Electrochemically Stable Cobalt–Zinc Mixed Oxide/Hydroxide Hierarchical Porous Film Electrode for High-Performance Asymmetric Supercapacitor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of CoZn-MOF Supported on Nickel Foam
2.3. Preparation of CoZn-MOH Supported on Nickel Foam
2.4. Preparation of AC
2.5. Fabrication of Supercapacitor Electrodes
2.6. Characterization
2.7. Electrochemical Analysis
3. Results and Discussion
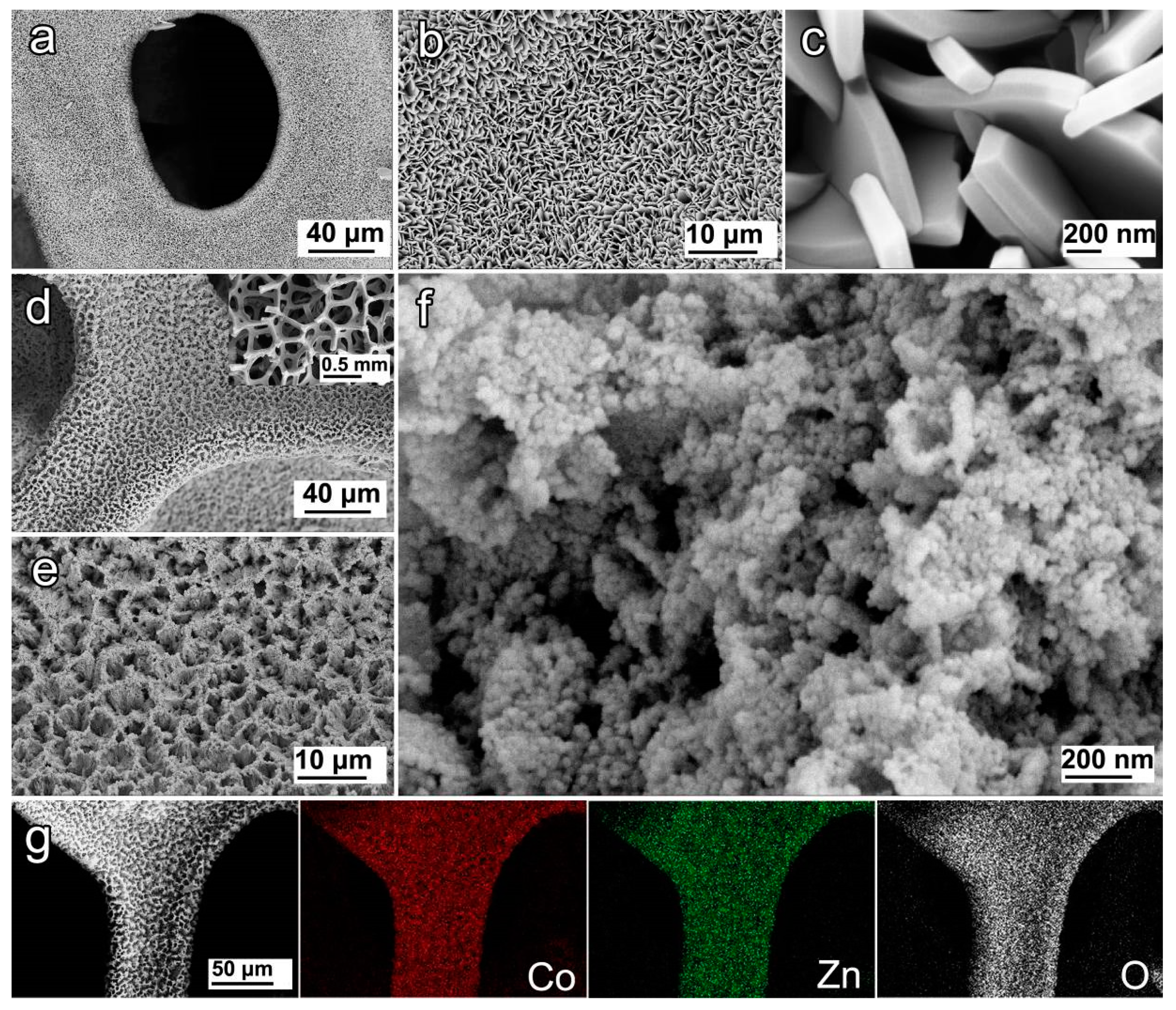
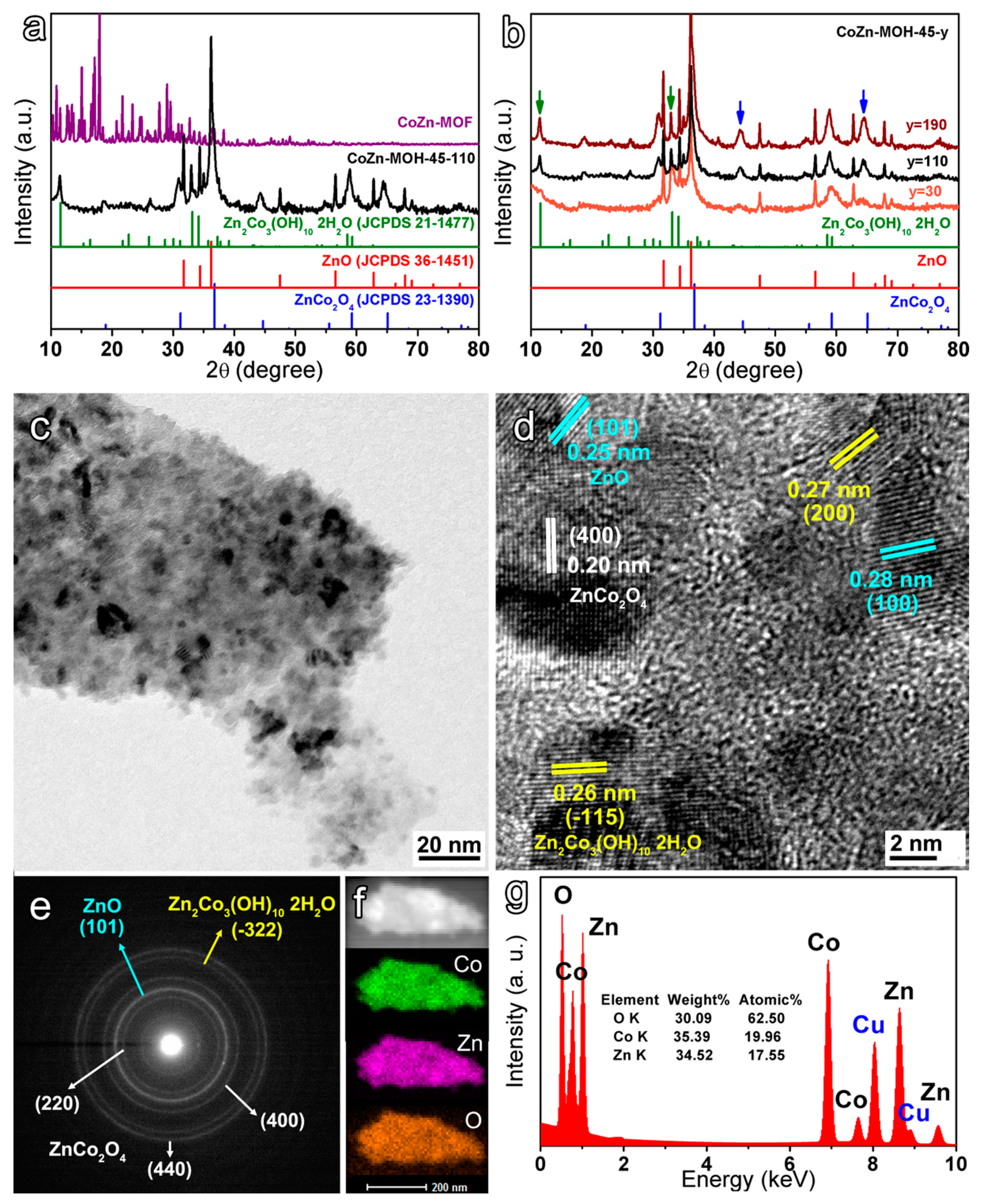
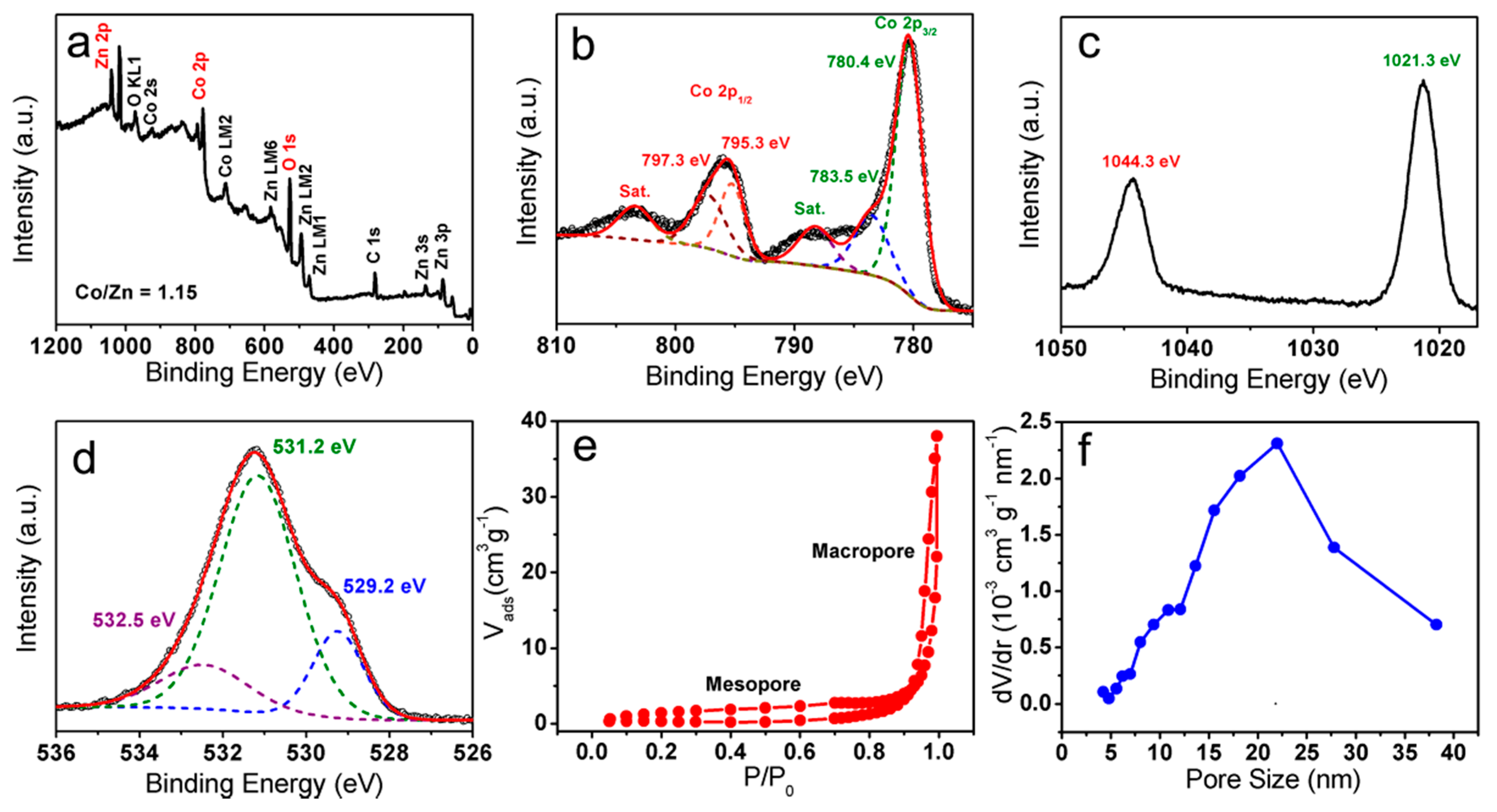
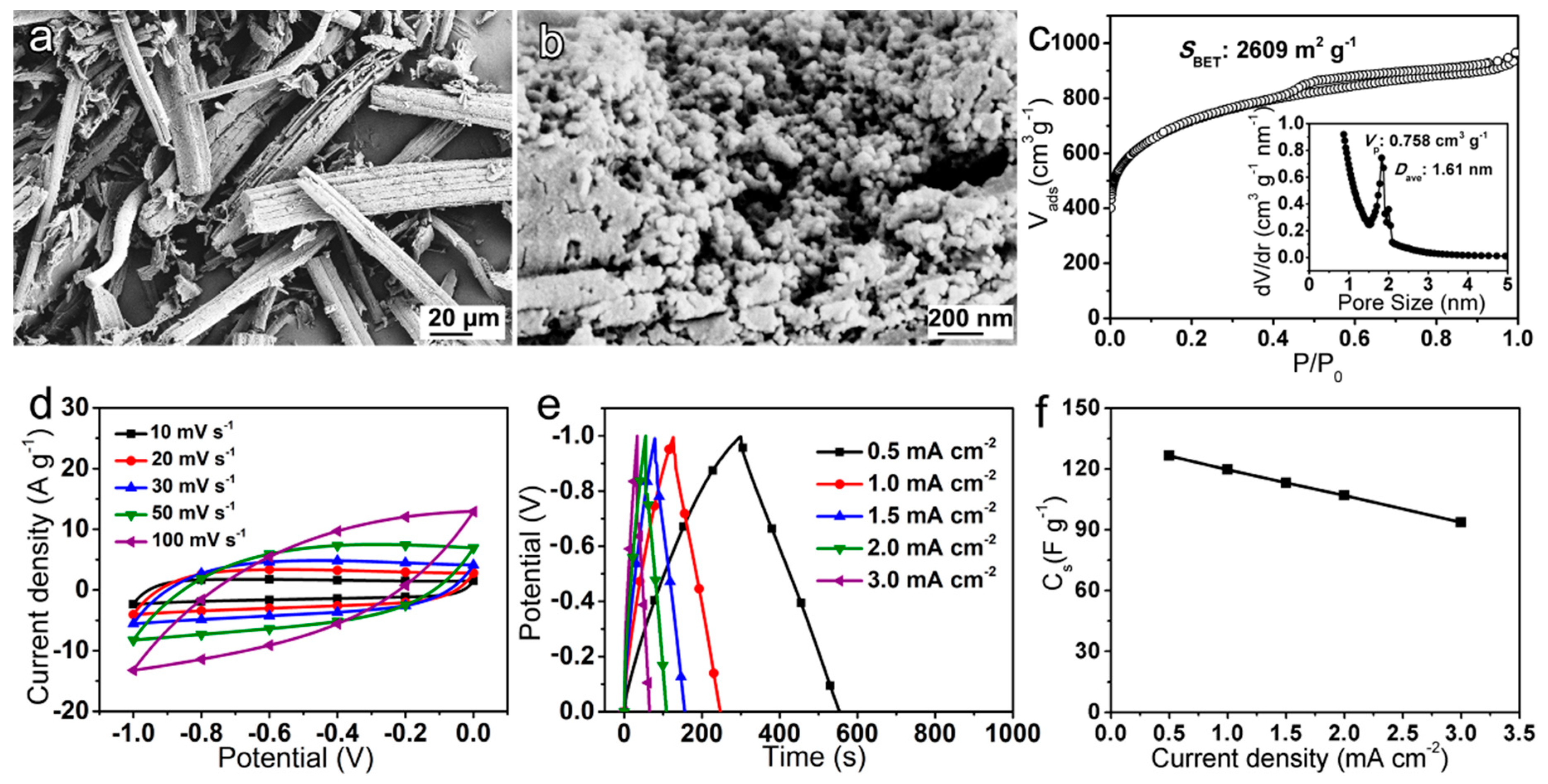
3.1. Morphology and Compositions
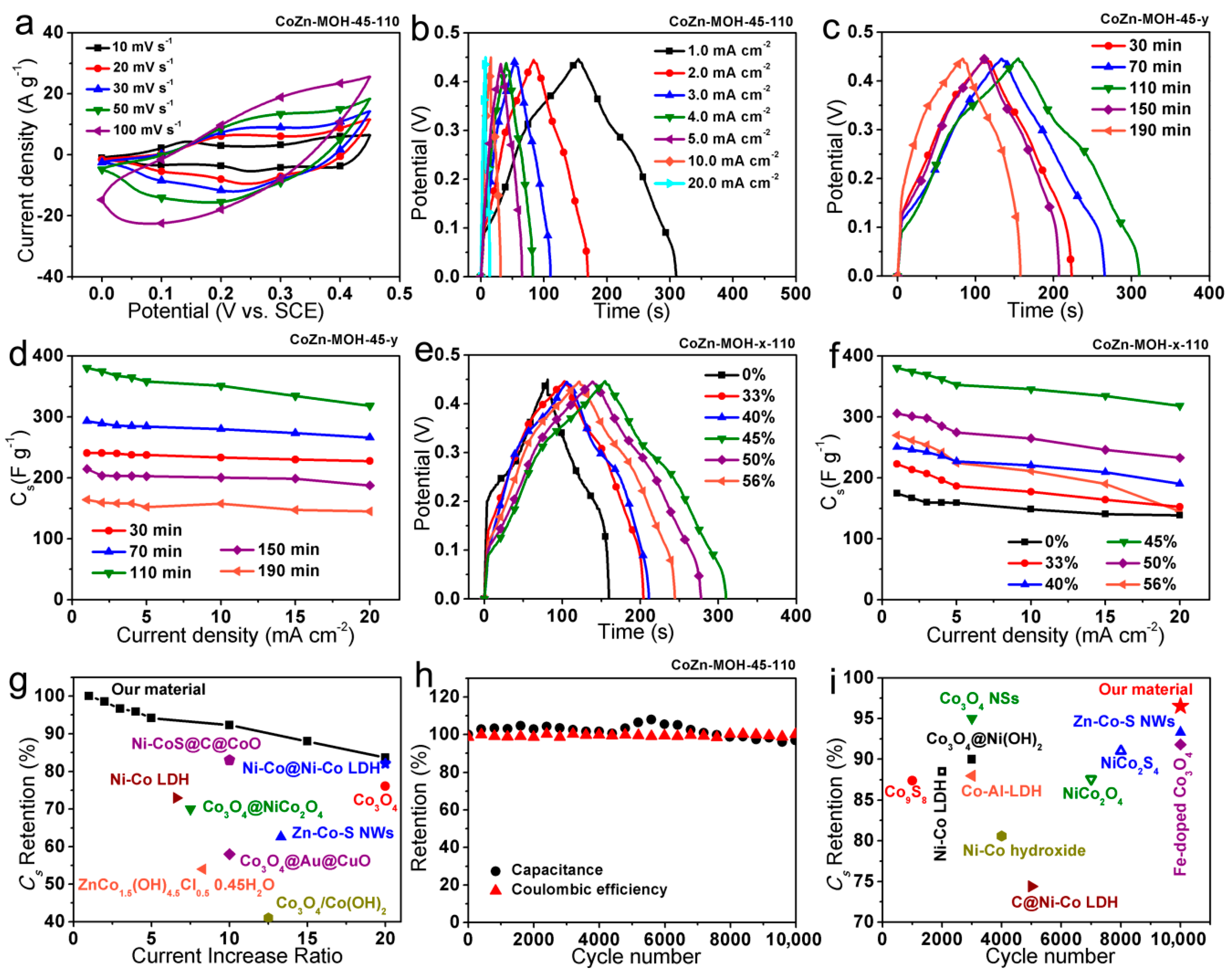
3.2. Electrochemical Properties of Electrodes
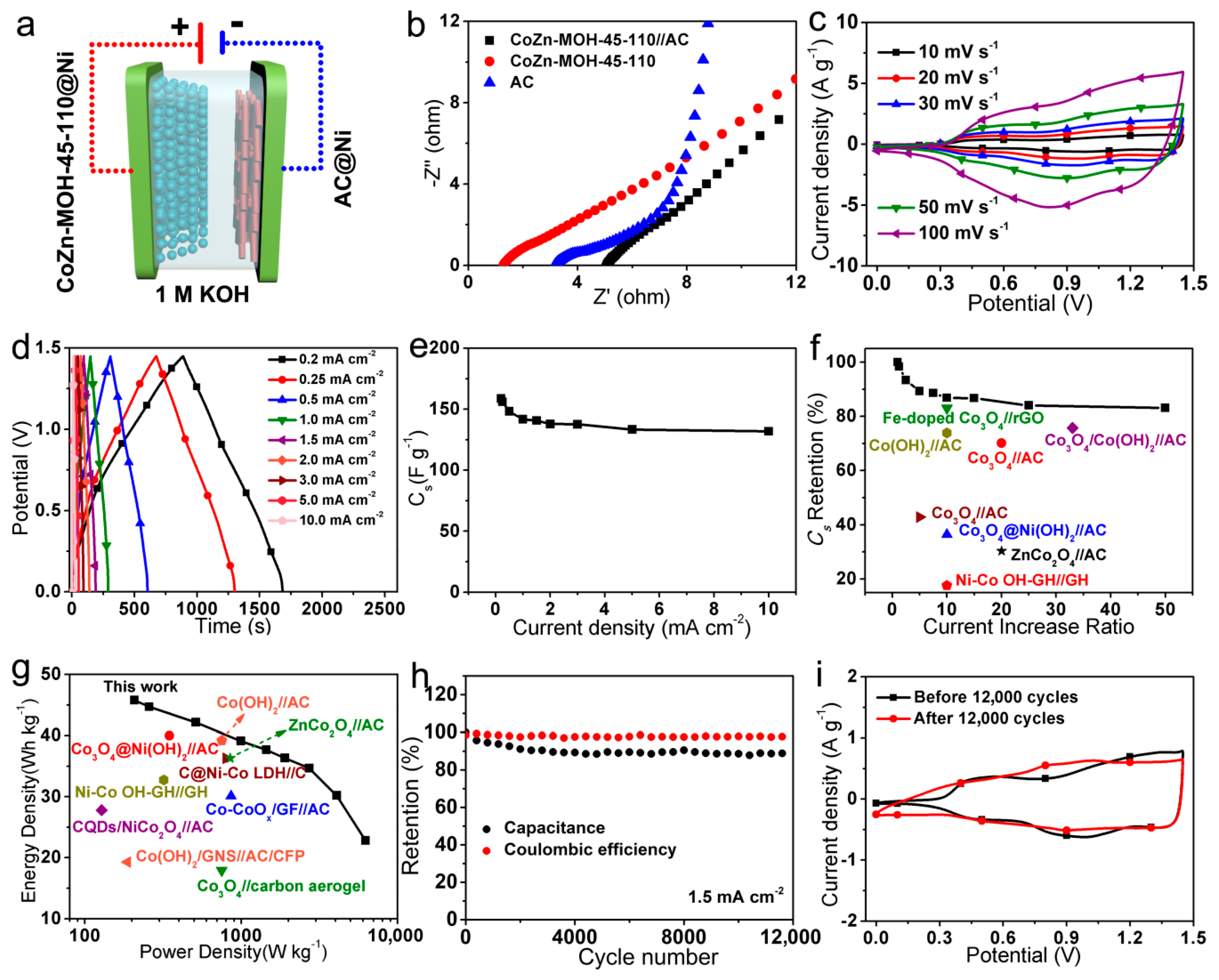
3.3. Electrochemical Performances of CoZn-MOH-45-110//AC ASC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ratajczak, P.; Suss, M.E.; Kaasik, F.; Béguin, F. Carbon Electrodes for Capacitive Technologies. Energy Storage Mater. 2019, 16, 126–145. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, S.; Lou, G.; Shen, Y.; Chen, H.; Shen, Z.; Zhao, S.; Zhang, J.; Chai, S.; Zou, Q. Review of Macroporous Materials as Electrochemical Supercapacitor Electrodes. J. Mater. Sci. 2017, 52, 11201–11228. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Wu, Y.; Zhu, X.; Lu, Y.; Yu, S.; Yang, C.; Chen, H.; Guan, C.; Li, L.; Shen, Z. Facile Activation of Commercial Carbon Felt as a Low-Cost Free-Standing Electrode for Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 42503–42512. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, D.; Shen, Z.; Bao, B.; Zhao, S.; Wu, L. Functional Biomass Carbons with Hierarchical Porous Structure for Supercapacitor Electrode Materials. Electrochim. Acta 2015, 180, 241–251. [Google Scholar] [CrossRef]
- Eskusson, J.; Rauwel, P.; Nerut, J.; Jänes, A. A Hybrid Capacitor Based on Fe3O4-Graphene Nanocomposite/Few-Layer Graphene in Different Aqueous Electrolytes. J. Electrochem. Soc. 2016, 163, A2768–A2775. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Lou, G.; Wu, Y.; Zhu, X.; Chen, H.; Shen, Z.; Fu, S.; Bao, B.; Wu, L. Synthesis of NiMn-LDH Nanosheet@Ni3S2 Nanorod Hybrid Structures for Supercapacitor Electrode Materials with Ultrahigh Specific Capacitance. Sci. Rep. 2018, 8, 5246. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Balamurugan, J.; Kim, N.H.; Lee, J.H. Hierarchical Zn–Co–S Nanowires as Advanced Electrodes for All Solid State Asymmetric Supercapacitors. Adv. Energy Mater. 2018, 8, 1702014. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Y.; Liu, J.; Huang, X.; Yuan, C.; Lou, X.W. Recent Advances in Metal Oxide-based Electrode Architecture Design for Electrochemical Energy Storage. Adv. Mater. 2012, 24, 5166–5180. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, S.; Chen, M.; Wu, L. Reduced Graphene Oxide-MnO2 Hollow Sphere Hybrid Nanostructures as High-Performance Electrochemical Capacitors. J. Mater. Chem. 2012, 22, 25207–25216. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Guan, C.; Wu, Y.; Yang, C.; Shen, Z.; Zou, Q. Energy-Saving Synthesis of MOF-Derived Hierarchical and Hollow Co(VO3)2-Co(OH)2 Composite Leaf Arrays for Supercapacitor Electrode Materials. ACS Appl. Mater. Interfaces 2018, 10, 18440–18444. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kang, J.; Choe, G.; Yim, S. Enhanced Energy Density of Supercapacitors Using Hybrid Electrodes Based on Fe2O3 and MnO2 Nanoparticles. Int. J. Electrochem. Sci. 2017, 12, 10015–10022. [Google Scholar] [CrossRef]
- Wu, C.; Chen, L.; Lou, X.; Ding, M.; Jia, C. Fabrication of Cobalt-Nickel-Zinc Ternary Oxide Nanosheet and Applications for Supercapacitor Electrode. Front. Chem. 2018, 6, 597. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Zhu, W.; Li, Z.; Zhu, J.; Zhu, X.; Pezzotti, G. Tailoring morphology of cobalt–nickel layered double hydroxide via different surfactants for high-performance supercapacitor. R. Soc. Open Sci. 2018, 5, 180867. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, S.; Xu, K.; Zhang, Y.; Zhang, L.; Lou, G.; Wu, Y.; Zhu, E.; Chen, H.; Shen, Z.; et al. Sustainable Activated Carbons from Dead Ginkgo Leaves for Supercapacitor Electrode Active Materials. Chem. Eng. Sci. 2018, 181, 36–45. [Google Scholar] [CrossRef]
- Yu, S.; Zhu, X.; Lou, G.; Wu, Y.; Xu, K.; Zhang, Y.; Zhang, L.; Zhu, E.; Chen, H.; Shen, Z.; et al. Sustainable Hierarchical Porous Biomass Carbons Enriched with Pyridinic and Pyrrolic Nitrogen for Asymmetric Supercapacitor. Mater. Des. 2018, 149, 184–193. [Google Scholar] [CrossRef]
- Yu, S.; Liu, D.; Zhao, S.; Bao, B.; Jin, C.; Huang, W.; Chen, H.; Shen, Z. Synthesis of wood derived nitrogen-doped porous carbon-polyaniline composites for supercapacitor electrode materials. RSC Adv. 2015, 5, 30943–30949. [Google Scholar] [CrossRef]
- Liu, D.; Yu, S.; Shen, Y.; Chen, H.; Shen, Z.; Zhao, S.; Fu, S.; Yu, Y.; Bao, B. Polyaniline Coated Boron Doped Biomass Derived Porous Carbon Composites for Supercapacitor Electrode Materials. Ind. Eng. Chem. Res. 2015, 54, 12570–12579. [Google Scholar] [CrossRef]
- Guan, C.; Liu, X.; Ren, W.; Li, X.; Cheng, C.; Wang, J. Rational Design of Metal-Organic Framework Derived Hollow NiCo2O4 Arrays for Flexible Supercapacitor and Electrocatalysis. Adv. Energy Mater. 2017, 7, 1602391. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, N.; Zhang, G.; Xu, M.; Lu, W.; Zhou, L.; Huang, H. Design of Hierarchical Ni-Co@Ni-Co Layered Double Hydroxide Core–Shell Structured Nanotube Array for High-Performance Flexible All-Solid-State Battery-Type Supercapacitors. Adv. Funct. Mater. 2017, 27, 1605307. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Cheng, H.; Li, N.; Ma, T.Y.; Su, Y.-Z. ZnCo2O4 Quantum Dots Anchored on Nitrogen-Doped Carbon Nanotubes as Reversible Oxygen Reduction/Evolution Electrocatalysts. Adv. Mater. 2016, 28, 3777–3784. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Song, D.; Pu, T.; Li, J.; Huang, B.; Wang, W.; Zhao, C.; Xie, L.; Chen, L. Two-dimensional porous ZnCo2O4 thin sheets assembled by 3D nanoflake array with enhanced performance for aqueous asymmetric supercapacitor. Chem. Eng. J. 2018, 336, 679–689. [Google Scholar] [CrossRef]
- Dupin, J.-C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Cao, L.; Xu, F.; Liang, Y.Y.; Li, H.L. Preparation of the Novel Nanocomposite Co(OH)2/Ultra-Stable Y Zeolite and Its Application as a Supercapacitor with High Energy Density. Adv. Mater. 2004, 16, 1853–1857. [Google Scholar] [CrossRef]
- Wang, R.; Yan, X.; Lang, J.; Zheng, Z.; Zhang, P. A hybrid supercapacitor based on flower-like Co(OH)2 and urchin-like VN electrode materials. J. Mater. Chem. A 2014, 2, 12724–12732. [Google Scholar] [CrossRef]
- Wei, G.; Zhou, Z.; Zhao, X.; Zhang, W.; An, C. Ultrathin Metal–Organic Framework Nanosheet-Derived Ultrathin Co3O4 Nanomeshes with Robust Oxygen-Evolving Performance and Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 23721–23730. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, L.; Chen, D.; Shen, G. Nanowire-assembled Co3O4@NiCo2O4 architectures for high performance all-solid-state asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 24981–24988. [Google Scholar] [CrossRef]
- Singh, A.K.; Sarkar, D. Substrate-integrated core–shell Co3O4@Au@CuO hybrid nanowires as efficient cathode materials for high-performance asymmetric supercapacitors with excellent cycle life. J. Mater. Chem. A 2017, 5, 21715–21725. [Google Scholar] [CrossRef]
- Pan, Z.; Jiang, Y.; Yang, P.; Wu, Z.; Tian, W.; Liu, L.; Song, Y.; Gu, Q.; Sun, D.; Hu, L. In Situ Growth of Layered Bimetallic ZnCo Hydroxide Nanosheets for High-Performance All-Solid-State Pseudocapacitor. ACS Nano 2018, 12, 2968–2979. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, L.; Chen, M.; Yan, Y.; Wu, L. Nickel–Cobalt Layered Double Hydroxide Nanosheets for High-Performance Supercapacitor Electrode Materials. Adv. Funct. Mater. 2014, 24, 934–942. [Google Scholar] [CrossRef]
- Pang, H.; Li, X.; Zhao, Q.; Xue, H.; Lai, W.-Y.; Hu, Z.; Huang, W. One-pot synthesis of heterogeneous Co3O4-nanocube/Co(OH)2-nanosheet hybrids for high-performance flexible asymmetric all-solid-state supercapacitors. Nano Energy 2017, 35, 138–145. [Google Scholar] [CrossRef]
- Lin, J.; Liu, Y.; Wang, Y.; Jia, H.; Chen, S.; Qi, J.; Qu, C.; Cao, J.; Fei, W.; Feng, J. Rational construction of nickel cobalt sulfide nanoflakes on CoO nanosheets with the help of carbon layer as the battery-like electrode for supercapacitors. J. Power Sources 2017, 362, 64–72. [Google Scholar] [CrossRef]
- Bai, X.; Liu, Q.; Liu, J.; Zhang, H.; Li, Z.; Jing, X.; Liu, P.; Wang, J.; Li, R. Hierarchical Co3O4@Ni(OH)2 core-shell nanosheet arrays for isolated all-solid state supercapacitor electrodes with superior electrochemical performance. Chem. Eng. J. 2017, 315, 35–45. [Google Scholar] [CrossRef]
- Xiang, K.; Xu, Z.; Qu, T.; Tian, Z.; Zhang, Y.; Wang, Y.; Xie, M.; Guo, X.; Ding, W.; Guo, X. Two dimensional oxygen-vacancy-rich Co3O4 nanosheets with excellent supercapacitor performances. Chem. Commun. 2017, 53, 12410–12413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wei, J.; Chen, L.; Tang, S.; Deng, M.; Du, Y. All-solid-state asymmetric supercapacitors based on Fe-doped mesoporous Co3O4 and three-dimensional reduced graphene oxide electrodes with high energy and power densities. Nanoscale 2017, 9, 15423–15433. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jiang, L.; Long, C.; Wei, T.; Fan, Z. Dual Support System Ensuring Porous Co–Al Hydroxide Nanosheets with Ultrahigh Rate Performance and High Energy Density for Supercapacitors. Adv. Funct. Mater. 2015, 25, 1648–1655. [Google Scholar] [CrossRef]
- Lai, F.; Miao, Y.-E.; Zuo, L.; Lu, H.; Huang, Y.; Liu, T. Biomass-Derived Nitrogen-Doped Carbon Nanofiber Network: A Facile Template for Decoration of Ultrathin Nickel-Cobalt Layered Double Hydroxide Nanosheets as High-Performance Asymmetric Supercapacitor Electrode. Small 2016, 12, 3235–3244. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.; Xie, S.; Liu, Y.; Liu, C. Flower-like nickel-cobalt hydroxides converted from phosphites for high rate performance hybrid supercapacitor electrode materials. Electrochim. Acta 2016, 210, 915–924. [Google Scholar] [CrossRef]
- Zheng, X.; Gu, Z.; Hu, Q.; Geng, B.; Zhang, X. Ultrathin porous nickel–cobalt hydroxide nanosheets for high-performance supercapacitor electrodes. RSC Adv. 2015, 5, 17007–17013. [Google Scholar] [CrossRef]
- Han, X.; Tao, K.; Wang, D.; Han, L. Design of a porous cobalt sulfide nanosheet array on Ni foam from zeolitic imidazolate frameworks as an advanced electrode for supercapacitors. Nanoscale 2018, 10, 2735–2741. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, G.; Zheng, J.; Ma, J.; Yang, C.; Wang, Q. Microwave synthesis of three-dimensional nickel cobalt sulfide nanosheets grown on nickel foam for high-performance asymmetric supercapacitors. J. Colloid Interface Sci. 2018, 516, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Fan, H.; Song, Y.; Cui, Y.; Wang, R. Interconnected hierarchical NiCo2O4 microspheres as high-performance electrode materials for supercapacitors. Dalton Trans. 2017, 46, 9201–9209. [Google Scholar] [CrossRef] [PubMed]
- Vidyadharan, B.; Aziz, R.A.; Misnon, I.I.; Anil Kumar, G.M.; Ismail, J.; Yusoff, M.M.; Jose, R. High energy and power density asymmetric supercapacitors using electrospun cobalt oxide nanowire anode. J. Power Sources 2014, 270, 526–535. [Google Scholar] [CrossRef]
- Chen, M.; Qu, G.; Yang, W.; Li, W.; Tang, Y. Celgard membrane-mediated ion diffusion for synthesizing hierarchical Co(OH)2 nanostructures for electrochemical applications. Chem. Eng. J. 2018, 350, 209–216. [Google Scholar] [CrossRef]
- Hwang, M.; Kang, J.; Seong, K.-D.; Kim, D.K.; Jin, X.; Antink, W.H.; Lee, C.; Piao, Y. Ni-Co hydroxide nanoneedles embedded in graphene hydrogel as a binder-free electrode for high-performance asymmetric supercapacitor. Electrochim. Acta 2018, 270, 156–164. [Google Scholar] [CrossRef]
- Park, C.; Hwang, J.; Hwang, Y.-T.; Song, C.; Ahn, S.; Kim, H.-S.; Ahn, H. Intense pulsed white light assisted fabrication of Co-CoOx core-shell nanoflakes on graphite felt for flexible hybrid supercapacitors. Electrochim. Acta 2017, 246, 757–765. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Zhu, M.; He, X. High-performance all-solid state asymmetric supercapacitor based on Co3O4 nanowires and carbon aerogel. J. Power Sources 2015, 282, 179–186. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Z.; Jing, M.; Hou, H.; Yang, Y.; Zhang, Y.; Yang, X.; Song, W.; Jia, X.; Ji, X. Porous NiCo2O4 spheres tuned through carbon quantum dots utilised as advanced materials for an asymmetric supercapacitor. J. Mater. Chem. A 2015, 3, 866–877. [Google Scholar] [CrossRef]
- Zhao, C.; Ren, F.; Xue, X.; Zheng, W.; Wang, X.; Chang, L. A high-performance asymmetric supercapacitor based on Co(OH)2/graphene and activated carbon electrodes. J. Electroanal. Chem. 2016, 782, 98–102. [Google Scholar] [CrossRef]
- Chen, H.; Hu, L.; Yan, Y.; Che, R.; Chen, M.; Wu, L. One-Step Fabrication of Ultrathin Porous Nickel Hydroxide-Manganese Dioxide Hybrid Nanosheets for Supercapacitor Electrodes with Excellent Capacitive Performance. Adv. Energy Mater. 2013, 3, 1636–1646. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, S.; Wu, L. Porous Nickel Hydroxide–Manganese Dioxide-Reduced Graphene Oxide Ternary Hybrid Spheres as Excellent Supercapacitor Electrode Materials. ACS Appl. Mater. Interfaces 2014, 6, 8621–8630. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zhu, X.; Zhu, E.; Lou, G.; Wu, Y.; Lu, Y.; Wang, H.; Song, J.; Tao, Y.; Pei, G.; et al. Electrochemically Stable Cobalt–Zinc Mixed Oxide/Hydroxide Hierarchical Porous Film Electrode for High-Performance Asymmetric Supercapacitor. Nanomaterials 2019, 9, 345. https://doi.org/10.3390/nano9030345
Yang H, Zhu X, Zhu E, Lou G, Wu Y, Lu Y, Wang H, Song J, Tao Y, Pei G, et al. Electrochemically Stable Cobalt–Zinc Mixed Oxide/Hydroxide Hierarchical Porous Film Electrode for High-Performance Asymmetric Supercapacitor. Nanomaterials. 2019; 9(3):345. https://doi.org/10.3390/nano9030345
Chicago/Turabian StyleYang, Hanbin, Xinqiang Zhu, Enhui Zhu, Gaobo Lou, Yatao Wu, Yingzhuo Lu, Hanyu Wang, Jintao Song, Yingjie Tao, Gu Pei, and et al. 2019. "Electrochemically Stable Cobalt–Zinc Mixed Oxide/Hydroxide Hierarchical Porous Film Electrode for High-Performance Asymmetric Supercapacitor" Nanomaterials 9, no. 3: 345. https://doi.org/10.3390/nano9030345
APA StyleYang, H., Zhu, X., Zhu, E., Lou, G., Wu, Y., Lu, Y., Wang, H., Song, J., Tao, Y., Pei, G., Chu, Q., Chen, H., Ma, Z., Song, P., & Shen, Z. (2019). Electrochemically Stable Cobalt–Zinc Mixed Oxide/Hydroxide Hierarchical Porous Film Electrode for High-Performance Asymmetric Supercapacitor. Nanomaterials, 9(3), 345. https://doi.org/10.3390/nano9030345




