Utilizing Infrared Spectroscopy to Analyze the Interfacial Structures of Ionic Liquids/Al2O3 and Ionic Liquids/Mica Mixtures under High Pressures
Abstract
:1. Introduction
2. Materials and Methods
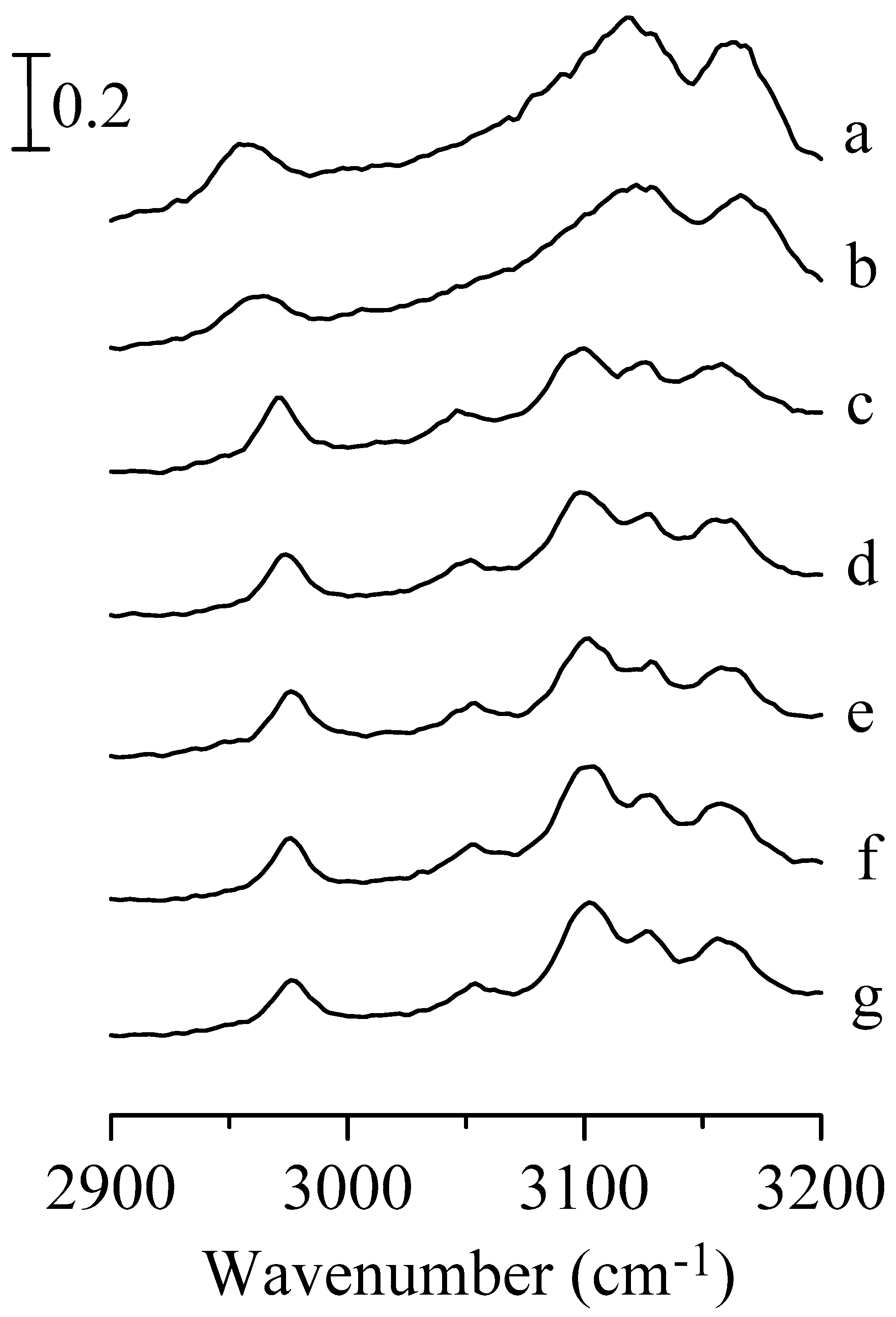
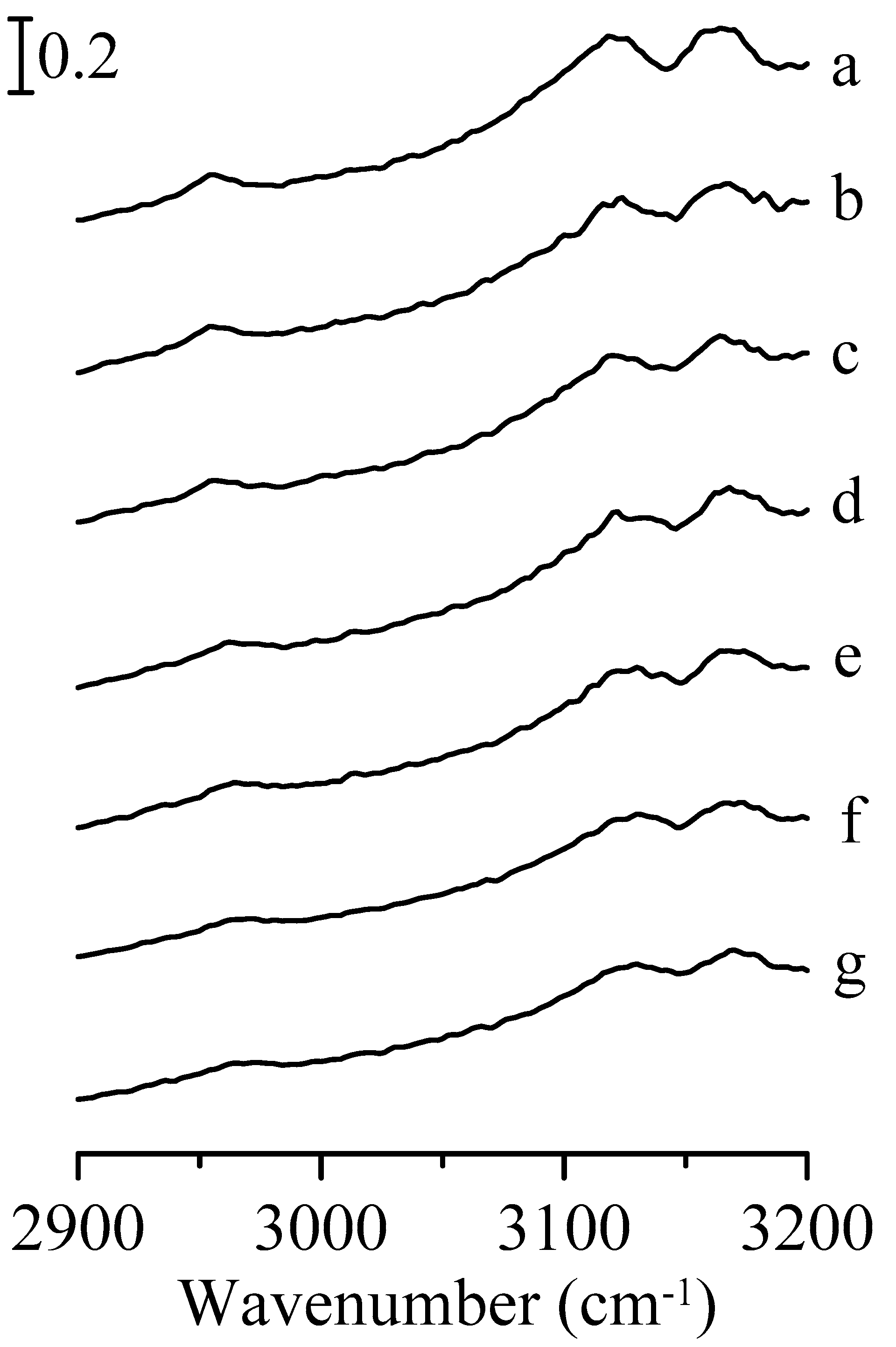
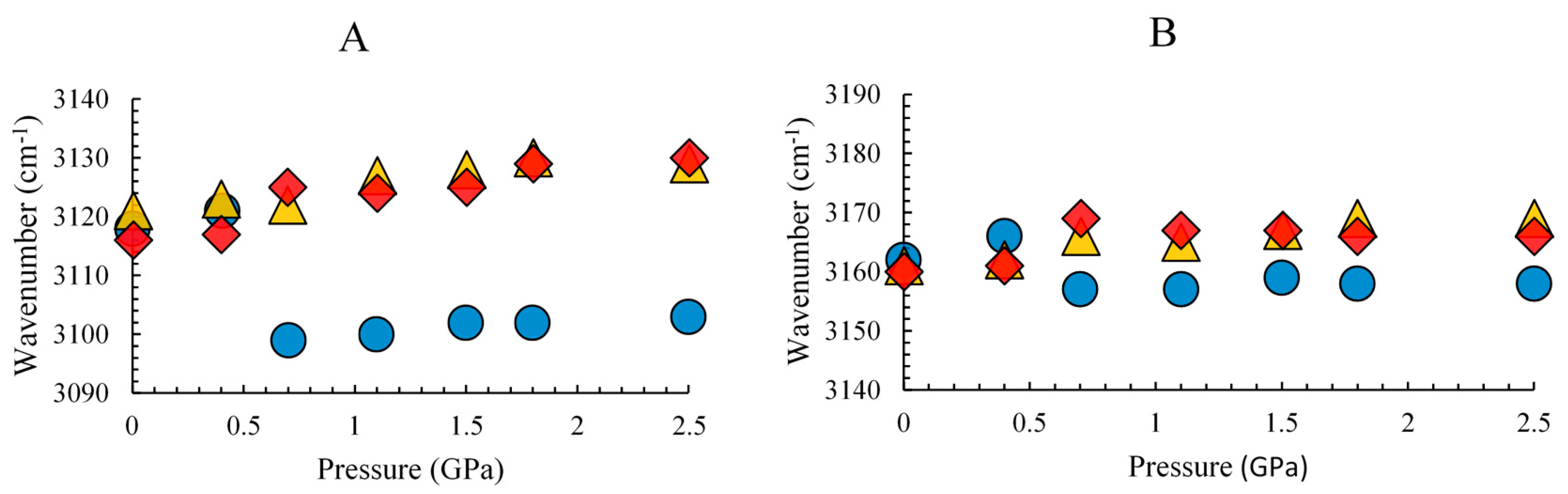
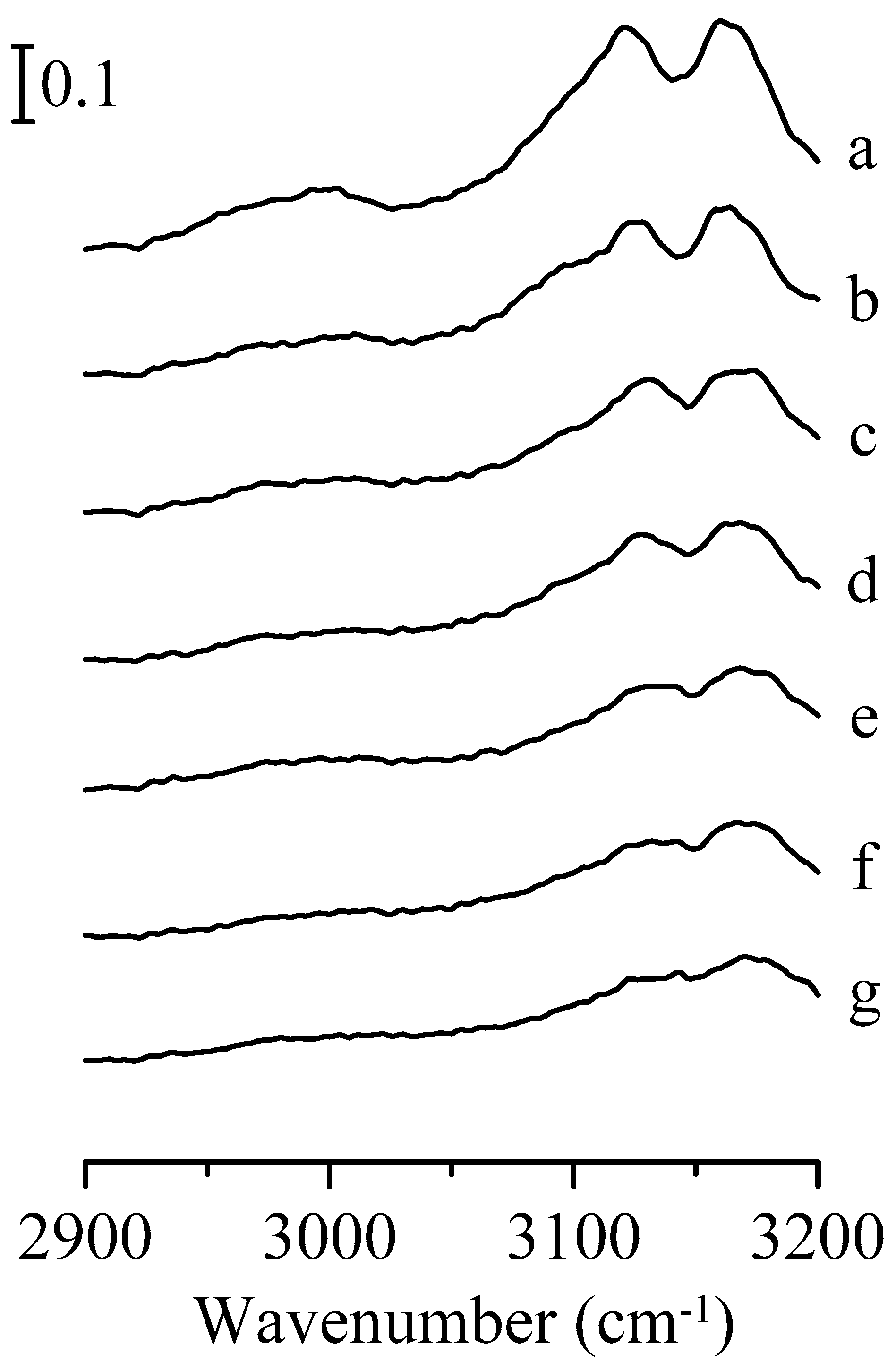
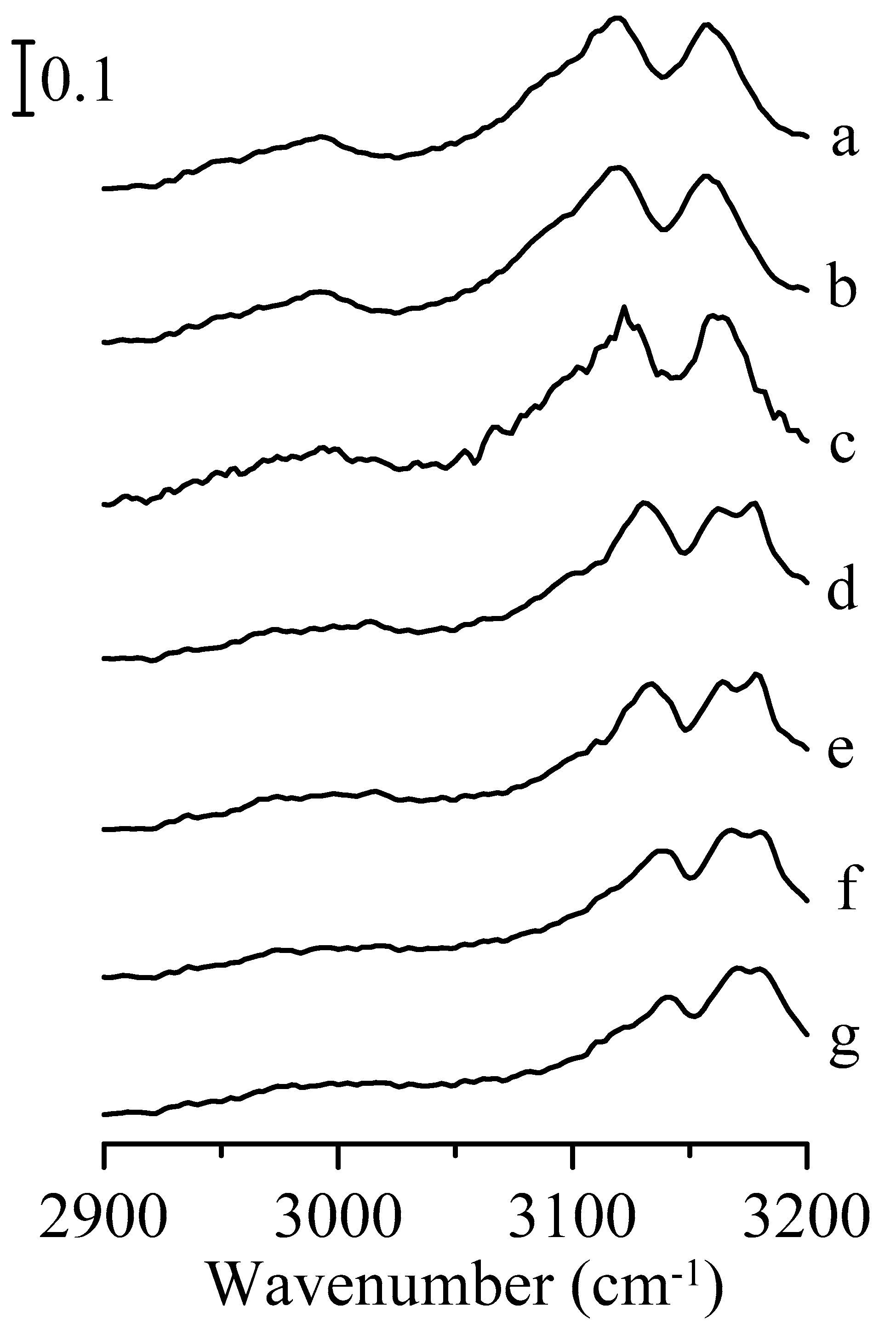
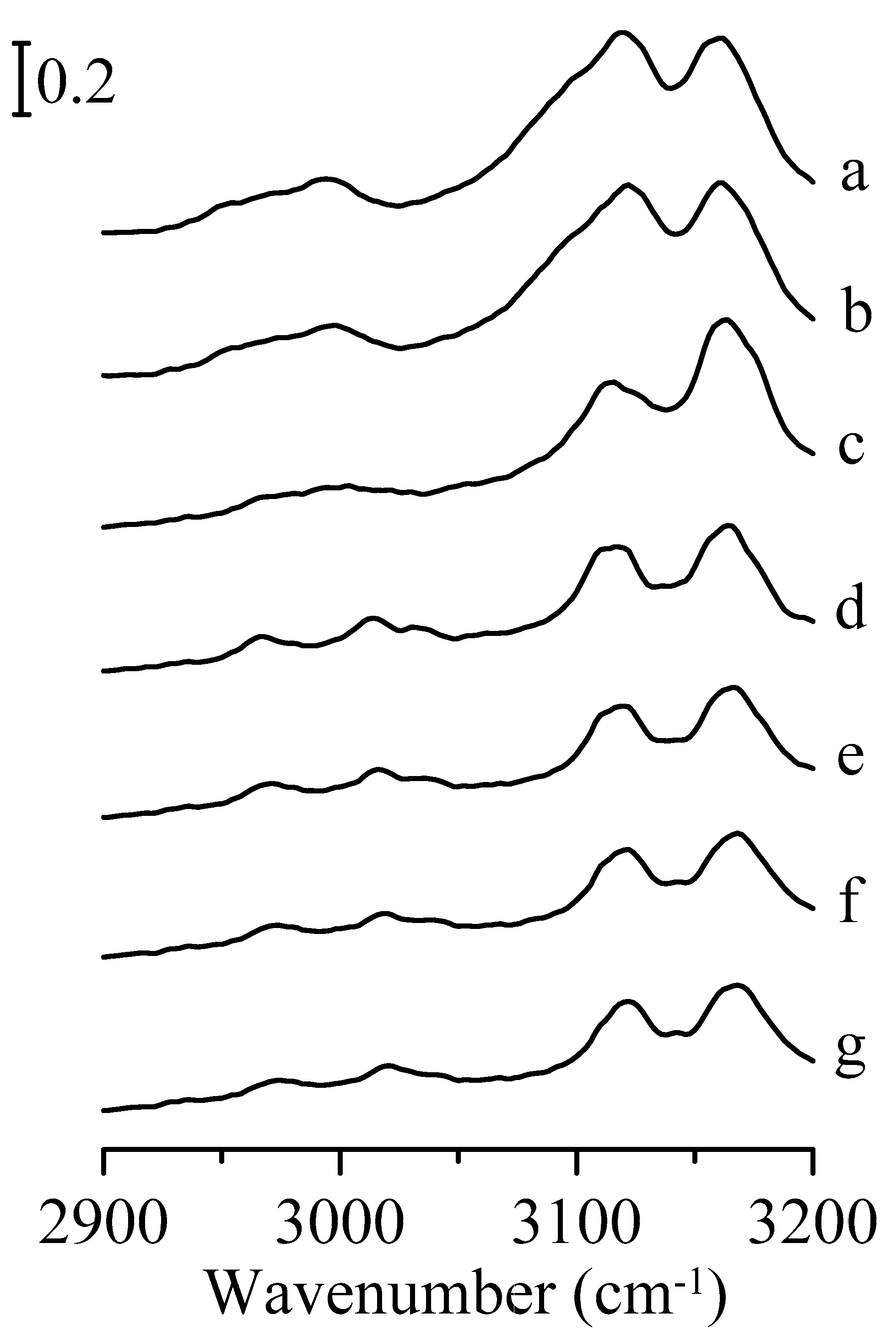
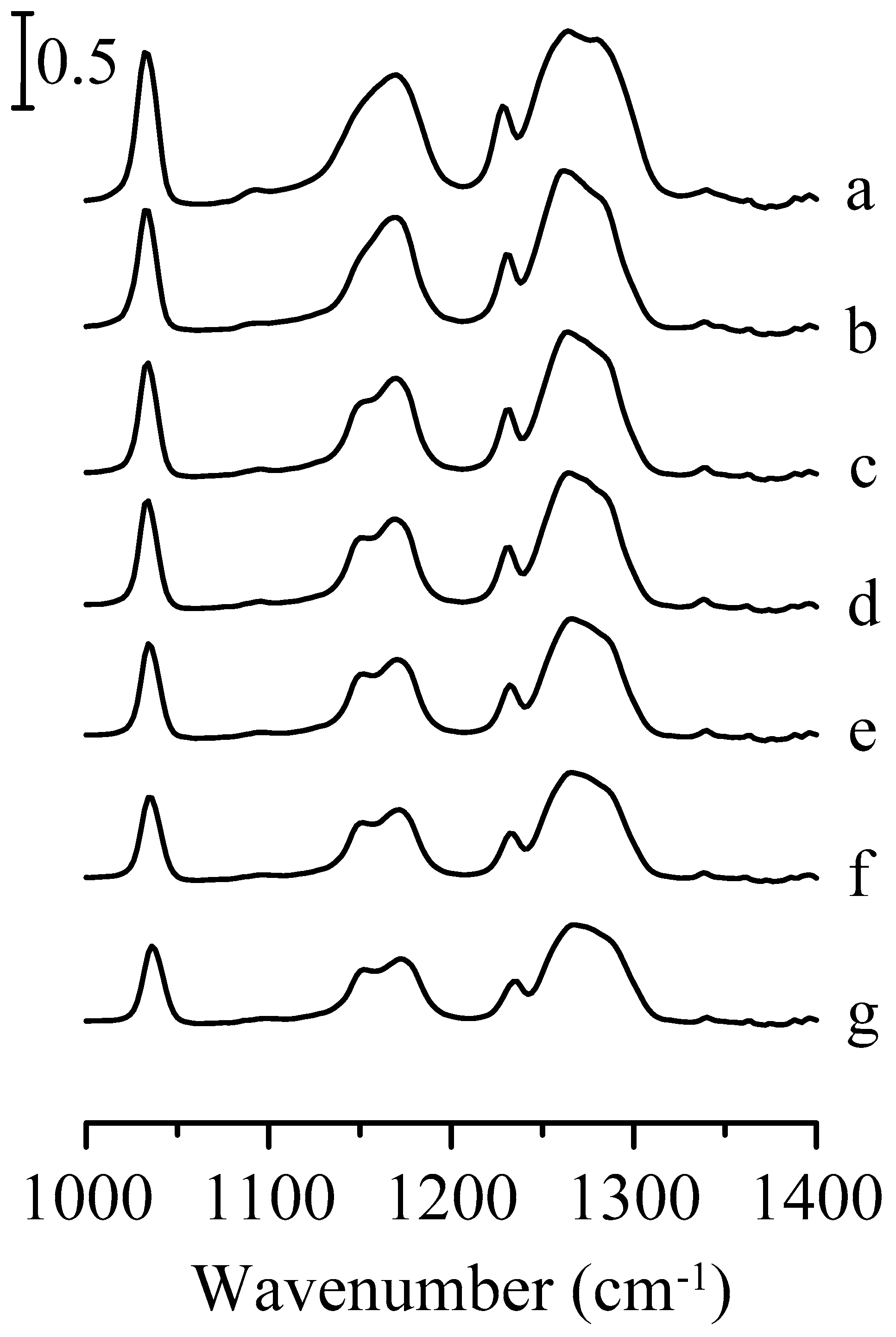
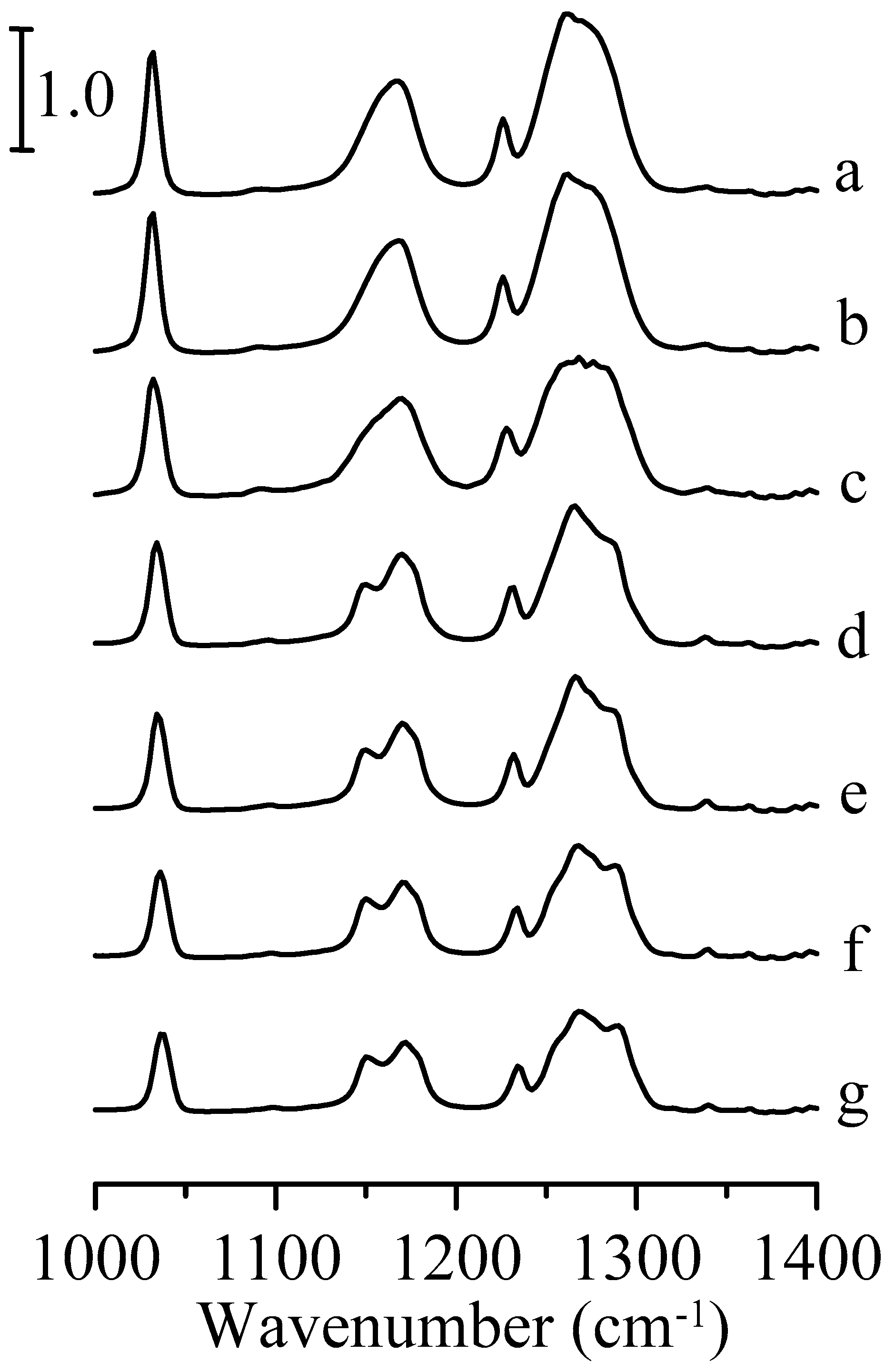
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dos Santos Barbosa, A.; dos Santos Barbosa, A.; Barbosa, T.L.A.; Rodrigues, M.G. Synthesis of Zeolite Membrane (Nay/Alumina): Effect of Precursor of Ceramic Support and Its Application in the Process of Oil–Water Separation. Sep. Purif. Technol. 2018, 200, 141–154. [Google Scholar] [CrossRef]
- Okon, E.; Shehu, H.; Gobina, E. Evaluation of the Performance of A-Alumina Nano-Porous Ceramic Composite Membrane for Esterification Applications in Petroleum Refinery. Catal. Today 2018, 310, 146–156. [Google Scholar] [CrossRef]
- Garbarino, G.; Vijayakumar, R.P.P.; Riani, P.; Finocchio, E.; Busca, G. Ethanol and Diethyl Ether Catalytic Conversion over Commercial Alumina and Lanthanum-Doped Alumina: Reaction Paths, Catalyst Structure and Coking. Appl. Catal. B Environ. 2018, 236, 490–500. [Google Scholar] [CrossRef]
- Tan, Y.; Abdullah, A.; Hameed, B. Catalytic Fast Pyrolysis of Durian Rind Using Silica-Alumina Catalyst: Effects of Pyrolysis Parameters. Bioresour. Technol. 2018, 264, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Gergely, A.; Pászti, Z.; Bertóti, I.; Török, T.; Mihály, J.; Kálmán, E. Novel Zinc-Rich Epoxy Paint Coatings with Hydrated Alumina and Carbon Nanotubes Supported Polypyrrole for Corrosion Protection of Low Carbon Steel: Part Ii: Corrosion Prevention Behavior of the Hybrid Paint Coatings. Mater. Corros. 2013, 64, 1091–1103. [Google Scholar] [CrossRef]
- Gergely, A.; Bertóti, I.; Török, T.; Pfeifer, É.; Kálmán, E. Corrosion Protection with Zinc-Rich Epoxy Paint Coatings Embedded with Various Amounts of Highly Dispersed Polypyrrole-Deposited Alumina Monohydrate Particles. Prog. Org. Coat. 2013, 76, 17–32. [Google Scholar] [CrossRef]
- Saptiama, I.; Kaneti, Y.V.; Suzuki, Y.; Suzuki, Y.; Tsuchiya, K.; Sakae, T.; Takai, K.; Fukumitsu, N.; Alothman, Z.A.; Hossain, M.S.A. Mesoporous Alumina as an Effective Adsorbent for Molybdenum (Mo) toward Instant Production of Radioisotope for Medical Use. Bull. Chem. Soc. Jpn. 2017, 90, 1174–1179. [Google Scholar] [CrossRef]
- Su, Y.; Hu, L.; Fan, H.; Song, J.; Zhang, Y. Surface Engineering Design of Alumina/Molybdenum Fibrous Monolithic Ceramic to Achieve Continuous Lubrication from Room Temperature to 800 C. Tribol. Lett. 2017, 65, 47. [Google Scholar] [CrossRef]
- Jani, A.M.M.; Kempson, I.M.; Losic, D.; Voelcker, N.H. Dressing in Layers: Layering Surface Functionalities in Nanoporous Aluminum Oxide Membranes. Angew. Chem. Int. Ed. Engl. 2010, 49, 7933–7937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Q.; Li, G. Fabrication of Novel Nanoporous Array Anodic Alumina Solid-Phase Microextraction Fiber Coating and Its Potential Application for Headspace Sampling of Biological Volatile Organic Compounds. Anal. Chim. Acta 2012, 727, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Claussen, J.C.; Hengenius, J.B.; Wickner, M.M.; Fisher, T.S.; Umulis, D.M.; Porterfield, D.M. Effects of Carbon Nanotube-Tethered Nanosphere Density on Amperometric Biosensing: Simulation and Experiment. J. Phys. Chem. C 2011, 115, 20896–20904. [Google Scholar] [CrossRef]
- Kumeria, T.; Rahman, M.M.; Santos, A.; Ferré-Borrull, J.; Marsal, L.F.; Losic, D. Structural and Optical Nanoengineering of Nanoporous Anodic Alumina Rugate Filters for Real-Time and Label-Free Biosensing Applications. Anal. Chem. 2014, 86, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Santos, A. Nanoporous Anodic Alumina Photonic Crystals: Fundamentals, Developments and Perspectives. J. Mater. Chem. C 2017, 5, 5581–5599. [Google Scholar] [CrossRef]
- Kumeria, T.; Rahman, M.M.; Santos, A.; Ferreé-Borrull, J.; Marsal, L.F.; Losic, D. Nanoporous Anodic Alumina Rugate Filters for Sensing of Ionic Mercury: Toward Environmental Point-of-Analysis Systems. ACS Appl. Mater. Interfaces 2014, 6, 12971–12978. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Patra, N.; Cingolani, R. Use of Ionic Liquid in Fabrication, Characterization, and Processing of Anodic Porous Alumina. Nanoscale Res. Lett. 2009, 4, 865. [Google Scholar] [CrossRef] [PubMed]
- Griffin, L.R.; Browning, K.L.; Clarke, S.M.; Smith, A.M.; Perkin, S.; Skoda, M.; Norman, S.E. Direct Measurements of Ionic Liquid Layering at a Single Mica–Liquid Interface and in Nano-Films between Two Mica–Liquid Interfaces. Phys.Chem. Chem. Phys. 2017, 19, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Jurado, L.A.; Kim, H.; Rossi, A.; Arcifa, A.; Schuh, J.K.; Spencer, N.D.; Leal, C.; Ewoldt, R.H.; Espinosa-Marzal, R.M. Effect of the Environmental Humidity on the Bulk, Interfacial and Nanoconfined Properties of an Ionic Liquid. Phys. Chem. Chem. Phys. 2016, 18, 22719–22730. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Kozbial, A.; Li, L. What Causes Extended Layering of Ionic Liquids on the Mica Surface? Chem. Sci. 2015, 6, 3478–3482. [Google Scholar] [CrossRef] [PubMed]
- Perkin, S.; Crowhurst, L.; Niedermeyer, H.; Welton, T.; Smith, A.M.; Gosvami, N.N. Self-Assembly in the Electrical Double Layer of Ionic Liquids. ChemComm 2011, 47, 6572–6574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Rouha, M.; Feng, G.; Lee, S.S.; Docherty, H.; Fenter, P.; Cummings, P.T.; Fulvio, P.F.; Dai, S.; McDonough, J. Nanoscale Perturbations of Room Temperature Ionic Liquid Structure at Charged and Uncharged Interfaces. ACS Nano 2012, 6, 9818–9827. [Google Scholar] [CrossRef] [PubMed]
- Nanbu, N.; Sasaki, Y.; Kitamura, F. In situ FT-IR Spectroscopic Observation of a Room-Temperature Molten Salt|Gold Electrode Interphase. Electrochem. Commun. 2003, 5, 383–387. [Google Scholar] [CrossRef]
- Zhou, W.; Inoue, S.; Iwahashi, T.; Kanai, K.; Seki, K.; Miyamae, T.; Kim, D.; Katayama, Y.; Ouchi, Y. Double Layer Strucutre and Adsorption/Desorption Hysteresis of Neat Ionic Liquid on Pt Electrode Surface- an in-situ IR-Visible Sum-Frequency Generation Spectroscopic Study. Electrochem. Commun. 2010, 12, 672–675. [Google Scholar] [CrossRef]
- Yuan, Y.-X.; Niu, T.-C.; Xu, M.-M.; Yao, J.-L.; Gu, R.-A. Probing the Adsorption of Methylimidazole at Ionic Liquids/Cu Electrode Interface by Surface-Enhanced Raman Scattering Spectroscopy. J. Raman Spectrosc. 2010, 41, 516–523. [Google Scholar] [CrossRef]
- Motobayashi, K.; Minami, K.; Nishi, N.; Sakka, T.; Osawa, M. Hysteresis of Potential-Dependent Changes in Ion Density and Structure of an Ionic Liquid on a Gold Electrode: In Situ Observation by Surface-Enhanced Infrared Absorption Spectroscopy. J. Phys. Chem. Lett. 2013, 4, 3110–3114. [Google Scholar] [CrossRef]
- Motobayashi, K.; Osawa, M. Recent Advances in Spectroscopic Investigations on Ionic Liquid/Electrode Interfaces. Curr. Opin. Electrochem. 2018, 8, 147–155. [Google Scholar] [CrossRef]
- Uysal, A.; Zhou, H.; Feng, G.; Lee, A.A.; Li, S.; Cummings, P.T.; Fulvio, P.F.; Dai, S.; McDonough, J.K.; Gogotsi, Y.; et al. Interfacial Ionic Liquid: Connecting Static and Dynamic Structures. J. Phys. Condens. Matter 2015, 27, 032101. [Google Scholar] [CrossRef] [PubMed]
- Motobayashi, K.; Nishi, N.; Inoue, Y.; Minami, K.; Sakka, T.; Osawa, M. Potential-Induced Restructuring Dynamics of Ionic Liquids on a Gold Electrode: Steric Effect of Constituent Ions Studied by Surface-Enhanced Infrared Absorption Spectroscopy. J. Electroanal. Chem. 2017, 800, 126–133. [Google Scholar] [CrossRef]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis; Wiley-VCH: Weinheim, Germany, 2008; pp. 1–367. [Google Scholar]
- Zhang, S.; Zhang, J.; Zhang, Y.; Deng, Y. Nanoconfined Ionic Liquids. Chem. Rev. 2016, 117, 6755–6833. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Hung, T.C.; Chang, S.C.; Jiang, J.C.; Lin, S.H. Interactions of Silica Nanoparticles and Ionic Liquids Probed by High Pressure Vibrational Spectroscopy. J. Phys. Chem. C 2011, 115, 11962–11967. [Google Scholar] [CrossRef]
- Chang, H.-C.; Jiang, J.-C.; Tsai, W.-C.; Chen, G.-C.; Lin, S.H. Hydrogen Bond Stabilization in 1, 3-Dimethylimidazolium Methyl Sulfate and 1-Butyl-3-Methylimidazolium Hexafluorophosphate Probed by High Pressure: The Role of Charge-Enhanced C−H⋅⋅⋅O Interactions in the Room-Temperature Ionic Liquid. J. Phys. Chem. B 2006, 110, 3302–3307. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, M.V.; Lynden-Bell, R. Probing the Neutral Graphene–Ionic Liquid Interface: Insights from Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2012, 14, 2552–2556. [Google Scholar] [CrossRef] [PubMed]
- Ravula, S.; Baker, S.N.; Kamath, G.; Baker, G.A. Ionic Liquid-Assisted Exfoliation and Dispersion: Stripping Graphene and Its Two-Dimensional Layered Inorganic Counterparts of Their Inhibitions. Nanoscale 2015, 7, 4338–4353. [Google Scholar] [CrossRef]
- Chang, H.-C.; Wang, T.-H.; Burba, C.M. Probing Structures of Interfacial 1-Butyl-3-Methylimidazolium Trifluoromethanesulfonate Ionic Liquid on Nano-Aluminum Oxide Surfaces Using High-Pressure Infrared Spectroscopy. Appl. Sci. 2017, 7, 855. [Google Scholar] [CrossRef]
- Mele, A.; Tran, C.D.; De Paoli Lacerda, S.H. The Structure of a Room-Temperature Ionic Liquid with and without Trace Amounts of Water: The Role of C–H⋅⋅⋅O and C–H⋅⋅⋅F Interactions in 1-N-Butyl-3-Methylimidazolium Tetrafluoroborate. Angew. Chem. Int. Ed. Engl. 2003, 115, 4500–4502. [Google Scholar] [CrossRef]
- Chang, H.-C.; Hsu, D.-T. Interactions of Ionic Liquids and Surfaces of Graphene Related Nanoparticles under High Pressures. Phys. Chem. Chem. Phys. 2017, 19, 12269–12275. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Zhang, R.-L.; Hsu, D.-T. The Effect of Pressure on Cation–Cellulose Interactions in Cellulose/Ionic Liquid Mixtures. Phys. Chem. Chem. Phys. 2015, 17, 27573–27578. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Jiang, J.-C.; Kuo, M.-H.; Hsu, D.-T.; Lin, S.H. Pressure-Enhanced Surface Interactions between Nano-Tio2 and Ionic Liquid Mixtures Probed by High Pressure Ir Spectroscopy. Phys. Chem. Chem. Phys. 2015, 17, 21143–21148. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Chang, S.-C.; Hung, T.-C.; Jiang, J.-C.; Kuo, J.-L.; Lin, S.H. A High-Pressure Study of the Effects of Tio2 Nanoparticles on the Structural Organization of Ionic Liquids. J. Phys. Chem. C 2011, 115, 23778–23783. [Google Scholar] [CrossRef]
- Jonas, J.; Jonas, A. High-Pressure NMR Spectroscopy of Proteins and Membranes. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 287–318. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Moffatt, D. The Uncoupled OH or OD Stretch in Water as an Internal Pressure Gauge for High-Pressure Infrared Spectroscopy of Aqueous Systems. Appl. Spectrosc. 1987, 41, 1070–1072. [Google Scholar] [CrossRef]
- Wong, P.; Moffatt, D.; Baudais, F. Crystalline Quartz as an Internal Pressure Calibrant for High-Pressure Infrared Spectroscopy. Appl. Spectrosc. 1985, 39, 733–735. [Google Scholar] [CrossRef]
- Gu, Y.; Kar, T.; Scheiner, S. Fundamental Properties of the C−H⋅⋅⋅O Interaction: Is It a True Hydrogen Bond? J. Am. Chem. Soc. 1999, 121, 9411–9422. [Google Scholar] [CrossRef]
- Masunov, A.; Dannenberg, J.; Contreras, R.H. C–H Bond-Shortening Upon Hydrogen Bond Formation: Influence of an Electric Field. J. Phys. Chem. A 2001, 105, 4737–4740. [Google Scholar] [CrossRef]
- Terraschke, H.; Olchowka, J.; Geringer, E.; Rodrigues, A.V.; Wickleder, C. Facile Ionic Liquid-Assisted Strategy for Direct Precipitation of Eu2+-Activated Nanophosphors under Ambient Conditions. Small 2018, 14, 1703707. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.G.; Yang, M.H.; Jung, S.C.; Lee, K.G.; Kim, J.-G.; Park, H.S.; Park, T.J.; Lee, S.B.; Han, Y.-K.; Huh, Y.S. Enhanced Pseudocapacitance of Ionic Liquid/Cobalt Hydroxide Nanohybrids. ACS Nano 2013, 7, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Koßmann, S.; Thar, J.; Kirchner, B.; Hunt, P.A.; Welton, T. Cooperativity in Ionic Liquids. J. Chem. Phys. 2006, 124, 174506. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Jiang, J.-C.; Lin, S.H.; Weng, N.-H.; Chao, M.-C. Evidence for C−H⋅⋅⋅O Interaction of Acetone and Deuterium Oxide Probed by High-Pressure. J. Chem. Phys. 2001, 115, 3215–3218. [Google Scholar] [CrossRef]
- Köddermann, T.; Wertz, C.; Heintz, A.; Ludwig, R. Ion-Pair Formation in the Ionic Liquid 1-Ethyl-3-Methylimidazolium Bis (Triflyl) Imide as a Function of Temperature and Concentration. ChemPhysChem 2006, 7, 1944–1949. [Google Scholar] [CrossRef] [PubMed]
- Grondin, J.; Lassegues, J.-C.; Cavagnat, D.; Buffeteau, T.; Johansson, P.; Holomb, R. Revisited Vibrational Assignments of Imidazolium-Based Ionic Liquids. J. Raman Spectrosc. 2011, 42, 733–743. [Google Scholar] [CrossRef]
- Wulf, A.; Fumino, K.; Ludwig, R. Comment on “New Interpretation of the CH stretching Vibrations in Imidazolium-Based Ionic Liquids”. J. Phys. Chem. A 2010, 114, 685–686. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Chatzipapadopoulos, S.; Kerle, D.; Friedriszik, F.; Lutgens, M.; Lochbrunner, S.; Kuhn, O.; Ludwig, R. Hydrogen Bonding in Ionic Liquids Probed by Linear and Nonlinear Vibrational Spectroscopy. New J. Phys. 2012, 14, 105026. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-H.; Chang, H.-C.; Fu, Y.-P. Utilizing Infrared Spectroscopy to Analyze the Interfacial Structures of Ionic Liquids/Al2O3 and Ionic Liquids/Mica Mixtures under High Pressures. Nanomaterials 2019, 9, 373. https://doi.org/10.3390/nano9030373
Chang Y-H, Chang H-C, Fu Y-P. Utilizing Infrared Spectroscopy to Analyze the Interfacial Structures of Ionic Liquids/Al2O3 and Ionic Liquids/Mica Mixtures under High Pressures. Nanomaterials. 2019; 9(3):373. https://doi.org/10.3390/nano9030373
Chicago/Turabian StyleChang, Yen-Hsu, Hai-Chou Chang, and Yen-Pei Fu. 2019. "Utilizing Infrared Spectroscopy to Analyze the Interfacial Structures of Ionic Liquids/Al2O3 and Ionic Liquids/Mica Mixtures under High Pressures" Nanomaterials 9, no. 3: 373. https://doi.org/10.3390/nano9030373
APA StyleChang, Y.-H., Chang, H.-C., & Fu, Y.-P. (2019). Utilizing Infrared Spectroscopy to Analyze the Interfacial Structures of Ionic Liquids/Al2O3 and Ionic Liquids/Mica Mixtures under High Pressures. Nanomaterials, 9(3), 373. https://doi.org/10.3390/nano9030373





