. Wood Sci. Technol. 2010, 44, 515–522. [Google Scholar] [CrossRef]
- Sahin, H.T.; Mantanis, G.I. Nano-based surface treatment effects on swelling, water sorption and hardness of wood. Maderas Ciencia y Technologia 2011, 13, 41–48. [Google Scholar] [CrossRef]
- Moya, R.; Berrocal, A.; Zuniga, A.; Baudrit, J.; Noguera, S.C. Effect of silver nanoparticles on white rot wood decay and some physical properties of three tropical wood species. Wood Fiber Sci. 2014, 16, 1–12. [Google Scholar]
- Mantanis, G.I.; Terzi, E.; Kartal, S.N.; Papadopoulos, A.N. Evaluation of mold, decay and termite resistance of pine wood treated with zinc and copper based nanocompounds. Int. Biodeterioration Biodegradation 2014, 90, 140–144. [Google Scholar] [CrossRef]
- Reinprecht, L.; Vidholdova, Z.; Kozienka, M. Decay inhibition of lime wood with zinc oxide nanoparticles used in combination with acrylic resin. Acta Facultatis Xylologiae Zvolen 2015, 57, 43–52. [Google Scholar]
- Reinprecht, L.; Vidholdova, Z. Biological resistance and application properties of particleboards containing nano-zinc oxide. Adv. Mater. Sci. Eng. 2018, 2018, 2680121. [Google Scholar] [CrossRef]
- Kartal, S.N.; Green, F.; Clausen, C.A. Do the unique properties of nanometals affect leachability or efficacy against fungi and termites? Int. Biodetereriorationand Biodegradation 2009, 63, 490–495. [Google Scholar] [CrossRef]
- Huang, H.H.; Lin, C.; Hsu, K. Comparison of resistance improvement to fungal growth on green and conventional building materials by nano-metal impregnation. Build. Environ. 2015, 93, 119–127. [Google Scholar] [CrossRef]
- Terzi, E.; Kartal, S.; Yilgor, N.; Rauktari, L.; Yoshimura, T. Role of various nano-particles in preservation of fungal decay, mold growth and termite attack in wood and their effect on weathering properties and water repellency. Int. Biodeterioration Biodegradation 2016, 107, 77–87. [Google Scholar] [CrossRef]
- Bak, M.; Nemeth, R. Effect of different nanoparticle treatments on the decay resistance of wood. BioResources 2018, 13, 7886–7899. [Google Scholar] [CrossRef]
- Zhang, Z.; MacMullen, J.; Dhakal, H.N.; Radulovic, J.; Herodotou, C.; Totomis, M.; Bennett, N. Biofouling resistance of titanium dioxide and zinc oxide nanoparticulate silane/siloxane exterior facade treatments. Build. Environ. 2013, 59, 47–55. [Google Scholar] [CrossRef]
- Goffredo, G.B.; Citterio, B.; Biavasco, F.; Stazi, F.; Barkeli, S.; Munafo, P. Nanotechnology on wood: The effect of photocatalytic nanocoatings againstAspergillus nige. J. Cult. Herit. 2017, 27, 125–136. [Google Scholar] [CrossRef]
- Evans, P.; Matsunaga, H.; Kiguchi, M. Large scale application of nanaotechnology for wood protection. Nature Nanotechnology 2018, 3, 577–587. [Google Scholar] [CrossRef]
- Khoee, S.; Haslemi, A. Synthesis and characterization of IUdR loaded PEG/PCL/PEG polymersome in mixed DCM/DMF solvent: Experimental and molecular dynamics insights into the role of solvent composition and star architecture in drug dispersion and diffusion. Eur. J. Pharm. Sci. 2018, 114, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, L.; Heiden, P.; Laks, P. Use of nanaparticles for controlled release of biocides in solid wood. J. Appl. Polym. Sci. 2001, 79, 458–465. [Google Scholar] [CrossRef]
- Liu, Y.; Laks, P.; Heiden, P. Controlled release of biocides in solid wood. Efficacy against brown rot wood decay fungus. J. Appl. Polym. Sci. 2002, 86, 596–607. [Google Scholar] [CrossRef]
- Havrlik, M.; Pyranova, P. Protection of wooden materials against biological attack by using nanotechnology. Acta Polytechnika 2015, 55, 101–108. [Google Scholar] [CrossRef]
- Soltani, M.; Najafi, A.; Bakar, E. Water repellent effect and dimensional stability of beech wood impregnated with nano-zinc oxide. BioResources 2013, 8, 6280–6287. [Google Scholar] [CrossRef]
- Bauer, F.; Mehnert, R. UV curable acrylate nanoparticles: Properties and applications. J. Polym. Res. 2005, 12, 483–491. [Google Scholar] [CrossRef]
- Polo, A.; Diamanti, M.V.; Bjarnsholt, T.; Hoiby, N.; Villa, F.; Pedeferri, M.P.; Cappitelli, F. Effects of Photoactivated Titanium Dioxide Nanopowders and Coating on Plank-tonic and Biofilm Growth of Pseudomonas aeruginosa. Photochem. Photobiol. 2011, 87, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Gladis, F.; Eggert, A.; Karsten, U.; Schumann, R. Prevention of Biofilm Growth on Man-Made Surfaces: Evaluation of Antialgal Activity of Two Biocides and Photocatalytic Nanoparticles. Biofouling 2010, 26, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Clausen, C.A.; Kartal, S.N.; Arango, R.A.; Green, F. The Role of Particle Size of Particulate Nano-zinc OxideWood Preservatives on Termite Mortality and Leach Resistance. Nanoscale Res. Lett. 2011, 6, 427–432. [Google Scholar] [CrossRef]
- Lykidis, C.; Mantanis, G.; Adamopoulos, S.; Kalafata, K.; Arabatzis, I. Effects of Nano-sized Zinc Oxide and ZincBorate Impregnation on Brown Rot Resistance of BlackPine (Pinus nigra L.) Wood. Wood Mater. Sci. Eng. 2013, 8, 242–244. [Google Scholar] [CrossRef]
- Shi, X.; Yuan, L.; Sun, X.; Chang, C.; Sun, J. Controllable Synthesis of 4ZnOB2O3·H2O Nano-/Microstructures with Different Morphologies: Influence of Hydrothermal Reaction Parameters and Formation Mechanism. J. Phys. Chem. C 2008, 112, 3558–3567. [Google Scholar] [CrossRef]
- Shi, X.; Li, B.; Qin, G.; Tian, S. Mechanism of Antifungal Action of Borate Against Colletotrichum gloeosporioides Related to Mitochondrial Degradation in Spores. Postharvest Biol. Technol. 2012, 67, 138–143. [Google Scholar] [CrossRef]
- Wu, J.; Ma, G.; Li, P.; Ling, L.; Wang, B. Surface Modification of Nanosilica with Acrylsilane-Containing Tertiary Amine Structure and Their Effect on the Properties of UV-Curable Coating. J. Coat. Technol. Res. 2014, 11, 387–395. [Google Scholar] [CrossRef]
- Greenwood, P. Nano-particle Reinforced Latex Dispersions with Modified Colloidal Silica. JCT Coat. Tech. 2008, 5, 44–51. [Google Scholar]
- Vu, C.; Laferte, O. Silica Nanoparticles in the Optimisation of Scratch and Abrasion Resistance of High Performance UV Multi-layer Coatings. Eur. Coat. J. 2006, 6, 48–61. [Google Scholar]
- Landry, V.; Riedl, B.; Blanchet, P. Alumina and Zirconia Acrylate Nanocomposites Coatings for Wood Flooring: Photocalorimetric Characterization. Prog. Org. Coat. 2008, 61, 76–82. [Google Scholar] [CrossRef]
- Landry, V.; Riedl, B.; Blanchet, P. Dispersion is the Key to Performance. Eur. Coat. J. 2009, 7, 30–41. [Google Scholar]
- Landry, V.; Blanchet, P.; Riedl, B. Mechanical and Optical Properties of Clay-Based Nanocomposites Coatings for Wood Flooring. Prog. Org. Coat. 2010, 67, 381–388. [Google Scholar] [CrossRef]
- Malucelli, G.; Alongi, J.; Gioffredi, E.; Lazzari, M. Thermal, Rheological, and Barrier Properties of Waterborne Acrylic Nanocomposite Coatings Based on Boehmite or Organo-modified Montmorillonite. J. Therm. Anal. Calorim. 2012, 111, 1303–1310. [Google Scholar] [CrossRef]
- Milligan, W.O.; McAtee, J.L. Crystal Structure of c-AlOOH and c-ScOOH. J. Phys. Chem. 1956, 60, 273–277. [Google Scholar] [CrossRef]
- Ghaemy, M.; Bekhradnia, S. Thermal and photocuring of an acrylat-based coating resin reinforced with nanosilica. J. Coat. Technol. Res. 2012, 9, 569–578. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose Nanocrystals vs. Cellulose Nanofibrils: A Comparative Study on Their Microstructures and Effects as Polymer Reinforcing Agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]

| Property | Advantage | Disadvantage |
|---|---|---|
| Hydrofobicity | Nanocomposite coatings create rough hydrophobic surface without affecting the softness and abrasion resistance of the wood. The impregnation of nanomaterials reduces the pore size and space available within the cell wall, which is used for the absorption of water molecules. | Although nanotechnology is widely integrated in wood treatment, recently there are increasing critiques and discussions concerning the potential health and environmental risks of nanomaterials. Past experience shows that new technologies may not be more environmentally superior than traditional ones either due to the possibility of shifting the negative impacts outside the impact category or even the late identification of their impacts. Even if the incomplete knowledge or a lack of information about nanomaterials delays the study, addressing the potential impact of nano-based treatments using tools like a life-cycle assessment will help to avoid mistakes like in the past when new technological innovations were released prior to an impact assessment. |
| UV-protection | A surface modification with nano-sized inorganic fillers has found many applications as they are nontoxic and stable under exposure to UV. Their large surface to volume ratio makes them effective towards improving poor the photoresistance property of the wood. | |
| Fire performance | Wood is composed of cellulose, hemicelluloses, and lignin, which can make it undergo thermal degradation when it is in contact with a source of ignition. Although the fire performance of wood is improved using different fire retardants, there have been a low efficiency, leaching, and a high environmental and health risk while utilizing many of these chemicals. The utilization of nanoparticles alone or in combination with other fire-retardant chemicals can reduce the ignitability of the wood and limit the leaching of fire-retardant chemicals. | |
| Antimicrobial | Nanoparticles increase the decay resistance of wood by reducing the moisture availability in the wood either by preventing the absorption of the moisture or by blocking the flow path of liquid water. | |
| Mechanical properties | The mechanical properties of wood depend on environmental agents, its isotropic properties, and sometimes on the type of treatment used to improve wood attributes. Impregnating the wood with nanoparticles enhances its hardness by filling the cavities of the wood. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulos, A.N.; Bikiaris, D.N.; Mitropoulos, A.C.; Kyzas, G.Z. Nanomaterials and Chemical Modifications for Enhanced Key Wood Properties: A Review. Nanomaterials 2019, 9, 607. https://doi.org/10.3390/nano9040607
Papadopoulos AN, Bikiaris DN, Mitropoulos AC, Kyzas GZ. Nanomaterials and Chemical Modifications for Enhanced Key Wood Properties: A Review. Nanomaterials. 2019; 9(4):607. https://doi.org/10.3390/nano9040607
Chicago/Turabian StylePapadopoulos, Antonios N., Dimitrios N. Bikiaris, Athanasios C. Mitropoulos, and George Z. Kyzas. 2019. "Nanomaterials and Chemical Modifications for Enhanced Key Wood Properties: A Review" Nanomaterials 9, no. 4: 607. https://doi.org/10.3390/nano9040607







