A Facile and Efficient Protocol for Preparing Residual-Free Single-Walled Carbon Nanotube Films for Stable Sensing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Spray Ink
2.2. Spray Deposition and Post Treatments
2.3. Scanning Electron Microscopy
2.4. X-Ray Photoelectron Spectroscopy
2.5. Sheet Resistance Measurement
2.6. Transmission Measurement
2.7. Fourier-Transform Infrared Spectroscopy
2.8. Gas Measurement Setup
2.9. Screen Printing of the Sensor Electrodes
3. Results and Discussion
3.1. Film Characterization
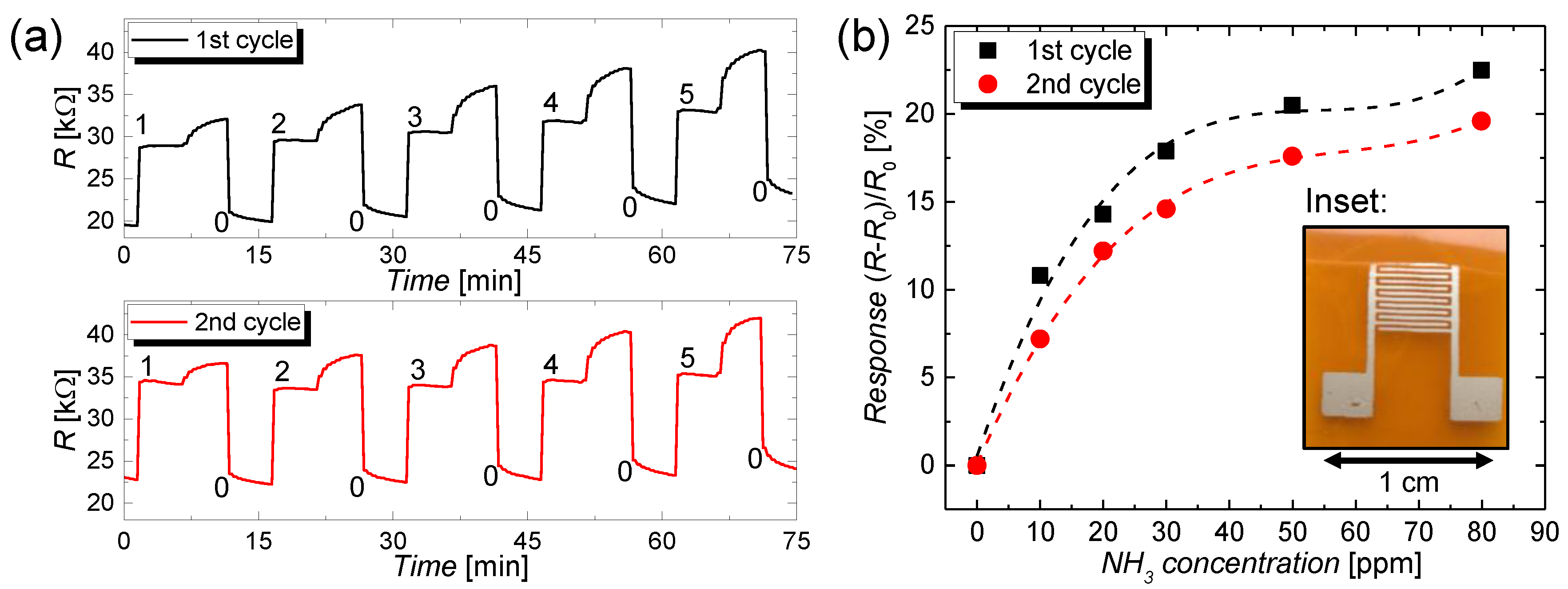
3.2. Sensing Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube-polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Wang, J. Carbon-nanotube based electrochemical biosensors: A review. Electroanalysis 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Khare, R.; Bose, S. Carbon Nanotube Based Composites—A Review. J. Miner. Mater. Charact. Eng. 2005, 4, 31. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature 1996, 381, 678–680. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Lorents, D.C. Mechanical and thermal properties of carbon nanotubes. Carbon 1995, 33, 925–930. [Google Scholar] [CrossRef]
- Demczyk, B.G.; Wang, Y.M.; Cumings, J.; Hetman, M.; Han, W.; Zettl, A.; Ritchie, R.O. Direct mechanical measurement of the tensile strength and elastic modulus of multiwalled carbon nanotubes. Mater. Sci. Eng. A 2002, 334, 173–178. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Zhou, O.Z. Applications of Carbon Nanotubes. In Carbon Nanotubes; Springer: Berlin/Heidelberg, Germany, 2001; pp. 391–425. [Google Scholar]
- Dürkop, T.; Getty, S.A.; Cobas, E.; Fuhrer, M.S. Extraordinary Mobility in Semiconducting Carbon Nanotubes. Nano Lett. 2004, 4, 35–39. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Ye, Q.; Cinke, M.; Han, J.; Meyyappan, M. Carbon nanotube sensors for gas and organic vapor detection. Nano Lett. 2003, 3, 929–933. [Google Scholar] [CrossRef]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A stretchable carbon nanotube strain sensor for human-motion detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Che, G.; Lakshmi, B.B.; Fisher, E.R.; Martin, C.R. Carbon nanotubule membranes for electrochemical energy storage and production. Nature 1998, 393, 346–349. [Google Scholar] [CrossRef]
- Frackowiak, E.; Béguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, T.; Kim, Y.S.; Choi, H.S.; Lim, H.J.; Yang, S.J.; Park, C.R. Surface modifications for the effective dispersion of carbon nanotubes in solvents and polymers. Carbon 2012, 50, 3–33. [Google Scholar] [CrossRef]
- Harris, P.J.F. Carbon nanotube composites. Int. Mater. Rev. 2004, 49, 31–43. [Google Scholar] [CrossRef]
- Mane, S.; Chatterjee, S. An Electrochemical Comparison of Single-Walled and Multi-Walled Carbon Nanotubes Utilizing Paeonol as the Model Drug. ChemistrySelect 2018, 3, 6406–6413. [Google Scholar] [CrossRef]
- Journet, C.; Maser, W.K.; Bernier, P.; Loiseau, A.; Lamy de la Chapelle, M.; Lefrant, S.; Deniard, P.; Lee, R.; Fischer, J.E. Large-scale production of single-walled carbon nanotubes by the electric-arc technique. Nature 1997, 388, 756–758. [Google Scholar] [CrossRef]
- Sadeghian, Z. Large-scale production of multi-walled carbon nanotubes by low-cost spray pyrolysis of hexane. New Carbon Mater. 2009, 24, 33–38. [Google Scholar] [CrossRef]
- Takahashi, T.; Tsunoda, K.; Yajima, H.; Ishii, T. Dispersion and purification of single-wall carbon nanotubes using carboxymethylcellulose. Jpn. J. Appl. Phys. 2004, 43, 3636. [Google Scholar] [CrossRef]
- Tummala, N.R.; Striolo, A. SDS Surfactants on carbon nanotubes: Aggregate morphology. ACS Nano 2009, 3, 595–602. [Google Scholar] [CrossRef]
- Chen, L.; Xie, H.; Li, Y.; Yu, W. Carbon nanotubes with hydrophilic surfaces produced by a wet-mechanochemical reaction with potassium hydroxide using ethanol as solvent. Mater. Lett. 2009, 63, 45–47. [Google Scholar] [CrossRef]
- Kónya, Z.; Vesselenyi, I.; Niesz, K.; Kukovecz, A.; Demortier, A.; Fonseca, A.; Delhalle, J.; Mekhalif, Z.; Nagy, J.B.; Koós, A.A.; et al. Large scale production of short functionalized carbon nanotubes. Chem. Phys. Lett. 2002, 360, 429–435. [Google Scholar] [CrossRef]
- Ikeda, A.; Hamano, T.; Hayashi, K.; Kikuchi, J.I. Water-solubilization of nucleotides-coated single-walled carbon nanotubes using a high-speed vibration milling technique. Org. Lett. 2006, 8, 1153–1156. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.; Lee, J.; Jang, M.; Shin, M.; Ahn, J.; Park, T.; Yi, W. Characteristic features of stone-wales defects in single-walled carbon nanotube; Adsorption, dispersion, and field emission. J. Nanomater. 2010, 2010, 3. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, D.R.; Hong, J.; Jung, D.; Shim, S.E. Significance of the dispersion stability of carbon nanotubes on the thermal conductivity of nylon 610 nanocomposite. J. Dispers. Sci. Technol. 2010, 31, 1230–1235. [Google Scholar] [CrossRef]
- Usrey, M.L.; Chaffee, A.; Jeng, E.S.; Strano, M.S. Application of polymer solubility theory to solution phase dispersion of single-walled carbon nanotubes. J. Phys. Chem. C 2009, 113, 9532–9540. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.T.; Feng, Q.P.; Xie, X.M.; Wang, X.H.; Ye, X.Y. Dispersion and noncovalent modification of multiwalled carbon nanotubes by various polystyrene-based polymers. J. Appl. Polym. Sci. 2008, 109, 3525–3532. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.; Li, K. The high dispersion of DNA-multiwalled carbon nanotubes and their properties. Anal. Biochem. 2009, 387, 267–270. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L. Temperature and pH-responsive “smart” carbon nanotube dispersions. Methods Mol. Biol. 2010, 625, 27–38. [Google Scholar] [PubMed]
- Umeyama, T.; Kawabata, K.; Tezuka, N.; Matano, Y.; Miyato, Y.; Matsushige, K.; Tsujimoto, M.; Isoda, S.; Takano, M.; Imahori, H. Dispersion of carbon nanotubes by photo- and thermal-responsive polymers containing azobenzene unit in the backbone. Chem. Commun. 2010, 46, 5969–5971. [Google Scholar] [CrossRef]
- Tseng, C.H.; Wang, C.C.; Chen, C.Y. Functionalizing carbon nanotubes by plasma modification for the preparation of covalent-integrated epoxy composites. Chem. Mater. 2007, 19, 308–315. [Google Scholar] [CrossRef]
- Okpalugo, T.I.T.; Papakonstantinou, P.; Murphy, H.; McLaughlin, J.; Brown, N.M.D. Oxidative functionalization of carbon nanotubes in atmospheric pressure filamentary dielectric barrier discharge (APDBD). Carbon 2005, 43, 2951–2959. [Google Scholar] [CrossRef]
- Riou, I.; Bertoncini, P.; Bizot, H.; Mevellec, J.Y.; Buléon, A.; Chauvet, O. Carboxymethylcellulose/Single Walled Carbon Nanotube Complexes. J. Nanosci. Nanotechnol. 2009, 9, 6176–6180. [Google Scholar] [CrossRef] [PubMed]
- Hajian, A.; Fu, Q.; Berglund, L.A. Recyclable and superelastic aerogels based on carbon nanotubes and carboxymethyl cellulose. Compos. Sci. Technol. 2018, 159, 1–10. [Google Scholar] [CrossRef]
- Young, B.R.; Aminayi, P. Single-walled carbon nanotube (SWNT)-carboxymethylcellulose (CMC) dispersions in aqueous solution and electronic transport properties when dried as thin film conductors. J. Dispers. Sci. Technol. 2018, 39, 1613–1626. [Google Scholar] [CrossRef]
- Zhao, X.; Cai, Z.; Zhao, D.; Liu, W.; Han, B. Aggregation and stabilization of multiwalled carbon nanotubes in aqueous suspensions: Influences of carboxymethyl cellulose, starch and humic acid. RSC Adv. 2016, 6, 67260–67270. [Google Scholar]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, A.; Abdellah, A.; Scarpa, G.; Lugli, P. Fabrication of carbon nanotube thin films on flexible substrates by spray deposition and transfer printing. Carbon 2013, 61, 72–79. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Abdellah, A.; Scarpa, G.; Lugli, P. Metallic nanoparticles functionalizing carbon nanotube networks for gas sensing applications. Nanotechnology 2014, 25, 055208. [Google Scholar] [CrossRef] [PubMed]
- Loghin, F.; Colasanti, S.; Weise, A.; Falco, A.; Abdelhalim, A.; Lugli, P.; Abdellah, A. Scalable spray deposition process for highly uniform and reproducible CNT-TFTs. Flex. Print. Electron. 2016, 1, 045002. [Google Scholar] [CrossRef]
- Abdellah, A.; Fabel, B.; Lugli, P.; Scarpa, G. Spray deposition of organic semiconducting thin-films: Towards the fabrication of arbitrary shaped organic electronic devices. Org. Electron. Phys. Mater. Appl. 2010, 11, 1031–1038. [Google Scholar] [CrossRef]
- Abdellah, A.; Abdelhalim, A.; Loghin, F.; Kohler, P.; Ahmad, Z.; Scarpa, G.; Lugli, P. Flexible carbon nanotube based gas sensors fabricated by large-scale spray deposition. IEEE Sens. J. 2013, 13, 4014–4021. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Winkler, M.; Loghin, F.; Zeiser, C.; Lugli, P.; Abdellah, A. Highly sensitive and selective carbon nanotube-based gas sensor arrays functionalized with different metallic nanoparticles. Sens. Actuators B Chem. 2015, 220, 1288–1296. [Google Scholar] [CrossRef]
- Falco, A.; Rivadeneyra, A.; Loghin, F.C.; Salmeron, J.F.; Lugli, P.; Abdelhalim, A. Towards low-power electronics: Self-recovering and flexible gas sensors. J. Mater. Chem. A 2018, 6, 7107–7113. [Google Scholar] [CrossRef]
- Bobinger, M.; Mock, J.; La Torraca, P.; Becherer, M.; Lugli, P.; Larcher, L. Tailoring the Aqueous Synthesis and Deposition of Copper Nanowires for Transparent Electrodes and Heaters. Adv. Mater. Interfaces 2017, 4, 1700568. [Google Scholar] [CrossRef]
- Mock, J.; Bobinger, M.; Bogner, C.; Lugli, P.; Becherer, M. Aqueous Synthesis, Degradation, and Encapsulation of Copper Nanowires for Transparent Electrodes. Nanomaterials 2018, 8, 767. [Google Scholar] [CrossRef]
- Wagner, C.D.; Davis, L.E.; Zeller, M.V.; Taylor, J.A.; Raymond, R.H.; Gale, L.H. Empirical atomic sensitivity factors for quantitative analysis by electron spectroscopy for chemical analysis. Surf. Interface Anal. 1981, 3, 211–225. [Google Scholar] [CrossRef]
- Wagner, C.D. Sensitivity factors for XPS analysis of surface atoms. J. Electron Spectrosc. Relat. Phenom. 1983, 32, 99–102. [Google Scholar] [CrossRef]
- Dhayal, M.; Ratner, D.M. XPS and SPR analysis of glycoarray surface density. Langmuir 2009, 25, 2181–2187. [Google Scholar] [CrossRef]
- Liu, F.; Li, W.; Guo, H.; Li, B.; Bai, Z.; Hu, R. XPS study on the change of carbon-containing groups and sulfur transformation on coal surface. J. Fuel Chem. Technol. 2011, 39, 81–84. [Google Scholar]
- Bi, X.; Ren, X.; Huang, Z.; Yu, M.; Kreidler, E.; Wu, Y. Investigating dendrites and side reactions in sodium–oxygen batteries for improved cycle lives. Chem. Commun. 2015, 51, 7665–7668. [Google Scholar] [CrossRef]
- Kundu, S.; Wang, Y.; Xia, W.; Muhler, M. Thermal stability and reducibility of oxygen-containing functional groups on multiwalled carbon nanotube surfaces: A quantitative high-resolution xps and TPD/TPR study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Seifert, M.; Vargas, J.E.B.; Bobinger, M.; Sachsenhauser, M.; Aron, W.; Roche, S.; Garrido, J.A. Role of Grain Boundaries in Tailoring Electronic Properties of Polycrystalline Graphene by Chemical Functionalization Supplementary Information Defect generation. 2D Mater. 2015, 2, 024008. [Google Scholar] [CrossRef]
- Knipe, S.W.; Mycroft, J.R.; Pratt, A.R.; Nesbitt, H.W.; Bancroff, G.M. X-ray photoelectron spectroscopic study of water adsorption on iron sulphide minerals. Geochim. Cosmochim. Acta 1995, 59, 1079–1090. [Google Scholar] [CrossRef]
- Reiche, S.; Blume, R.; Zhao, X.C.; Su, D.; Kunkes, E.; Behrens, M.; Schlögl, R. Reactivity of mesoporous carbon against water—An in-situ XPS study. Carbon 2014, 77, 175–183. [Google Scholar] [CrossRef]
- Biswal, D.R.; Singh, R.P. Characterisation of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohydr. Polym. 2004, 57, 379–387. [Google Scholar] [CrossRef]
- Pushpamalar, V.; Langford, S.J.; Ahmad, M.; Lim, Y.Y. Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohydr. Polym. 2006, 64, 312–318. [Google Scholar] [CrossRef]
- Cao, Q.; Rogers, J.A. Ultrathin films of single-walled carbon nanotubes for electronics and sensors: A review of fundamental and applied aspects. Adv. Mater. 2009, 21, 29–53. [Google Scholar] [CrossRef]
- Bobinger, M.R.; Romero, F.J.; Salinas-Castillo, A.; Becherer, M.; Lugli, P.; Morales, D.P.; Rodríguez, N.; Rivadeneyra, A. Flexible and robust laser-induced graphene heaters photothermally scribed on bare polyimide substrates. Carbon 2019, 144, 116–126. [Google Scholar] [CrossRef]
- Albrecht, A.; Bobinger, M.; Salmer, F.; Becherer, M.; Cheng, G.; Lugli, P.; Rivadeneyra, A. Over-Stretching Tolerant Conductors on Rubber Films by Inkjet-Printing Silver Nanoparticles for Wearables. Polymers 2018, 10, 1413. [Google Scholar] [CrossRef]
- Merilampi, S.; Laine-Ma, T.; Ruuskanen, P. The characterization of electrically conductive silver ink patterns on flexible substrates. Microelectron. Reliab. 2009, 49, 782–790. [Google Scholar] [CrossRef]
- Faddoul, R.; Reverdy-Bruas, N.; Blayo, A. Formulation and screen printing of water based conductive flake silver pastes onto green ceramic tapes for electronic applications. Mater. Sci. Eng. B 2012, 177, 1053–1066. [Google Scholar] [CrossRef]
- Zaporotskova, I.V.; Boroznina, N.P.; Parkhomenko, Y.N.; Kozhitov, L.V. Carbon nanotubes: Sensor properties. A review. Mod. Electron. Mater. 2017, 2, 95–105. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Star, A. Carbon nanotube gas and vapor sensors. Angew. Chem. Int. Ed. 2008, 47, 6550–6570. [Google Scholar] [CrossRef]
- Star, A.; Joshi, V.; Skarupo, S.; Thomas, D.; Gabriel, J.C.P. Gas sensor array based on metal-decorated carbon nanotubes. J. Phys. Chem. B 2006, 110, 21014–21020. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Huh, J.S. Behavior of single-walled carbon nanotube-based gas sensors at various temperatures of treatment and operation. Sens. Actuators B Chem. 2006, 117, 426–430. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef]
- Lu, Y.; Li, J.; Han, J.; Ng, H.T.; Binder, C.; Partridge, C.; Meyyappan, M. Room temperature methane detection using palladium loaded single-walled carbon nanotube sensors. Chem. Phys. Lett. 2004, 391, 344–348. [Google Scholar] [CrossRef]
- Tsai, M.H.; Lin, H.M.; Tsai, W.L.; Hwu, Y. Examine the gas absorption properties of single wall carbon nanotube bundles by X-ray absorption techniques. Rev. Adv. Mater. Sci. 2003, 5, 302–305. [Google Scholar]
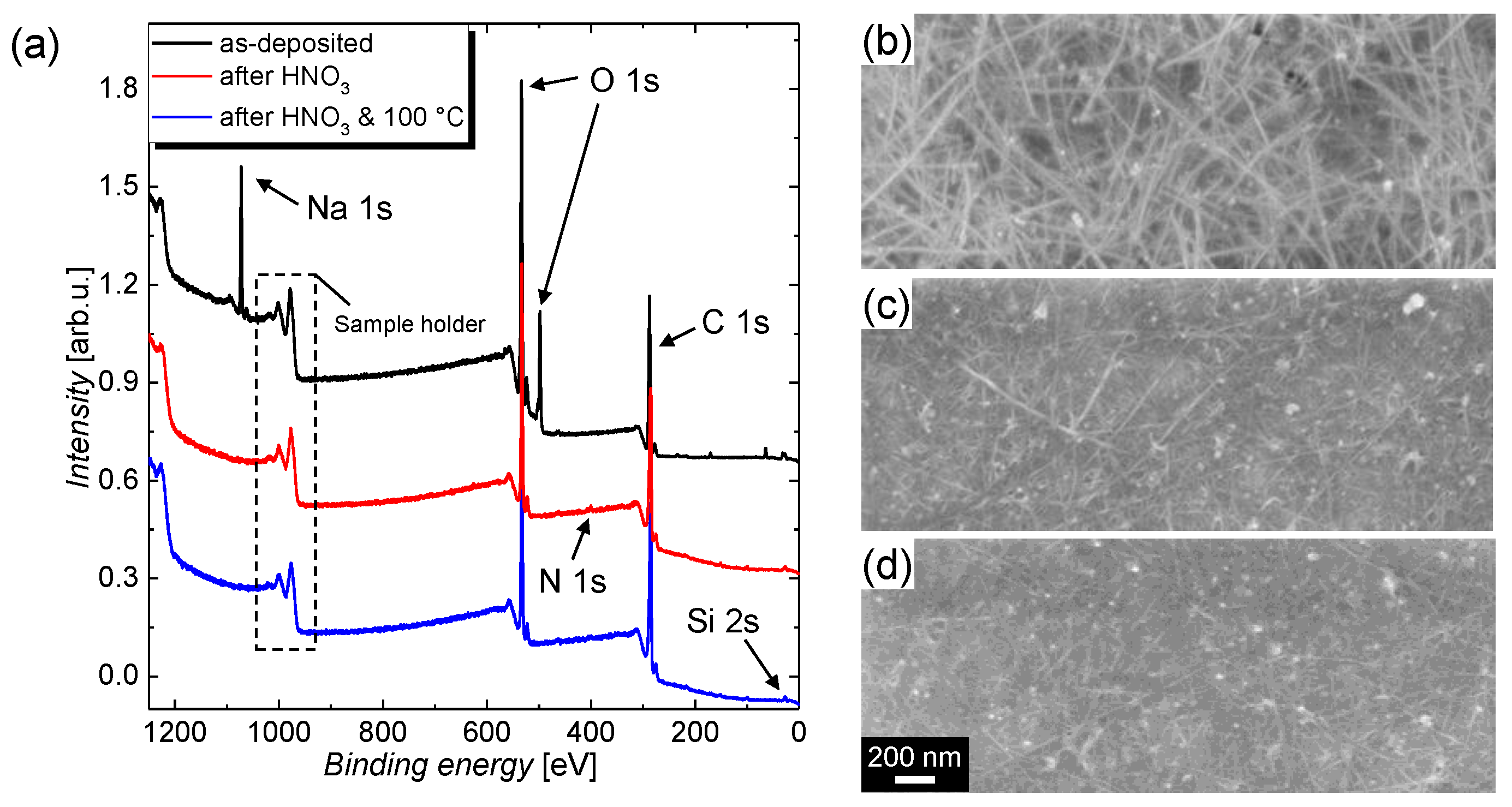
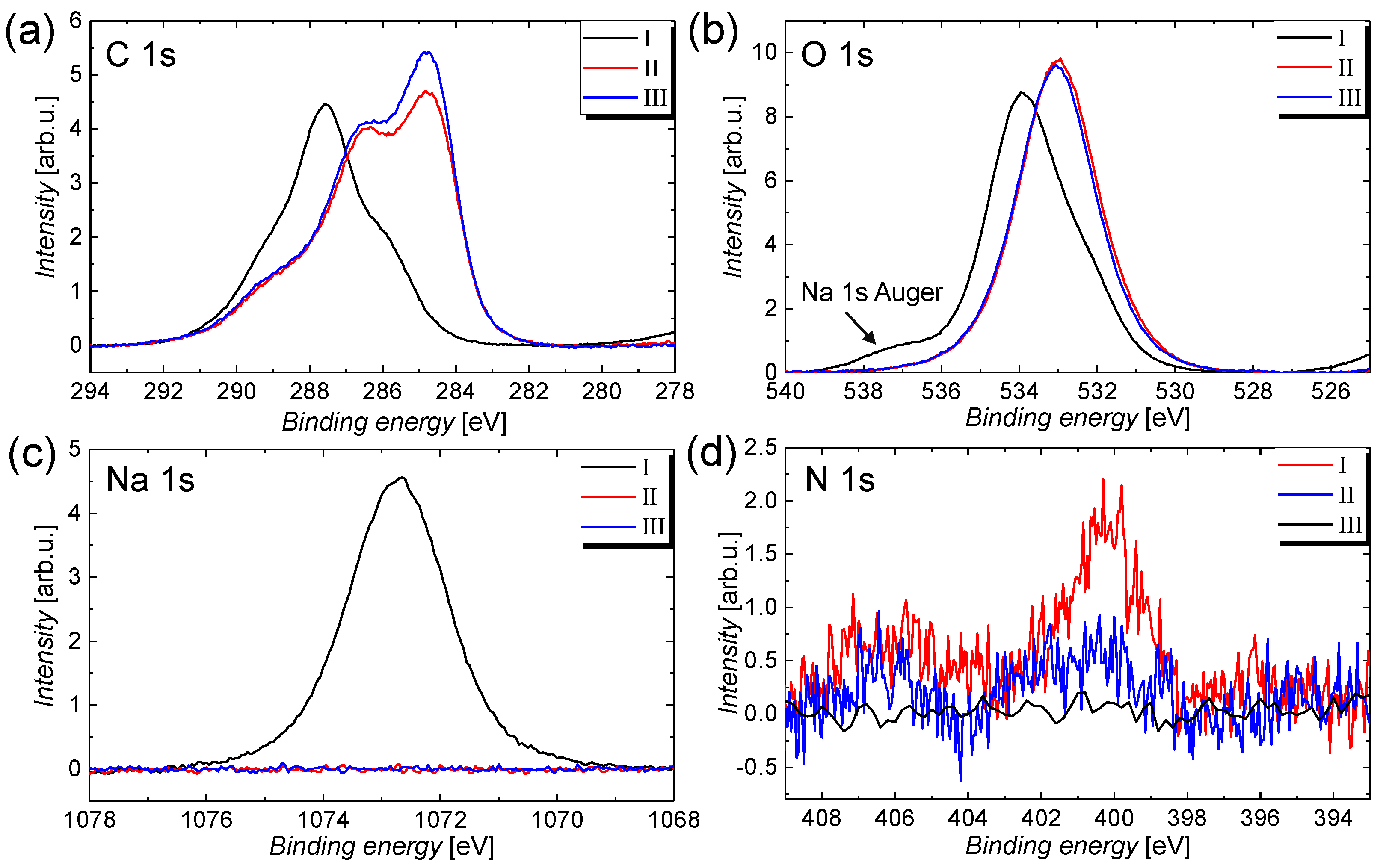
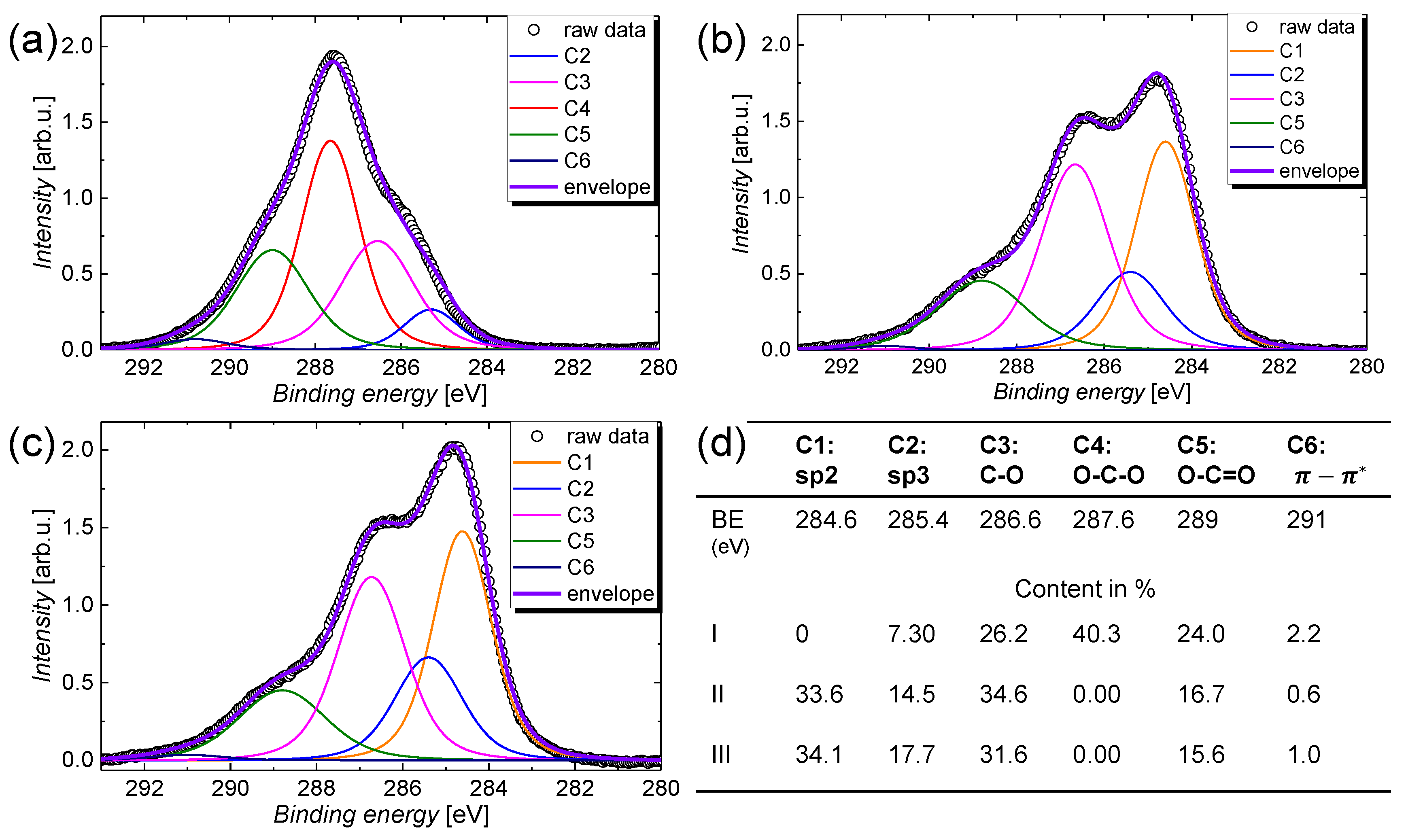
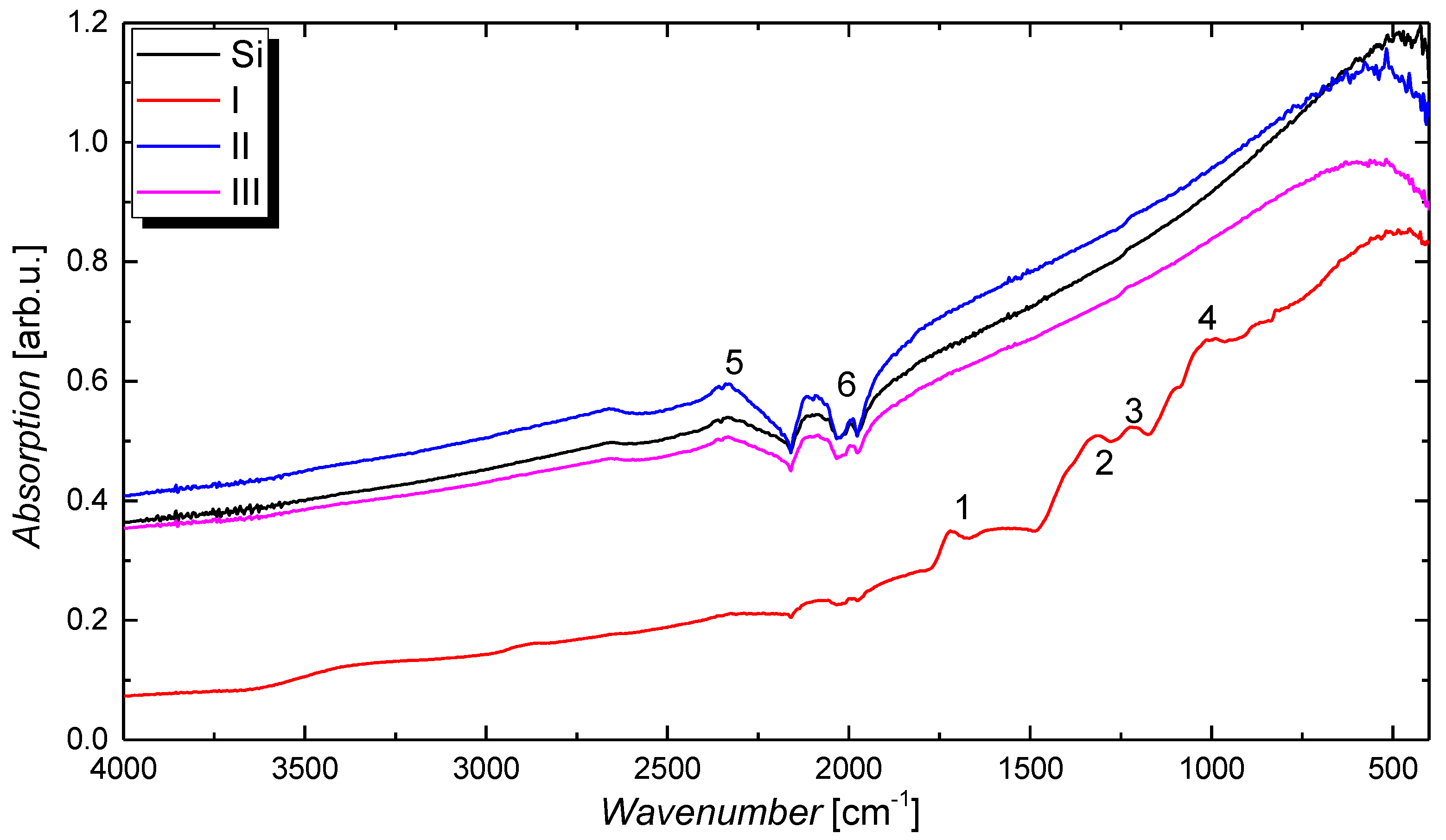
| Treatment | C 1s | O 1s | Na 1s | Si 2s | N 1s |
|---|---|---|---|---|---|
| Content in % | |||||
| I | 50.6 | 45.4 | 3.70 | 0.30 | 0 |
| II | 63.2 | 34.5 | 0.0 | 0.55 | 1.75 |
| III | 65.3 | 33.4 | 0.0 | 0.73 | 0.58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loghin, F.; Rivadeneyra, A.; Becherer, M.; Lugli, P.; Bobinger, M. A Facile and Efficient Protocol for Preparing Residual-Free Single-Walled Carbon Nanotube Films for Stable Sensing Applications. Nanomaterials 2019, 9, 471. https://doi.org/10.3390/nano9030471
Loghin F, Rivadeneyra A, Becherer M, Lugli P, Bobinger M. A Facile and Efficient Protocol for Preparing Residual-Free Single-Walled Carbon Nanotube Films for Stable Sensing Applications. Nanomaterials. 2019; 9(3):471. https://doi.org/10.3390/nano9030471
Chicago/Turabian StyleLoghin, Florin, Almudena Rivadeneyra, Markus Becherer, Paolo Lugli, and Marco Bobinger. 2019. "A Facile and Efficient Protocol for Preparing Residual-Free Single-Walled Carbon Nanotube Films for Stable Sensing Applications" Nanomaterials 9, no. 3: 471. https://doi.org/10.3390/nano9030471
APA StyleLoghin, F., Rivadeneyra, A., Becherer, M., Lugli, P., & Bobinger, M. (2019). A Facile and Efficient Protocol for Preparing Residual-Free Single-Walled Carbon Nanotube Films for Stable Sensing Applications. Nanomaterials, 9(3), 471. https://doi.org/10.3390/nano9030471






