Abstract
A novel strategy, ion diffusion method controlled by ion exchange membrane combining with agar hydrogel template, was reported for the synthesis of Mn3O4 nanoparticles without any oxidizing agents. X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS) and Brunauere-Emmette-Teller (BET) isotherm were carried out to characterize the structure, morphology, pore size and distribution and specific surface area of the as-prepared nanomaterials. It is shown that the morphology and size of Mn3O4 nanoparticles can be controlled by the concentration of agar hydrogel. All the specific capacitances of the Mn3O4 samples prepared with agar hydrogel template are much higher than that of Mn3O4 prepared without any template agent. The Mn3O4 sample prepared at 1.5 g L−1 of agar hydrogel solution exhibits a highest specific capacitance of 183.0 F g−1 at the current density of 0.5 A g−1, which is increased by 293% compared with that of Mn3O4 synthesized without any template agent. The results indicate that the ion diffusion method controlled by ion exchange membrane combining with agar hydrogel template is a convenient and effective approach for preparing inorganic nanomaterials.
1. Introduction
As a new type of energy storage device, supercapacitors have the advantages of long cycle life, high power density, high safety and environmental friendliness [1,2,3,4], and have been applied in many fields [5]. According to their energy storage mechanism, supercapacitors can be classified as pseudocapacitors, electrical double layer capacitors (EDLCs) and hybrid supercapacitors [6]. Pseudocapacitors can achieve energy storage and release through highly reversible adsorption/desorption processes or fast redox reactions of the electrode active materials in the electrolyte. As the electrochemical reactions of pseudocapacitors occur both on the surface and inside of electrode, the energy density and specific capacitance of pseudocapacitors are usually much higher than that of other electrochemical capacitors [7,8].
Electrode active materials largely determine the performance of supercapacitors [9], and the morphology and structure of electrode materials have a great influence on the behavior of pseudocapacitor [10]. The reported pseudocapacitive materials mainly include transition metal hydroxides and oxides [11,12], carbon based electrode materials and conducting polymers [13] such as RuO2 [14], MnOx (x = 2 or 3/4) [15] and polythiophene derivatives (PTh) [16]. Transition metal oxides undergo rapid reversible redox reaction and exhibit an excellent pseudocapacitive performance. However, some of the transition metal oxides, for example RuO2, are rare and expensive, so they are not widely used in commercial application [2]. Therefore, Manganese oxide has become the suitable substitute of noble metal oxides and a popular research topic in recent years due to its abundant resources, environmental benignity, high theoretical specific capacity and capacitance retention [17,18].
Among the manganese oxide, MnO2 and Mn3O4 are two manganese oxides used as electrodes for supercapacitor. The spinel structured Mn3O4 are thermodynamically stable, which could avoid the structural collapse caused by proton intercalation and deintercalation in the electrode reaction process, showing excellent cycling stability and capacity retention [19]. The fact that Mn3O4 has only a single and stable hausmannite structure at room temperature makes the preparation of phase-pure Mn3O4 nanocrystals relatively easy. All these factors are advantageous to Mn3O4 as electrode materials for supercapacitor. Various methods such as hydrothermal method, solvothermal method and chemical bath deposition etc. have been successfully used to prepare Mn3O4 nanomaterials [20,21,22]. However, Mn3O4 has the disadvantages of low specific surface area, poor electronic conductivity, easy agglomeration in the preparation process and thus limiting its practical application. Various approaches have been developed to enhance its electrochemical performance such as reducing the particle size, and forming mesoporous structure [23,24]. Zhang et al. [25] have successfully prepared various Mn3O4 nanorods with different microstructures and nanostructures, and the Mn3O4 electrode has a specific capacitance of 136.5 F g−1 at a current density of 0.1 A g−1. Liu et al. have synthesized Mn3O4 solid nanospheres with a specific capacitance of 150 F g−1 at a current density of 0.3 A g−1 in 1 M Na2SO4 [26]. In our previous work [27], we doped Co ions in the Mn3O4 to prepare the Co(OH)2/Mn3O4 nanocomposites via a facile ion diffusion method, which effectively improved the aggregation behavior of Mn3O4 nanoparticles and increased the conductivity and specific capacitance.
The template method for preparing nanomaterials has the advantage of high repetition rate, controllable morphology, structure and size of the synthetic materials. It is classified as hard template method and soft template method [28]. In recent years, hydrogel has become an ideal template for preparing nanomaterials [29]. Hydrogel has a sponge-like three-dimensional network structure composed of polymer network and solvent. The unique three-dimensional network structure of hydrogel can provide the space for the nucleation and growth of inorganic nanoparticles, and the sizes of nanomaterials can be adjusted by modulating the pore size of the three-dimensional network of the polymer hydrogel [30,31]. At present, most of the hydrogel templates used for preparing nanomaterials are crosslinked polyacrylamide compounds, and inorganic salts solution is generally used as reactant and medium. The inorganic nanoparticles/hydrogel complex is obtained by the free radical polymerization and crosslinking reaction of acrylamide in the existence of crosslinking agent, and then the wet gel is dried and calcined to get inorganic nanomaterials [32,33,34]. This method takes the advantages of solid phase method and sol-gel method, which can mix various reactant ions uniformly at atomic level in aqueous solution, does not require the expensive alkoxides as reactants, and can control well the stoichiometric ratio of the product. The disadvantage of this method is that the synthesis process involves complex polymerization and crosslinking reaction, so it is difficult to modulate the pore size of the three-dimensional network [28]. Therefore, suitable commercial hydrogel has been chosen and used as a template combining with other synthetic strategies to control the synthesis of inorganic nanomaterials. Liu et al. [31] reported a method for preparing hydroxyapatite/dense hydrogel nanocomposites closely similar to the structure of bone. This method includes the promoting cations and anions diffusing respectively into the dense hydrogel templates under a direct current field, meeting, nucleating and growing process of hydroxyapatite nanoparticles. Their research not only contributes to the preparation of bone substitute materials, but also provides a new idea and method for preparing nanomaterials with hydrogel template. The disadvantage is that the concentration of hydrogels cannot be adjusted, and nor can the size of nanomaterials be controlled.
Agar hydrogels are easy to prepare without by-products and can be directly obtained by mixing agar with hot deionized water. The size of the three-dimensional network structure of the hydrogel can be controlled by changing the concentration of the agar hydrogel. In our previous work [35,36], a novel ion diffusion method controlled by ion exchange membrane was used for synthesizing RuO2·nH2O and Ni(OH)2 nanomaterials. In this paper, we combine ion diffusion method with agar hydrogel template method directly to synthesize Mn3O4 nanomaterials without adding any oxidizing agents. The Mn3O4 nanoparticles can directly nucleate and grow in the three-dimensional network structure of agar hydrogel and then the template is removed by calcination to obtain pure Mn3O4 nanoparticles. By changing the concentration of agar hydrogel and thus controlling the morphology and size of Mn3O4 nanoparticles, the electrochemical performance of Mn3O4 nanoparticles has been significantly improved.
2. Materials and Methods
2.1. Materials
Manganese sulfate (MnSO4) and sodium sulfate (Na2SO4) were purchased from Tianjin Fuchen Chemical Reagents (Tianjin, China), sodium hydroxide (NaOH) was purchased from Beijing Chemical Works (Beijing, China), and agar (biochemical reagent) was purchased from Tianjin Dingshengxin Chemical Industry Co. Ltd. (Tianjin, China). All reagents were analytical grade and used without further purification.
2.2. Synthesis
The Mn3O4 nanoparticles were prepared with the reaction device reported in our previous work [35]. The reaction device is shown in Figure 1, the cation exchange membrane is fixed between the groove chamber a and b, and anion exchange membrane is fixed between groove chamber b and c. 50 mL of 0.5 mol L−1 Manganese sulfate was added into groove chamber a, and 50 mL of 0.6 mol L−1 Sodium hydroxide was added into groove chamber c. Then 100 mL different concentrations of agar hydrogel (1.0, 1.5, 2.0, 2.5 g L−1) were respectively added into groove chamber b. The reaction was carried out at 30 °C for 12 h. Due to the concentration difference, Mn2+ and OH− diffused into groove chamber c through cation exchange membrane and anion exchange membrane, respectively. Mn2+ reacted with OH− in the groove chamber c and a brown-black product was obtained by centrifugation. The resulting Mn3O4 was repeatedly rinsed with deionized water and ethanol and dried at 70 °C for 12 h, following by calcination at 350 °C for 2 h to obtain pure Mn3O4. Mn3O4 nanoparticles prepared with different concentrations of agar hydrogel were named M1.0, M1.5, M2.0 and M2.5, respectively. For example, M1.5 represents the sample prepared at 1.5 g L−1 of agar hydrogel solution. Mn3O4 sample was also prepared in the same manner without adding any hydrogel template agent and named M0.
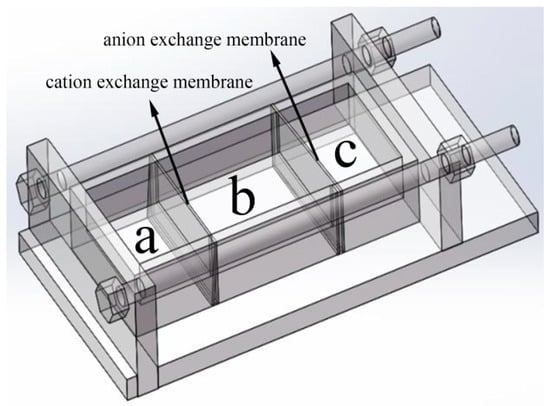
Figure 1.
The ion diffusion reaction device controlled by ion exchange membrane.
2.3. Characterization
The phase structure and crystallinity of the samples were identified by X-ray diffraction (XRD, ULTIMAIV RIGAKU, Tokyo, Japan) in the range of 10–80° with a scan rate of 10°/min. The morphology of the samples was investigated using S-4800 field emission scanning electron microscopy (FESEM, Hitachi, Tokyo, Japan) and transmission electron microscopy (TEM, Tecnai G2 F20, Hillsborough, OR, USA). The valence states of Mn in the samples was characterized by X-ray photoelectron spectroscopy (XPS, PHI QUANTERA-II, ULVAC-PHI, INC., Tokyo, Japan) using amonochromatic Al K X-ray source (h = 1486.6 eV). The BELSORP-max specific surface area and pore size distribution instrument (ANKERSMID B.V. Holland, Nijverdal, Netherlands) were used to determine the pore size distribution and specific surface area.
2.4. Electrode Preparation and Electrochemical Characterization
80 wt % active materials (Mn3O4), 15 wt % acetylene black and 5 wt % polytetrafluorethlene (PTFE) were blended with a few drops of ethanol and then stirred to form well-mixed slurry to prepare working electrode. The resulting slurry was evenly coated on the current collector (nickel foam) with an area of 1 cm2 and dried at 70 °C for 12 h, and the mass of the whole material loading on nickel foam was about 8 mg. Finally, the electrode was pressed at 10 MPa. Cyclic voltammetry (CV), galvanostatic charge-discharge (GCD) and electrochemical impedance spectroscopy (EIS) tests were studied using CHI760E electrochemical workstation and 1 mol L−1 Na2SO4 aqueous solution was used as electrolyte. The CV and GCD analyses were performed in the potential window range of −0.2–0.8 V. EIS measurement was tested between 0.01 Hz and 100 kHz. The electrode test was carried out in a three-electrode system with nickel foam coated with Mn3O4 nanomaterials as the working electrode, saturated calomel electrode (SCE) as the reference electrode, and platinum foil as the counter electrode.
3. Results and Discussion
3.1. Morphology and Structure
Figure 2 shows XRD pattern of Mn3O4 samples. The diffraction peaks at 18.0°, 29.0°, 32.5°, 36.2°, 44.6°, 53.9°, 58.8°, 60.0° and 64.7° correspond to (101), (112), (103), (211), (220), (312), (321), (224) and (314) planes of Mn3O4, respectively. All the diffraction peaks are indexed to the tetragonal hausmannite Mn3O4 (JCPDS 01-1127). No other impurity peaks appear, which reveals that pure hausmannite is obtained [37]. The diffraction peaks of Mn3O4 crystals become weaker and wider obviously in the existence of agar hydrogel template agent, indicating lower crystallinity or smaller size of Mn3O4.
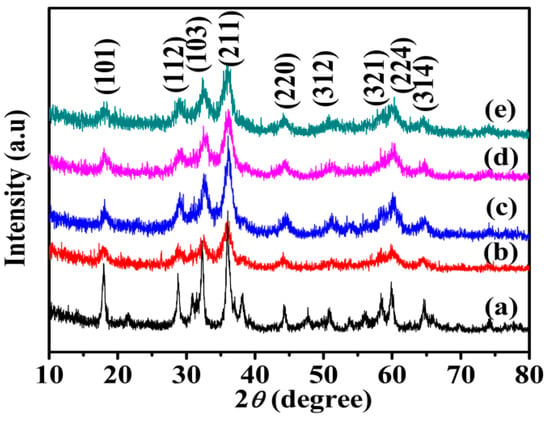
Figure 2.
X-ray diffraction (XRD) patterns of Mn3O4 samples synthesized with different concentrations of agar hydrogel. (a) M0, (b) M1.0, (c) M1.5, (d) M2.0, (e) M2.5.
Figure 3a shows that M0 is constructed from irregularly tetragonal bipyramids and flaky particles with size of 30–150 nm. With increasing concentration of agar hydrogel (Figure 3b–d), the morphology of Mn3O4 nanoparticles gradually becomes irregularly spherical shape, the size of Mn3O4 nanoparticles becomes smaller and more uniform. M2.5 (Figure 3e) shows more regularly spherical shape and the minimal size of 10–20 nm. When the concentration of agar hydrogel is lower (M1.0 and M1.5), the aggregation behavior of Mn3O4 nanoparticles is improved. However, with the increase of concentration of agar hydrogel (M2.0 and M2.5), the structure of Mn3O4 nanoparticles becomes more compact. As shown in Figure 3c, M1.5 looks looser and exhibits irregularly spherical particles with the size of about 20–30 nm and the existence of abundant mesopores, which is advantageous for the full contact between electrolyte and active materials, and the enhancement of the capacitance.
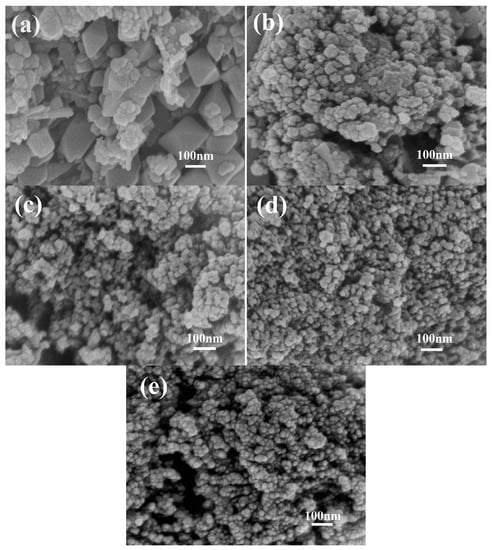
Figure 3.
Scanning electron microscopy (SEM) images of Mn3O4 samples synthesized with different concentrations of agar hydrogel. (a) M0, (b) M1.0, (c) M1.5, (d) M2.0, (e) M2.5.
Figure 4 shows the TEM images of Mn3O4 nanomaterials synthesized with different concentrations of agar hydrogel, it is found that the agar hydrogel could effectively change the size and morphology of Mn3O4 nanomaterials. M0 (Figure 4a) shows the irregularly tetragonal bipyramids and flaky particles with size of 30–150 nm assembled by finer Mn3O4 nanoparticles. Figure 4b shows the TEM image of M1.5 before calcination, the Mn3O4 nanoparticles are covered with agar hydrogel template, and cannot be observed clearly. After the agar hydrogel template is removed by calcination, M1.5 (Figure 4c) shows that the irregularly spherical particles are constructed from smaller primary particles with size of about 5 nm, and there are many mesopores of different sizes in M1.5. When increasing the concentration of agar hydrogel, the morphology of M2.5 becomes more compact. The results of TEM are consistent with those of SEM and the enhancement of the capacitance.
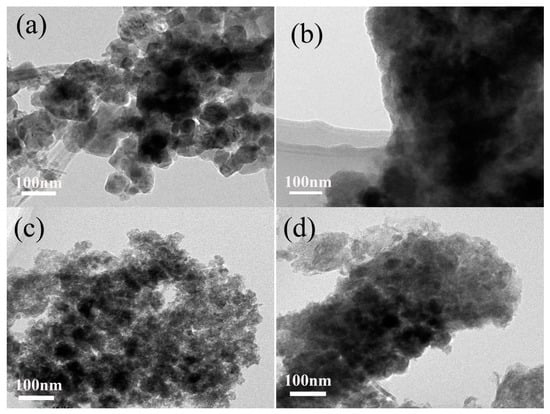
Figure 4.
Transmission electron microscopy (TEM) images of Mn3O4 samples. (a) M0, (b) M1.5 (before calcination), (c) M1.5, (d) M2.5.
The SEM and TEM results suggest that agar hydrogel with three-dimensional network plays an important role in the size and shape-controlled synthesis process of Mn3O4 nanoparticles. The suitable concentration of agar hydrogel can effectively regulate the morphology, particle size and distribution of Mn3O4, and form more mesopores.
Figure 5a shows the XPS survey scan analysis of M1.5 in the binding energy range 0–1200 eV. Except for the contaminant carbon, no significant impurities are founded. As shown in Figure 5b, the Mn 2p3/2 spectrum shows two distinct peaks at 641.59 and 643.42 eV, which are consistent with the binding energy of Mn(II) to Mn(III) in Mn2+(Mn3+)2O4 [27]. The O 1s peak of Mn3O4 splits into two peaks with the binding energy 530.09 and 531.47 eV in Figure 5c, which are in agreement with the analysis of O 1s in Mn3O4 [38]. All the results prove that pure Mn3O4 is obtained, which is consistent with the XRD analysis. The mole ratio of total manganese to the oxygen (Mn/O) calculated from Figure 5b,c is 1.54, which is slightly higher than the theoretical value (1.33). It is presumed that the excess oxygen is due to the residual oxygen produced by incomplete carbonization of agar hydrogel.
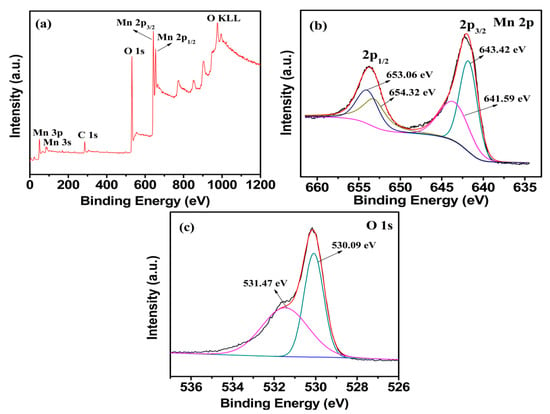
Figure 5.
X-ray photoelectron spectroscopy (XPS) spectra of the (a) survey scan, (b) Mn 2p, (c) O 1s electron XPS spectra for M1.5.
The BET surface area and pore size distributions of as-prepared Mn3O4 particles synthesized at different concentrations of agar hydrogel were investigated by N2 adsorption-desorption measurement. As shown in Figure 6, according to the International Union of Pure and Applied Chemistry (IUPAC) classification of adsorption and desorption isotherms, all five isotherm profiles can be classed as type IV with a hysteresis loop in the relative pressure range of 0.55–0.75, which indicate that the samples have the adsorption properties of porous materials [39]. For M1.0, M1.5, M2.0 and M2.5 samples, the hysteresis loop shifts to lower relative pressure, indicating smaller pores size. The Barrett-Joyner-Halenda (BJH) pore size distribution curves demonstrate the existence of more abundant mesopores and relatively homogeneous pore distribution for the Mn3O4 samples synthesized using agar hydrogel template. Table 1 exhibits the results of the measured BET specific surface area and the BJH pore size distribution of the samples, the entire samples prepared using agar hydrogel template (M1.0, M1.5, M2.0 and M2.5) have much higher specific area than that prepared without using any template (M0). For M1.0, it is suggested that the largest specific area of 75.7 m2 g−1 comes from the more micropores and smaller mesopores formed by the assembly of finer Mn3O4 nanoparticles, the micropores and smaller mesopores are disadvantageous to the diffusion of electrolytes. M2.5 has the smallest specific surface area of 49.6 m2 g−1, due to the close packing of Mn3O4 nanoparticles. M1.5 has the largest average pore diameter of 15.9 nm, higher specific area and larger pore volume resulting from the abundant and larger mesopores (Figure 6c inset). These results are consistent with that of SEM and TEM.
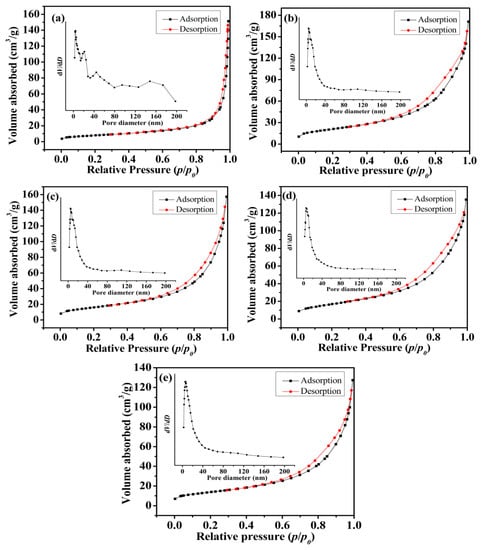
Figure 6.
Nitrogen adsorption-desorption isotherms and the pore size distribution (insert) of Mn3O4 samples. (a) M0, (b) M1.0, (c) M1.5, (d) M2.0, (e) M2.5.

Table 1.
BET surface area and pore volume of as-prepared Mn3O4.
3.2. Electrochemical Performance of Mn3O4 Electrode
In order to investigate the capacitive performance of the as-prepared Mn3O4, CV and GCD tests were employed. Figure 7a shows the CV curves of Mn3O4 nanoparticles synthesized with different concentrations of agar hydrogel at the scan rate of 5 mV s−1. All the CV curves of the as-prepared materials exhibit a symmetrical quasi-rectangular shape, which reveals the pseudocapacitive behaviors and good reversibility [40,41]. The pseudocapacity of manganese oxide is attributed to the redox exchange of protons or cations in the electrolyte as the following equation:
where, MnOa(OH)b and MnOa−n(OH)b+n represent the high oxidation state and low oxidation state of manganese, respectively. The electrochemical performance of the electrode materials is positively related to the area enclosed by the CV curves. Therefore, it can be concluded that M1.5 has the largest specific capacitance from Figure 7a. The specific capacitances of the Mn3O4 prepared using agar hydrogel template agent were higher than that of M0. The higher specific capacitance of Mn3O4 synthesized with agar hydrogel template result from the higher specific area and the presence of more mesopores. The higher specific surface area can increase the contact area between the electrode active materials and electrolyte, and the more charges can be transferred into the inside of electrode material through the electrochemical reaction at the interface, which makes the active materials to be fully reacted and thus greatly improving the electrochemical properties [42,43].
MnOa(OH)b + nH+ ne− ⇔ MnOa−n(OH)b+n
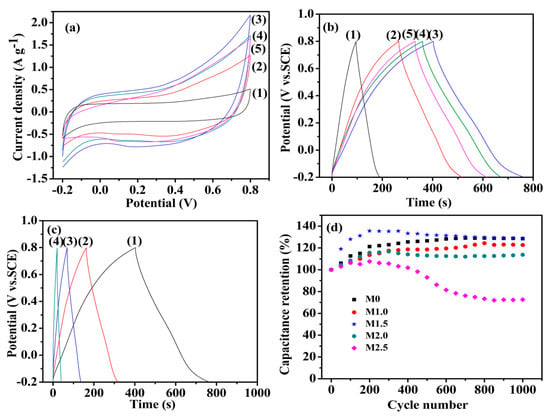
Figure 7.
Cyclic voltammetry (CV) curves (a) at 5 mV s−1 and galvanostatic charge-discharge (GCD) curves (b) at 0.5 A g−1 (1) M0, (2) M1.0, (3) M1.5, (4) M2.0, (5) M2.5; (c) Galvanostatic charge-discharge curves of M1.5 electrode at (1) 0.5 A g−1, (2)1.0 A g−1, (3) 2.0 A g−1, (4) 5.0 A g−1; (d) The relation between capacitance retention and the charge-discharge cycle times of Mn3O4 samples.
The GCD curves of Mn3O4 electrodes at the current density 0.5 A g−1 are shown in Figure 7b, the specific capacitance of Mn3O4 electrode can be calculated from the GCD curves according to the following equation:
where C (F g−1) is the specific capacitance; I (A) is the discharge current; △t (s) is the discharge time; m (g) is the mass of active material; △E (V) is the GCD potential range. According to the discharge curves, it can be calculated that the specific capacitance values of M0, M1.0, M1.5, M2.0 and M2.5 are 46.5, 126.1, 183.0, 156.8 and 143.3 F g−1 at the current density 0.5 A g−1, respectively. The specific capacitance of M1.5 is higher than that of Mn3O4 nanomaterials reported in the literature [25,26]. It is suggested that the highest specific capacitance of M1.5 is due to the fact that M1.5 has more abundant mesopores, which will promote the diffusion of electrolyte ions into the interior of electrode active materials [27,44].
Figure 7c shows the GCD curves of M1.5 electrode at different current densities. The charging curves and discharging curves are approximately symmetrical, indicating that the electrode active material is relatively stable and highly reversible. The specific capacitance of M1.5 evaluated from the discharge curves are 183.0, 154.7, 134.8, 102.0 F g−1 at current densities of 0.5, 1.0, 2.0, 5.0 A g−1, respectively. When the current density is low, the Mn3O4 electrode materials can be sufficiently and effectively wetted by the electrolyte and more active sites of electrode materials are exposed to the electrolyte, which can increase the utilization of active materials and make them participate more fully in the redox reaction [37,44]. The specific capacitance shows a decreasing trend with the current densities increase, which is due to the gradually reduction of protons entering to the inside of active materials, and thus causing a transfer of redox reactions gradually from the inside of Mn3O4 nanoparticles to the surface of electrode [45,46]. The cyclic stability of Mn3O4 electrodes were employed in charge-discharge test at 2.0 A g−1 up to 1000 cycles. As shown in Figure 7d, all the Mn3O4 electrodes have an outstanding cycling performance except M2.5. The specific capacitance retention of Mn3O4 electrodes increases gradually and reaches a highest value, and thereafter slowly decreases, the phenomenon may be attributed to the following reasons. First, electrolyte ions gradually penetrate into the interior of electrode materials and open more pore of electrode materials at the beginning stage of charge-discharge cycles, which will enhance the faradic reactions [47]. Second, the electrode is gradually wetted, which will increase the active sites of electrode materials and promote the faraday reaction [48,49]. Third, the abundant mesoporous provide good buffer volume expansion capability and increase the cyclic stability of the electrodes [50]. Due to the smaller pore size of M2.5, the structure of M2.5 will be changed and thus formed a dense inner layer after repeated charge-discharge test, which is not conductive to the electrode reactions and the flowing of electrolyte, and lead to a rapid decrease of the capacitance retention [44]. However, the samples synthesized under lower agar hydrogel concentration maintain the same high capacitance retention as M0, which means that suitable concentration of agar hydrogel template will not influence the stability of Mn3O4 electrode.
EIS measurements were performed to further study the interfacial ion diffusion and charge transfer process of the Mn3O4 electrodes. As shown in Figure 8, the Nyquist plot of Mn3O4 electrodes is consisted of the approximate semicircle in high-frequency region and a straight line with certain slop in low-frequency region, which is consistent with the characteristics of pseudocapacitor. The electrode process is controlled by electrochemical polarization in high-frequency region, due to the internal resistance (Rs) and charge-transfer resistance (Rct) of the active materials redox reaction [44,51]. The intercept of the Nyquist plot in the real axis represents the Rs value, and the semicircle radius represents the Rct value. According to the Figure 8, the Rs values of M0, M1.0, M1.5, M2.0 and M2.5 are 2.205, 1.595, 1.507, 1.653 and 1.568 Ω cm2, respectively. The Rct values of M0, M1.0, M1.5, M2.0 and M2.5 are 1.794, 0.575, 0.708, 0.715 and 0.714 Ω cm2, respectively. The Rs and Rct value of M0 is the largest, and Rs value of M1.5 is the smallest, indicating that Mn3O4 with higher specific surface area and more abundant mesopores is advantageous to reduce the charge transfer resistance of the electrode/electrolyte interface [52]. In the low-frequency region, the electrode impedance plot is a straight line, showing that the electrolytes diffusion is the determining step of electrode process [53], which indicates the diffusion resistance (Warburg resistance Zw) of electrolyte into active materials and the level of proton transfer, the larger liner slope in the low-frequency region indicating the smaller Zw value, higher transfer rate of protons and electrolyte ions. The straight lines of M1.0, M1.5 and M2.0 at low frequency are much steeper than that of M0, indicating M0 has the larger Zw compared with M1.0, M1.5 and M2.0. M1.5 has the highest conductivity, which are advantageous for the improvement of chemical performance.
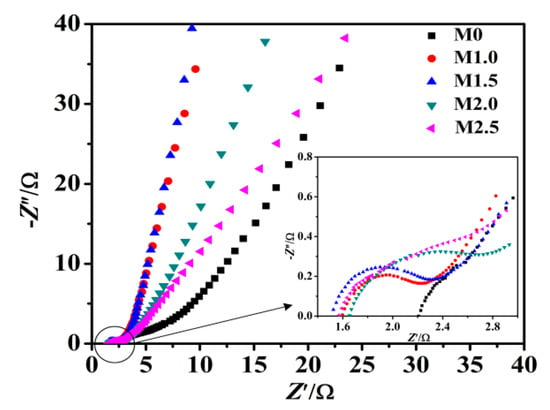
Figure 8.
Nyquist plots of as-prepared samples electrodes.
In a word, the synthetic strategy used in this work can control the morphology and particle size of the as-synthesized Mn3O4 nanoparticles by changing the concentration of agar hydrogel template, and endow the Mn3O4 nanoparticles with higher specific surface area and abundant mesoporous, which is conducive to improving the electrochemical performance. It should be pointed out that this part of the work is the first attempt to synthesize Mn3O4 nanoparticles using this novel synthetic strategy, and we will continue the synthesis of other inorganic nanomaterials, with a view to further improving this method and expanding its application field.
4. Conclusions
The irregularly spherical Mn3O4 nanoparticles were successfully synthesized via an ion diffusion method controlled by ion exchange membrane combining with agar hydrogel template without any oxidizing agents. It is discovered that the structure and electrochemical behavior of Mn3O4 nanoparticles are closely dependent on the concentration of agar hydrogel. The SEM and TEM analysis reveal that agar hydrogel template could adjust the morphology and size of Mn3O4 nanoparticles. BET analysis indicates that Mn3O4 synthesized with hydrogel template all have much higher specific area and abundant mesoporous than that synthesized without hydrogel template. Mn3O4 nanoparticles can directly nucleate and grow in the three-dimensional network structure of agar hydrogel, which can reduce the agglomeration of Mn3O4 nanoparticles and thus increase its specific area. Mn3O4 sample synthesized with 1.5 g L−1 of agar hydrogel solution (M1.5) has the highest specific capacitance of 183.0 F g−1 in 1.0 mol L−1 Na2SO4 electrolyte at the current density of 0.5 A g−1, which is increased by 293% compared with that of Mn3O4 synthesized without any template agent. The charge-discharge tests show that suitable concentration of agar hydrogel template will not influence the stability of Mn3O4 electrode.
The as-prepared Mn3O4 nanoparticles show high electrochemical performance, which is ascribed to the improvement of the conductivity and utilization of Mn3O4 nanomaterials. The ion diffusion method controlled by ion exchange membrane combining with agar hydrogel template is a novel approach for preparing inorganic nanomaterials, which has the advantages of mild reaction conditions, easy operation, energy saving and environmental friendliness.
Author Contributions
Q.Z. conceived the idea and guided the experiments; Q.X. performed the experiments and measurements; both authors analyzed the data, discussed the results and contributed to writing the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Han, K.H.; Huang, H.; Gong, Q.H.; Si, T.T.; Zhang, Z.L.; Zhou, G.W. Temperature induced hierarchical Tremella-like and Pinecone-like NiO microspheres for high-performance supercapacitor electrode materials. J. Mater. Sci. 2018, 53, 12477–12491. [Google Scholar] [CrossRef]
- Lu, W.J.; Huang, S.Z.; Miao, L.; Liu, M.X.; Zhu, D.Z.; Li, L.C.; Duan, H.; Xu, Z.J.; Gan, L.H. Synthesis of MnO2/N-doped ultramicroporous carbon nanospheres for high-performance supercapacitor electrodes. Chin. Chem. Lett. 2017, 28, 1324–1329. [Google Scholar] [CrossRef]
- Naderi, H.R.; Sobhani-Nasab, A.; Rahimi-Nasrabadi, M.; Ganjali, M.R. Decoration of nitrogen-doped reduced graphene oxide with cobalt tungstate nanoparticles for use in high-performance supercapacitors. Appl. Surf. Sci. 2017, 423, 1025–1034. [Google Scholar] [CrossRef]
- Wang, Y.L.; Chang, B.B.; Guan, D.X.; Pei, K.M.; Chen, Z.; Yang, M.S.; Dong, X.P. Preparation of nanospherical porous NiO by a hard template route and its supercapacitor application. Mater. Lett. 2014, 135, 172–175. [Google Scholar] [CrossRef]
- Tiruneh, S.N.; Kang, B.K.; Kwag, S.H.; Lee, Y.; Kim, M.; Yoon, D.H. Synergistically Active NiCo2S4 Nanoparticles Coupled with Holey Defect Graphene Hydrogel for High-Performance Solid-State Supercapacitors. Chem. Eur. J. 2018, 24, 3263–3270. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, B.Q. Facile synthesis and super capacitive behavior of SWNT/MnO2 hybrid films. Nano Energy 2012, 1, 479–487. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- He, Y.T.; Wang, L.X.; Jia, D.Z.; Zhao, Z.B.; Qiu, J.S. NiWO4/Ni/Carbon Composite Fibres for Supercapacitors with Excellent Cycling Performance. Electrochim. Acta 2016, 222, 446–454. [Google Scholar] [CrossRef]
- Li, J.; Wei, M.; Chu, W.; Wang, N. High-stable α-phase NiCo double hydroxide microspheres via microwave synthesis for supercapacitor electrode materials. Chem. Eng. J. 2017, 316, 277–287. [Google Scholar] [CrossRef]
- Wu, Q.F.; Hu, Z.H.; Liu, Y.F. A Novel Electrode Material of NiO Prepared by Facile Hydrothermal Method for Electrochemical Capacitor Application. J. Mater. Eng. Perform. 2013, 22, 2398–2402. [Google Scholar] [CrossRef]
- Zhang, G.X.; Xiao, X.; Li, B.; Gu, P.; Xue, H.G.; Pang, H. Transition metal oxides with one-dimensional/one-dimensional-analogue nanostructures for advanced supercapacitors. J. Mater. Chem. A 2017, 5, 8155–8186. [Google Scholar] [CrossRef]
- Li, N.; Huang, X.K.; Zhang, H.Y. High energy density transparent and flexible asymmetric supercapacitor based on a transparent metal hydroxides@graphene micro-structured film via a scalable gas-liquid diffusion method. J. Alloys Compd. 2017, 712, 194–203. [Google Scholar] [CrossRef]
- Lokhande, V.C.; Lokhande, A.C.; Lokhande, C.D.; Kim, J.H.; Ji, T. Supercapacitive composite metal oxide electrodes formed with carbon, metal oxides and conducting polymers. J. Alloys Compd. 2016, 682, 381–403. [Google Scholar] [CrossRef]
- Wu, Z.S.; Wang, D.W.; Ren, W.; Zhao, J.; Zhou, G.; Li, F.; Cheng, H.M. Anchoring Hydrous RuO2 on Graphene Sheets for High-Performance Electrochemical Capacitors. Adv. Funct. Mater. 2010, 20, 3595–3602. [Google Scholar] [CrossRef]
- Wei, W.F.; Cui, X.W.; Chen, W.X.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.F.; Cai, K.F.; Chen, Y.X.; Chen, L.D. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Li, W.; Xu, J.; Pan, Y.; An, L.; Xu, K.; Wang, G.; Yu, Z.; Yu, L.; Hu, J. A facile synthesis of α-MnO2 used as a supercapacitor electrode material: The influence of the Mn-based precursor solutions on the electrochemical performance. Appl. Surf. Sci. 2015, 357, 1747–1752. [Google Scholar] [CrossRef]
- Ge, J.; Yao, H.B.; Hu, W.; Yu, X.F.; Yan, Y.X.; Mao, L.B.; Li, H.H.; Li, S.S.; Yu, S.H. Facile dip coating processed graphene/MnO2 nanostructured sponges as high performance supercapacitor electrodes. Nano Energy 2013, 2, 505–513. [Google Scholar] [CrossRef]
- Xiao, X.C.; Wang, Y.; Chen, G.; Wang, L.H.; Wang, Y.D. Mn3O4/activated carbon composites with enhanced electrochemical performances for electrochemical capacitors. J. Alloys Compd. 2017, 703, 163–173. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, T.; Yan, C.Y.; Ma, J.; Li, C.Z. Hydrothermal synthesis of novel Mn3O4 nano-octahedrons with enhanced supercapacitors performances. Nanoscale 2010, 2, 2195–2198. [Google Scholar] [CrossRef]
- Ren, X.G.; An, J.W.; Yan, S.H.; Gao, L.Z.; Xu, S.M.; Wang, X.M.; Wei, G.Q. Assembly of Mn3O4/carbon Black Composite and Its Supercapacitor Application. Int. J. Electrochem. Sci. 2016, 11, 5080–5089. [Google Scholar] [CrossRef]
- Ulutas, C.; Erken, O.; Gunes, M.; Gumus, C. Effect of Annealing Temperature on The physical Properties of Mn3O4 Thin Film Prepared by Chemical Bath Deposition. Int. J. Electrochem. Sci. 2016, 11, 2835–2845. [Google Scholar] [CrossRef]
- Makgopa, K.; Raju, K.; Ejikeme, P.M.; Ozoemena, K.I. High-performance Mn3O4/onion-like carbon (OLC) nanohybrid pseudocapacitor: Unravelling the intrinsic properties of OLC against other carbon supports. Carbon 2017, 117, 20–32. [Google Scholar] [CrossRef]
- Qiao, Y.Q.; Sun, Q.J.; Sha, O.; Zhang, X.Y.; Tang, Y.F.; Shen, T.D.; Kong, L.X.; Gao, W.M. Synthesis of Mn3O4 nano-materials via CTAB/SDS vesicle templating for high performance supercapacitors. Mater. Lett. 2018, 210, 128–132. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, D.; Wei, Y.Q.; Li, A.Y.; Liu, B.; Yuan, Y.; Zhang, H.C.; Li, G.H.; Zhang, F. Fabrication of a 1D Mn3O4 nano-rod electrode for aqueous asymmetric supercapacitors and capacitive deionization. Inorg. Chem. Front. 2019, 6, 355–365. [Google Scholar] [CrossRef]
- Liu, Z.H.; Zhang, L.; Xu, G.C.; Zhang, L.; Jia, D.Z.; Zhang, C.Y. Mn3O4 hollow microcubes and solid nanospheres derived from a metal formate framework for electrochemical capacitor applications. RSC Adv. 2017, 7, 11129–11134. [Google Scholar] [CrossRef]
- Liu, H.; Xue, Q.; Zhao, J.S.; Zhang, Q. Enhanced supercapacitive performance of binary cooperative complementary Co(OH)2/Mn3O4 nanomaterials directly synthesized through ion diffusion method controlled by ion exchange membrane. Electrochim. Acta 2018, 260, 330–337. [Google Scholar] [CrossRef]
- Xie, Y.D.; Kocaefe, D.; Chen, C.Y.; Kocaefe, Y. Review of Research on Template Methods in Preparation of Nanomaterials. J. Nanomater. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Acharya, G.; Shin, C.S.; McDermott, M.; Mishra, H.; Park, H.; Kwon, I.C.; Park, K. The hydrogel template method for fabrication of homogeneous nano/microparticles. J. Control. Release 2010, 141, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N. Soft and flexible hydrogel templates of different sizes and various functionalities for metal nanoparticle preparation and their use in catalysis. Prog. Polym. Sci. 2013, 38, 1329–1356. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, D.C.; Tomsia, A.P.; Minor, A.M.; Song, X.Y.; Saiz, E. Three-Dimensional Biomimetic Mineralization of Dense Hydrogel Templates. J. Am. Chem. Soc. 2009, 131, 9937–9939. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.A.; Giersig, M.; Willig, F.; Antonietti, M. Porous “coral-like” TiO2 structures produced by templating polymer gels. Langmuir 1998, 14, 6333–6336. [Google Scholar] [CrossRef]
- Schattka, J.H.; Shchukin, D.G.; Jia, J.G.; Antonietti, M.; Caruso, R.A. Photocatalytic activities of porous titania and titania/zirconia structures formed by using a polymer gel templating. Chem. Mater. 2002, 14, 5103–5108. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Schattka, J.H.; Antonietti, M.; Caruso, R.A. Photocatalytic properties of porous metal oxide networks formed by nanoparticle infiltration in a polymer gel template. J. Phys. Chem. B 2003, 107, 952–957. [Google Scholar] [CrossRef]
- Hao, X.T.; Zhao, J.S.; Zhang, Q. Temperature-dependent textural and electrochemical properties of a ruthenium oxide capacitor prepared by exchange membrane controlled ion diffusion. Ceram. Int. 2016, 42, 9170–9177. [Google Scholar] [CrossRef]
- Zhao, J.S.; Zhang, Q. Synthesis of Ni(OH)2 Nanoflakes Through a Novel Ion Diffusion Method Controlled by Ion Exchange Membrane and Electrochemical Supercapacitive Properties. Electrochim. Acta 2015, 184, 47–57. [Google Scholar] [CrossRef]
- Gnana Sundara Raj, B.; Asiri, A.M.; Wu, J.J.; Anandan, S. Synthesis of Mn3O4 nanoparticles via chemical precipitation approach for supercapacitor application. J. Alloys Compd. 2015, 636, 234–240. [Google Scholar] [CrossRef]
- Hiremath, V.; Cho, M.; Seo, J.G. Self-assembled Mn3O4 nano-clusters over carbon nanotube threads with enhanced supercapacitor performance. New J. Chem. 2018, 42, 19608–19614. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, J.L. Degradation of sulfamethazine using Fe3O4-Mn3O4/reduced graphene oxide hybrid as Fenton-like catalyst. J. Hazard. Mater. 2017, 324, 653–664. [Google Scholar] [CrossRef]
- Ghaemi, M.; Ataherian, F.; Zolfaghari, A.; Jafari, S.M. Charge storage mechanism of sonochemically prepared MnO2 as supercapacitor electrode: Effects of physisorbed water and proton conduction. Electrochim. Acta 2008, 53, 4607–4614. [Google Scholar] [CrossRef]
- Chandra Sekhar, S.; Nagaraju, G.; Yu, J.S. Ant-cave structured MnCO3/Mn3O4 microcubes by biopolymer-assisted facile synthesis for high-performance pseudocapacitors. Appl. Surf. Sci. 2018, 435, 398–405. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Y.; Lian, J.B.; Xu, Y.G.; Bao, J.; Qiu, J.X.; Xu, L.; Xu, H.; Hua, M.Q.; Li, H.M. Morphology controlled preparation of ZnCo2O4 nanostructures for asymmetric supercapacitor with ultrahigh energy density. Energy 2017, 123, 296–304. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Lee, Y.R. Green synthesis of nitrogen-doped graphitic carbon sheets with use of Prunus persica for supercapacitor applications. Appl. Surf. Sci. 2017, 393, 276–286. [Google Scholar] [CrossRef]
- Sankar, K.V.; Kalpana, D.; Selvan, R.K. Electrochemical properties of microwave-assisted reflux-synthesized Mn3O4 nanoparticles in different electrolytes for supercapacitor applications. J. Appl. Electrochem. 2012, 42, 463–470. [Google Scholar] [CrossRef]
- Zeng, W.; Huang, Y.P.; Xiong, Y.; Wang, N.; Xu, C.; Huang, L.S. Gas bubble templated synthesis of Mn3O4-embedded hollow carbon nanospheres in ethanol flame for elastic supercapacitor. J. Alloys Compd. 2018, 731, 210–221. [Google Scholar] [CrossRef]
- Xu, J.A.; Gao, L.; Cao, J.Y.; Wang, W.C.; Chen, Z.D. Preparation and electrochemical capacitance of cobalt oxide (Co3O4) nanotubes as supercapacitor material. Electrochim. Acta 2010, 56, 732–736. [Google Scholar] [CrossRef]
- Sambath Kumar, K.; Cherusseri, J.; Thomas, J. Two-Dimensional Mn3O4 Nanowalls Grown on Carbon Fibers as Electrodes for Flexible Supercapacitors. ACS Omega 2019, 4, 4472–4480. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.F.; Hai, Z.Y.; Li, Y.K.; Kan, S.H.; Chen, J.M.; Chen, X.; Zhuiykov, S.; Cui, D.F.; Xue, C.Y. Facile-synthesized NiCo2O4@MnMoO4 with novel and functional structure for superior performance supercapacitors. Appl. Surf. Sci. 2018, 452, 413–422. [Google Scholar] [CrossRef]
- Xu, X.W.; Pei, L.Y.; Yang, Y.; Shen, J.F.; Ye, M.X. Facile synthesis of NiWO4/reduced graphene oxide nanocomposite with excellent capacitive performance for supercapacitors. J. Alloys Compd. 2016, 654, 23–31. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Long, B.; Cheng, F.Y.; Tang, J.J.; Sun, A.T.; Yang, J.; Jia, M.; Wang, H. N-doped carbon encapsulated porous MnO/Mn3O4 submicrospheres as high-performance anode for lithium-ion batteries. J. Electroanal. Chem. 2019, 838, 1–6. [Google Scholar] [CrossRef]
- Wu, T.H.; Chu, Y.H.; Hu, C.C.; Hardwick, L.J. Criteria appointing the highest acceptable cell voltage of asymmetric supercapacitors. Electrochem. Commun. 2013, 27, 81–84. [Google Scholar] [CrossRef]
- Arora, P.; Popov, B.N.; White, R.E. Electrochemical investigations of cobalt-doped LiMn2O4 as cathode material for lithium-ion batteries. J. Electrochem. Soc. 1998, 145, 807–815. [Google Scholar] [CrossRef]
- Wang, F.; Hu, S.S. Electrochemical reduction of dioxygen on carbon nanotubes-dihexadecyl phosphate film electrode. J. Electroanal. Chem. 2005, 580, 68–77. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).