In Vitro and In Vivo Studies of Hydrophilic Electrospun PLA95/β-TCP Membranes for Guided Tissue Regeneration (GTR) Applications
Abstract
1. Introduction
2. Materials and Methods
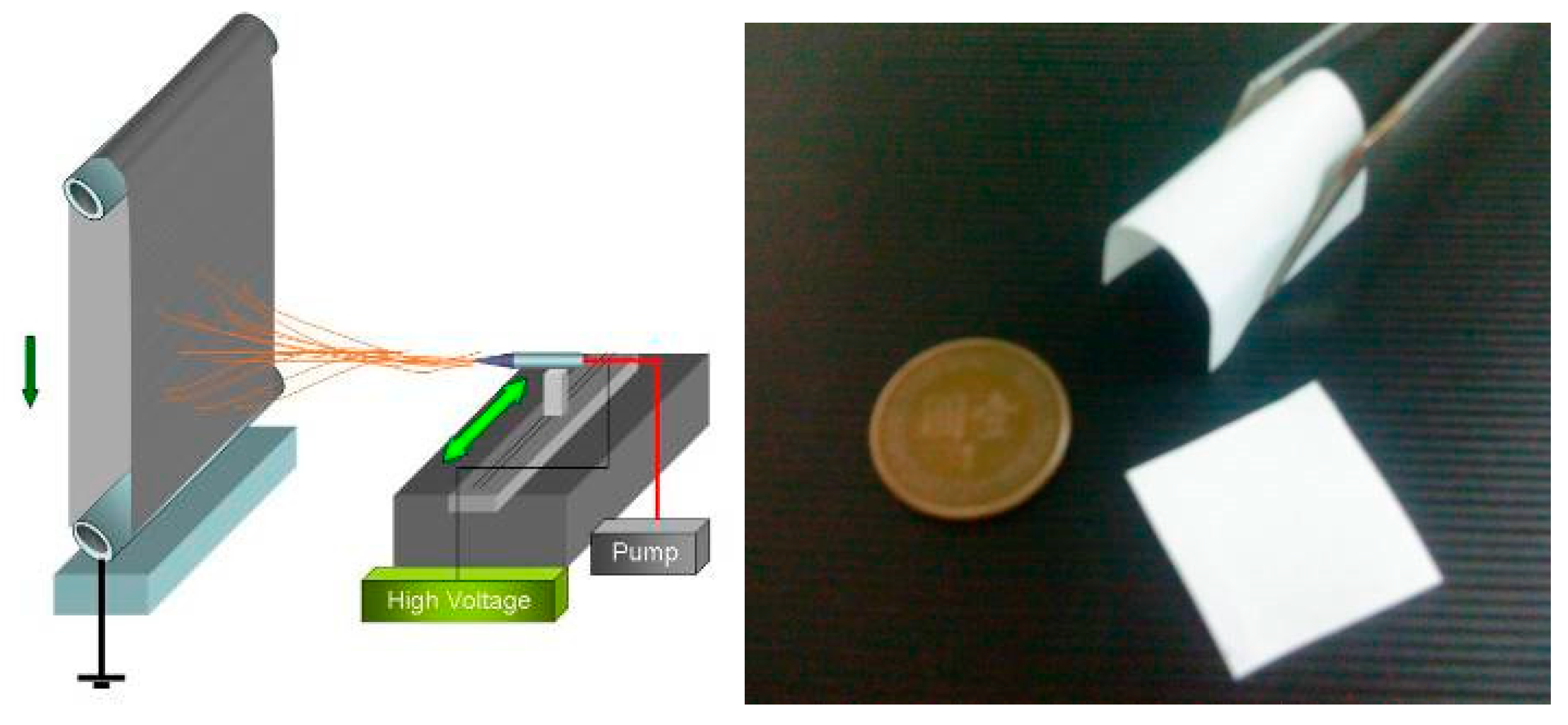
2.1. ES PLA95/β-TCP Fibrous Membranes
2.2. Cytotoxicity Testing
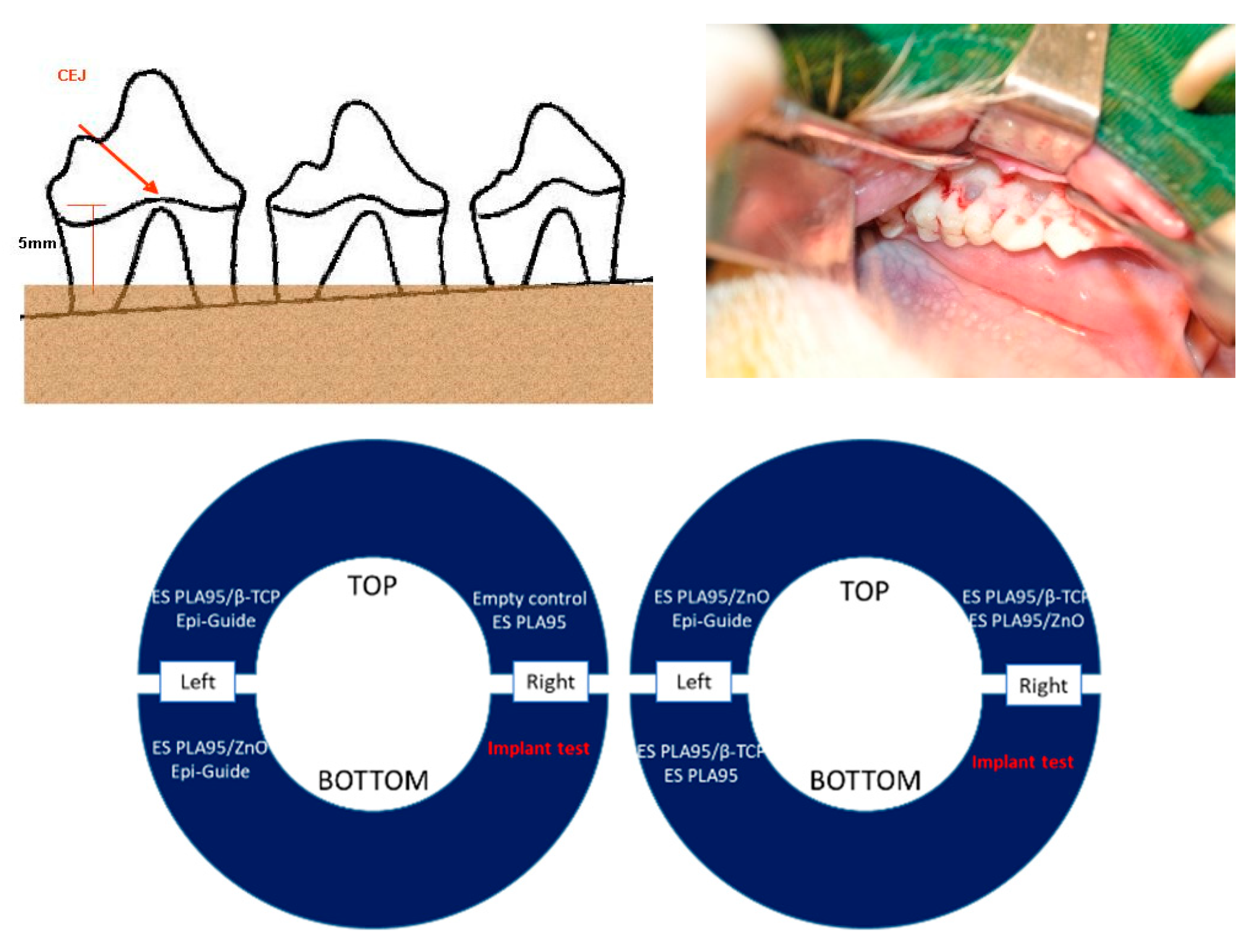
2.3. In Vivo Test (Animal Model)
2.4. Clinical Trial
2.5. Statistical Analysis
3. Results and Discussion
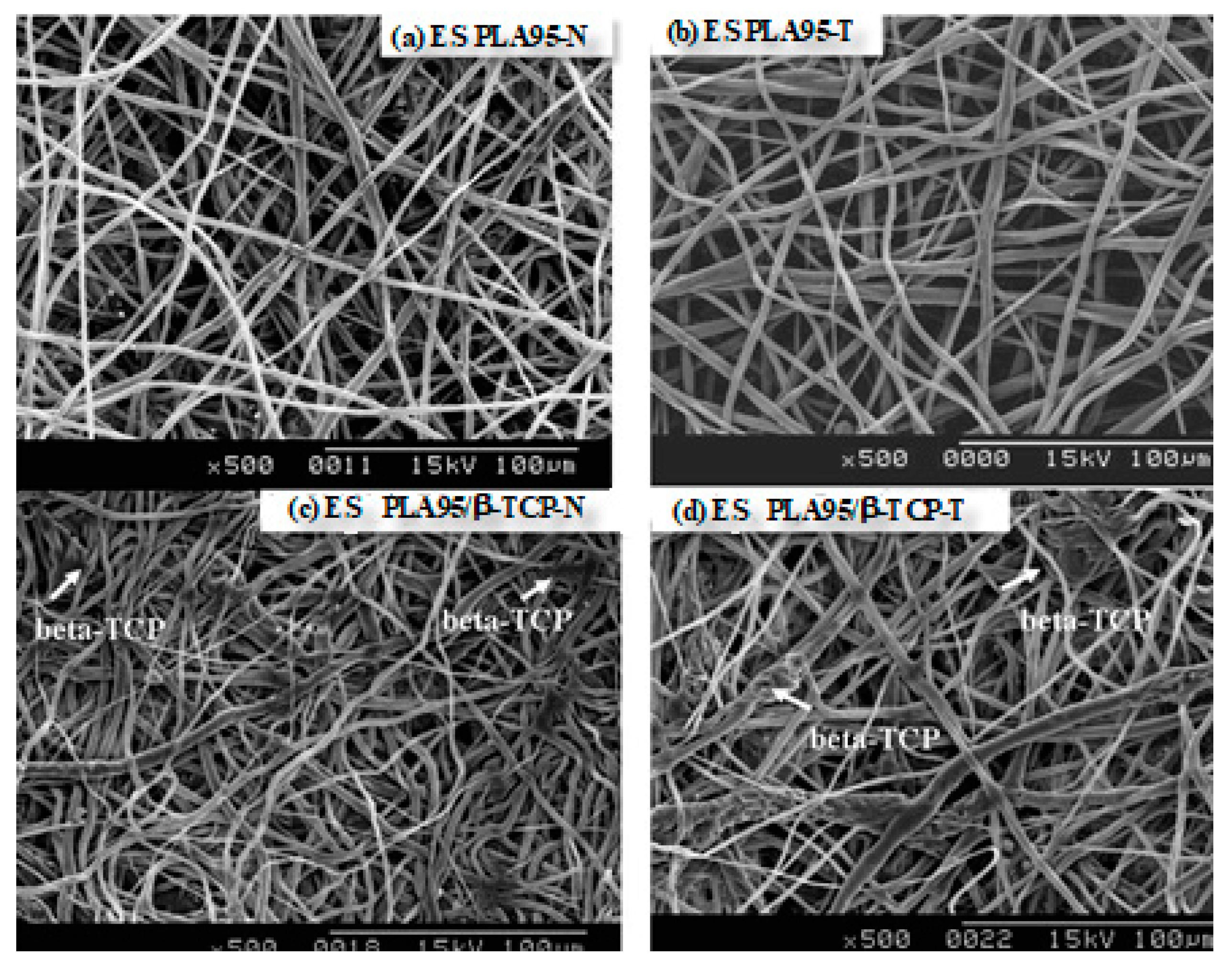
3.1. Morphology
3.2. Cytotoxicity Test
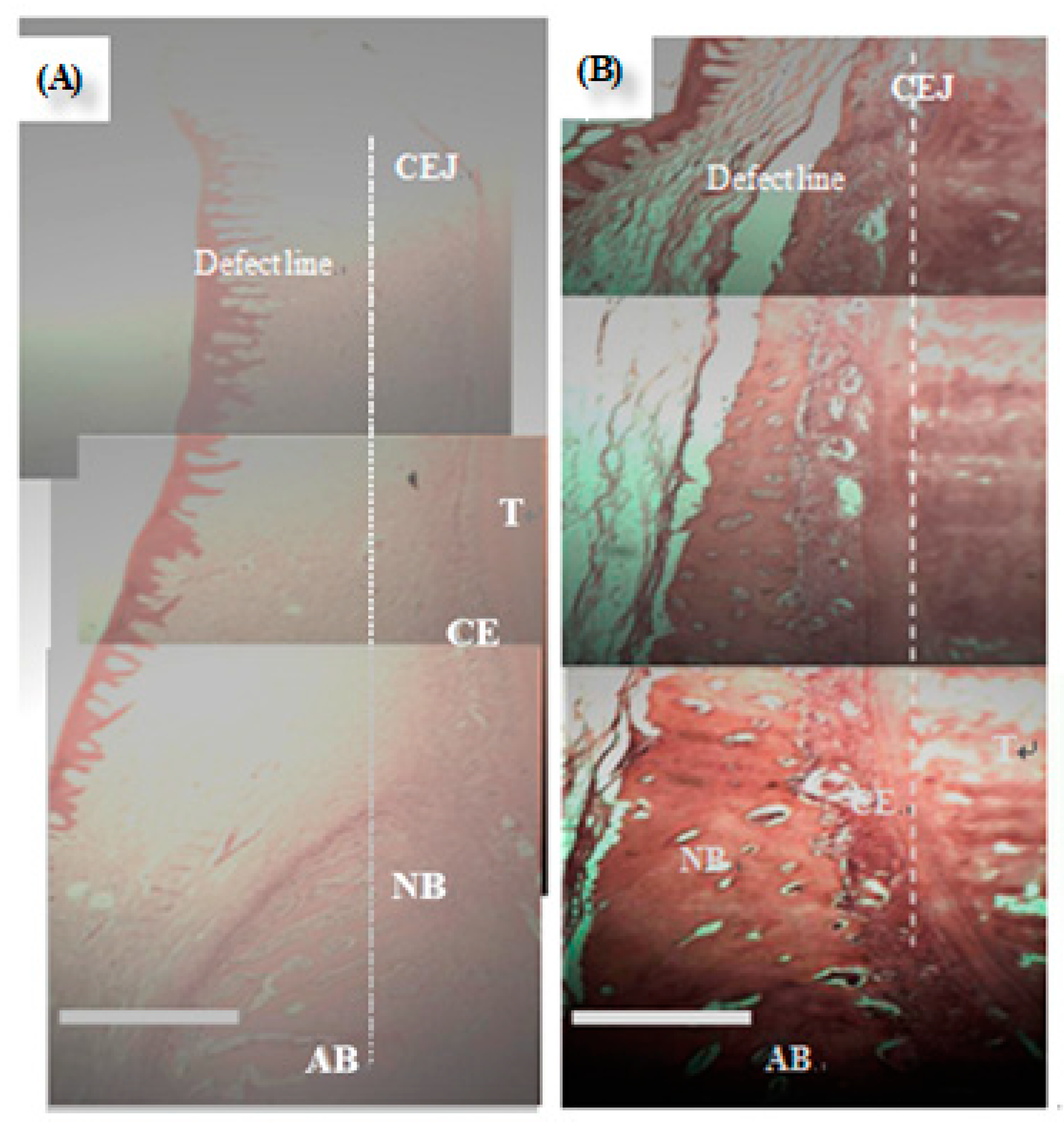
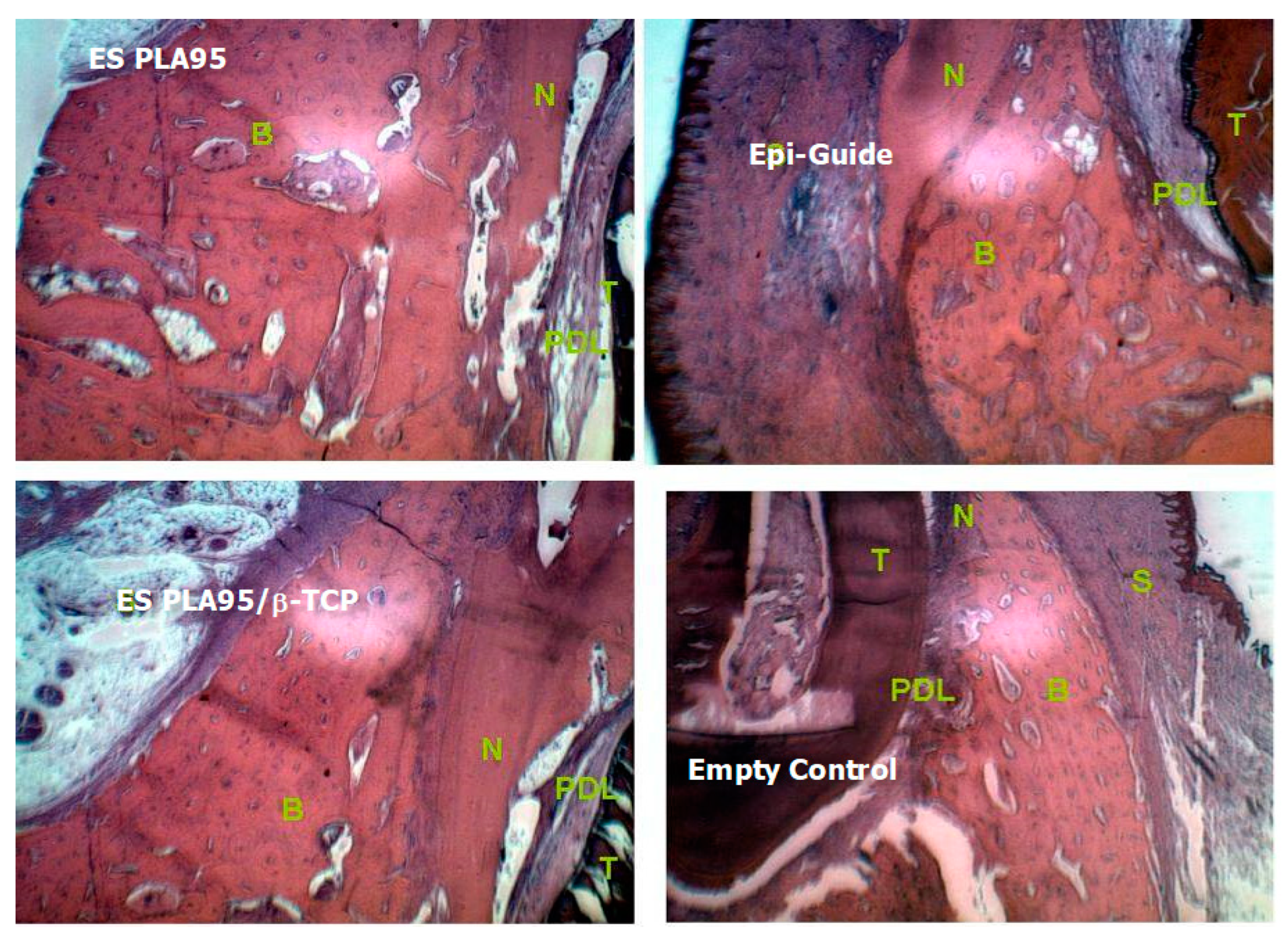
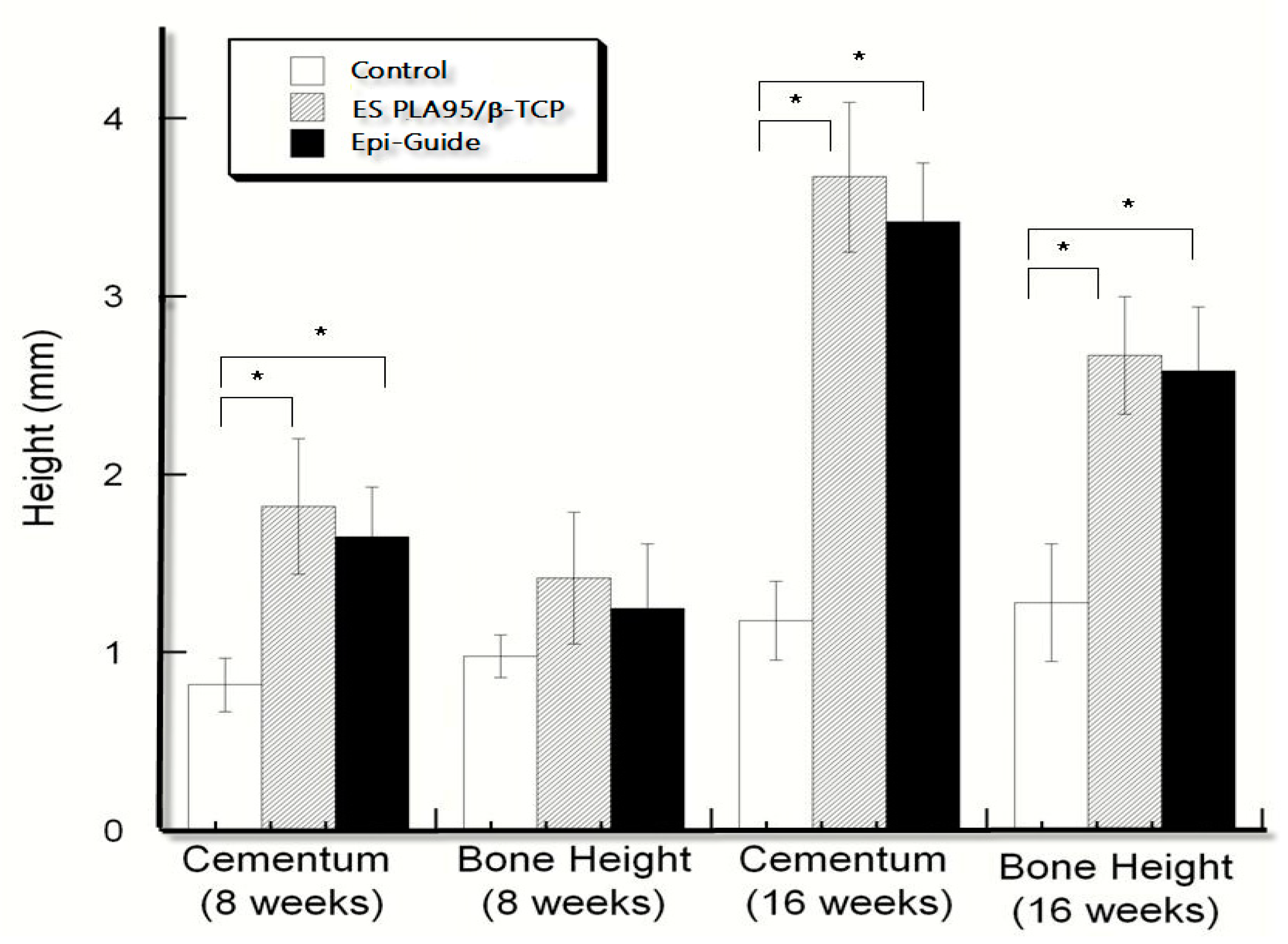
3.3. In Vivo Test (Animal Model)
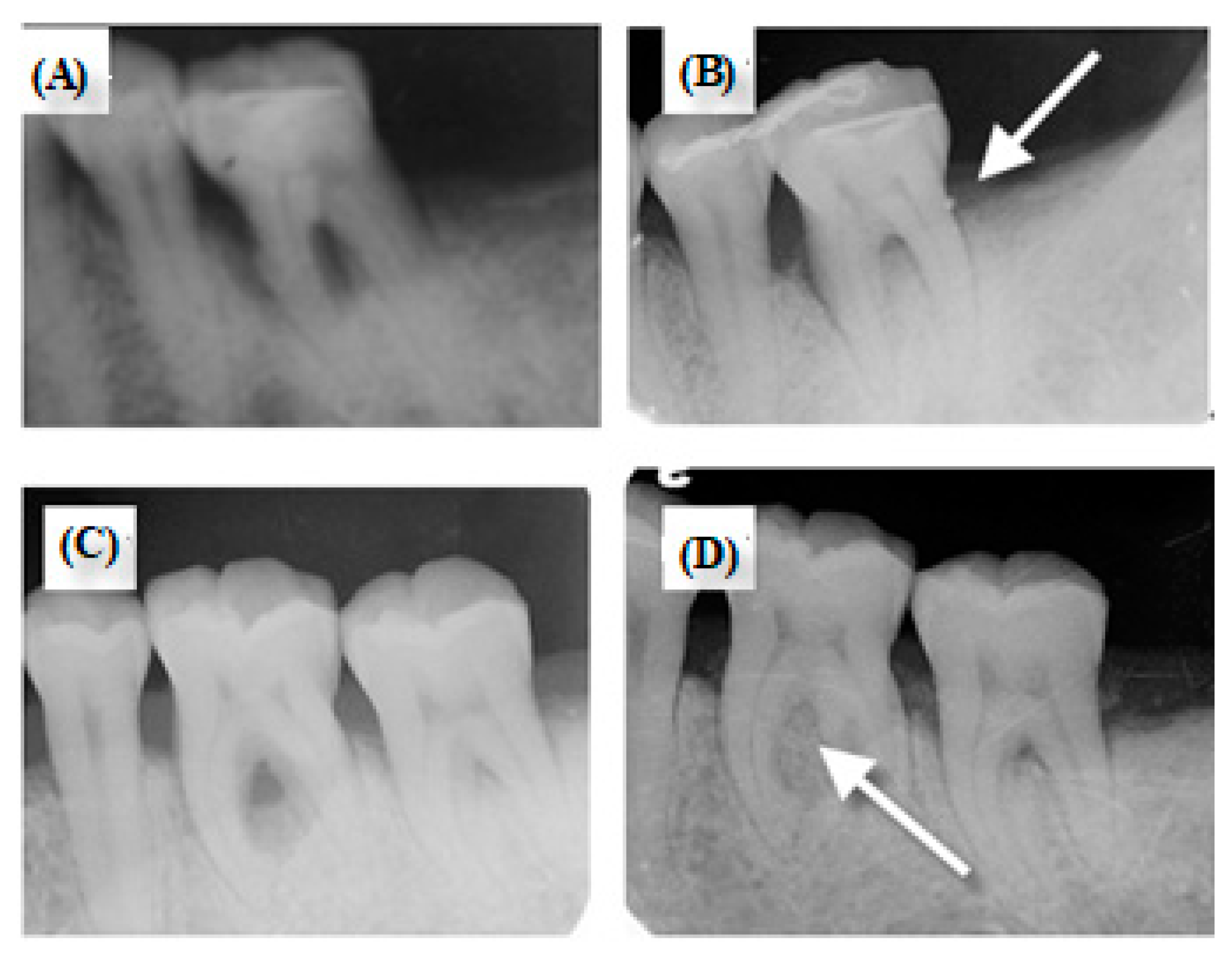
3.4. Clinical Trial
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slots, J.; MacDonald, E.S.; Nowzari, H. Infectious aspects of periodontal regeneration. Periodontol 2000 1999, 19, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Haffajee, A.D.; Socransky, S.S. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000 1994, 5, 78–111. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Stefanac, S.J. The Systemic Phase of Treatment. In Treatment Planning in Dentistry (Second Edition); Stefanac, S.J., Nesbit, S.P., Eds.; Mosby: Saint Louis, MO, USA, 2007; Chapter 5; pp. 91–111. [Google Scholar] [CrossRef]
- Buser, D.; Dahlin, C.; Schenk, R.K. Guided Bone Regeneration in Implant Dentistry; Quintessence Books: Batavia, IL, USA, 1994. [Google Scholar]
- Ronda, M.; Rebaudi, A.; Torelli, L.; Stacchi, C. Expanded vs. dense polytetrafluoroethylene membranes in vertical ridge augmentation around dental implants: A prospective randomized controlled clinical trial. Clin. Oral Implant. Res. 2014, 25, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Ruskin, J.; Higginbottom, F.; Hardwick, R.; Dahlin, C. Osseointegration of titanium implants in bone regenerated in membrane-protected defects: A histologic study in the canine mandible. Implant Dent. 1996, 5, 212. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Shi, B.; Miron, R.J. Membranes for guided tissue and bone regeneration. Ann. Oral Maxillofac. Surg. 2013, 1, 10. [Google Scholar] [CrossRef]
- Jo, Y.-Y.; Oh, J.-H. New Resorbable Membrane Materials for Guided Bone Regeneration. Appl. Sci. 2018, 8, 2157. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Chang, I.-L.; Yang, J.-C.; Lee, S.-Y.; Wang, L.-F.; Hu, H.-T.; Yang, S.-Y.; Lin, H.-C. Early clinical experience with resorbable poly-5D/95L-lactide (PLA95) plate system for treating distal radius fractures. J. Dent. Sci. 2013, 8, 44–52. [Google Scholar] [CrossRef]
- Lin, J.W.; Lin, C.-M.; Chiu, W.T. Clinical Experience with Bioresorbable Plates for Skull Flap Fixation. J. Dent. Sci. 2006, 1, 187–194. [Google Scholar]
- Lee, S.-Y.; Wang, L.-F.; Wang, Y.-C.; Chiou, S.-Y.; Lee, S.-A.; Yang, J.-C. Treatment of facial fractures using poly-5D/95L-lactide (PLA95) copolymer plates and screws. J. Dent. 2008, 3, 150–158. [Google Scholar]
- Ignatius, A.A.; Claes, L.E. In vitro biocompatibility of bioresorbable polymers: Poly(L, DL-lactide) and poly(l-lactide-co-glycolide). Biomaterials 1996, 17, 831–839. [Google Scholar] [CrossRef]
- Kikuchi, M.; Koyama, Y.; Takakuda, K.; Miyairi, H.; Shirahama, N.; Tanaka, J. In vitro change in mechanical strength of β-tricalcium phosphate/copolymerized poly-l-lactide composites and their application for guided bone regeneration. J. Biomed. Mater. Res. 2002, 62, 265–272. [Google Scholar] [CrossRef]
- Kikuchi, M.; Koyama, Y.; Yamada, T.; Imamura, Y.; Okada, T.; Shirahama, N.; Akita, K.; Takakuda, K.; Tanaka, J. Development of guided bone regeneration membrane composed of β-tricalcium phosphate and poly (l-lactide-co-glycolide-co-ε-caprolactone) composites. Biomaterials 2004, 25, 5979–5986. [Google Scholar] [CrossRef]
- Sui, G.; Yang, X.; Mei, F.; Hu, X.; Chen, G.; Deng, X.; Ryu, S. Poly-L-lactic acid/hydroxyapatite hybrid membrane for bone tissue regeneration. J. Biomed. Mater. Res. A 2007, 82, 445–454. [Google Scholar] [CrossRef]
- Jeong, S.I.; Ko, E.K.; Yum, J.; Jung, C.H.; Lee, Y.M.; Shin, H. Nanofibrous Poly(lactic acid)/Hydroxyapatite Composite Scaffolds for Guided Tissue Regeneration. Macromol. Biosci. 2008, 8, 328–338. [Google Scholar] [CrossRef]
- Kim, H.-W.; Song, J.-H.; Kim, H.-E. Nanofiber Generation of Gelatin–Hydroxyapatite Biomimetics for Guided Tissue Regeneration. Adv. Funct. Mater. 2005, 15, 1988–1994. [Google Scholar] [CrossRef]
- Wu, S.; Xiao, Z.; Song, J.; Li, M.; Li, W. Evaluation of BMP-2 Enhances the Osteoblast Differentiation of Human Amnion Mesenchymal Stem Cells Seeded on Nano-Hydroxyapatite/Collagen/Poly(l-Lactide). Int. J. Mol. Sci. 2018, 19, 2171. [Google Scholar] [CrossRef]
- Yang, F.; Both, S.K.; Yang, X.; Walboomers, X.F.; Jansen, J.A. Development of an electrospun nano-apatite/PCL composite membrane for GTR/GBR application. Acta Biomater. 2009, 5, 3295–3304. [Google Scholar] [CrossRef]
- Hu, H.-T.; Lee, S.-Y.; Chen, C.-C.; Yang, Y.-C.; Yang, J.-C. Processing and properties of hydrophilic electrospun polylactic acid/beta-tricalcium phosphate membrane for dental applications. Polym. Eng. Sci. 2013, 53, 833–842. [Google Scholar] [CrossRef]
- Gorgieva, S.; Tomaž, V.; Vanja, K. Processing of Biopolymers-Based, Efficiently Integrated Bilayer Membranes for Use in Guided Tissue Regeneration Procedures. IOP Conf. Ser. Mater. Sci. Eng. 2018, 460, 012036. [Google Scholar]
- Senthamizhan, A.; Balusamy, B.; Uyar, T. 1—Electrospinning: A versatile processing technology for producing nanofibrous materials for biomedical and tissue-engineering applications. In Electrospun Materials for Tissue Engineering and Biomedical Applications; Uyar, T., Kny, E., Eds.; Woodhead Publishing: Witney, Oxford, UK, 2017; pp. 3–41. [Google Scholar]
- Theron, S.A.; Zussman, E.; Yarin, A.L. Experimental investigation of the governing parameters in the electrospinning of polymer solutions. Polymer 2004, 45, 2017–2030. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Lim, Te.; Fujihara, K.; Teo, W.E.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005. [Google Scholar]
- Jung, R.E.; Glauser, R.; Scharer, P.; Hammerle, C.H.; Sailer, H.F.; Weber, F.E. Effect of rhBMP-2 on guided bone regeneration in humans. Clin. Oral Implant. Res. 2003, 14, 556–568. [Google Scholar] [CrossRef]
- Wikesjo, U.M.; Qahash, M.; Thomson, R.C.; Cook, A.D.; Rohrer, M.D.; Wozney, J.M.; Hardwick, W.R. rhBMP-2 significantly enhances guided bone regeneration. Clin. Oral Implant. Res. 2004, 15, 194–204. [Google Scholar] [CrossRef]
- Yin, L.; Yang, S.; He, M.; Chang, Y.; Wang, K.; Zhu, Y.; Liu, Y.; Chang, Y.; Yu, Z. Physicochemical and biological characteristics of BMP-2/IGF-1-loaded three-dimensional coaxial electrospun fibrous membranes for bone defect repair. J. Mater. Sci. Mater. Med. 2017, 28, 94. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Gill, A.S.; Sikri, P. Evaluation of the regenerative potential of 25% doxycycline-loaded biodegradable membrane vs biodegradable membrane alone in the treatment of human periodontal infrabony defects: A clinical and radiological study. Indian J. Dent. Res. 2008, 19, 116–123. [Google Scholar] [CrossRef]
- Lyons, L.C.; Weltman, R.L.; Moretti, A.J.; Trejo, P.M. Regeneration of degree ii furcation defects with a 4% doxycycline hyclate bioabsorbable barrier. J. Periodontol. 2008, 79, 72–79. [Google Scholar] [CrossRef]
- Zafiropoulos, G.K.; Hoffmann, O.; Kasaj, A.; Willershausen, B.; Weiss, O.; Van Dyke, T.E. Treatment of Intrabony Defects Using Guided Tissue Regeneration and Autogenous Spongiosa Alone or Combined With Hydroxyapatite/beta-Tricalcium Phosphate Bone Substitute or Bovine-Derived Xenograft. J. Periodontol. 2007, 78, 2216–2225. [Google Scholar] [CrossRef]
- Schwarz, F.; Herten, M.; Ferrari, D.; Wieland, M.; Schmitz, L.; Engelhardt, E.; Becker, J. Guided bone regeneration at dehiscence-type defects using biphasic hydroxyapatite + beta tricalcium phosphate (Bone Ceramic) or a collagen-coated natural bone mineral (BioOss Collagen): An immunohistochemical study in dogs. Int. J. Oral Maxillofac. Surg. 2007, 36, 1198–1206. [Google Scholar] [CrossRef]
- Coonts, B.A.; Whitman, S.L.; O’Donnell, M.; Polson, A.M.; Bogle, G.; Garrett, S.; Swanbom, D.D.; Fulfs, J.C.; Rodgers, P.W.; Southard, G.L.; et al. Biodegradation and biocompatibility of a guided tissue regeneration barrier membrane formed from a liquid polymer material. J. Biomed. Mater. Res. 1998, 42, 303–311. [Google Scholar] [CrossRef]
- Da Silva Pereira, S.L.; Sallum, A.W.; Casati, M.Z.; Caffesse, R.G.; Weng, D.; Nociti, F.H., Jr.; Sallum, E.A. Comparison of bioabsorbable and non-resorbable membranes in the treatment of dehiscence-type defects. A histomorphometric study in dogs. J. Periodontol. 2000, 71, 1306–1314. [Google Scholar] [CrossRef]
- Magnusson, I.; Batich, C.; Collins, B.R. New Attachment Formation Following Controlled Tissue Regeneration Using Biodegradable Membranes. J. Periodontol. 1988, 59, 1–6. [Google Scholar] [CrossRef]
- Pitaru, S.; Tal, H.; Soldinger, M.; Grosskopf, A.; Noff, M. Partial Regeneration of Periodontal Tissues Using Collagen Barriers. J. Periodontol. 1988, 59, 380–386. [Google Scholar] [CrossRef]
| Extracts of Test Item and Controls | Observation | Grade | Reactivity |
|---|---|---|---|
| Membrane extract, reagent blank, and negative control extract (N = 3) | Discrete intracytoplasmic granules; no cell lysis; no reduction of cell growth | 0 | None |
| Positive control (N = 3) | Complete destruction of the cell layers | 4 | Severe |
| Time | Membranes | Cementum Height (Mean ± SD) mm | Bone Height (Mean ± SD) mm |
|---|---|---|---|
| 8 weeks | Empty control | 0.82 ± 0.15 | 0.98 ± 0.12 |
| ES PLA95/β-TCP | 1.82 ±0.38 | 1.42 ± 0.37 | |
| Epi-Guide® membrane | 1.65 ± 0.28 | 1.25 ± 0.36 | |
| 16 weeks | Empty control | 1.18 ± 0.22 *,# | 1.28 ± 0.33 *,# |
| ES PLA95/β-TCP | 3.67 ± 0.42 * | 2.67 ± 0.33 * | |
| Epi-Guide® membrane | 3.42 ±0.33 # | 2.58 ± 0.36 # |
| Clinical Index | N | Before | After | p-Value |
|---|---|---|---|---|
| Median (Q1–Q3) | ||||
| Probing depth (PD) mm | 9 | 6.3 (6–7) | 3 (2.7–4.5) | 0.0039 * |
| Plaque index (PI)% | 9 | 16 (10–16) | 16 (16–16) | 0.62 |
| Gingival index (GI) | 9 | 1 (1–1) | 0.5 (0.5–0.5) | 0.015 * |
| Bleeding on probing (BOP)% | 9 | 17 (16–33) | 0 (0–16) | 0.031 * |
| Gingival recession (GR) mm | 9 | 0 (0–0) | 2 (0–2) | 0.25 |
| Mobility (MOB) | 9 | 0 (0–1) | 0 (0–1) | 1.00 |
| Clinical attachment level (CAL = PD + GR) mm | 9 | 7 (7–8) | 5 (4–5) | 0.0039 * |
| Clinical Index | N | Before | After | p-Value |
|---|---|---|---|---|
| Median (Q1–Q3) | ||||
| Probing depth (PD) mm | 11 | 5.75 (5–8) | 3.5 (3–5) | 0.001* |
| Plaque index (PI)% | 11 | 16 (10–16) | 10 (0–16) | 0.125 |
| Gingival index (GI) | 11 | 1 (1–1) | 0.5 (0–0.5) | 0.0098* |
| Bleeding on probing (BOP)% | 11 | 17 (16–50) | 16 (0–16) | 0.1250 |
| Gingival recession (GR) mm | 11 | 0 (0–2) | 2 (1–3) | 0.0078* |
| Mobility (MOB) | 11 | 0 (0–1) | 0 (0–1) | 0.7500 |
| Clinical attachment level (CAL = PD + GR) mm | 11 | 8 (6–9) | 5 (5–8) | 0.0020* |
| Clinical Index | Epi-Guide® Group | ES PLA95/β-TCP Group | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Median | Q1 | Q3 | N | Median | Q1 | Q3 | ||
| Probing depth (PD) mm | 9 | 2 | 3.7 | 2 | 11 | 2.5 | 3 | 1.5 | 0.85 |
| Plaque index (PI)% | 9 | 0 | 0 | 0 | 11 | 0 | 16 | 0 | 0.33 |
| Gingival index (GI) | 9 | 0.5 | 0.5 | 0.5 | 11 | 0.5 | 0.5 | 0.5 | 0.24 |
| Bleeding on probing(BOP) % | 9 | 16 | 17 | 0 | 11 | 0 | 34 | 0 | 0.60 |
| Gingival recession (GR) mm | 9 | 0 | 0 | 2 | 11 | 1 | 0 | 2 | 0.31 |
| Mobility (MOB) | 9 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | 1.00 |
| Clinical attachment level (CAL = PD + GR) mm | 9 | 2 | 4 | 2 | 11 | 2 | 3 | 1 | 0.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-C.; Lee, S.-Y.; Teng, N.-C.; Hu, H.-T.; Huang, P.-C.; Yang, J.-C. In Vitro and In Vivo Studies of Hydrophilic Electrospun PLA95/β-TCP Membranes for Guided Tissue Regeneration (GTR) Applications. Nanomaterials 2019, 9, 599. https://doi.org/10.3390/nano9040599
Chen C-C, Lee S-Y, Teng N-C, Hu H-T, Huang P-C, Yang J-C. In Vitro and In Vivo Studies of Hydrophilic Electrospun PLA95/β-TCP Membranes for Guided Tissue Regeneration (GTR) Applications. Nanomaterials. 2019; 9(4):599. https://doi.org/10.3390/nano9040599
Chicago/Turabian StyleChen, Chien-Chung, Sheng-Yang Lee, Nai-Chia Teng, Hsin-Tai Hu, Pei-Chi Huang, and Jen-Chang Yang. 2019. "In Vitro and In Vivo Studies of Hydrophilic Electrospun PLA95/β-TCP Membranes for Guided Tissue Regeneration (GTR) Applications" Nanomaterials 9, no. 4: 599. https://doi.org/10.3390/nano9040599
APA StyleChen, C.-C., Lee, S.-Y., Teng, N.-C., Hu, H.-T., Huang, P.-C., & Yang, J.-C. (2019). In Vitro and In Vivo Studies of Hydrophilic Electrospun PLA95/β-TCP Membranes for Guided Tissue Regeneration (GTR) Applications. Nanomaterials, 9(4), 599. https://doi.org/10.3390/nano9040599






