Effect of Nb3+ Substitution on the Structural, Magnetic, and Optical Properties of Co0.5Ni0.5Fe2O4 Nanoparticles
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Materials and Instruments
2.2. Procedure
3. Results and Discussion
3.1. XRD Analysis
3.2. Spectral Analysis
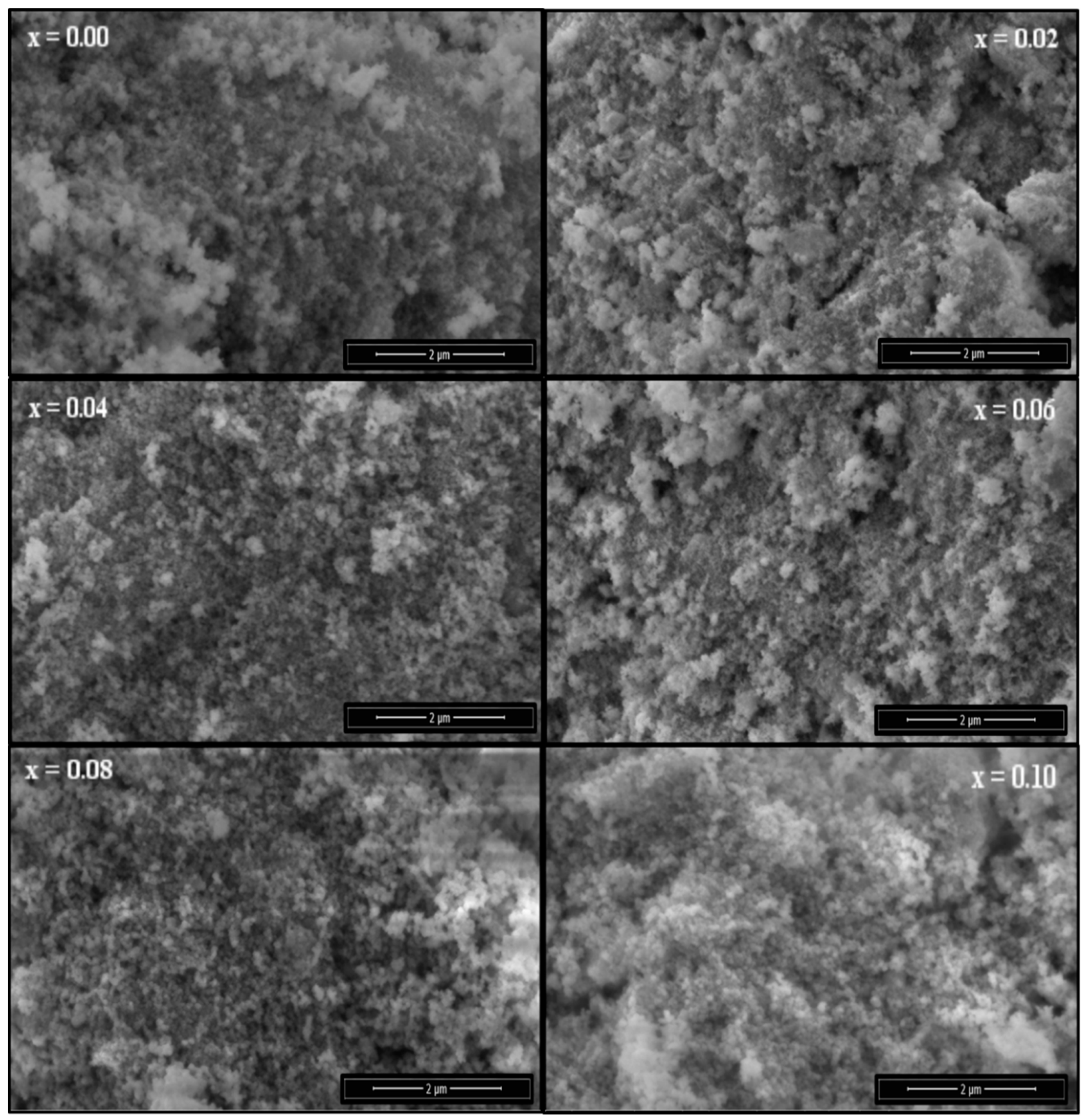
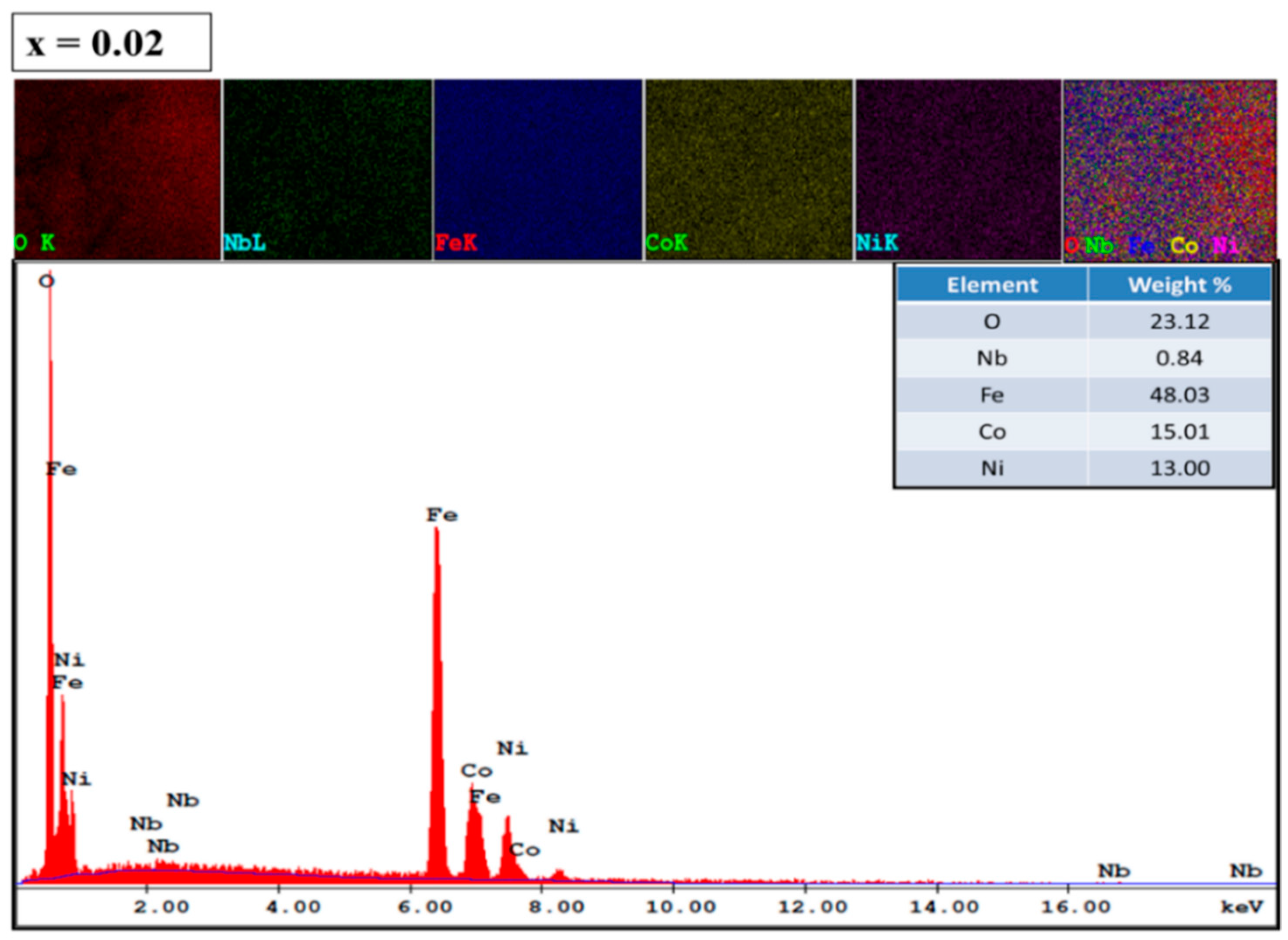
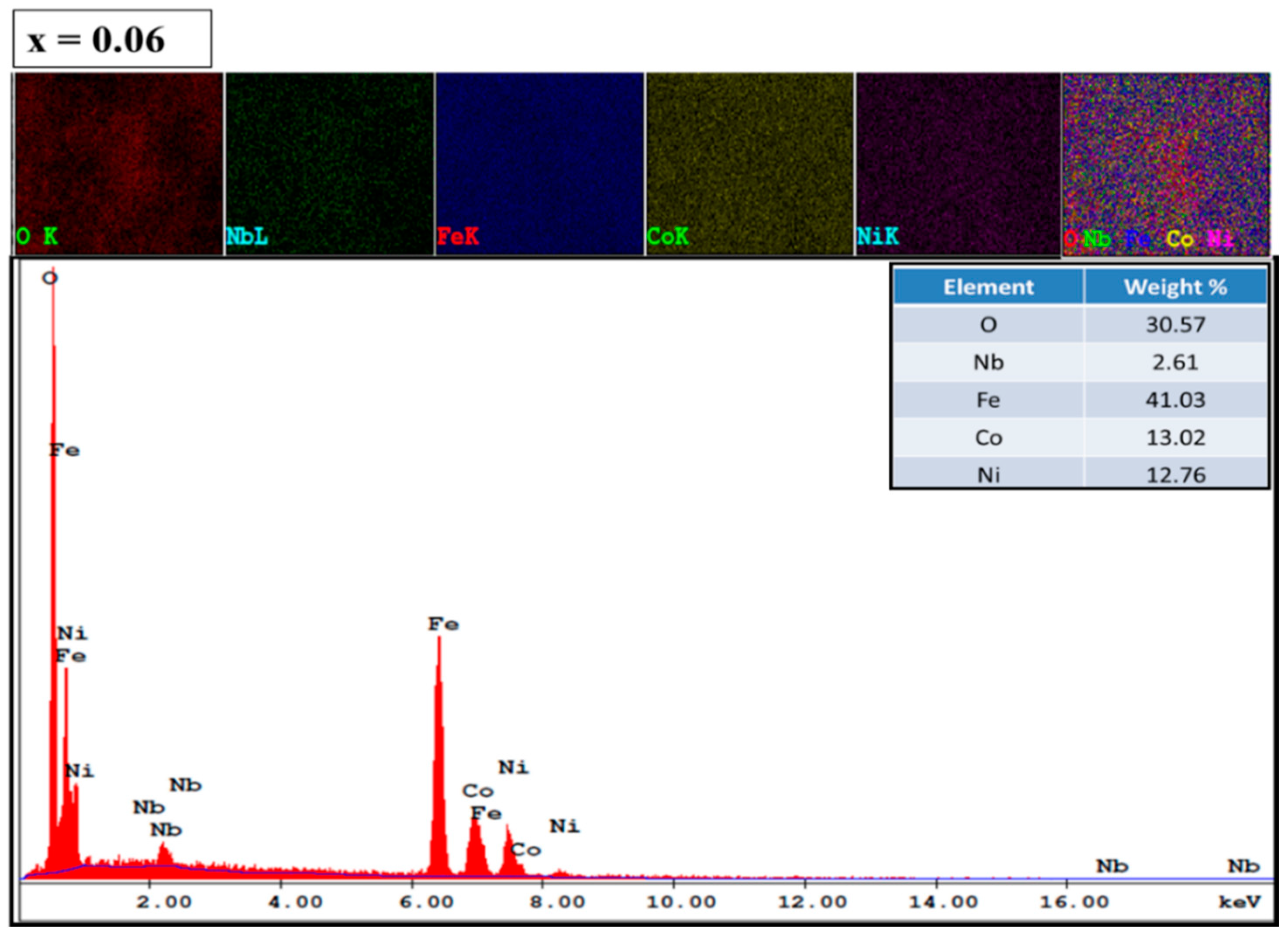
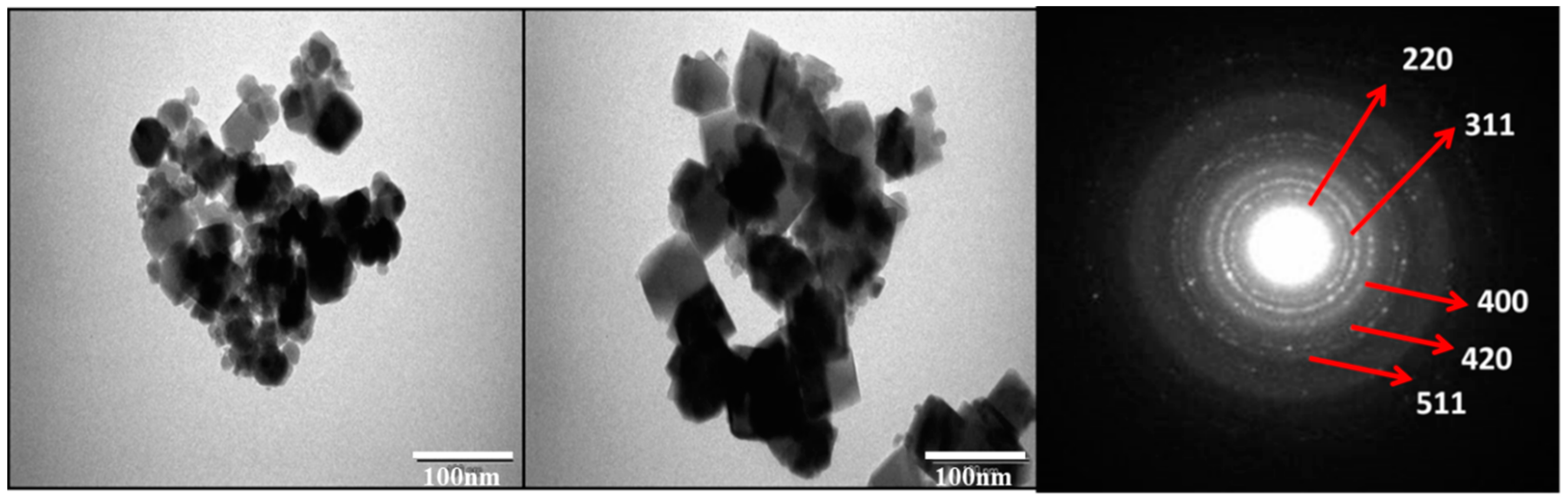
3.3. Morphological Analysis
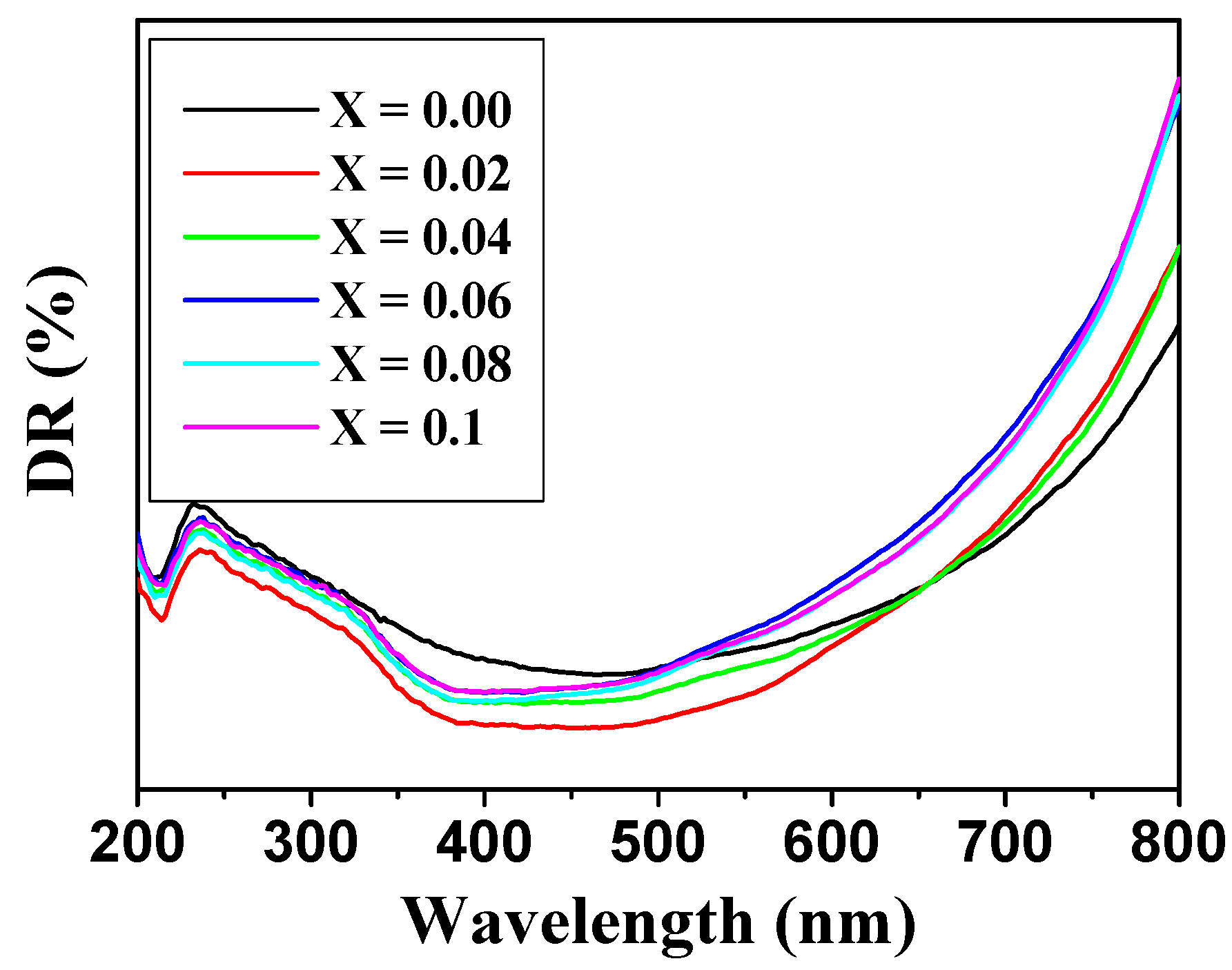
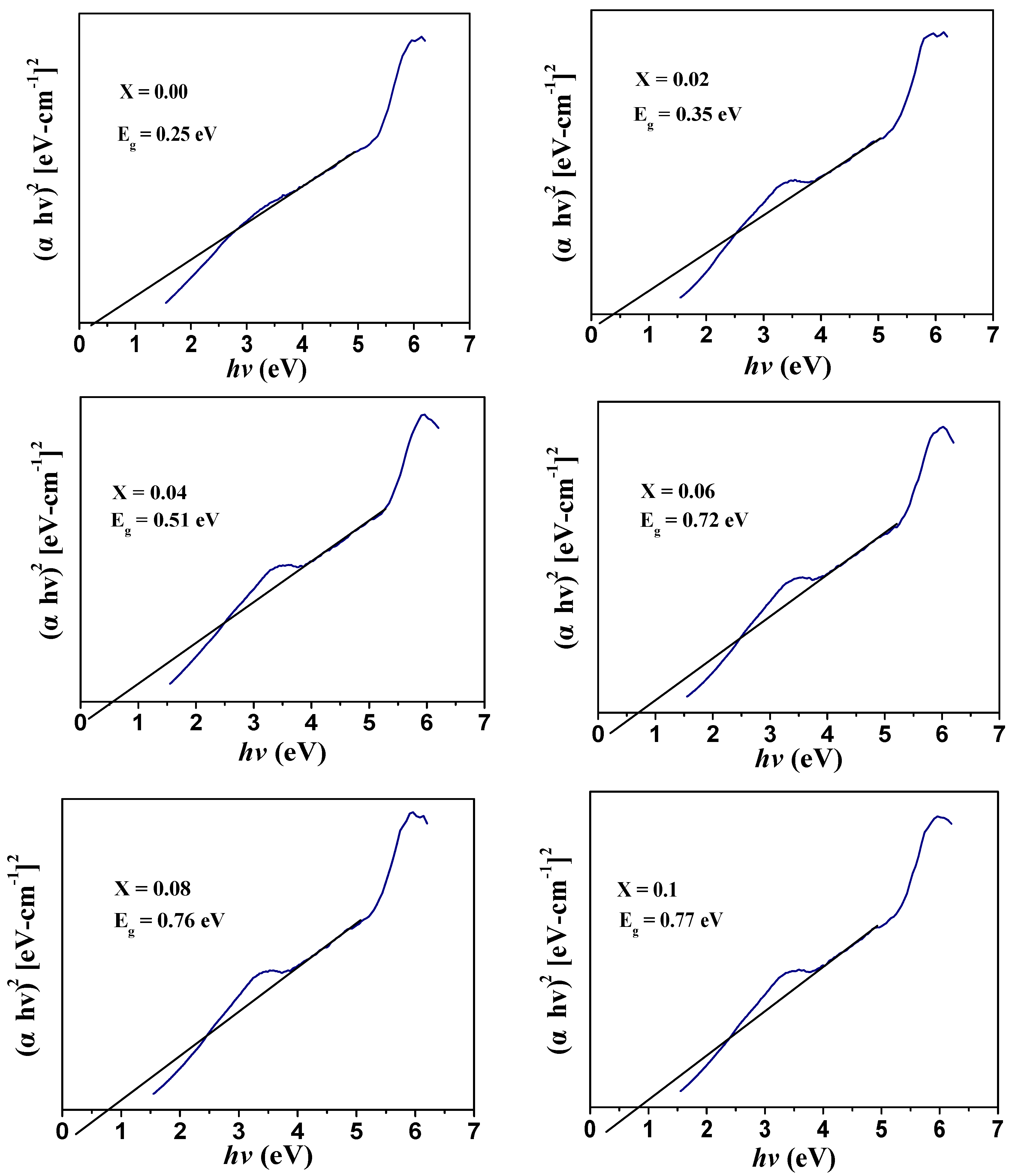
3.4. Optical Analysis
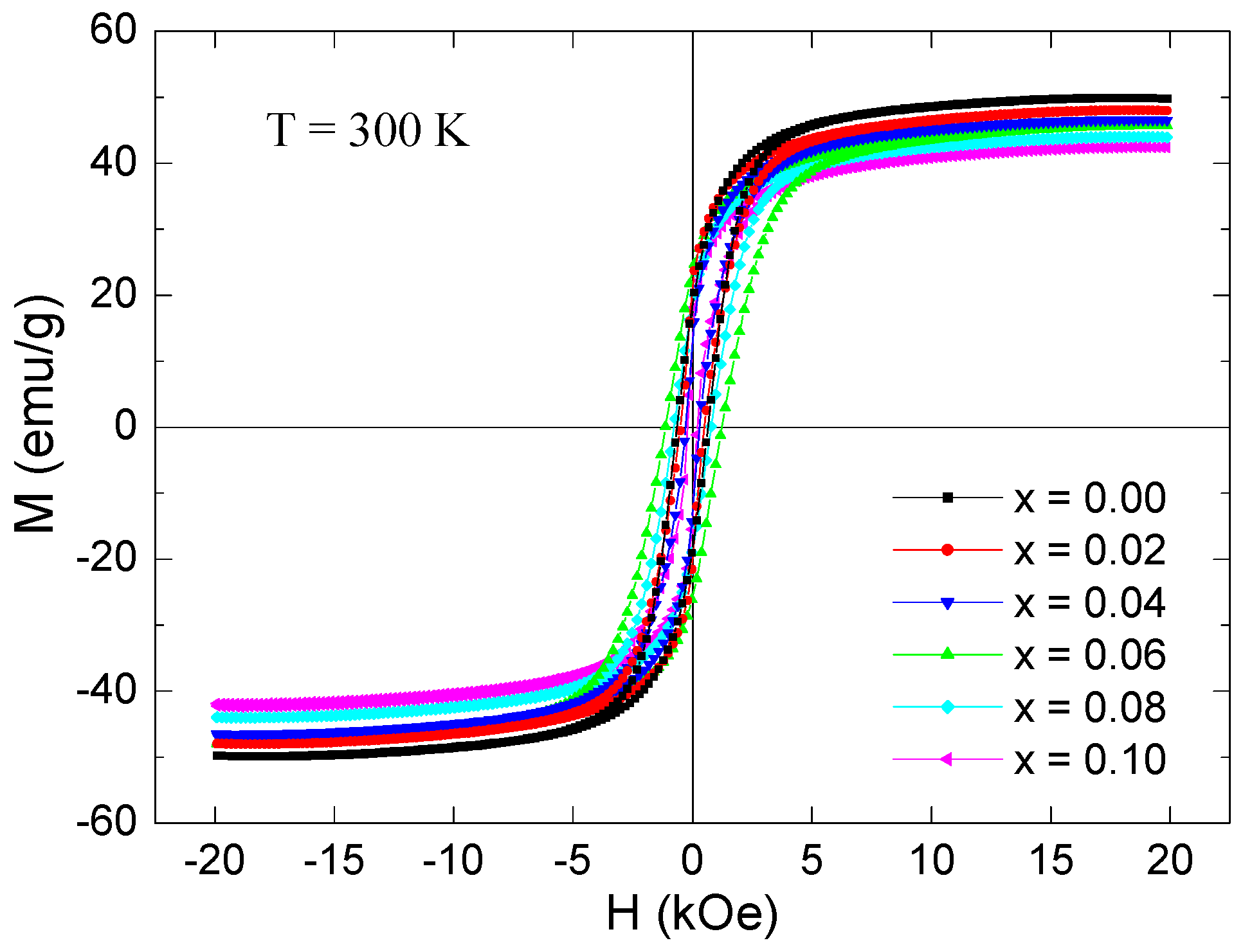
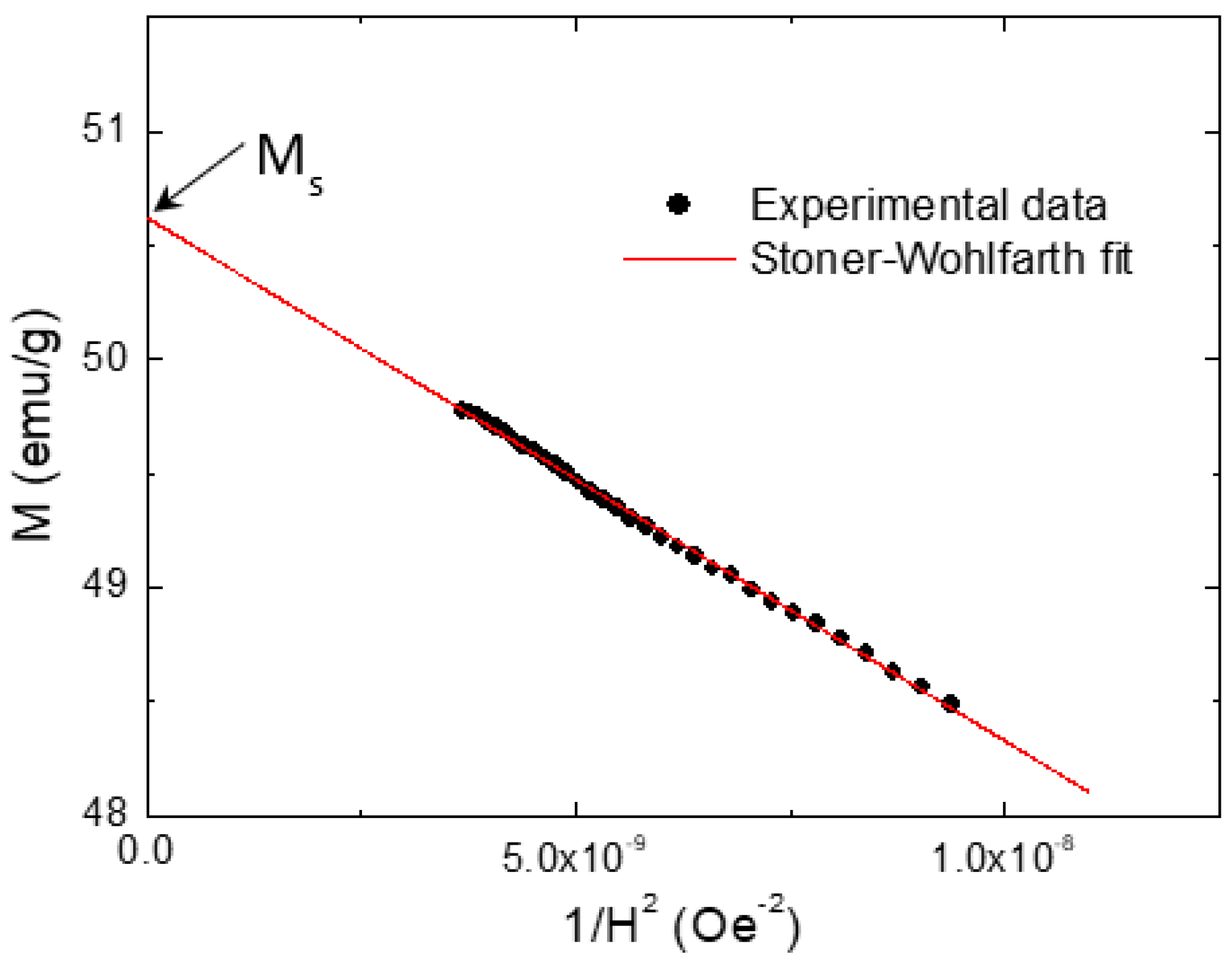
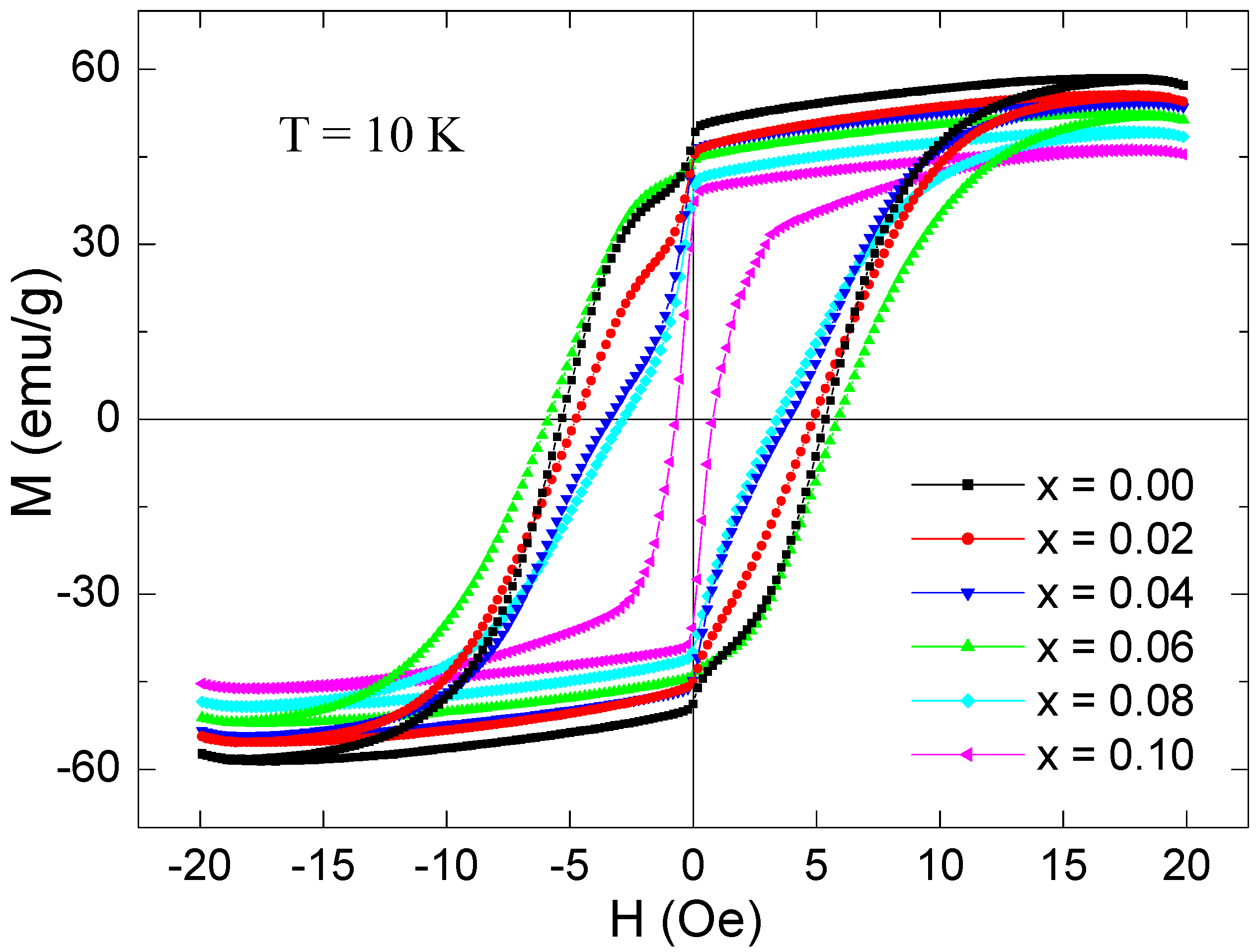
3.5. Magnetization Investigations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Q.; Zhang, Z.J. Size-dependent super paramagnetic properties of spinel ferrite nanocrystallites. Appl. Phys. Lett. 1998, 73, 3156. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, H.; Yu, L.; Cui, Y.; Zhao, X.; Feng, S. Magnetic properties of resubstituted Ni–Mn ferrite nanocrystallites. J. Mater. Sci. 2007, 42, 686–691. [Google Scholar] [CrossRef]
- Kumar, E.R.; Kamzin, A.S.; Janani, K. Effect of annealing on particle size, microstructure and gas sensing properties of Mn substituted CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 2016, 417, 122–129. [Google Scholar] [CrossRef]
- Kumar, E.R.; Jayaprakash, R.; Patel, R. Structural and morphological studies of manganese substituted CoFe2O4 and NiFe2O4 nanoparticles. Superlattices Microstruct. 2013, 62, 277–284. [Google Scholar] [CrossRef]
- Dascalu, G.; Popescu, T.; Feder, M.; Caltun, O.F. Structural, electric and magnetic properties of CoFe1.8RE0.2O4 (RE = Dy, Gd, La) bulk materials. J. Magn. Magn. Mater. 2013, 333, 69–74. [Google Scholar] [CrossRef]
- Shah, M.S.; Ali, K.; Ali, I.; Mahmood, A.; Ramay, S.M.; Farid, M.T. Structural and magnetic properties of praseodymium substituted barium-based spinel ferrites. Mater. Res. Bull. 2018, 98, 77–82. [Google Scholar] [CrossRef]
- Junaid, M.; Khan, M.A.; Iqbal, F.; Murtaza, G.; Akhtar, M.N.; Ahmad, M.; Shakir, I.; Warsi, M.F. Structural, spectral, dielectric and magnetic properties of Tb–Dy doped Li-Ni nano-ferrites synthesized via micro-emulsion route. J. Magn. Magn. Mater. 2016, 419, 338–344. [Google Scholar] [CrossRef]
- Boda, N.; Boda, G.; Naidu, K.C.B.; Srinivas, M.; Batoo, K.M.; Ravinder, D.; Reddy, A.P. Effect of rare earth elements on low temperature magnetic properties of Ni and Co-ferrite nanoparticles. J. Magn. Magn. Mater. 2019, 473, 228–235. [Google Scholar] [CrossRef]
- Kokare, M.K.; Jadhav, N.A.; Singh, V.; Rathod, S.M. Effect of Sm3+ substitution on the structural and magnetic properties of Ni-Co nanoferrites. Opt. Laser Technol. 2019, 112, 107–116. [Google Scholar] [CrossRef]
- Karimi, Z.; Mohammadifar, Y.; Shokrollahi, H.; Asl, S.K.; Yousefi, G.; Karimi, L. Magnetic and structural properties of nano sized Dy-doped cobalt ferrite synthesized by co-precipitation. J. Magn. Magn. Mater. 2014, 361, 150–156. [Google Scholar] [CrossRef]
- Ghone, D.M.; Mathe, V.L.; Patankar, K.K.; Kaushik, S.D. Microstructure, lattice strain, magnetic and magnetostriction properties of holmium substituted cobalt ferrites obtained by co-precipitation method. J. Alloys Compd. 2018, 739, 52–61. [Google Scholar] [CrossRef]
- Wu, X.; Ding, Z.; Song, N.; Li, L.; Wang, W. Effect of the rare-earth substitution on the structural, magnetic and adsorption properties in cobalt ferrite nanoparticles. Ceram. Int. 2016, 42, 4246–4255. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Ansari, S.A.; Kumar, D.R. Structural, morphological, magnetic properties and cation distribution of Ce and Sm co-substituted nano crystalline cobalt ferrite. Mater. Chem. Phys. 2018, 208, 248–257. [Google Scholar] [CrossRef]
- Amir, M.; Baykal, A.; Sertkol, M.; Sözeri, H. Microwave Assisted Synthesis and Characterization of CoxZn1−xCr0.5Fe0.5O4 nanoparticles. J. Inorg. Organomet. Polym. 2015, 25, 747–754. [Google Scholar] [CrossRef]
- Baykal, A.; Elmal, A.Z.; Sertkol, M.; Sözeri, H. Structural and Magnetic Properties of NiCrxFe2−xO4 nanoparticles Synthesized via Microwave Method. J. Supercond. Nov. Magn. 2015, 28, 3405–3410. [Google Scholar] [CrossRef]
- Güner, S.; Baykal, A.; Amir, M.; Güngüneş, H.; Geleri, M.; Sözeri, H.; Shirsath, S.E.; Sertkol, M. Synthesis and characterization of oleylamine capped MnxFe1−xFe2O4 nanocomposite: Magneto-optical properties, cation distribution and hyperfine interactions. J. Alloys Compd. 2016, 888, 675–686. [Google Scholar] [CrossRef]
- Chen, R.; Wang, W.; Zhao, X.; Zhang, Y.; Wu, S.; Li, F. Rapid hydrothermal synthesis of magnetic CoxNi1−xFe2O4 nanoparticles and their application on removal of Congo red. Chem. Eng. J. 2014, 242, 226–233. [Google Scholar] [CrossRef]
- Maaz, K.; Khalid, W.; Mumtaz, A.; Hasanain, S.K.; Liu, J.; Duan, J.L. Magnetic characterization of Co1−xNixFe2O4 (0 < x < 1) nanoparticles prepared by co-precipitation route. Phys. E Low-Dimens. Syst. Nanostruct. 2009, 41, 593–599. [Google Scholar]
- Salunkhe, A.B.; Khot, V.M.; Phadatare, M.R.; Thorat, N.D.; Joshi, R.S.; Yadav, H.M.; Pawar, S.H. Low temperature combustion synthesis and magnetostructural properties of Co–Mn nanoferrites. J. Magn. Magn. Mater. 2014, 352, 91–98. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Ali, S.; Baykal, A.; Ercan, I.; Sozeri, H. Nd3+ Ion-Substituted Co1−2xNixMnxFe2−yNdyO4 nanoparticles: Structural, Morphological, and Magnetic Investigations. J. Inorg. Organomet. Polym. Mater. 2018, 1–91. [Google Scholar] [CrossRef]
- Yadav, R.S.; Havlica, J.; Masilko, J.; Kalina, L.; Wasserbauer, J.; Hajdúchová, M.; Enev, V.; Kuřitka, I.; Kožáková, Z. Impact of Nd3+ in CoFe2O4 spinel ferrite nanoparticles on cation distribution, structural and magnetic properties. J. Magn. Magn. Mater. 2016, 399, 109–117. [Google Scholar] [CrossRef]
- Tahar, L.B.; Artus, M.; Ammar, S.; Smiri, L.S.; Herbst, F.; Vaulay, M.J.; Richard, V.; Grenèche, J.M.; Villain, F.; Fiévet, F. Magnetic properties of CoFe1.9RE0.1O4 nanoparticles (RE = La, Ce, Nd, Sm, Eu, Gd, Tb, Ho) prepared in polyol. J. Magn. Magn. Mater. 2008, 320, 3242–3250. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, H.; Zhao, X.; Yu, L.; Cui, Y.; Feng, S. Magnetic properties of CoFe2O4 ferrite doped with rare earth ion. Mater. Lett. 2006, 60, 1–6. [Google Scholar] [CrossRef]
- Karaoglu, E.; Baykal, A.; Erdemi, H.; Alpsoy, L.; Sozeri, H. Synthesis and characterization of dl-thioctic acid (DLTA)–Fe3O4 nanocomposite. J. Alloys Compd. 2011, 509, 9218–9225. [Google Scholar] [CrossRef]
- Garlyyev, B.; Durmus, Z.; Kemikli, N.; Sozeri, H.; Baykal, A.; Ozturk, R. Synthesis and magnetic properties of a porphine-based photosynthesizer with magnetic nano-carriers. Polyhedron 2011, 30, 2843–2848. [Google Scholar] [CrossRef]
- Temizel, E.; Ayan, E.; Senel, M.; Erdemi, H.; Yavuz, M.S.; Kavas, H.; Baykal, A.; Öztürk, R. Synthesis, conductivity and magnetic properties of poly(N-pyrrole phosphonic acid)–Fe3O4 nanocomposite. Mater. Chem. Phys. 2011, 131, 284–291. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Korkmaz, A.D.; Guner, S.; Sertkol, M.; Shirsath, S.E.; Baykal, A. Structural, optical and magnetic properties of Tm3+ substituted cobalt spinel ferrites synthesized via sonochemical approach. Ultrason.–Sonochem. 2019. [Google Scholar] [CrossRef]
- Almessiere, M.A. Magnetic and structural characterization of Nb3+-substituted CoFe2O4 nanoparticles. Ceram. Int. 2019. [Google Scholar] [CrossRef]
- Li, X.; Hou, Y.; Zhao, Q.; Wang, L. A general, one-step and template-free synthesis of sphere-like zinc ferrite nanostructures with enhanced photocatalytic activity for dye degradation. J. Colloid Interface Sci. 2011, 358, 102. [Google Scholar] [CrossRef] [PubMed]
- Stoner, E.C.; Wohlfarth, E.P. A Mechanism of Magnetic Hysteresis in Heteregeneous Alloys. Philos. Trans. R. Soc. A 1948, 240, 599–642. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Korkmaz, A.D.; Slimani, Y.; Nawaz, M.; Ali, S.; Baykal, A. Magneto-optical properties of rare earth metals substituted Co-Zn spinel nanoferrites. Ceram. Int. 2019, 45, 3449–3458. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Baykal, A. Exchange spring magnetic behavior of Sr0.3Ba0.4Pb0.3Fe12O19/(CuFe2O4)x nanocomposites fabricated by a one-pot citrate sol-gel combustion method. J. Alloys Compd. 2018, 762, 389–397. [Google Scholar] [CrossRef]
- Duong, G.V.; Hanh, N.; Linh, D.V.; Groessinger, R.; Weinberger, P.; Schafler, E.; Zehetbauer, M. Monodispersed nanocrystalline Co1−xZnxFe2O4 particles by forced hydrolysis: Synthesis and characterization. J. Magn. Magn. Mater. 2007, 311, 46–50. [Google Scholar] [CrossRef]
- Slimani, Y.; Güngüneş, H.; Nawaz, M.; Manikandan, A.; el Sayed, H.S.; Almessiere, M.A.; Sözeri, H.; Shirsath, S.E.; Ercan, I.; Baykal, A. Magneto-optical and microstructural properties of spinel cubic copper ferrites with Li-Al co-substitution. Ceram. Int. 2018, 44, 14242–14250. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Baykal, A. Structural and magnetic properties of Ce doped strontium hexaferrite. Ceram. Int. 2018, 44, 9000. [Google Scholar] [CrossRef]
- Chen, F.X.; Jia, J.T.; Xu, Z.G.; Zhou, B.; Liao, C.S.; Yan, C.H.; Chen, L.Y.; Zhao, H.B. Microstructure, magnetic, and magneto-optical properties of chemical synthesized Co-RE (RE = Ho, Er, Tm, Yb, Lu) ferrite nanocrystalline films. J. Appl. Phys. 1999, 86, 2727. [Google Scholar] [CrossRef]
- Kim, D.H.; Nikles, D.E.; Johnson, D.T.; Brazel, C.S. Heat generation of aqueously dispersed CoFe2O4 nanoparticles as heating agents for magnetically activated drug delivery and hyperthermia. J. Magn. Magn. Mater. 2008, 320, 2390–2396. [Google Scholar] [CrossRef]
- Deraz, N.M. Effects of magnesia addition on structural, morphological and magnetic properties of nano-crystalline nickel ferrite system. Ceram. Int. 2012, 38, 511–516. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Jamil, Y.; Tabasuum, A.; Hussain, T. Refinement in the structural and magnetic properties of Co0.5Ni0.5Fe2O4 and its application as laser micro-propellant using ablation confinement. J. Magn. Magn. Mater. 2015, 384, 302–307. [Google Scholar] [CrossRef]
- Sepelak, V.; Bergmann, I.; Feldhoff, A.; Heitjans, P.; Krumeich, F.; Menzel, D.; Litterst, F.J.; Campbell, S.J.; Becker, K.D. Nanocrystalline Nickel Ferrite, NiFe2O4: Mechanosynthesis, Nonequilibrium Cation Distribution, Canted Spin Arrangement, and Magnetic Behavior. J. Phys. Chem. C 2007, 111, 5026–5033. [Google Scholar] [CrossRef]
- Slimani, Y.; Almessiere, M.A.; Nawaz, M.; Baykal, A.; Akhtar, S.; Ercan, I.; Belenli, I. Effect of bimetallic (Ca, Mg) substitution on magneto-optical properties of NiFe2O4 nanoparticles. Ceram. Int. 2018. [Google Scholar] [CrossRef]
- Coey, J.M.D. Noncollinear spin arrangement in ultrafine ferrimagnetic crystallites. Phys. Rev. Lett. 1971, 27, 1140–1142. [Google Scholar] [CrossRef]
- Amir, M.; Gungunes, H.; Slimani, Y.; Tashkandi, N.; el Sayed, H.S.; Aldakheel, F.; Sertkol, M.; Sozeri, H.; Manikandan, A.; Ercan, I.; et al. Mossbauer studies and magnetic properties of cubic CuFe2O4 nanoparticles. J. Supercond. Nov. Magn. 2018. [Google Scholar] [CrossRef]
- Lin, Q.; He, Y.; Xu, J.; Lin, J.; Guo, Z.; Yang, F. Effects of Al3+ Substitution on Structural and Magnetic Behavior of CoFe2O4 Ferrite Nanomaterials. Nanomaterials 2018, 8, 750. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; He, Y.; Du, X.; Lin, Q.; Yang, H.; Shen, H. Structural and Magnetic Studies of Cr3+ Substituted Nickel Ferrite Nanomaterials Prepared by Sol-Gel Auto-Combustion. Crystals 2018, 8, 384. [Google Scholar] [CrossRef]
- Ali, I.; Islam, M.U.; Awan, M.S.; Ahmad, M.; Ashiq, M.N.; Naseem, S. Effect of Tb3+ substitution on the structural and magnetic properties of M-type hexaferrites synthesized by sol–gel auto-combustion technique. J. Alloy. Compd. 2013, 550, 564–572. [Google Scholar] [CrossRef]
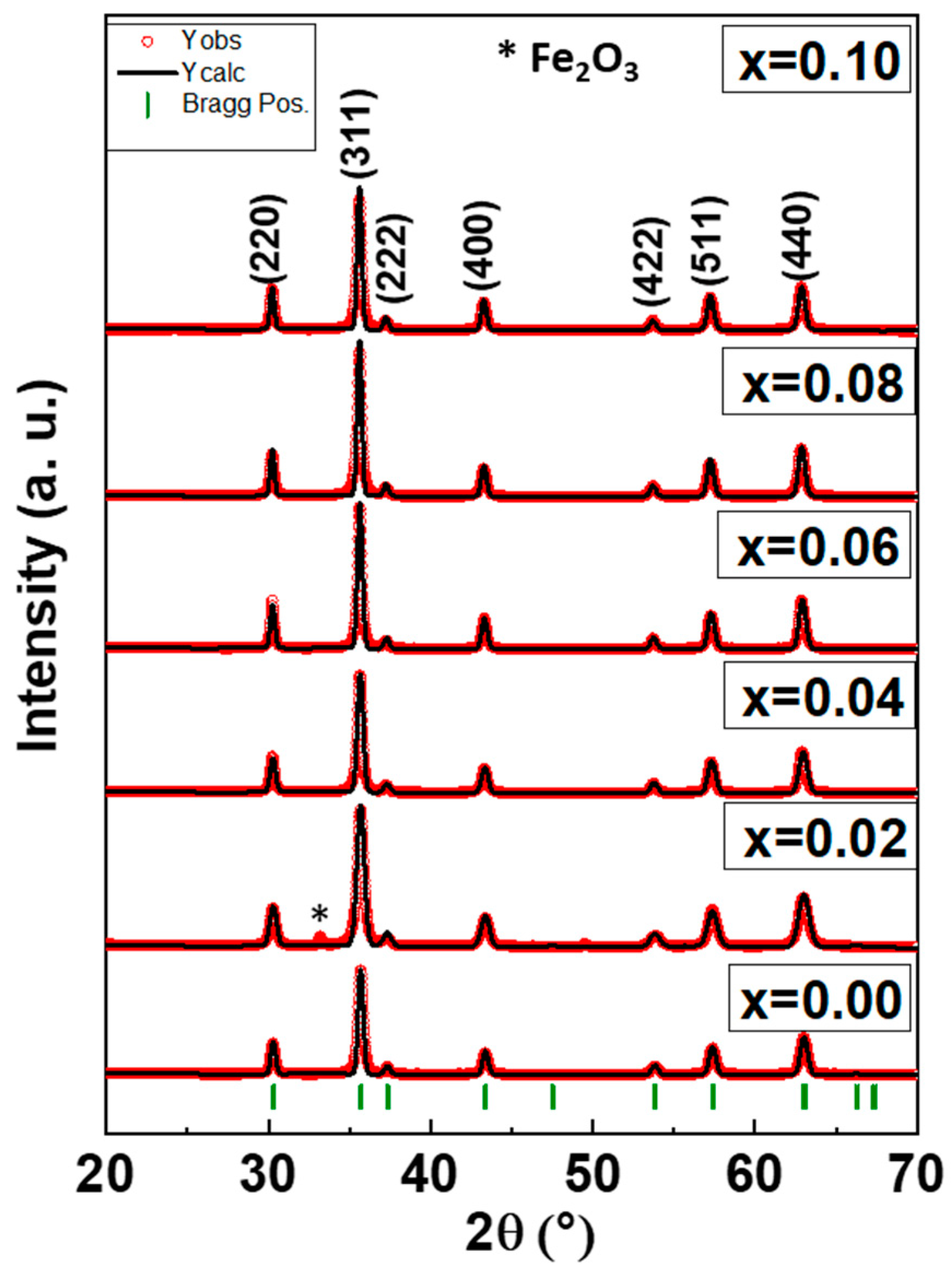

| x | a (Å) | V (Å)3 | DXRD (nm) ± 0.09 | χ2(chi2) | RBragg |
|---|---|---|---|---|---|
| 0.00 | 8.345 (1) | 581.15 | 22.86 | 1.75 | 12.67 |
| 0.02 | 8.345 (9) | 581.33 | 18.71 | 1.32 | 3.12 |
| 0.04 | 8.352 (3) | 582.67 | 24.18 | 1.52 | 3.99 |
| 0.06 | 8.356 (6) | 583.57 | 27.59 | 1.32 | 2.48 |
| 0.08 | 8.362 (3) | 584.76 | 26.86 | 1.77 | 7.88 |
| 0.10 | 8.362 (4) | 584.77 | 24.70 | 1.32 | 8.77 |
| x | Mmax,20 (emu/g) | Ms (emu/g) | Mr (emu/g) | Ka (Erg/g) | SQR | Hc (Oe) | |
|---|---|---|---|---|---|---|---|
| 0.00 | 49.77 | 50.62 | 18.47 | 1.96 × 105 | 0.365 | 648.11 | 2.13 |
| 0.02 | 47.98 | 48.81 | 20.9 | 1.74 × 105 | 0.428 | 509.9 | 2.06 |
| 0.04 | 46.57 | 47.36 | 13 | 1.78 × 105 | 0.274 | 286.11 | 2.00 |
| 0.06 | 45.68 | 46.52 | 23.66 | 2.16 × 105 | 0.509 | 1129.92 | 1.97 |
| 0.08 | 43.93 | 44.71 | 18.5 | 1.77 × 105 | 0.414 | 775.67 | 1.90 |
| 0.10 | 42.36 | 43.15 | 13.48 | 1.63 × 105 | 0.312 | 207.31 | 1.84 |
| x | Mmax,20 (emu/g) | Ms (emu/g) | Mr (emu/g) | Ka (Erg/g) | SQR | Hc (Oe) | |
|---|---|---|---|---|---|---|---|
| 0.00 | 57.22 | 57.96 | 48.09 | 4.30 × 105 | 0.830 | 5335.84 | 2.43 |
| 0.02 | 54.44 | 55.25 | 44.47 | 3.98 × 105 | 0.805 | 4760.39 | 2.33 |
| 0.04 | 53.53 | 54.26 | 44.24 | 3.78 × 105 | 0.815 | 3440.9 | 2.29 |
| 0.06 | 51.23 | 52.05 | 44.08 | 5.24 × 105 | 0.847 | 5882.24 | 2.21 |
| 0.08 | 48.39 | 48.62 | 34.11 | 3.57 × 105 | 0.702 | 2848 | 2.07 |
| 0.10 | 45.36 | 45.71 | 34.94 | 2.67 × 105 | 0.764 | 708.93 | 1.95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almessiere, M.A.; Slimani, Y.; Sertkol, M.; Nawaz, M.; Sadaqat, A.; Baykal, A.; Ercan, I.; Ozçelik, B. Effect of Nb3+ Substitution on the Structural, Magnetic, and Optical Properties of Co0.5Ni0.5Fe2O4 Nanoparticles. Nanomaterials 2019, 9, 430. https://doi.org/10.3390/nano9030430
Almessiere MA, Slimani Y, Sertkol M, Nawaz M, Sadaqat A, Baykal A, Ercan I, Ozçelik B. Effect of Nb3+ Substitution on the Structural, Magnetic, and Optical Properties of Co0.5Ni0.5Fe2O4 Nanoparticles. Nanomaterials. 2019; 9(3):430. https://doi.org/10.3390/nano9030430
Chicago/Turabian StyleAlmessiere, Munirah. A., Yassine Slimani, Murat Sertkol, Muhammed Nawaz, Ali Sadaqat, Abdulhadi Baykal, Ismail Ercan, and Bekir Ozçelik. 2019. "Effect of Nb3+ Substitution on the Structural, Magnetic, and Optical Properties of Co0.5Ni0.5Fe2O4 Nanoparticles" Nanomaterials 9, no. 3: 430. https://doi.org/10.3390/nano9030430
APA StyleAlmessiere, M. A., Slimani, Y., Sertkol, M., Nawaz, M., Sadaqat, A., Baykal, A., Ercan, I., & Ozçelik, B. (2019). Effect of Nb3+ Substitution on the Structural, Magnetic, and Optical Properties of Co0.5Ni0.5Fe2O4 Nanoparticles. Nanomaterials, 9(3), 430. https://doi.org/10.3390/nano9030430







