Fast Microwave Synthesis of Hierarchical Porous Carbons from Waste Palm Boosted by Activated Carbons for Supercapacitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Porous Carbons
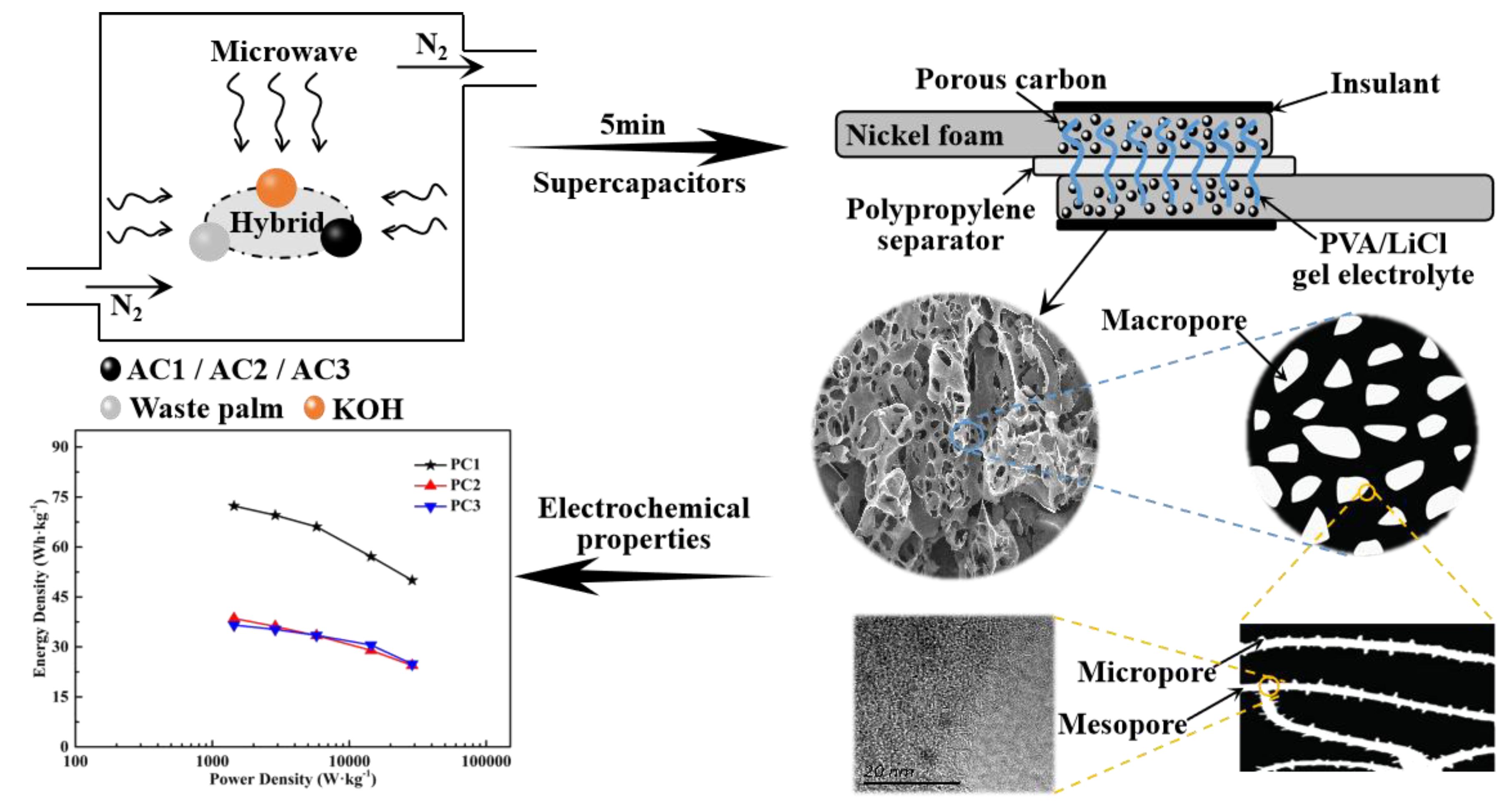
2.3. Fabrication of Solid-State Symmetric Supercapacitors
2.4. Characterizations and Measurements
3. Results and Discussion
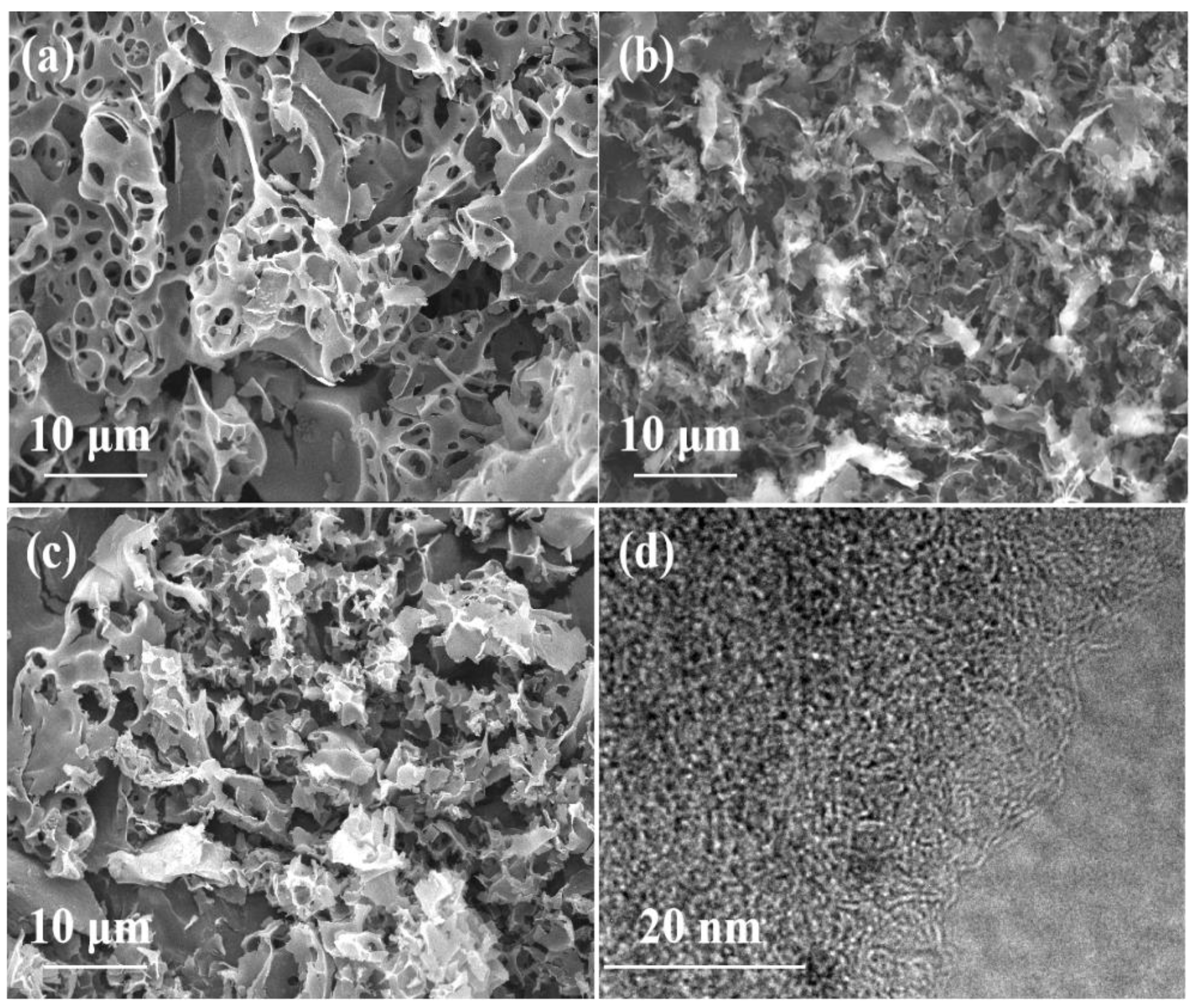
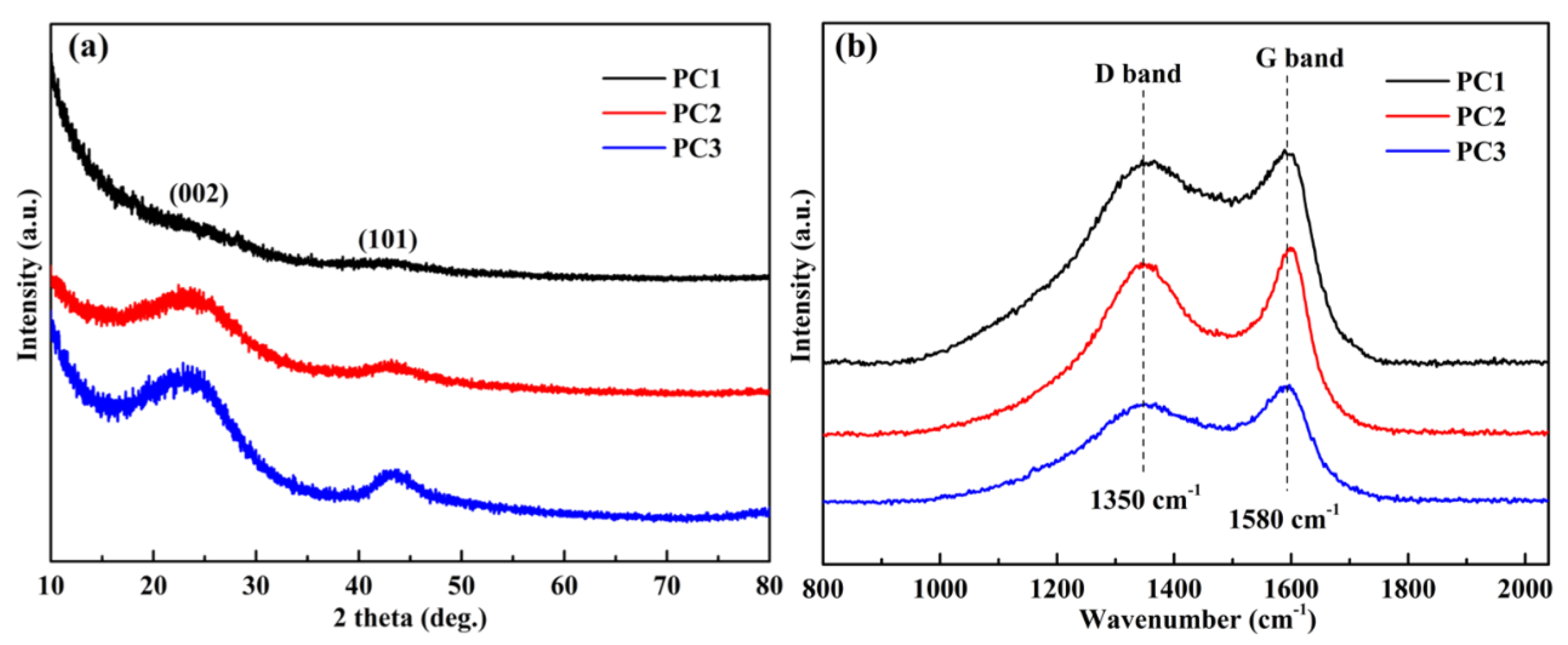
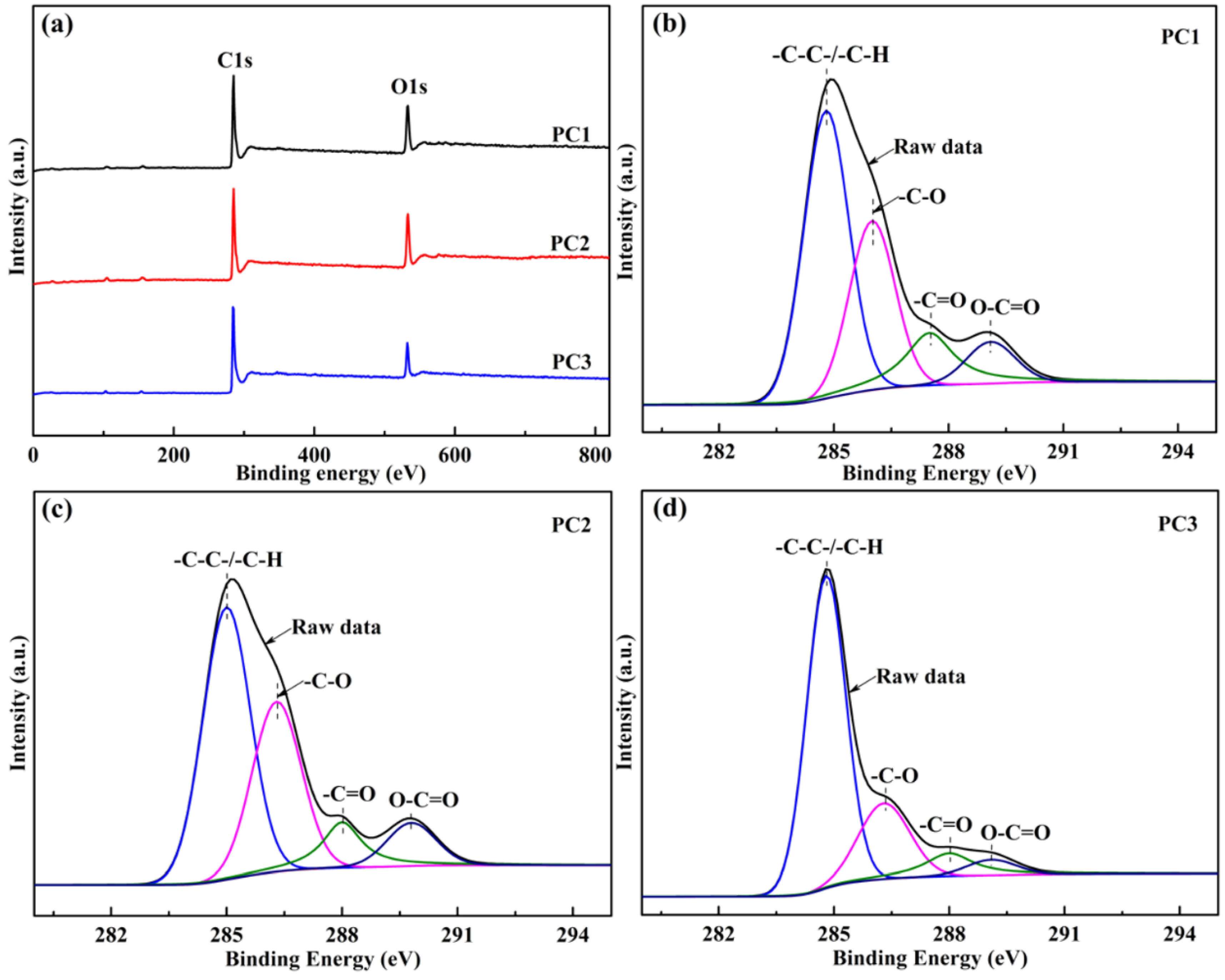
3.1. Morphological and Structural Characterization
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Xia, Y. Recent Progress in Supercapacitors: From Materials Design to System Construction. Adv. Mater. 2013, 25, 5336–5342. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lee, P.S.; Li, C. 3D carbon based nanostructures for advanced supercapacitors. Energy Environ. Sci. 2013, 6, 41–53. [Google Scholar] [CrossRef]
- Peng, X.; Peng, L.; Wu, C.; Xie, Y. Two dimensional nanomaterials for flexible supercapacitors. Chem. Soc. Rev. 2014, 43, 3303–3323. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, F.; Liu, M.; Lu, G.Q.; Cheng, H. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage. Angew. Chem. Int. Ed. 2008, 47, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Poh, C.K.; Chen, J.S.; Xu, G.; Wang, D.; Li, Q.; Lin, J.; Lou, X.W.D. Nitrogen-containing microporous carbon nanospheres with improved capacitive properties. Energy Environ. Sci. 2011, 4, 717–724. [Google Scholar] [CrossRef]
- Feng, H.; Hu, H.; Dong, H.; Xiao, Y.; Cai, Y.; Lei, B.; Liu, Y.; Zheng, M. Hierarchical structured carbon derived from bagasse wastes: A simple and efficient synthesis route and its improved electrochemical properties for high-performance supercapacitors. J. Power Sources 2016, 302, 164–173. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, Y.; Lu, W.; Zhang, H.; Song, Z.; Luo, D.; Gan, L.; Liu, M.; Sun, D. A novel synthesis of hierarchical porous carbons from interpenetrating polymer networks for high performance supercapacitor electrodes. Carbon 2017, 111, 667–674. [Google Scholar] [CrossRef]
- Salanne, M.; Rotenberg, B.; Naoi, K.; Kaneko, K.; Taberna, P.L.; Grey, C.P.; Dunn, B.; Simon, P. Efficient storage mechanisms for building better supercapacitors. Nat. Energy 2016, 1, 16070. [Google Scholar] [CrossRef]
- Xu, F.; Tang, Z.; Huang, S.; Chen, L.; Liang, Y.; Mai, W.; Zhong, H.; Fu, R.; Wu, D. Facile synthesis of ultrahigh-surface-area hollow carbon nanospheres for enhanced adsorption and energy storage. Nat. Commun. 2015, 6, 7221. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, Z.; Zhong, X.; Huang, X.; Weiss, N.O.; Huang, Y.; Duan, X. Holey graphene frameworks for highly efficient capacitive energy storage. Nat. Commun. 2014, 5, 4554. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Zhi, C.; Wang, X.; Tang, D.; Xu, Y.; Weng, Q.; Jiang, X.; Mitome, M.; Golberg, D.; et al. Three-dimensional strutted graphene grown by substrate-free sugar blowing for high-power-density supercapacitors. Nat. Commun. 2013, 4, 2905. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lian, J.; Hou, L.; Zhang, J.; Gou, H.; Xia, M.; Zhao, Y.; Strobel, T.A.; Tao, L.; Gao, F. Ultrahigh volumetric capacitance and cyclic stability of fluorine and nitrogen co-doped carbon microspheres. Nat. Commun. 2015, 6, 8503. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, Y.; Sun, L.; Li, J.; Liu, C.; Ren, W.; Li, F.; Gao, L.; Chen, J.; Liu, F.; et al. Elemental superdoping of graphene and carbon nanotubes. Nat. Commun. 2016, 7, 10921. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Chen, I.; Liu, F.; Yang, C.; Bi, H.; Xu, F.; Huang, F. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 2015, 350, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Hyeon, T. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Li, Y.; Lu, C.; Zhang, S.; Su, F.; Shen, W.; Zhou, P.; Ma, C. Nitrogen- and oxygen-enriched 3D hierarchical porous carbon fibers: Synthesis and superior supercapacity. J. Mater. Chem. A 2015, 3, 14817–14825. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, H.; Yu, H. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Titirici, M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; Del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef] [PubMed]
- Sumathi, S.; Bhatia, S.; Lee, K.T.; Mohamed, A.R. Optimization of microporous palm shell activated carbon production for flue gas desulphurization: Experimental and statistical studies. Bioresour. Technol. 2009, 100, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Preparation of oil palm (Elaeis) empty fruit bunch activated carbon by microwave-assisted KOH activation for the adsorption of methylene blue. Desalination 2011, 275, 302–305. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, T.; Li, N.; Wang, P.; Abulikemu, G. Modification of bamboo-based activated carbon using microwave radiation and its effects on the adsorption of methylene blue. Appl. Surf. Sci. 2010, 256, 3309–3315. [Google Scholar] [CrossRef]
- Gorka, J.; Zawislak, A.; Choma, J.; Jaroniec, M. KOH activation of mesoporous carbons obtained by soft-templating. Carbon 2008, 46, 1159–1161. [Google Scholar] [CrossRef]
- Singh, G.; Kim, I.Y.; Lakhi, K.S.; Srivastava, P.; Naidu, R.; Vinu, A. Single step synthesis of activated bio-carbons with a high surface area and their excellent CO2 adsorption capacity. Carbon 2017, 116, 448–455. [Google Scholar] [CrossRef]
- He, X.; Geng, Y.; Qiu, J.; Zheng, M.; Long, S.; Zhang, X. Effect of activation time on the properties of activated carbons prepared by microwave-assisted activation for electric double layer capacitors. Carbon 2010, 48, 1662–1669. [Google Scholar] [CrossRef]
- Girgis, B.S.; El-Hendawy, A. Porosity development in activated carbons obtained from date pits under chemical activation with phosphoric acid. Microporous Mesoporous Mater. 2002, 52, 105–117. [Google Scholar] [CrossRef]
- Wang, T.; Tan, S.; Liang, C. Preparation and characterization of activated carbon from wood via microwave-induced ZnCl2 activation. Carbon 2009, 47, 1880–1883. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, T.; Wang, P.; Guo, L. Preparation and characterization of activated carbon from bamboo by microwave-induced phosphoric acid activation. Ind. Crop Prod. 2010, 31, 233–238. [Google Scholar] [CrossRef]
- Mao, H.; Zhou, D.; Hashisho, Z.; Wang, S.; Chen, H.; Wang, H.H.; Lashaki, M.J. Microporous activated carbon from pinewood and wheat straw by microwave-assisted KOH treatment for the adsorption of toluene and acetone vapors. RSC Adv. 2015, 5, 36051–36058. [Google Scholar] [CrossRef]
- Yun, Y.S.; Cho, S.Y.; Shim, J.; Kim, B.H.; Chang, S.; Baek, S.J.; Huh, Y.S.; Tak, Y.; Park, Y.W.; Park, S.; et al. Microporous Carbon Nanoplates from Regenerated Silk Proteins for Supercapacitors. Adv. Mater. 2013, 25, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Talapaneni, S.N.; Lee, J.H.; Je, S.H.; Buyukcakir, O.; Kwon, T.; Polychronopoulou, K.; Choi, J.W.; Coskun, A. Chemical Blowing Approach for Ultramicroporous Carbon Nitride Frameworks and Their Applications in Gas and Energy Storage. Adv. Funct. Mater. 2017, 27, 1604658. [Google Scholar] [CrossRef]
- Lee, Y.J.; Talapaneni, S.N.; Coskun, A. Chemically Activated Covalent Triazine Frameworks with Enhanced Textural Properties for High Capacity Gas Storage. ACS Appl. Mater. Interfaces 2017, 9, 30679–30685. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Guo, Y.; Li, Y.; Yin, H.; Zhang, S.; Yang, B.; Dong, X. Graphitized hierarchical porous carbon nanospheres: Simultaneous activation/graphitization and superior supercapacitance performance. J. Mater. Chem. A 2015, 3, 9565–9577. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Kohandehghan, A.; Li, Z.; Cui, K.; Tan, X.; Stephenson, T.J.; King’Ondu, C.K.; Holt, C.M.B.; Olsen, B.C.; et al. Interconnected Carbon Nanosheets Derived from Hemp for Ultrafast Supercapacitors with High Energy. ACS Nano 2013, 7, 5131–5141. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, D.; Yan, T.; Wen, X.; Zhang, J.; Shi, L.; Zhong, Q. Three-dimensional macroporous graphene architectures as high performance electrodes for capacitive deionization. J. Mater. Chem. A 2013, 1, 11778–11789. [Google Scholar] [CrossRef]
- Rufford, T.E.; Hulicova-Jurcakova, D.; Khosla, K.; Zhu, Z.; Lu, G.Q. Microstructure and electrochemical double-layer capacitance of carbon electrodes prepared by zinc chloride activation of sugar cane bagasse. J. Power Sources 2010, 195, 912–918. [Google Scholar] [CrossRef]
- Xu, B.; Duan, H.; Chu, M.; Cao, G.; Yang, Y. Facile synthesis of nitrogen-doped porous carbon for supercapacitors. J. Mater. Chem. A 2013, 1, 4565–4570. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, C.; Yu, D.; Sun, L.; Yang, C.; Zhang, H.; Sun, Y.; Besenbacher, F.; Yu, M. One-step production of O-N-S co-doped three-dimensional hierarchical porous carbons for high-performance supercapacitors. Nano Energy 2018, 47, 547–555. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, X.; Shi, S.; Nguyen, T.; Chen, M. Synergistical enhancement of the electrochemical properties of lignin-based activated carbon using NH3 center dot H2O dielectric barrier discharge plasma. RSC Adv. 2017, 7, 7392–7400. [Google Scholar] [CrossRef]
- Wu, Z.; Parvez, K.; Winter, A.; Vieker, H.; Liu, X.; Han, S.; Turchanin, A.; Feng, X.; Muellen, K. Layer-by-Layer Assembled Heteroatom-Doped Graphene Films with Ultrahigh Volumetric Capacitance and Rate Capability for Micro-Supercapacitors. Adv. Mater. 2014, 26, 4552–4558. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, Z.; Liang, H.; Yao, W.; Yu, Z.; Yu, S. Flexible all-solid-state high-power supercapacitor fabricated with nitrogen-doped carbon nanofiber electrode material derived from bacterial cellulose. Energy Environ. Sci. 2013, 6, 3331–3338. [Google Scholar] [CrossRef]
- Li, X.; Wei, B. Supercapacitors based on nanostructured carbon. Nano Energy 2013, 2, 159–173. [Google Scholar] [CrossRef]
- Pietrzak, W.; Kawa-Rygielska, J. Simultaneous saccharification and ethanol fermentation of waste wheat-rye bread at very high solids loading: Effect of enzymatic liquefaction conditions. Fuel 2015, 147, 236–242. [Google Scholar] [CrossRef]
- Alabadi, A.; Razzaque, S.; Yang, Y.; Chen, S.; Tan, B. Highly porous activated carbon materials from carbonized biomass with high CO2 capturing capacity. Chem. Eng. J. 2015, 281, 606–612. [Google Scholar] [CrossRef]
- Ma, C.; Li, Y.; Shi, J.; Song, Y.; Liu, L. High-performance supercapacitor electrodes based on porous flexible carbon nanofiber paper treated by surface chemical etching. Chem. Eng. J. 2014, 249, 216–225. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Bandosz, T.J. Combined Effect of Nitrogen- and Oxygen-Containing Functional Groups of Microporous Activated Carbon on its Electrochemical Performance in Supercapacitors. Adv. Funct. Mater. 2009, 19, 438–447. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Z.; Peng, H.; Qin, Y.; Li, G.; Chen, K. Nitrogen- and oxygen-containing activated carbon nanotubes with improved capacitive properties. RSC Adv. 2014, 4, 5524–5530. [Google Scholar] [CrossRef]
- Yang, S.; Zhi, L.; Tang, K.; Feng, X.; Maier, J.; Muellen, K. Efficient Synthesis of Heteroatom (N or S)-Doped Graphene Based on Ultrathin Graphene Oxide-Porous Silica Sheets for Oxygen Reduction Reactions. Adv. Funct. Mater. 2012, 22, 3634–3640. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, X.; Mao, S.; Bo, Z.; Kim, H.; Cui, S.; Lu, G.; Feng, X.; Chen, J. Crumpled Nitrogen-Doped Graphene Nanosheets with Ultrahigh Pore Volume for High-Performance Supercapacitor. Adv. Mater. 2012, 24, 5610–5616. [Google Scholar] [CrossRef] [PubMed]
- Bichat, M.P.; Raymundo-Pinero, E.; Beguin, F. High voltage supercapacitor built with seaweed carbons in neutral aqueous electrolyte. Carbon 2010, 48, 4351–4361. [Google Scholar] [CrossRef]
- Frackowiak, E.; Beguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Cai, T.; Xing, W.; Liu, Z.; Zeng, J.; Xue, Q.; Qiao, S.; Yan, Z. Superhigh-rate capacitive performance of heteroatoms-doped double shell hollow carbon spheres. Carbon 2015, 86, 235–244. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Yang, K.; Lu, W.; Yu, J.; Gao, J.; Liao, G.; Qu, Y.; Wang, X.; Li, X.; et al. Hierarchical Porous Carbon Microspheres Derived from Biomass-Corncob as Ultra-High Performance Supercapacitor Electrode. Int. J. Electrochem. Sci. 2017, 12, 5604–5617. [Google Scholar] [CrossRef]
- Yang, S.; Wu, X.; Chen, C.; Hu, W.; Wang, X. Spherical α-Ni(OH)2 nanoarchitecture grown on graphene as advanced electrochemical pseudocapacitor materials. Chem. Commun. 2012, 48, 2773–2775. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, D.; Luo, C.; Fu, Q.; Pan, C. Highly porous graphitic biomass carbon as advanced electrode materials for supercapacitors. Green Chem. 2017, 19, 4132–4140. [Google Scholar] [CrossRef]

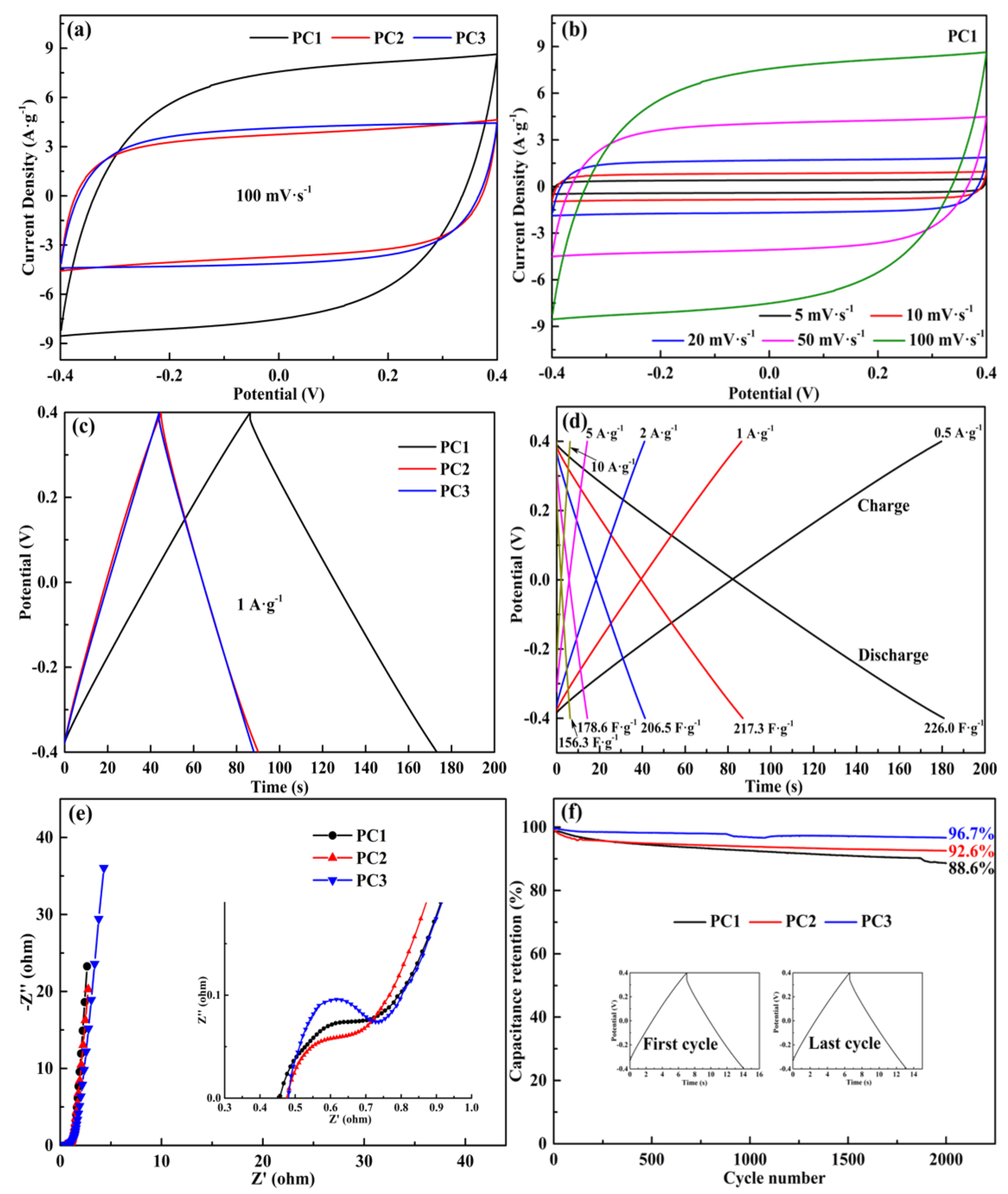
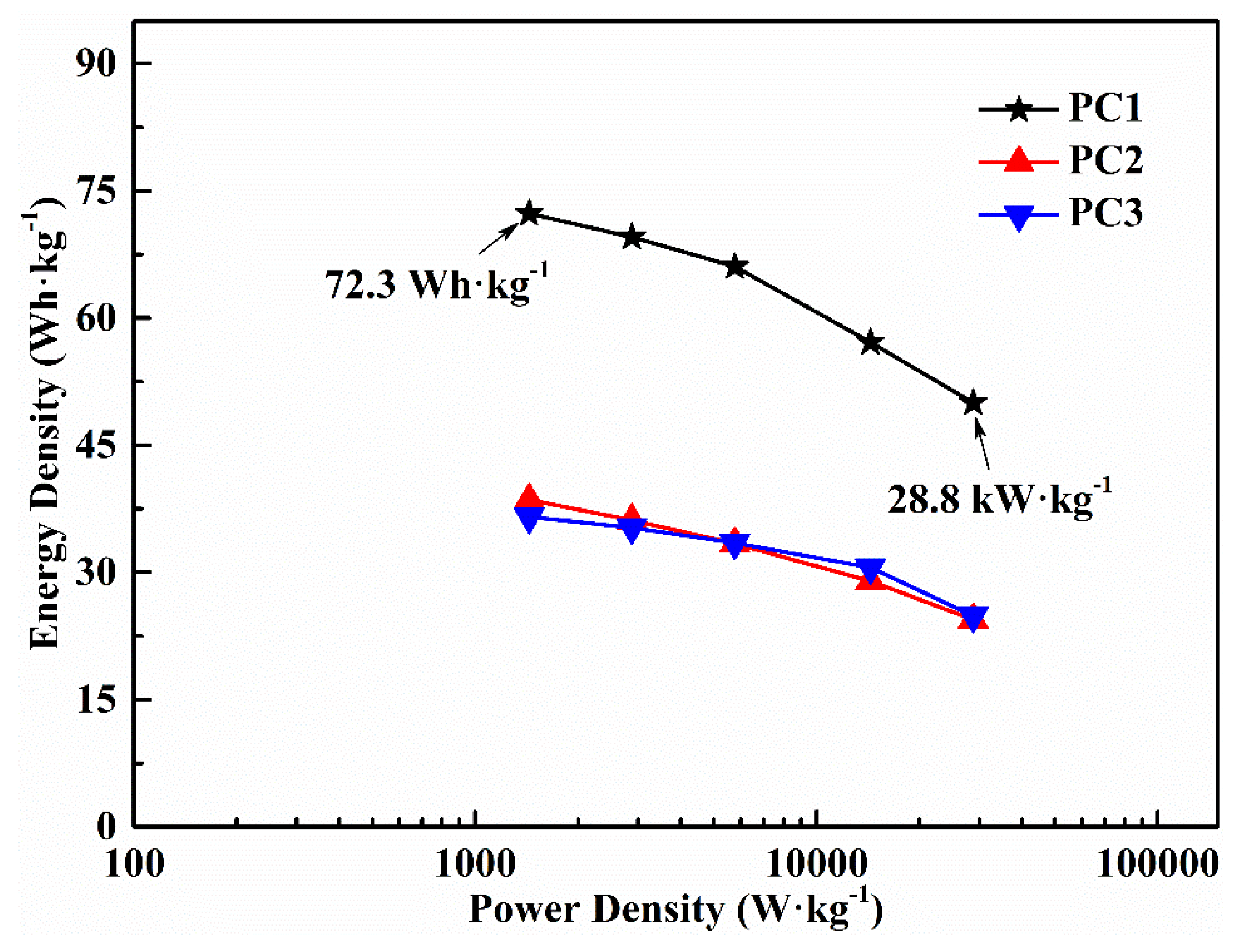
| Sample | SBET (m2·g−1) | Vta (Vmica) (cm3·g−1) | Mesoporosity (%) | Specific Capacitance b (F·g−1) | Capacitance Retention (%) |
|---|---|---|---|---|---|
| PC1 | 1573 | 0.815(0.573) | 29.69 | 226.0 | 88.6 |
| PC2 | 822 | 0.717(0.295) | 58.86 | 120.5 | 92.6 |
| PC3 | 1349 | 0.635(0.550) | 13.39 | 114.3 | 96.7 |
| Samples | ID/IG | Surface Atomic Elements (%) | Chemical Groups in C1s Region (%) | |||||
|---|---|---|---|---|---|---|---|---|
| C | N | O | -C-C- or -C-H | -C-O | -C=O | O-C=O | ||
| PC1 | 0.98 | 82.31 | 0.13 | 17.56 | 48.76 | 29.06 | 14.25 | 7.93 |
| PC2 | 1.02 | 80.54 | 0.15 | 19.31 | 49.51 | 31.99 | 10.11 | 8.39 |
| PC3 | 0.87 | 86.35 | 0.96 | 12.69 | 65.91 | 20.92 | 8.92 | 4.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Chen, W.; Hong, S.; Pan, M.; Jiang, M.; Wu, Q.; Mei, C. Fast Microwave Synthesis of Hierarchical Porous Carbons from Waste Palm Boosted by Activated Carbons for Supercapacitors. Nanomaterials 2019, 9, 405. https://doi.org/10.3390/nano9030405
Liu C, Chen W, Hong S, Pan M, Jiang M, Wu Q, Mei C. Fast Microwave Synthesis of Hierarchical Porous Carbons from Waste Palm Boosted by Activated Carbons for Supercapacitors. Nanomaterials. 2019; 9(3):405. https://doi.org/10.3390/nano9030405
Chicago/Turabian StyleLiu, Chaozheng, Weimin Chen, Shu Hong, Mingzhu Pan, Min Jiang, Qinglin Wu, and Changtong Mei. 2019. "Fast Microwave Synthesis of Hierarchical Porous Carbons from Waste Palm Boosted by Activated Carbons for Supercapacitors" Nanomaterials 9, no. 3: 405. https://doi.org/10.3390/nano9030405
APA StyleLiu, C., Chen, W., Hong, S., Pan, M., Jiang, M., Wu, Q., & Mei, C. (2019). Fast Microwave Synthesis of Hierarchical Porous Carbons from Waste Palm Boosted by Activated Carbons for Supercapacitors. Nanomaterials, 9(3), 405. https://doi.org/10.3390/nano9030405







