Hydrated Salt/Graphite/Polyelectrolyte Organic-Inorganic Hybrids for Efficient Thermochemical Storage
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
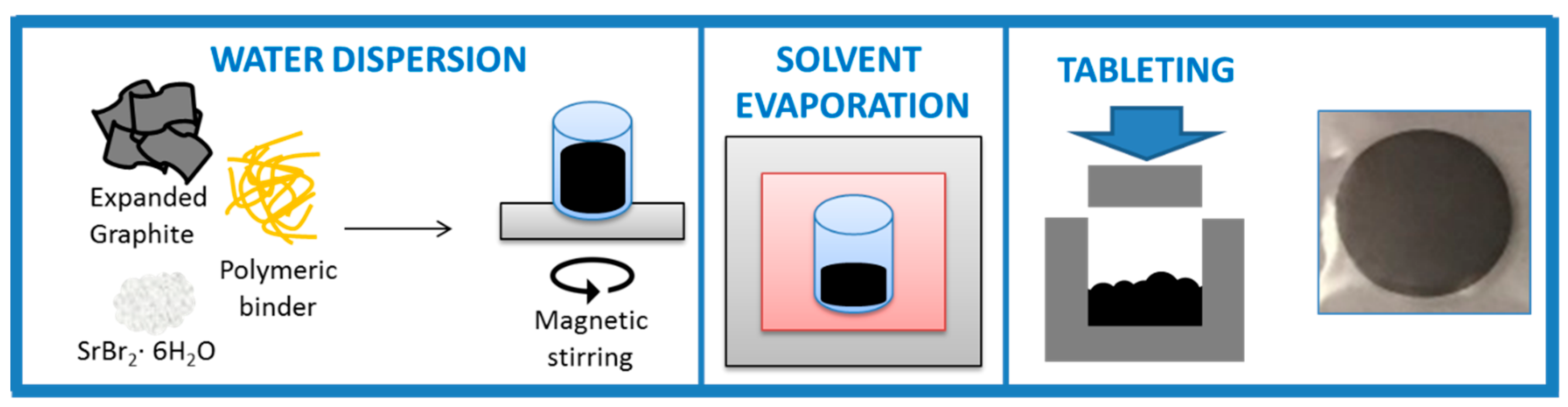
2.2. TCM Composite Manufacturing
2.3. Characterization
3. Results and Discussion
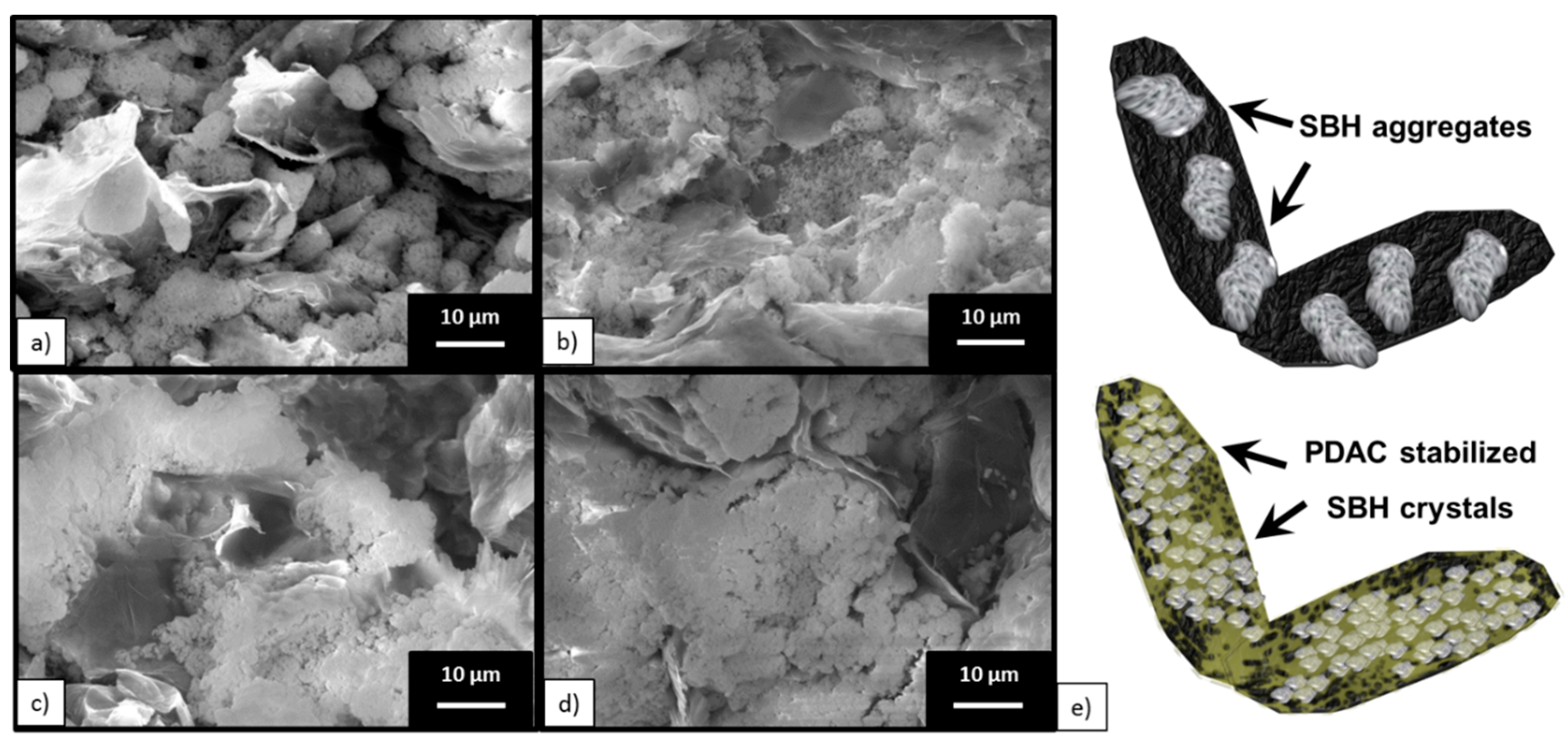
3.1. Morphology Analysis
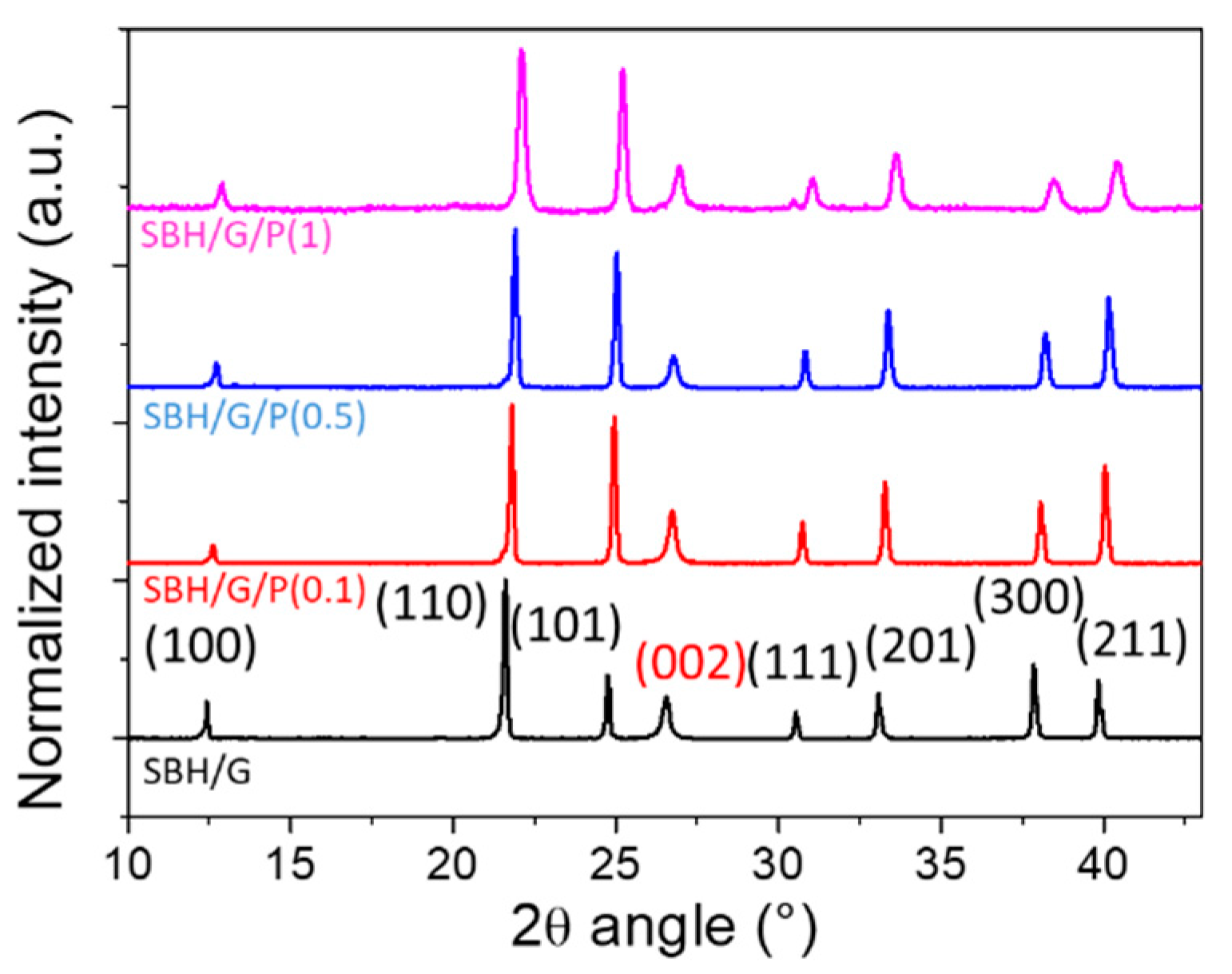
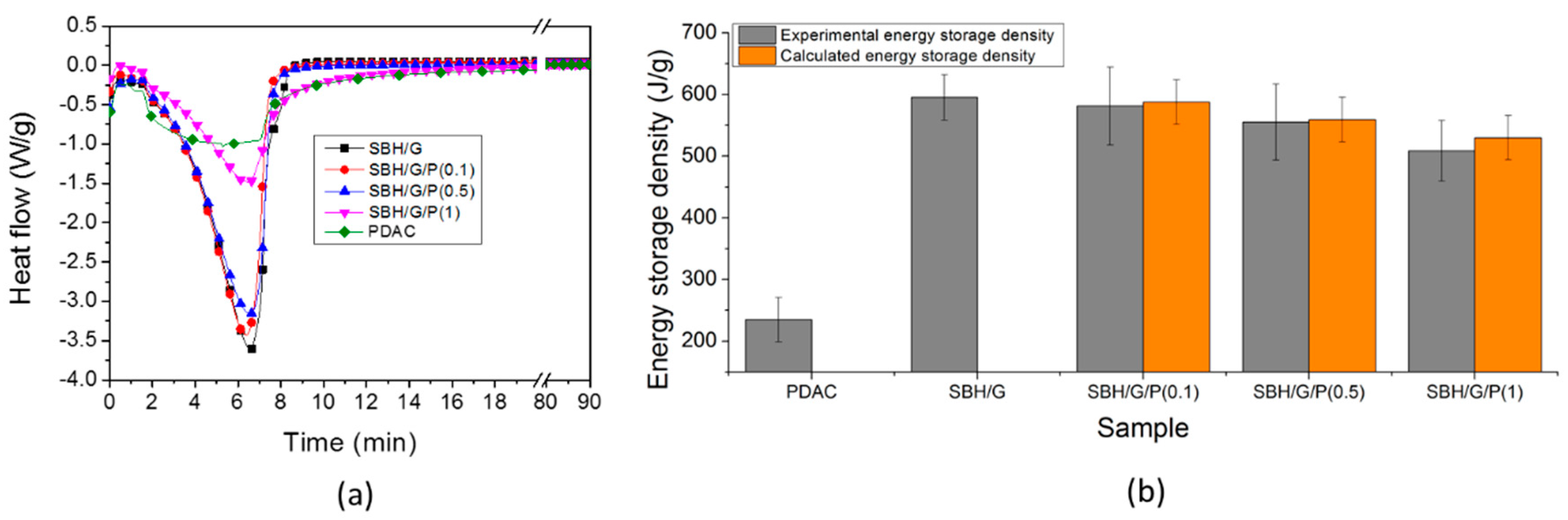
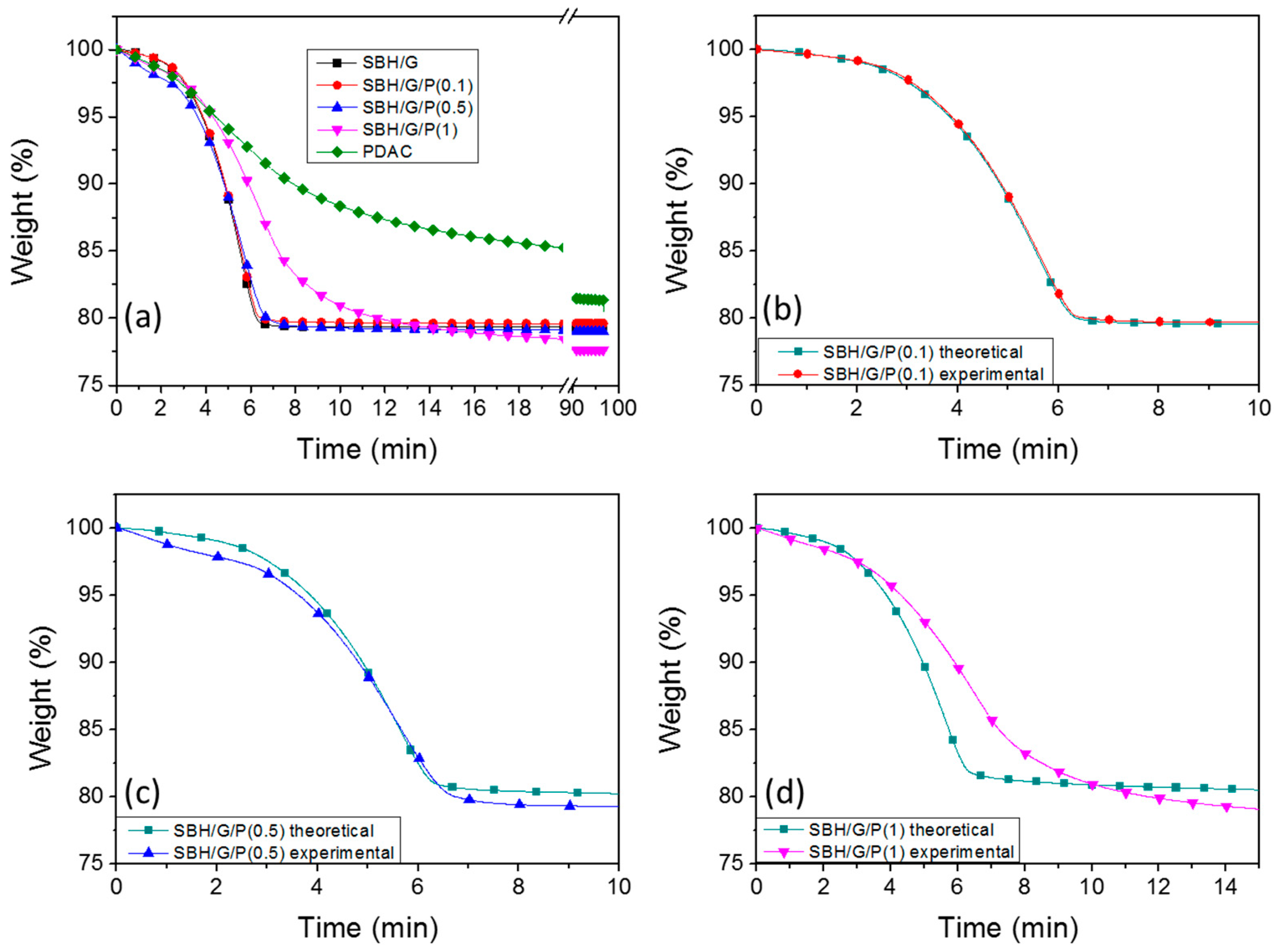
3.2. Thermal Properties
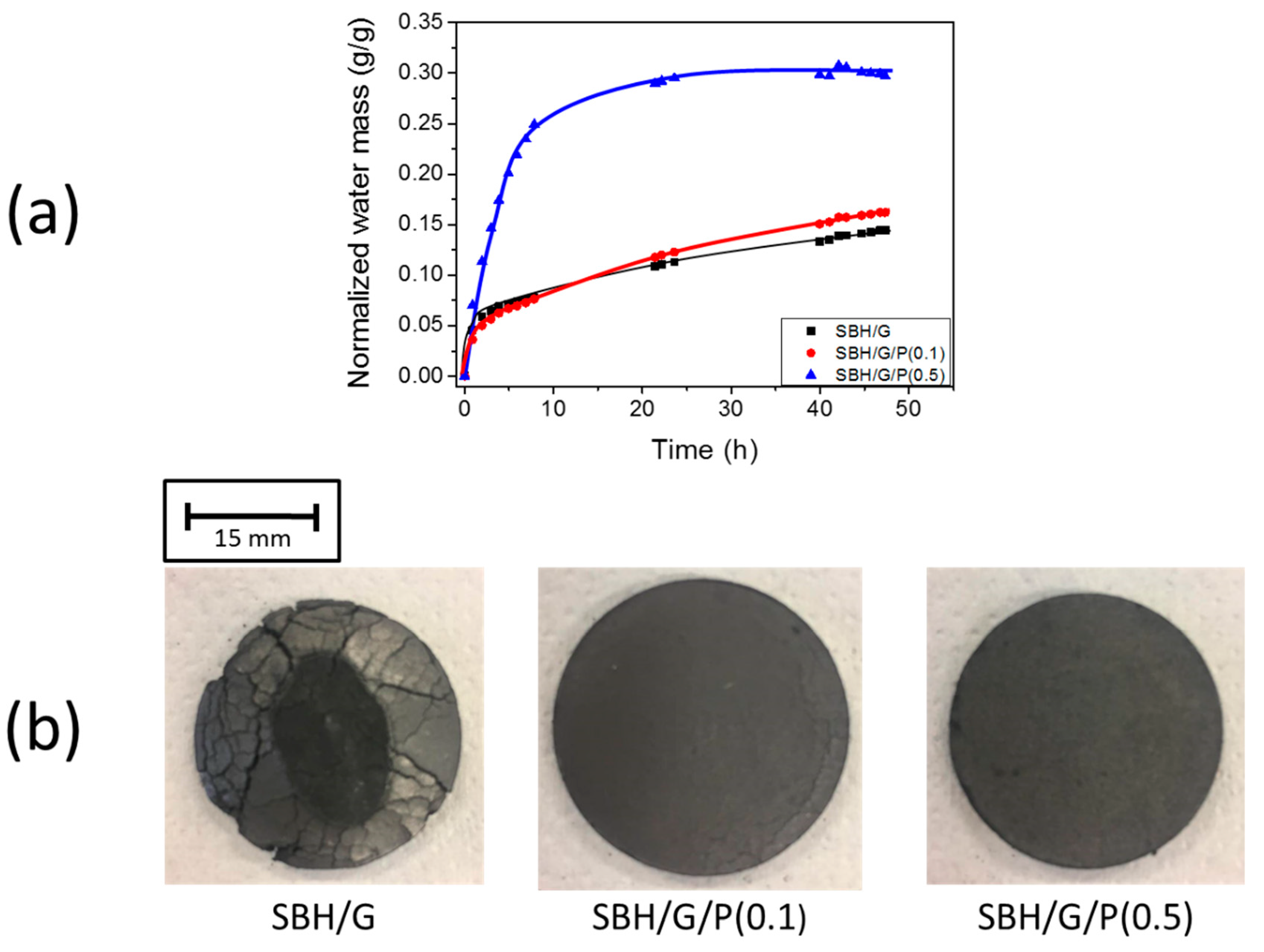
3.3. Composite Tabs Hydration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, X. Energy Solutions to Combat Global Warming; Springer: New York, NY, USA, 2017; Volume 33, ISBN 978-3-319-26948-1. [Google Scholar]
- Arce, P.; Medrano, M.; Gil, A.; Oró, E.; Cabeza, L.F. Overview of thermal energy storage (TES) potential energy savings and climate change mitigation in Spain and Europe. Appl. Energy 2011, 88, 2764–2774. [Google Scholar] [CrossRef]
- Ammar, Y.; Joyce, S.; Norman, R.; Wang, Y.; Roskilly, A.P. Low grade thermal energy sources and uses from the process industry in the UK. Appl. Energy 2012, 89, 3–20. [Google Scholar] [CrossRef]
- Bellocchi, S.; Leo, G.; Manno, M.; Pentimalli, M.; Salvatori, M.; Zaccagnini, A. Adsorbent materials for low-grade waste heat recovery: Application to industrial pasta drying processes. Energy 2017, 140, 729–745. [Google Scholar] [CrossRef]
- Yes ßiller, N.; Hanson, J.L.; Yee, E.H. Waste heat generation: A comprehensive review. Waste Manag. 2015, 42, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Wahlroos, M.; Pärssinen, M.; Rinne, S.; Syri, S.; Manner, J. Future views on waste heat utilization—Case of data centers in Northern Europe. Renew. Sustain. Energy Rev. 2018, 82, 1749–1764. [Google Scholar] [CrossRef]
- Cot-Gores, J.; Castell, A.; Cabeza, L.F. Thermochemical energy storage and conversion: A-state-of-the-art review of the experimental research under practical conditions. Renew. Sustain. Energy Rev. 2012, 16, 5207–5224. [Google Scholar] [CrossRef]
- Heier, J.; Bales, C.; Martin, V. Combining thermal energy storage with buildings—A review. Renew. Sustain. Energy Rev. 2015, 42, 1305–1325. [Google Scholar] [CrossRef]
- Saffari, M.; de Gracia, A.; Fernández, C.; Belusko, M.; Boer, D.; Cabeza, L.F. Optimized demand side management (DSM) of peak electricity demand by coupling low temperature thermal energy storage (TES) and solar PV. Appl. Energy 2018, 211, 604–616. [Google Scholar] [CrossRef]
- Islam, M.P.; Morimoto, T. Advances in low to medium temperature non-concentrating solar thermal technology. Renew. Sustain. Energy Rev. 2018, 82, 2066–2093. [Google Scholar] [CrossRef]
- Sarbu, I. A Comprehensive Review of Thermal Energy Storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Scapino, L.; Zondag, H.A.; Van Bael, J.; Diriken, J.; Rindt, C.C.M. Sorption heat storage for long-term low-temperature applications: A review on the advancements at material and prototype scale. Appl. Energy 2017, 190, 920–948. [Google Scholar] [CrossRef]
- Vasta, S.; Brancato, V.; La Rosa, D.; Palomba, V.; Restuccia, G.; Sapienza, A.; Frazzica, A. Adsorption Heat Storage: State of the Art and Future Perspectives. Nanomaterials 2018, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Wang, R.Z.; Wang, L.W. Sorption thermal storage for solar energy. Prog. Energy Combust. Sci. 2013, 39, 489–514. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Schmidt, T.; Rammelberg, H.U.; Watts, B.A.; Ruck, W.K.L. A systematic multi-step screening of numerous salt hydrates for low temperature thermochemical energy storage. Appl. Energy 2014, 124, 1–16. [Google Scholar] [CrossRef]
- Donkers, P.A.J. A review of salt hydrates for seasonal heat storage in domestic applications. Appl. Energy 2017, 199, 1–26. [Google Scholar] [CrossRef]
- Richter, M.; Habermann, E.M.; Siebecke, E.; Linder, M. A systematic screening of salt hydrates as materials for a thermochemical heat transformer. Thermochim. Acta 2018, 659, 136–150. [Google Scholar] [CrossRef]
- Kiyabu, S.; Lowe, J.S.; Ahmed, A.; Siegel, D.J. Computational Screening of Hydration Reactions for Thermal Energy Storage: New Materials and Design Rules. Chem. Mater. 2018, 30, 2006–2017. [Google Scholar] [CrossRef]
- Fopah-Lele, A.; Tamba, J.G. A review on the use of SrBr2·6H2O as a potential material for low temperature energy storage systems and building applications. Sol. Energy Mater. Sol. Cells 2017, 164, 175–187. [Google Scholar] [CrossRef]
- Fopah-Lele, A.; Rohde, C.; Neumann, K.; Tietjen, T.; Rönnebeck, T.; N’Tsoukpoe, K.E.; Osterland, T.; Opel, O.; Ruck, W.K. Lab-scale experiment of a closed thermochemical heat storage system including honeycomb heat exchanger. Energy 2016, 114, 225–238. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Aristov, Y.I. Composites “salt inside porous matrix” for adsorption heat transformation: A current state-of-the-art and new trends. Int. J. Low-Carbon Technol. 2012, 7, 288–302. [Google Scholar] [CrossRef]
- Korhammer, K.; Druske, M.-M.; Fopah-Lele, A.; Rammelberg, H.U.; Wegscheider, N.; Opel, O.; Osterland, T.; Ruck, W. Sorption and thermal characterization of composite materials based on chlorides for thermal energy storage. Appl. Energy 2016, 162, 1462–1472. [Google Scholar] [CrossRef]
- Gaeini, M.; Rouws, A.L.; Salari, J.W.O.; Zondag, H.A.; Rindt, C.C.M. Characterization of microencapsulated and impregnated porous host materials based on calcium chloride for thermochemical energy storage. Appl. Energy 2018, 212, 1165–1177. [Google Scholar] [CrossRef]
- Cammarata, A.; Verda, V.; Sciacovelli, A.; Ding, Y. Hybrid strontium bromide-natural graphite composites for low to medium temperature thermochemical energy storage: Formulation, fabrication and performance investigation. Energy Convers. Manag. 2018, 166, 233–240. [Google Scholar] [CrossRef]
- Lu, J.; Do, I.; Fukushima, H.; Lee, I.; Drzal, L.T. Stable aqueous suspension and self-assembly of graphite nanoplatelets coated with various polyelectrolytes. J. Nanomater. 2010, 2010, 2. [Google Scholar] [CrossRef]
- Qin, C.; Feng, Y.; An, H.; Han, J.; Cao, C.; Feng, W. Tetracarboxylated Azobenzene/Polymer Supramolecular Assemblies as High-Performance Multiresponsive Actuators. ACS Appl. Mater. Interfaces 2017, 9, 4066–4073. [Google Scholar] [CrossRef] [PubMed]
- Donkers, P.A.J.; Pel, L.; Adan, O.C.G. Experimental studies for the cyclability of salt hydrates for thermochemical heat storage. J. Energy Storage 2016, 5, 25–32. [Google Scholar] [CrossRef]
- Gustavsson, M.; Karawacki, E.; Gustafsson, S.E. Thermal conductivity, thermal diffusivity, and specific heat of thin samples from transient measurements with hot disk sensors. Rev. Sci. Instrum. 1994, 65, 3856–3859. [Google Scholar] [CrossRef]
- JCPDS-ICDD. Available online: http://www.icdd.com/ (accessed on 10 December 2018).
- Inoue, M.; Hirasawa, I. The relationship between crystal morphology and XRD peak intensity on CaSO4·2H2O. J. Cryst. Growth 2013, 380, 169–175. [Google Scholar] [CrossRef]
- Fopah, A.; Kuznik, F.; Opel, O.; Ruck, W.K.L. Performance analysis of a thermochemical based heat storage as an addition to cogeneration systems. Energy Convers. Manag. 2015, 106, 1327–1344. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Wang, R.Z.; Zhang, Y.N.; Yu, N. Development of SrBr2 composite sorbents for a sorption thermal energy storage system to store low-temperature heat. Energy 2016, 115, 129–139. [Google Scholar] [CrossRef]
| Sample Name | Weight Ratio | ||
|---|---|---|---|
| G | SBH | PDAC | |
| Expanded Natural Graphite | Strontium Bromide Hexahydrate | Polyelectrolyte Binder | |
| SBH/G | 1 | 5 | 0 |
| SBH/G/P 0.1) | 1 | 5 | 0.1 |
| SBH/G/P(0.5) | 1 | 5 | 0.5 |
| SBH/G/P(1) | 1 | 5 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salviati, S.; Carosio, F.; Saracco, G.; Fina, A. Hydrated Salt/Graphite/Polyelectrolyte Organic-Inorganic Hybrids for Efficient Thermochemical Storage. Nanomaterials 2019, 9, 420. https://doi.org/10.3390/nano9030420
Salviati S, Carosio F, Saracco G, Fina A. Hydrated Salt/Graphite/Polyelectrolyte Organic-Inorganic Hybrids for Efficient Thermochemical Storage. Nanomaterials. 2019; 9(3):420. https://doi.org/10.3390/nano9030420
Chicago/Turabian StyleSalviati, Sergio, Federico Carosio, Guido Saracco, and Alberto Fina. 2019. "Hydrated Salt/Graphite/Polyelectrolyte Organic-Inorganic Hybrids for Efficient Thermochemical Storage" Nanomaterials 9, no. 3: 420. https://doi.org/10.3390/nano9030420
APA StyleSalviati, S., Carosio, F., Saracco, G., & Fina, A. (2019). Hydrated Salt/Graphite/Polyelectrolyte Organic-Inorganic Hybrids for Efficient Thermochemical Storage. Nanomaterials, 9(3), 420. https://doi.org/10.3390/nano9030420






