1. Introduction
Among the most interesting and promising nanomaterials are colloidal semiconducting quantum dots (QDs). These nanostructures have found already several commercial applications in displays [
1], light concentrators [
2], photovoltaics [
3] and as optical probes in various bio-applications [
4,
5]. The main reasons for their still growing success are: broad absorption band (several hundreds of nm), narrow emission band (below 40 nm), high quantum yield (up to 95%), possibility of emission band tuning over a wide range of wavelengths (350–2000 nm) and high resistivity of optical properties on external physico-chemical conditions, e.g., pH, temperature or power of the excitation beam [
6]. QDs have a high surface to volume ratio, which can be controlled by QDs size but also by the nanostructures shape. This high surface area equips them with much more functional groups compared to organic compounds [
7]. This makes QDs much more reactive and thus more effective in biological sensing [
8].
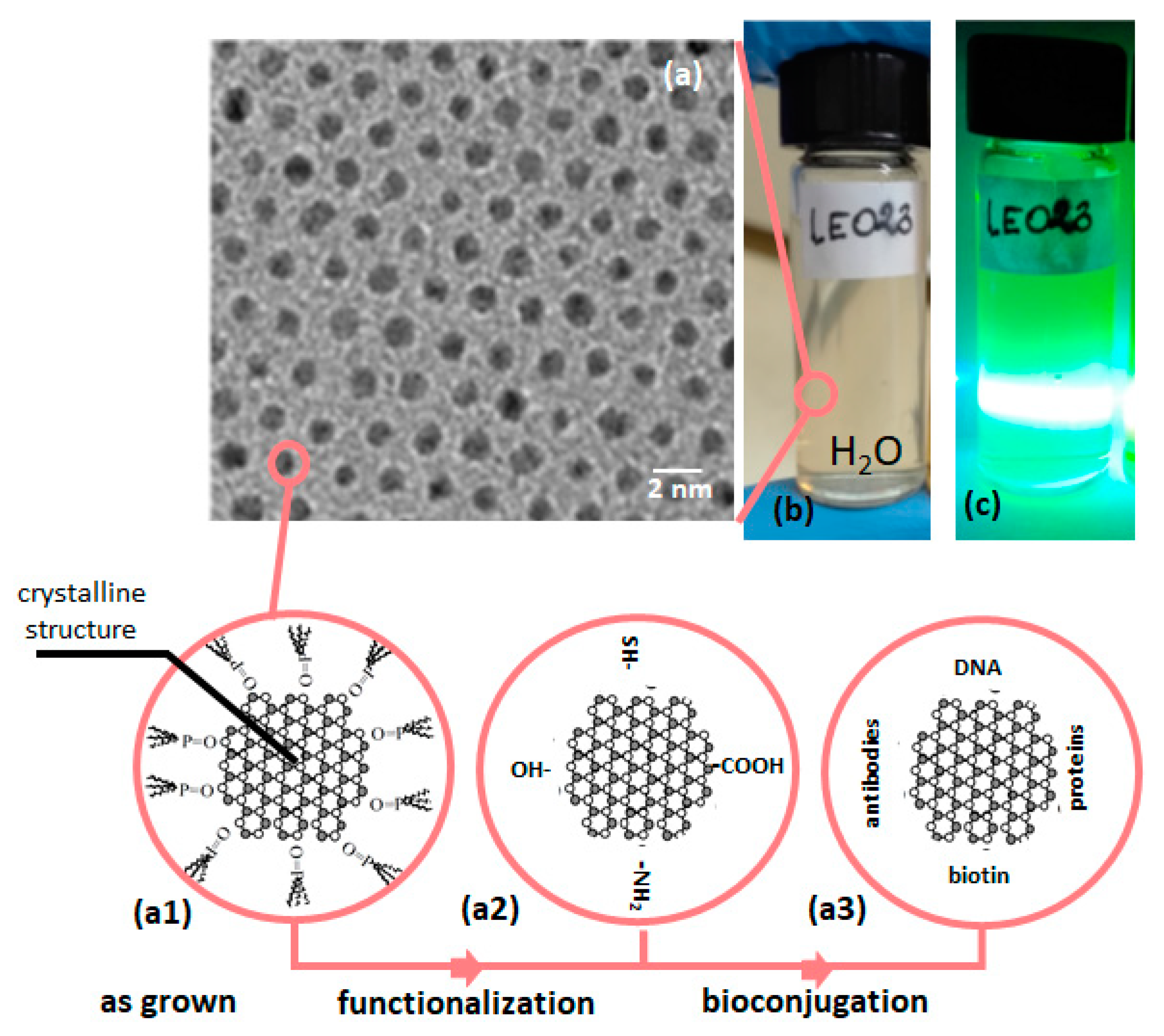
The high quality QDs are typically grown as hydrophobic structures. In consequence, to make them useful for biological or medical application, additional post growth treatment is usually needed. This treatment includes QDs functionalization and in many cases bioconjugation (see
Figure 1). One serious reason why QDs still do not dominate over organic markers (i.e., Green Fluorescent Protein, Rhodamine) lies in the absence of widely tested and already accepted protocols for QDs functionalization and bioconjugation [
9]. There is also another reason to not use the QDs in biological sensing, especially in case of in vivo imaging, namely their toxicity. The QDs toxicity is a subject widely discussed recently in the literature [
10]. The main reason affecting QDs toxicity is an aggregation of nanostructures in cells, organs, tissue etc. [
11]. This is an even more serious problem than the chemical toxicity, due to dissociation of Cd atoms from CdSe/CdS QDs, and this is true for any types of nanostructures: semiconducting, dielectric or metallic nanostructures. Nevertheless, fortunately the QDs toxicity becomes a much less serious problem when the QDs are used for external sensing or for some in vitro applications when we can use their potential without barriers.
Concluding the above discussion, it can be seen that real benefits coming from extraordinary properties of QDs must be always compared to drawbacks of using inorganic probes in biological systems. In other words, for some specific applications QDs are an excellent choice, or very bad idea [
12,
13,
14]. Among the applications where incontestably the advantages of QDs are utilized are sensing systems.
In this review, we present the main physical and chemical mechanisms used for detection of various species (bacteria, cells, nucleic acids, molecules, ions, etc.) with utilizing of QDs. We present the most successful examples of QDs applications in biology and medicine as optical and electrochemical sensors. Finally, we focus on the perspectives for further development in this field.
2. Comparison between Optical Properties of Organic Dyes and QDs
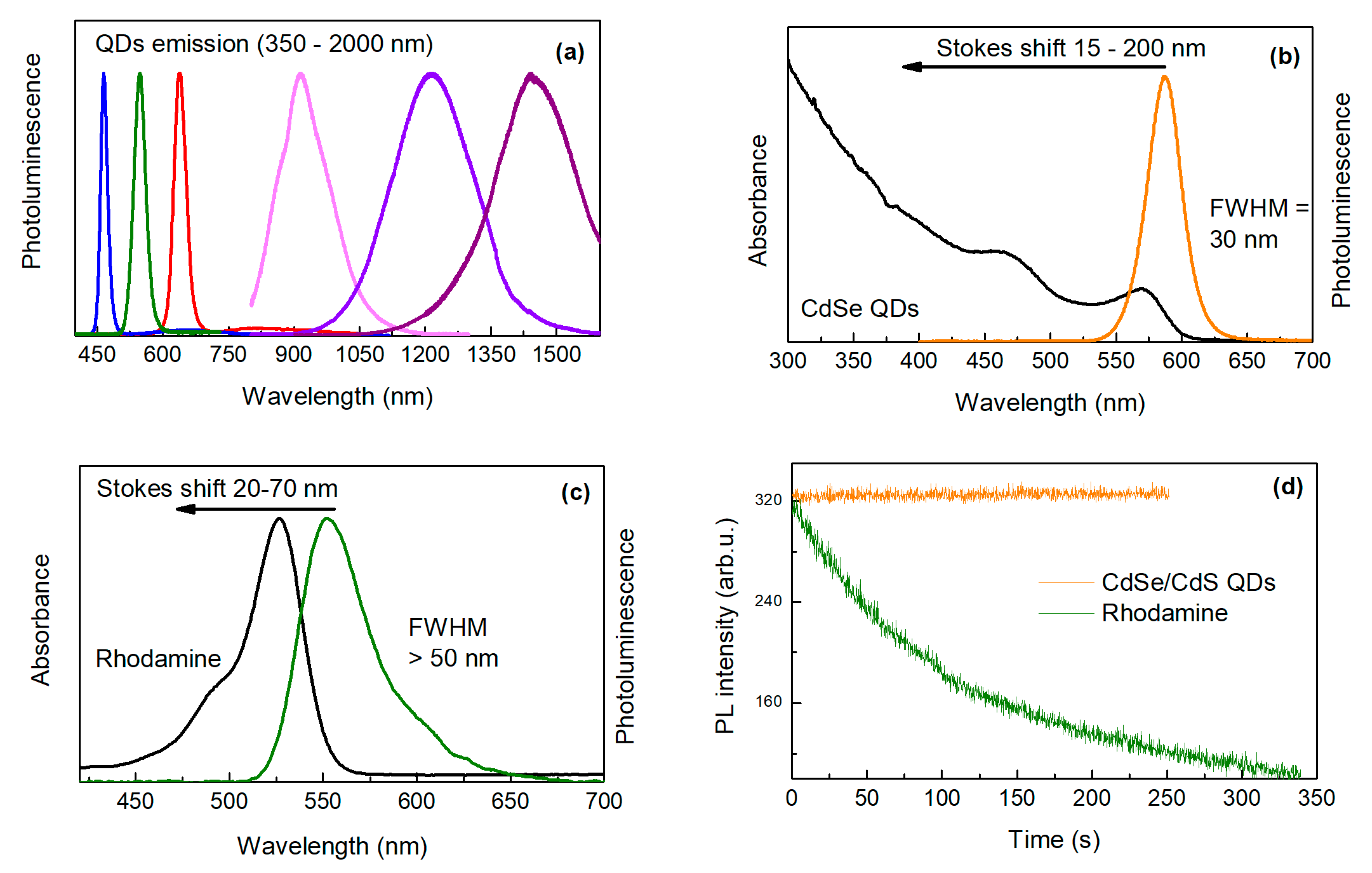
In comparison to organic dyes, QDs have the spectral position of absorption and emission dependent on their size (so-called Quantum Size Effect) [
15]. During synthesis, this effect enables continuous tuning of the emission peak position in a wide range of wavelengths (
Figure 2a). Moreover, the broad absorption of QDs allows free selection of the excitation wavelength and thus straightforward separation of the excitation and emission signal (
Figure 2b,c) [
16]. The fluorescence lifetimes of organic dyes are commonly too short for efficient temporal discrimination of short-lived autofluorescence of biological objects. In the case of QDs, the emission decay time can be tuned or selected with a proper choice of QDs composition (giving times up to several microseconds). This enables straightforward temporal discrimination of the signal from cellular autofluorescence and scattered excitation light by time-gated measurements, thereby enhancing detection sensitivity [
17]. In contrast to conventional dyes, QDs emitting different colors (and functionalized with different groups) can be simultaneously excited by a single excitation wavelength. This makes QDs suitable for multiplex testing by simultaneously detecting multiple signals [
18]. Moreover, QDs characterize with extremely high chemical stability and photostability (stability against chemical reactions induced by the incoming radiation). In addition, QDs are free from photobleaching [
19] (
Figure 2d) what is one of their most important advantages.
3. Fundamentals of QDs Sensing Phenomena
The unique optical properties of QDs make them attractive fluorophores that can be used both in vitro and in vivo in various biological studies, where traditional fluorescent labels based on organic molecules do not provide long-term stability, high enough intensity or where simultaneous detection of many signals is needed [
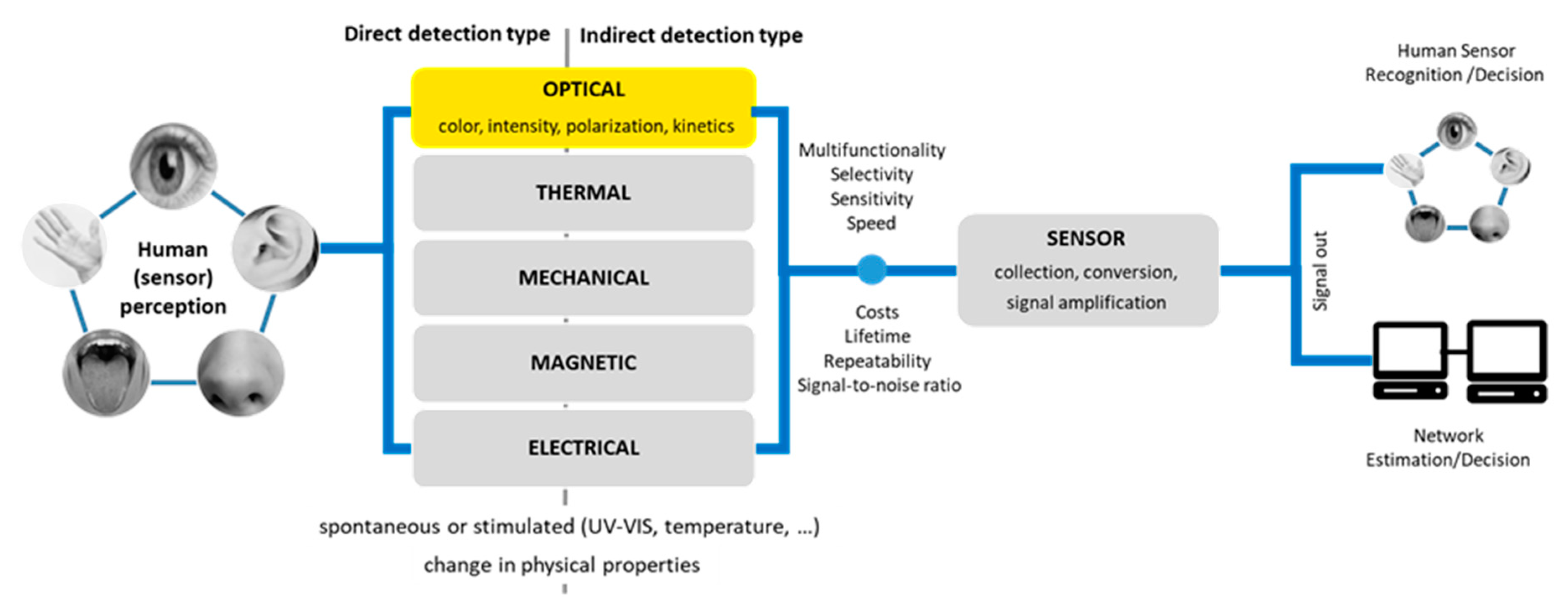
7]. In sensors, the signal detection bases on a registration of the change in one of the physical properties (optical, thermal, mechanical, magnetic, electrical) of sensing material induced by the interaction with the analyte. Changing in optical properties of QDs like emission color, intensity, polarization or emission kinetics can be used as the principle in optical sensors system (
Figure 3). In addition, obtained changes can be recorded directly by human senses or indirectly
via the signal transformation, amplification, and visualization. All these factors determine sensors construction and their mechanism of action in the detection of various substances [
20,
21].
4. Basic Strategies for Analyte Detection
The use of QDs for sensors construction requires adjusting their optical properties adequately to the needs that arise their shape, size, the color of emission, position of the absorption band. Moreover, to get specificity of QDs in their sensing action, the surface modification—called functionalization—must be applied first [
22,
23]. Functionalization is the process of attaching, exchanging already attached chemical molecules present on the surface of quantum dots. Chemical and physical methods used for this purpose, include processes such as exchange of ligands, silanisation, the creation of additional coatings or dendrimeric structures [
24]. The presence of ligands at the QDs surfaces affect their size, shape and physico-chemical properties, e.g., surface charge and chemical reactivity. Surface modifications allow the control of colloidal stability of QDs and their dispersion in non-polar environments (organic solvents in which they are most commonly synthesized) and polar (e.g., water, in which solubility is necessary for biological and medical applications). Moreover, the surface attached ligands determine the possibility of QDs conjugation to biological molecules (bioconjugation) or to determine their potential in applications where QDs must be embedded within the matrix [
25,
26].
In order to achieve high selectivity of QDs sensor, QDs are coupled to various vectors specific for an analyte. Wales et al. constructed a sensor for the selective detection of dicofol, a substance used to kill mites. For this purpose, they used CdS QDs with glutathione on their surface, whose both amino- and carboxyl- functional groups interact with chloride groups in the dicofol structure, thus leading to an increase in fluorescence intensity, which was directly proportional to the dicofol concentration in the studied sample [
27,
28].
The QDs-based sensors can be designed in several ways, depending on demands regarding their sensitivity, types of detected analytes, costs or complexity of their preparation.
Figure 4 shows the examples of preparation protocols used in QDs-based optical sensors.
In all cases, the protocol starts with the appropriate modification of QDs surface selectivity. As a result, QDs are targeted to determine a particular analyte. An important aspect is also the preparation of substrates which can take an active part in the detection protocol. Strategies (a) and (b) differ in Stages III and IV, which occur in reverse order. While in Strategy (a) Step III is the deposition of QDs, in Strategy (b) it is Step IV. This stage can be made using methods such as layer-by-layer [
29], sol-gel [
30] or electrochemical method [
31]. Stage IV in Strategy (a) and III in Strategy (b) are a conjugation of the analyte, which may be possible thanks to the previously prepared and targeted substrate. Jie et al. proposed the coupling of the analyte with the previously prepared substrate, based on CdSe nanocomposites, using antibodies selective for an antigen called human IgG [
32].
The final step in all strategies is QDs stimulation, which is used to detect the analyte. As a result, both the qualitative and quantitative assessment of the presence of the designated substance is possible. It is also possible to combine the first two strategies, resulting in Strategy (c), which uses the Förster resonance energy transfer between optical centers (QDs + QDs or dye).
The presented strategies differ in the number of steps that complicate detection and require a lot of user experience.
5. Physico-Chemical Mechanisms Used for Analyte Detection
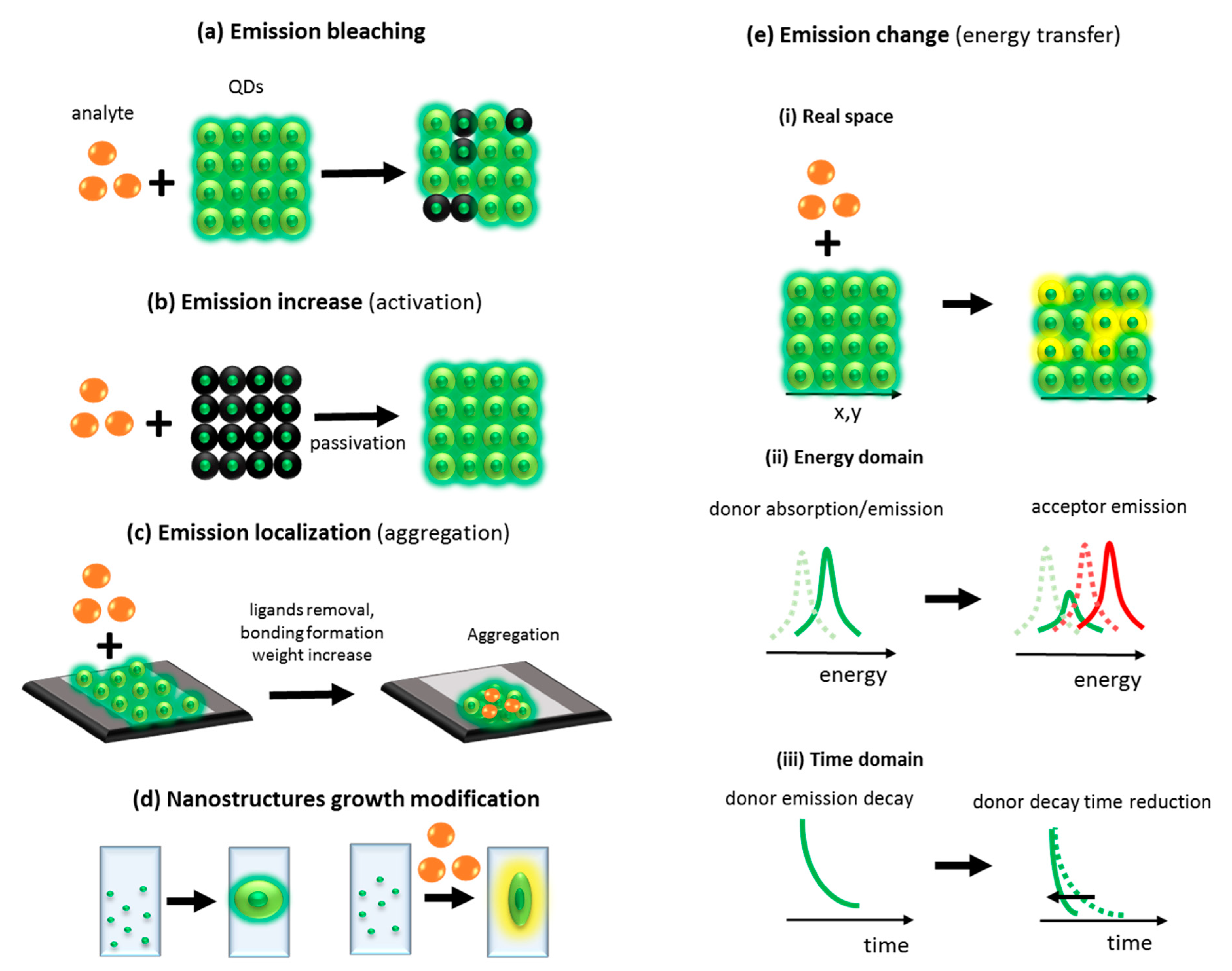
One of the most popular mechanisms used for detection of analyte relies on emission quenching from QDs. In this mechanism, due to the interaction of the QDs surface with the analyte, the QDs emission intensity decreases (
Figure 5a) [
33]. Another mechanism relies on an increase of QDs emission due to passivation of QDs surface by analyte (
Figure 5b), e.g., addition of bovine serum albumin or nucleic acids resulted in increasing emission from CdS dots coated with mercaptoacetic acid [
34].
The third mechanism, which can be used for analyte detection, is stimulated aggregation (
Figure 5c). In this mechanism, due to an interaction of the analyte with the QDs surface, the surface ligands are detached and QDs aggregate. The aggregation can be also induced by analyte stimulated bonds formation between functionalized QDs [
35].
There is also a very rarely used mechanism of analyte detection based on modification of the nanostructures’ growth process by introduction of the analyte during the nanostructures’ growth. Due to this perturbation, the nanostructures can have different emission or other properties which can be detected (
Figure 5d). There is also the fifth mechanism commonly used for analyte detection based on changes in QDs optical properties. The changes come from excitation energy transfer from QDs to other optical center (QDs or dye). In consequences, the color of emission changes or emission decay time of donor is reduced (
Figure 5e) [
36,
37].
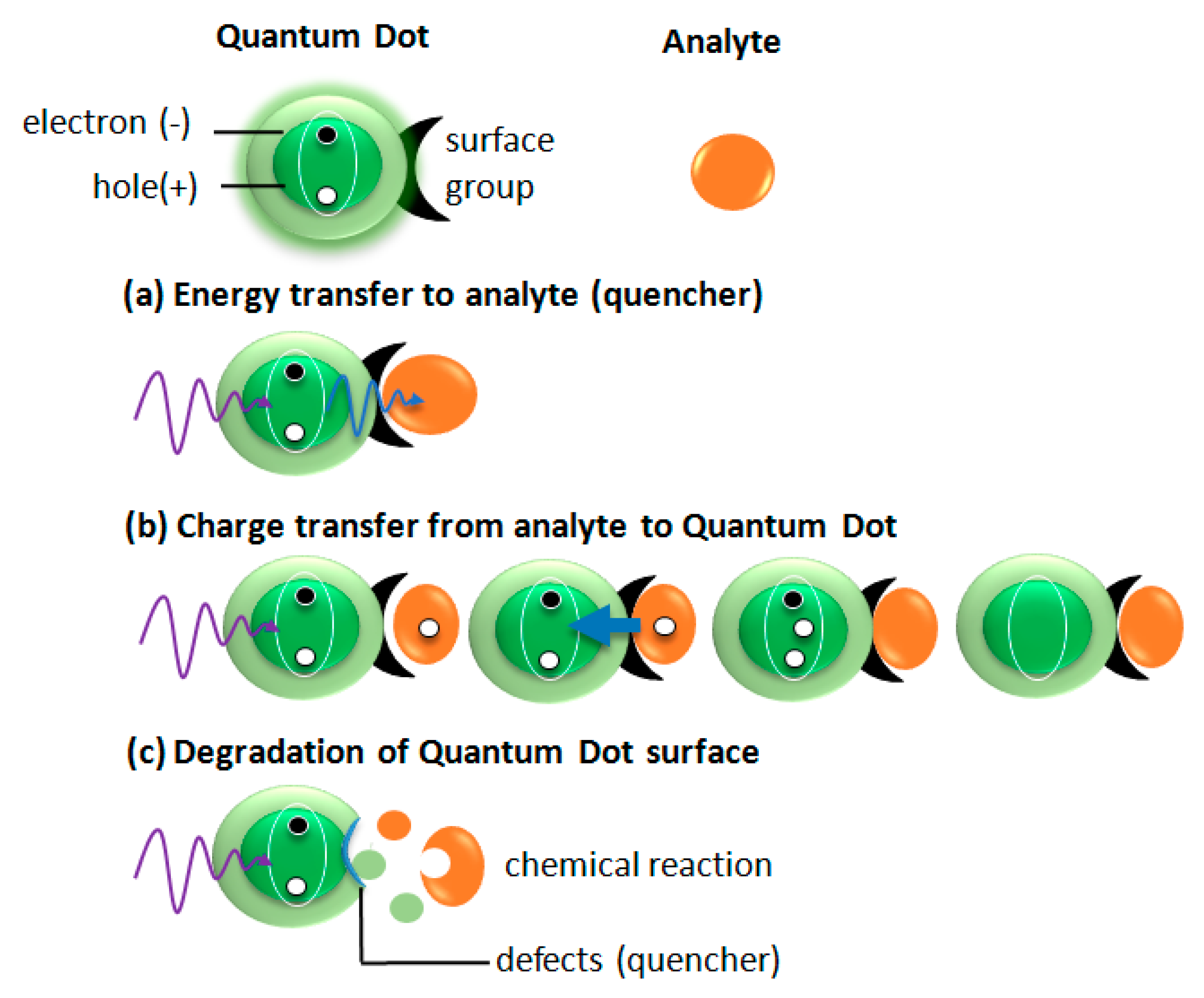
5.1. Emission Bleaching
One of the basic mechanisms of an analyte detection is emission bleaching (
Figure 5a). This mechanism can be induced by different physical phenomena schematically shown in
Figure 6. In the first example, the absorption band of the analyte overlaps with the emission band of QDs. In such a case, the emission from QDs is absorbed by an analyte, which results in bleaching QDs emission. (
Figure 6a). Another mechanism (
Figure 6b) bases on a charge transfer from an analyte to QDs. When the additional electron or hole appears in an excited QDs the Auger processes lead to QDs ionization or charging. In both cases, the dot became non-emissive (dark). The third mechanism, which is responsible for QDs emission quenching under the interaction with the analyte is analyte-induced degradation of the QDs surface providing to emission decrease. As an example, thiol coated ZnS QDs in the presence of peptides showed a significant emission quenching [
38].
5.2. Emission Change—Nonradiative Förster Resonance Energy Transfer (FRET)
When two optically active centers (donor and acceptor) are in close proximity to each other, (typically 1–10 nm) and an absorption spectrum of the acceptor overlaps an emission spectrum of the donor, the non-radiative transfer of the excitation energy from the donor to acceptor appears. This phenomenon is called FRET (Förster Resonance Energy Transfer). For the experiments using the FRET approach, photostable emitters must be used, characterized by a quantum efficiency greater than 0.1 and a high brightness (one in which the absorbance coefficient Ɛ is greater than 50.000 M
−1·cm
−1) [
39]. All these conditions are perfectly fulfilled by the quantum dots. The effectiveness of FRET is inversely proportional to the distance between the donor and acceptor and defined as:
where:
KFRET—FRET efficiency;
R—distance between donor and acceptor;
R0—distance at which the FRET efficiency is equal to 0.5 [
40].
The highest sensitivity of FRET signal is for a distance between the donor and acceptor in the range from 0.5R
0 to 1.5R
0 [
40]. For years researchers have been using the FRET mechanism to monitor intracellular interactions, due to its sensitivity to molecular rearrangements in the 1–10 nm range (this is the scale correlating with the size of biological macromolecules and the possibility of creating bonds between them) [
41].
The universality of FRET method allows its use in nanosystems as well [
42]. FRET yield is typically measured by observing one of the three parameters of the fluorescent donor: fluorescence intensity, spectral response or average fluorescence lifetime. Moreover, FRET has found application in many sensing systems giving the possibility of applying it to three analyte detection strategies.
Figure 7 shows different processes which can be detected with use of FRET. The first mechanism uses analyte as optically active acceptor. In this case, the analyte attachment as well analyte removal can be observed as a change in the optical signal. The other strategy uses the analyte as the emission quencher and was also discussed in the previous paragraph [
43]. The third strategy is more complex and uses a multistep energy-transfer phenomenon. Detection using FRET between the QDs, as donors, directed to a linker with an acceptor, associated, e.g., with a receptor protein, is widely used to study the receptor-ligand interactions and changes in protein conformation after binding to the target analyte [
44]. Thanks to this, in analytics consisting of several acceptors, QDs can interact with only one of them, which significantly improves the efficiency and sensitivity of the FRET method [
45].
5.3. Analyte Stimulated QDs Aggregation
The colloidal QDs solution is sensitive to the presence of additional charges either on QDs surface or in the solvent, which may result in QDs aggregation. The charges may be introduced or induced by an analyte, which ultimately is manifested by QDs aggregation [
46,
47,
48]. In the absence of analyte, a fluorescence comes from the whole volume of the QDs solution, while after aggregation caused by the analyte, the emission is localized [
19]. This type of stimulation belongs to qualitative tests.
6. Photoelectrochemical and Electrochemical Methods of Analyte Detection
The chemical detection method is usually signaled by the following ways: competition binding assay of labelled and unlabeled analytes, using labelled molecules specific for immobilized analytes, sandwich formation or enzyme immunoassay, where enzymatically active substrate is added that changes color or fluorescence after interaction with enzyme-related analyzes [
49].
Mo et al. [
50] used a redox mechanism in the detection of hydroquinone in water samples. They have observed that ZnS QDs cannot react with hydroquinone. When hydroquinone and K
2S
2O
8 were added into ZnSe QDs solution, no new photoluminescence (PL) peak was observed. Comparing with the pure ZnSe QDs solution, the PL intensity of the mixture decreased. This result reveals that hydroquinone oxidation product can efficiently quench the fluorescence emission of ZnSe QDs by energy transfer in electrochemiluminescence mechanism.
The development of new, reliable, fast and efficient methods for detecting anthropogenic and natural substances, both organic and inorganic, is a huge challenge for modern analytical chemistry and diagnostics. An alternative to such methods are electrochemical strategies using semiconducting quantum dots. The growing interest in the construction of electrochemical devices using quantum dots results from their aforementioned properties. Due to these features, small changes in the external environment lead to great changes in particle properties and electron transfer. Based on these significant changes, quantum dots are prone to engaging in heterogeneous redox chemistry with the surrounding environment. QDs are also used as carriers of biomacromolecules in bioanalytics. For this purpose, the chemical functionalization of QDs is carried out by means of a functional cap layer that allows the molecules to be trapped. The immobilization of biomolecules (e.g., the enzyme catalyzing the redox reaction) on the surface of semiconductor QDs causes QDs to promote direct electron transfer between biomolecules and the surface of the electrode, which significantly affects the operation of the system by enhancing the sensitivity due to signal amplification. A tremendous increase of development of electrochemical sensors based on QDs has been observed over the past decades due to the simplicity of implementation, high selectivity, and specificity of the system, low cost and the possibility of miniaturization [
51]. Moreover, research carried out by Bard et al. revealed that CdS QDs could also act as multi-electron donors or acceptors at a given potential due to trapping of holes and electrons within the particle [
52]. On the other hand, the surface structures of QDs also play a key role in determining the properties of the particles [
53].
Liu et al. [
54] described an electrochemical assay strategy for specific recognition of tumor cells. For this purpose, gold nanoparticles (AuNPs) have been assembled onto the indium tin oxide (ITO) substrate to create a specific, biocompatible interface to effective capture of tumor cells. CdSe/ZnS QDs labelled on the cell surface have been used as an amplified signal during the square wave stripping voltammetry (SWSV). The developed biosensing platform shown good analytical performance with a broad linear range, good selectivity and low limit of detection (LOD).
Electrochemiluminescence (ECL) is a method which aims to convert electric energy into radiation energy, in which electrochemically generated intermediate products undergo a high energy electron transfer reaction to generate excited states, resulting in the emission of a measurable luminescence signal [
55]. As a form of luminescence (light emission without heat), ECL is characterized by the fact that light emission occurs when an appropriate potential is applied to the electrode, as a result of which the oxidation or reduction reaction takes place. There are several features that distinguish ECL from other techniques, e.g., chemiluminescence (CL). It is clear that the electrochemical reaction that takes place allows for precise time control. This means that the emission of light can be delayed to the desired moment, e.g., an immune reaction or an enzymatic reaction. Another advantage of ECL is the ability to control the location of the reaction, which means that there is the possibility of limiting the emission of light to a specific area relative to the detector. Electrochemiluminescence may occur as a result of two independent processes: annihilation of ions and co-reactant ECL. The annihilation of ions consists in creating states of excited molecules due to the transfer of electrons between radical ions on the surface of the electrode. The ECL co-reactant is due to the use of anode or cathodic potential in a solution containing phosphor and co-agent molecules. Depending on the potential application, the phosphor or co-reactant molecules can be reduced or oxidized to form radical ions and medium compounds, followed by decomposition and formation of excited states that cause light emission [
56].
8. Conclusions and Perspectives
Quantum dots have remarkable optical properties, which make them among the most useful nanomaterials [
6]. They may be utilized in a wide range of applications, e.g., in new types of fluorescent probes and as active components of nanostructure-biomolecule complexes [
122]. Various schemes for the application of optical transduction QDs have been successfully tested, allowing a wide range of detection, high selectivity and sensitivity in the tested samples. The development of analytical methods for the detection of various chemical or biological compounds allows the use of QDs in sensors for determining the presence of ions, molecules and pH changes. The results of discussed studies lead to the improvement of existing detection devices and the design of new detection devices that allow more sensitive and faster analysis. Quantum dots-based detection technologies can be adapted to precision medical technologies by overturning point-of-care (POC) and personalized diagnostics. This engineering can supply high-throughput and mobile diagnostic platforms for screening pathogens and toxins immediately in field and POC clinical settings. Several of these technologies tender multiplexing capacities for simultaneous examination of multiple analytes with unexpectedly high sensitivity that can notably lower costs and detection time. Nevertheless, a universal sensor for different types of medicinal/or, i.e., food samples, is a challenge because of the inherent complexity of biological samples. Evaluation with numerous of biological (i.e., food, body fluids) samples and comparison with well-established techniques may assist to direct this challenge.
Among further fields that could exploit some of the advantages of QDs are fluorescent immunosensors designed as integrated devices. Due to the relevant improvement on the execution of fluorescent immunosensors and recent advances in miniaturization processes, it is believed that in the near future small and advanced fluorescent mobile analytical platforms, which combine steps of the immunoassay pathway, will be available. In conclusion, there is potential for further investigations of QDs in multiplex detection, particularly via continued miniaturization and integration into lab-on-chip platforms.