Effect of Carbon Nanoparticles on the Crystallization of Calcium Carbonate in Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
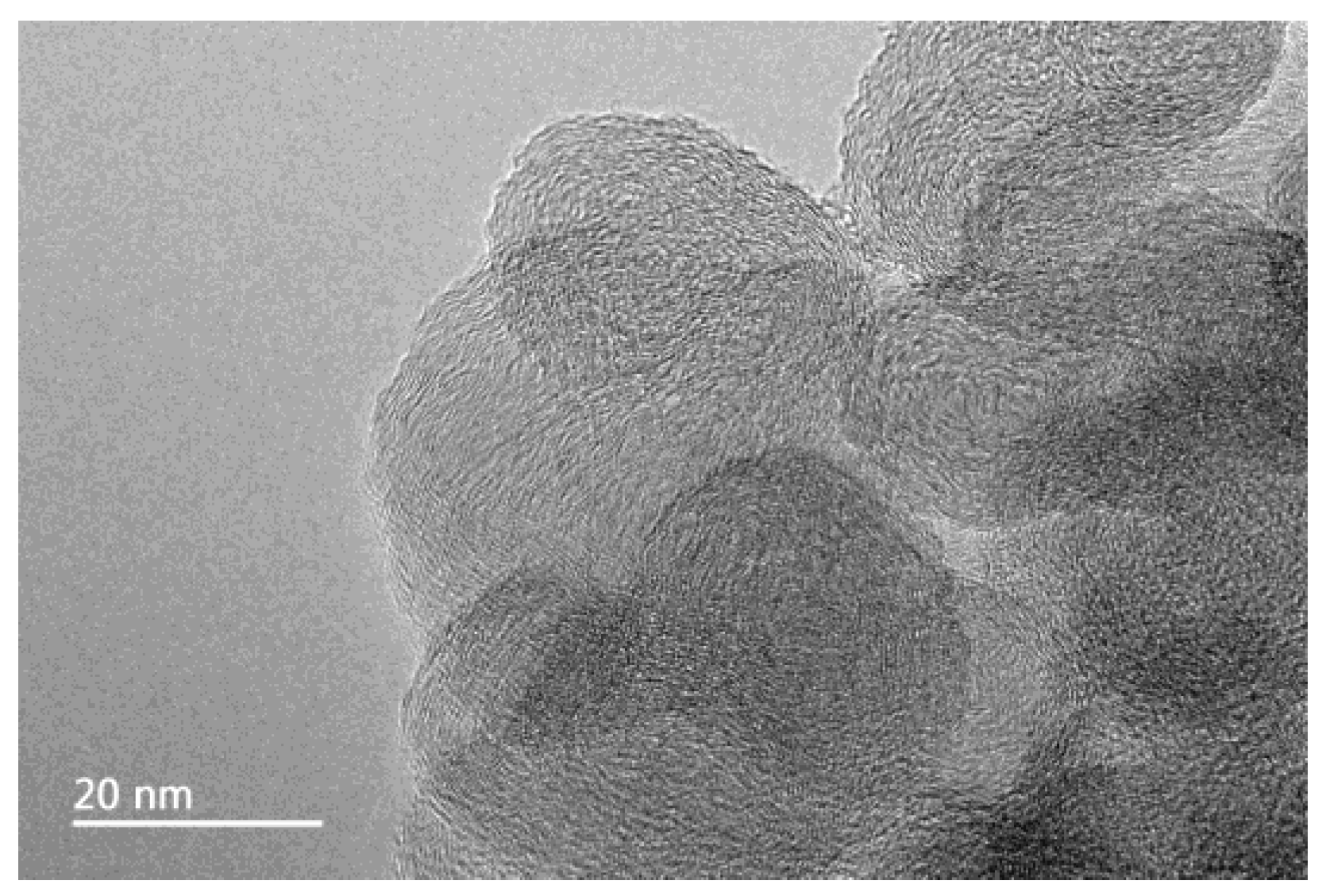
2.2. Particle Size Characterisation of Carbon Nanoparticles
2.3. The Preparation of Carbon Nanofluids
2.4. The Scale Inhibition Experiment of CaCO3
2.5. Solution Analysis
2.5.1. Determination of Residual Calcium Concentration in Solution
2.5.2. Determination of Critical Point of Calcium Carbonate Crystallization
2.6. Morphology and Structure Analysis of Calcium Carbonate Crystals
3. Results and Discussion
3.1. The Stability of Carbon Nanoparticles in Scale-Forming Solution
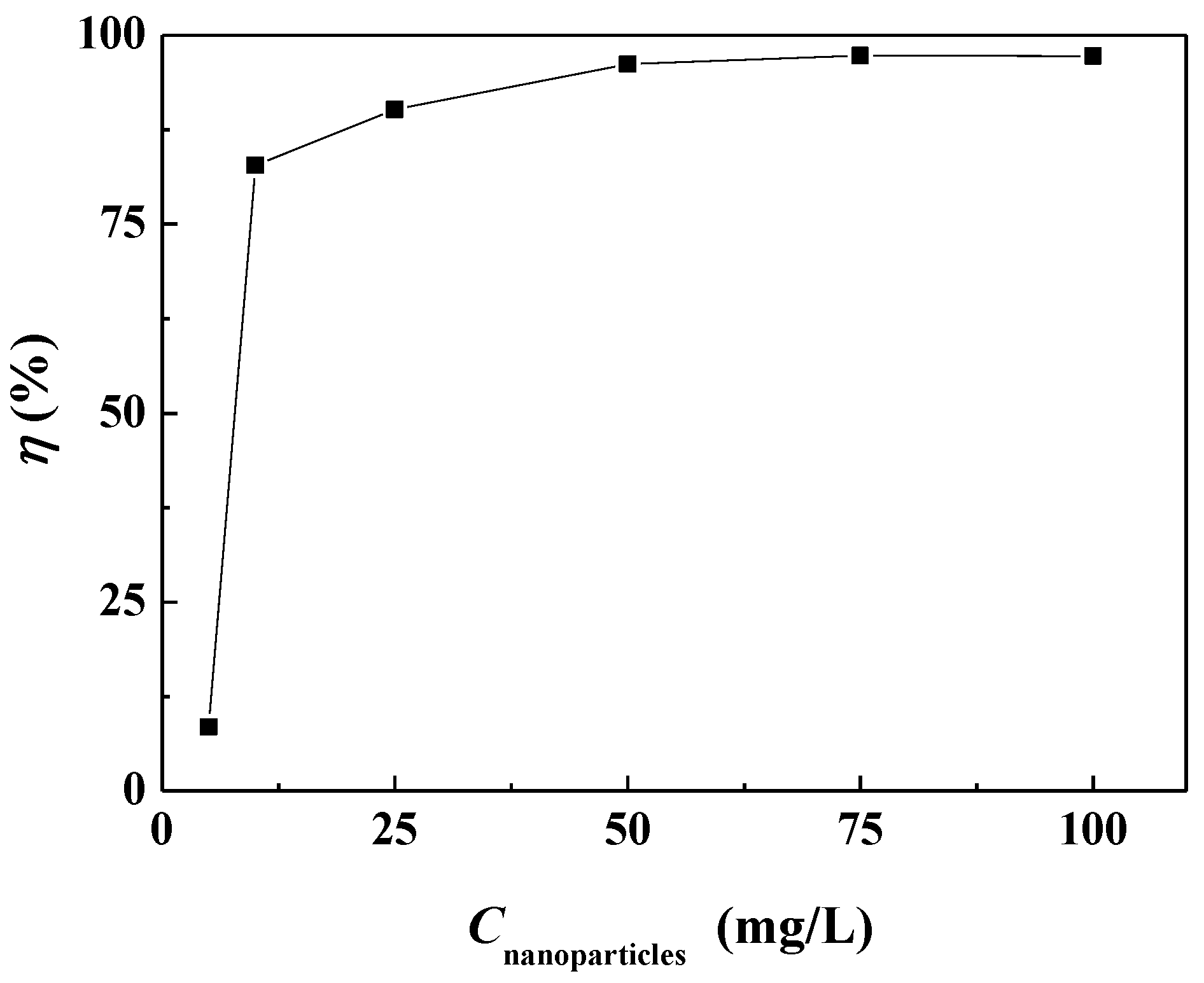
3.2. The Scale Inhibition Performance of Carbon Nanoparticles
3.3. Effect of Carbon Nanoparticles on the Concentration of Ca2+ in Solution
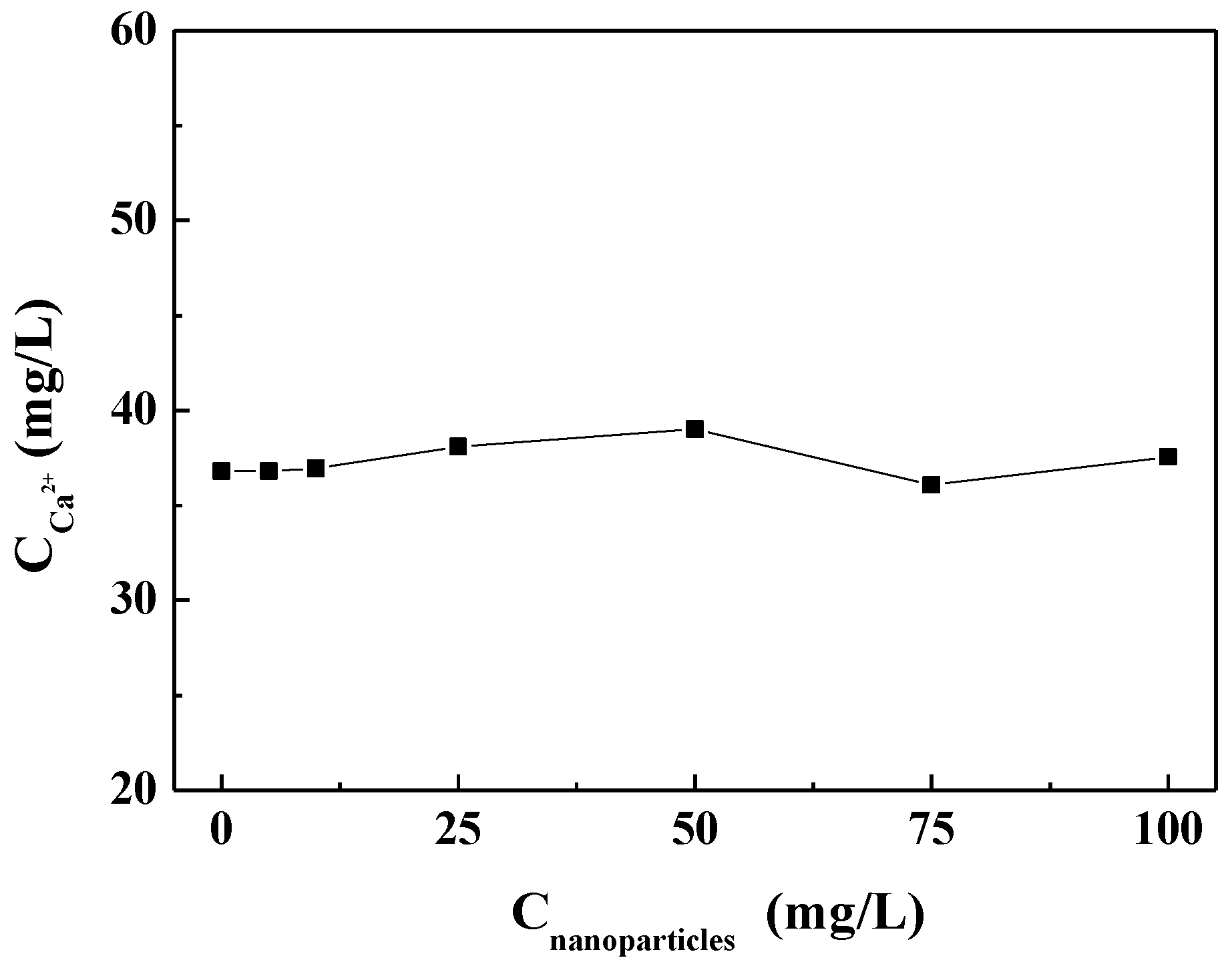
3.3.1. Effect of Carbon Nanoparticles on Residual Calcium Ion in Solution
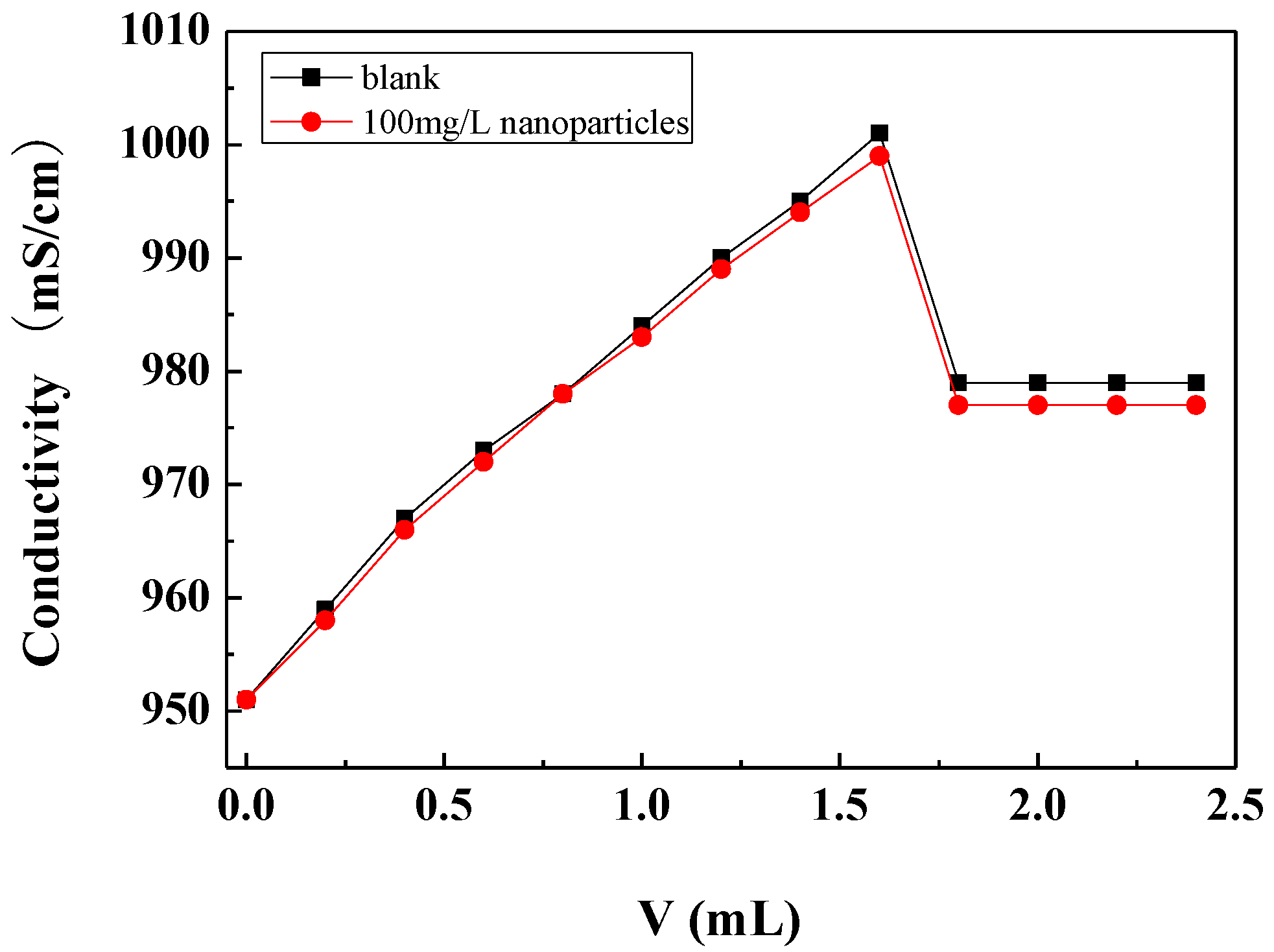
3.3.2. Effect of Carbon Nanoparticles on Critical Point of Calcium Carbonate Crystallization
3.4. Morphology and Structure Analysis of Calcium Carbonate Crystals
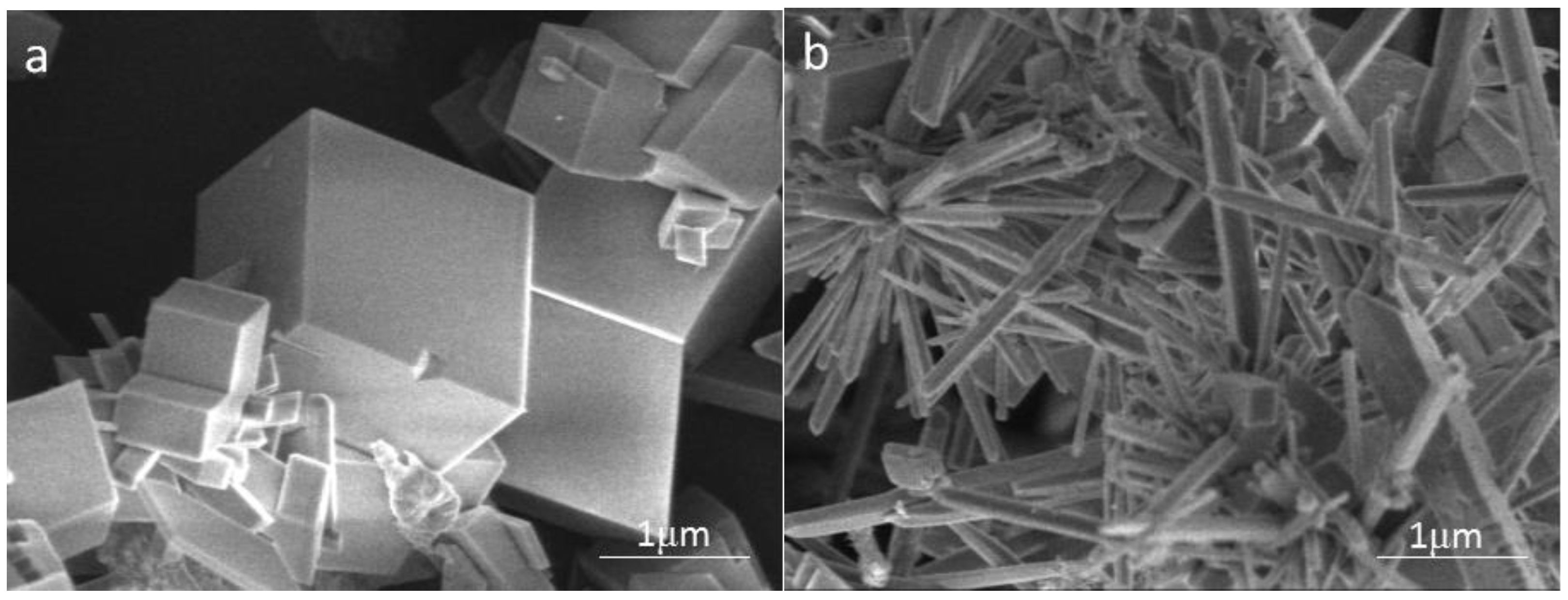
3.4.1. SEM Observation
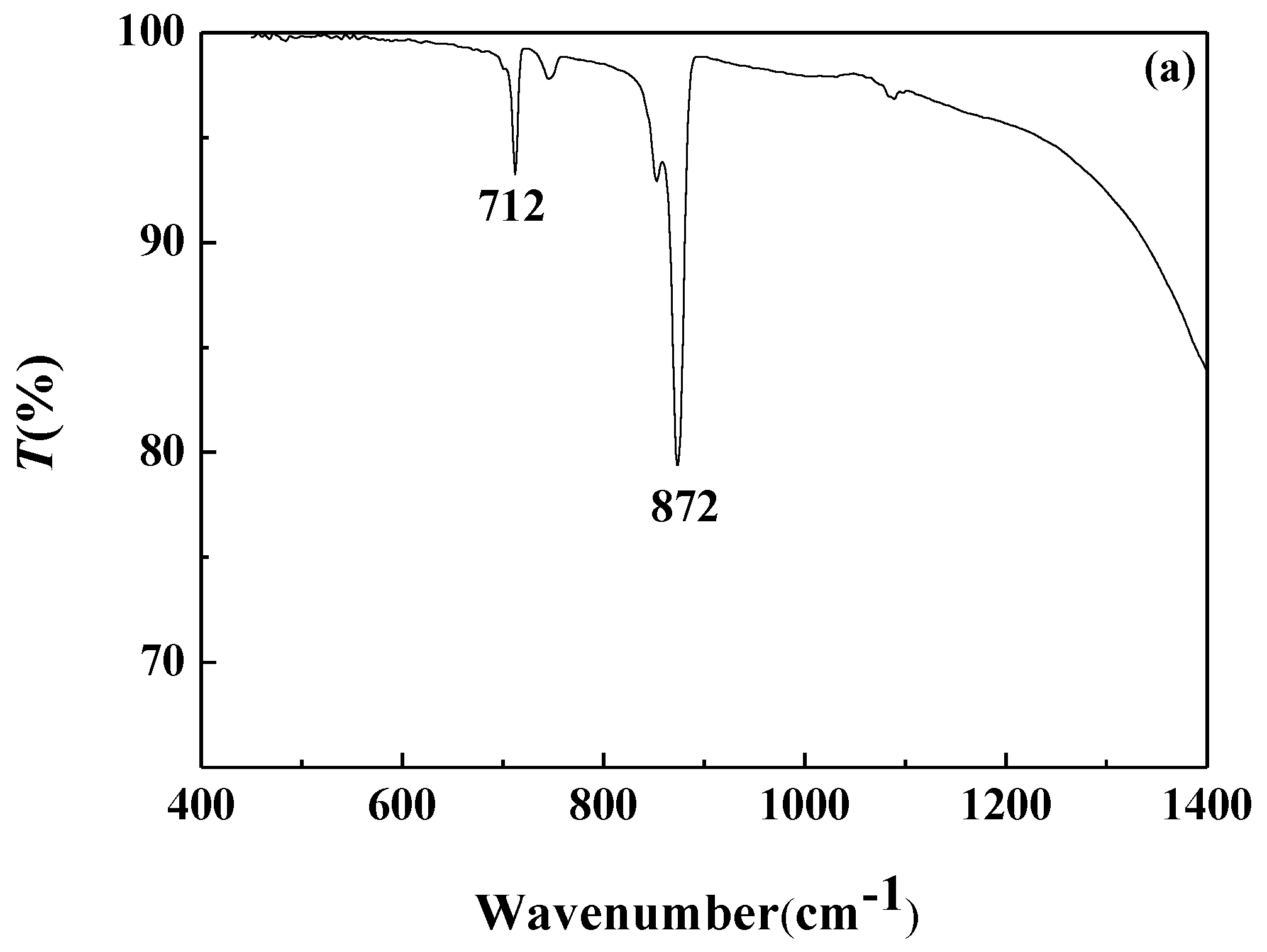
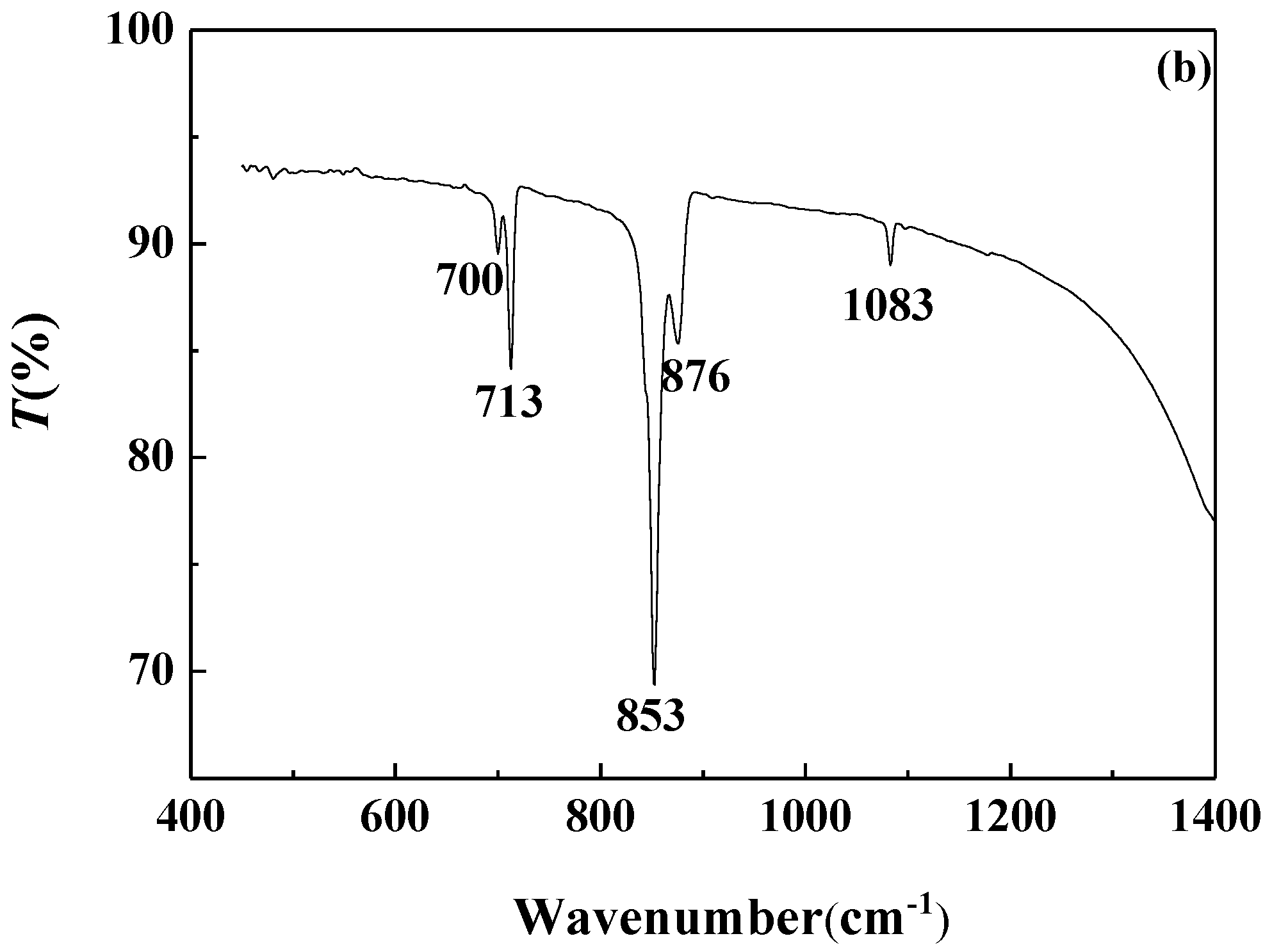
3.4.2. FTIR Analysis
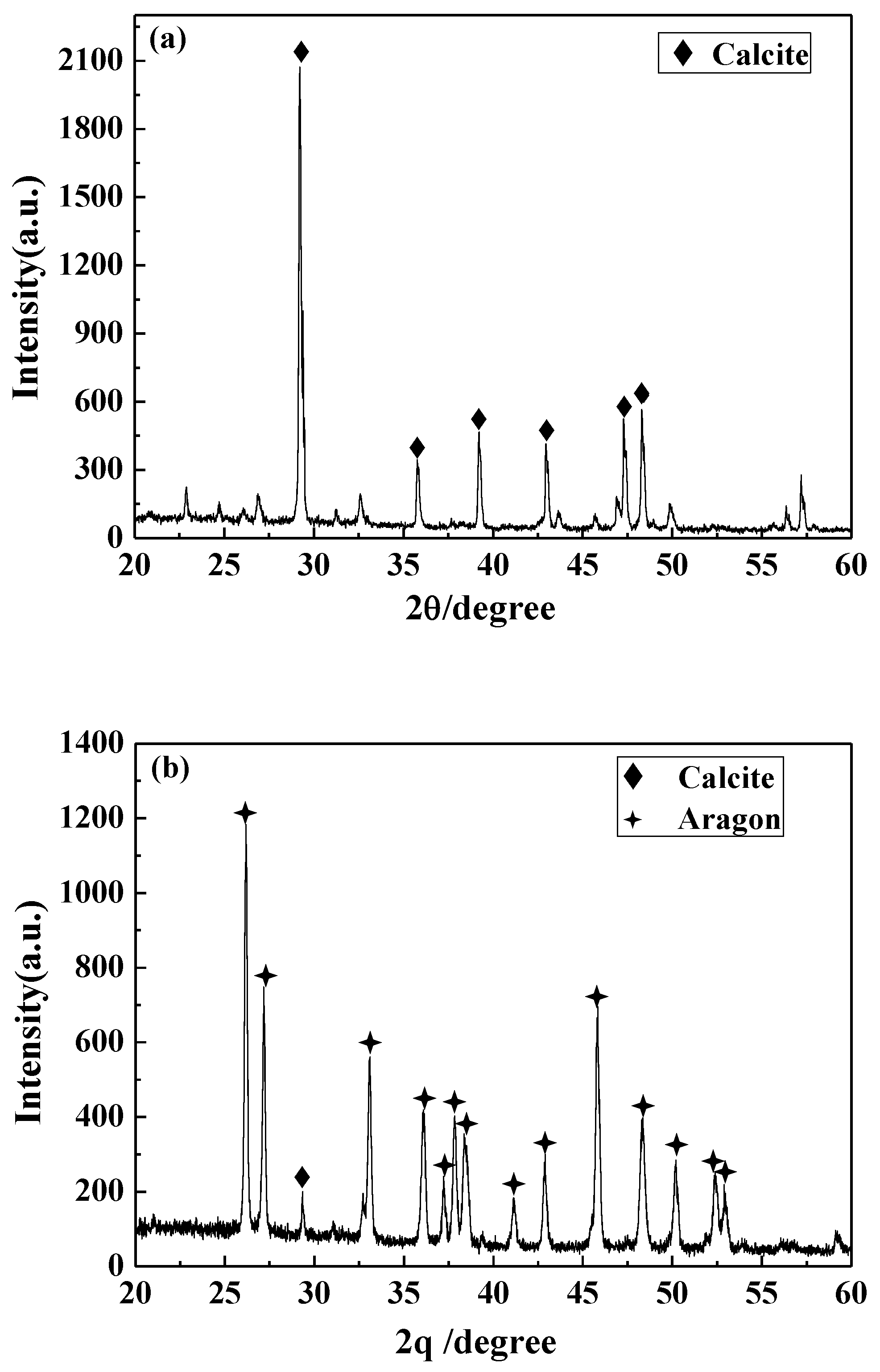
3.4.3. XRD Analysis
3.4.4. Effect of Carbon Nanoparticles on the Crystallization Process of Calcium Carbonate
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rubio-Castro, E.; Serna-González, M.; Ponce-Ortega, J.M.; El-Halwagi, M.M. Synthesis of cooling water systems with multiple cooling towers. Appl. Therm. Eng. 2013, 50, 957–974. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Z.; Xu, C.; Li, F.; Jin, H.; Shi, L.; Hu, H.Y. Water meta-cycle model and indicators for industrial processes—The pulp and paper case in China. Resour. Conserv. Recycl. 2018, 139, 228–236. [Google Scholar] [CrossRef]
- Alsadaie, S.M.; Mujtaba, I.M. Dynamic modelling of heat exchanger fouling in multistage flash (MSF) Desalination. Desalination 2017, 409, 47–65. [Google Scholar] [CrossRef]
- Rahmani, K.; Jadidian, R.; Haghtalab, S. Evaluation of inhibitors and biocides on the corrosion, scaling and biofouling control of carbon steel and copper–nickel alloys in a power plant cooling water system. Desalination 2016, 393, 174–185. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, Y.; Le, J.; Qian, M.; Huan, Y.; Yang, W.; Chen, Y. Highly effective scale inhibition performance of amino trimethylenephosphonic acid on calcium carbonate. Desalination 2017, 422, 165–173. [Google Scholar] [CrossRef]
- Smeets, P.J.; Cho, K.R.; Kempen, R.G.; Sommerdijk, N.A.; De Yoreo, J.J. Calcium carbonate nucleation driven by ion binding in a biomimetic matrix revealed by in situ electron microscopy. Nat. Mater. 2015, 14, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Piyadasa, C.; Ridgway, H.F.; Yeager, T.R.; Stewart, M.B.; Pelekani, C.; Gray, S.R.; Orbell, J.D. The application of electromagnetic fields to the control of the scaling and biofouling of reverse osmosis membranes—A review. Desalination 2017, 418, 19–34. [Google Scholar] [CrossRef]
- Zhao, J.D.; Liu, Z.A.; Zhao, E.J. Combined effect of constant high voltage electrostatic field and variable frequency pulsed electromagnetic field on the morphology of calcium carbonate scale in circulating cooling water systems. Water Sci. Technol. 2014, 70, 1074–1082. [Google Scholar] [CrossRef]
- Wang, J.; Liang, Y. Anti-fouling effect of axial alternating electromagnetic field on calcium carbonate fouling in U-shaped circulating cooling water heat exchange tube. Int. J. Heat Mass Transf. 2017, 115, 774–781. [Google Scholar] [CrossRef]
- Qin, Z.; Ren, X.; Shan, L.; Guo, H.; Geng, C.; Zhang, G.; Liang, Y. Nacrelike-structured multilayered polyelectrolyte/calcium carbonate nanocomposite membrane via Ca-incorporated layer-by-layer-assembly and CO2—Induced biomineralization. J. Membr. Sci. 2016, 498, 180–191. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Yao, Q.; Ma, S.; Wu, W.; Sun, W. Synthesis of fluorescent-tagged scale inhibitor and evaluation of its calcium carbonate precipitation performance. Desalination 2014, 340, 1–10. [Google Scholar] [CrossRef]
- Chaussemier, M.; Pourmohtasham, E.; Gelus, D.; Pécoul, N.; Perrot, H.; Lédion, J.; Horner, O. State of art of natural inhibitors of calcium carbonate scaling. A review article. Desalination 2015, 356, 47–55. [Google Scholar] [CrossRef]
- Yang, L.; Yang, W.; Xu, B.; Yin, X.; Chen, Y.; Liu, Y.; Huan, Y. Synthesis and scale inhibition performance of a novel environmental friendly and hydrophilic terpolymer inhibitor. Desalination 2017, 416, 166–174. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Gao, Y.; Fan, L.; Roddick, F.A.; Liu, Z. Antiscaling effect of polyaspartic acid and its derivative for RO membranes used for saline wastewater and brackish water desalination. Desalination 2017, 404, 224–229. [Google Scholar] [CrossRef]
- Yu, W.; Choi, S.U.S. The role of interfacial layers in the enhanced thermal conductivity of nanofluids: A renovated Hamilton–Crosser model. J. Nanopart. Res. 2004, 6, 355–361. [Google Scholar] [CrossRef]
- Eastman, J.A.; Choi, U.S.; Li, S.; Thompson, L.J.; Lee, S. Enhanced thermal conductivity through the development of nanofluids. MRS Proc. 1996, 457. [Google Scholar] [CrossRef]
- Eastman, J.A.; Choi, S.U.S.; Li, S.; Yu, W.; Thompson, L.J. Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl. Phys. Lett. 2001, 78, 718–720. [Google Scholar] [CrossRef]
- Khanafer, K.; Vafai, K.; Lightstone, M. Buoyancy-driven heat transfer enhancement in a two-dimensional enclosure utilizing nanofluids. Int. J. Heat Mass Transf. 2003, 46, 3639–3653. [Google Scholar] [CrossRef]
- Buongiorno, J. Convective transport in nanofluids. J. Heat Transf. 2006, 128, 240–250. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Mirlohi, A.; Nazari, M.A.; Ghasempour, R. A review of thermal conductivity of various nanofluids. J. Mol. Liq. 2018, 265, 181–188. [Google Scholar] [CrossRef]
- Singh, S.K.; Sarkar, J. Energy, exergy and economic assessments of shell and tube condenser using hybrid nanofluid as coolant. Int. Commun. Heat Mass Transf. 2018, 98, 41–48. [Google Scholar] [CrossRef]
- Xu, G.; Chen, W.; Deng, S.; Zhang, X.; Zhao, S. Performance evaluation of a nanofluid-based direct absorption solar collector with parabolic trough concentrator. Nanomaterials 2015, 5, 2131–2147. [Google Scholar] [CrossRef] [PubMed]
- Micali, F.; Milanese, M.; Colangelo, G.; de Risi, A. Experimental investigation on 4-strokes biodiesel engine cooling system based on nanofluid. Renew. Energy 2018, 125, 319–326. [Google Scholar] [CrossRef]
- Colangelo, G.; Favale, E.; Milanese, M.; de Risi, A.; Laforgia, D. Cooling of electronic devices: Nanofluids contribution. Appl. Therm. Eng. 2017, 127, 421–435. [Google Scholar] [CrossRef]
- Saidur, R.; Leong, K.Y.; Mohammad, H. A review on applications and challenges of nanofluids. Renew. Sust. Energ. Rev. 2011, 15, 1646–1668. [Google Scholar] [CrossRef]
- Chamsa-ard, W.; Brundavanam, S.; Fung, C.C.; Fawcett, D.; Poinern, G. Nanofluid types, their synthesis, properties and incorporation in direct solar thermal collectors: A review. Nanomaterials 2017, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Chen, Y.; Liang, X.; Xu, J.; Lee, C.; Liang, Q.; Deng, T. Dispersion stability of thermal nanofluids. Prog. Nat. Sci. 2017, 27, 531–542. [Google Scholar] [CrossRef]
- Hood, M.A.; Landfester, K.; Munoz-Espi, R. Chitosan nanoparticles affect polymorph selection in crystallization of calcium carbonate. Colloids Surf. A: Physicochem. Eng. Asp. 2018, 540, 48–52. [Google Scholar] [CrossRef]
- Ghorbani, N.; Wilson, M.C.T. Adsorption of polyphosphinocarboxylic acid (PPCA) scale inhibitor on carbon nanotubes (CNTs): A prospective method for enhanced oilfield scale prevention. J. Petrol. Sci. Eng. 2017, 150, 305–311. [Google Scholar] [CrossRef]
- Kiaei, Z.; Haghtalab, A. Experimental study of using Ca-DTPMP nanoparticles in inhibition of CaCO3 scaling in a bulk water process. Desalination 2014, 338, 84–92. [Google Scholar] [CrossRef]
- Al Nasser, W.N.; Shah, U.V.; Nikiforou, K.; Petrou, P.; Heng, J.Y.Y. Effect of silica nanoparticles to prevent calcium carbonate scaling using an in situ turbidimetre. Chem. Eng. Res. Des. 2016, 110, 98–107. [Google Scholar] [CrossRef]
- Gomez, V.; Correas, C.; Barron, A.R. Effect of carbon nanotubes on calcium carbonate/calcium silicate phase and morphology. Main Group Chem. 2017, 16, 57–65. [Google Scholar] [CrossRef]
- Anderson, R.E.; Barron, A.R. Effect of carbon nanomaterials on calcium carbonate crystallization. Main Group Chem. 2005, 4, 279–289. [Google Scholar] [CrossRef]
- Yoon, S.; In, I. Role of poly (N-vinyl-2-pyrrolidone) as stabilizer for dispersion of graphene via hydrophobic interaction. J. Mater. Sci. 2011, 46, 1316–1321. [Google Scholar] [CrossRef]
- Wang, J.; Liang, Y.; Chen, S. Influence of electromagnetic field intensity on the metastable zone width of CaCO3 crystallization in circulating water. J. Cryst. Growth 2016, 450, 56–60. [Google Scholar] [CrossRef]
- Drak, A.; Glucina, K.; Busch, M.; Hasson, D.; Laîne, J.M.; Semiat, R. Laboratory technique for predicting the scaling propensity of RO feed waters. Desalination 2000, 132, 233–242. [Google Scholar] [CrossRef]
- Valiakhmetova, A.; Sorbie, K.S.; Boak, L.S.; Shaw, S.S. Solubility and inhibition efficiency of phosphonate scale inhibitor_calcium_magnesium complexes for application in a precipitation-squeeze treatment. SPE Prod. Oper. 2017, 32, 343–350. [Google Scholar] [CrossRef]
- Huang, H.; Yao, Q.; Jiao, Q.; Liu, B.; Chen, H. Polyepoxysuccinic acid with hyper-branched structure as an environmentally friendly scale inhibitor and its scale inhibition mechanism. J. Saudi Chem. Soc 2019, 23, 61–74. [Google Scholar] [CrossRef]
- Abdel-Aal, N.; Sawada, K. Inhibition of adhesion and precipitation of CaCO3 by aminopolyphosphonate. J. Cryst. Growth 2003, 256, 188–200. [Google Scholar] [CrossRef]
- Legodi, M.A.; Waal, D.D.; Potgieter, J.H.; Potgieter, S.S. Rapid determination of CaCO3, in mixtures utilising FT-IR spectroscopy. Miner. Eng. 2001, 14, 1107–1111. [Google Scholar] [CrossRef]
- Shafiu Kamba, A.; Ismail, M.; Tengku Ibrahim, T.A.; Zakaria, Z.A.B. Synthesis and characterisation of, calcium carbonate aragonite nanocrystals from cockle shell powder (Anadara granosa). J. Nanomater. 2013, 2013, 95–107. [Google Scholar] [CrossRef]
- Liang, P.; Ying, Z.; Qiang, S.; Wang, D.; Xu, D. The effect of carboxymethyl chitosan on the precipitation of calcium carbonate. J. Cryst. Growth 2004, 261, 571–576. [Google Scholar] [CrossRef]
- Al-Roomi, Y.M.; Hussain, K.F. Potential kinetic model for scaling and scale inhibition mechanism. Desalination 2016, 393, 186–195. [Google Scholar] [CrossRef]


| Dispersion Conditions | PVP | Ultrasonic | Ultrasonic + PVP |
|---|---|---|---|
| Surface scale inhibition efficiency (η) | 0.12% | −0.33% | 0.46% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, C.; Wang, L.-T.; Sha, J.-Y.; Ge, H.-H. Effect of Carbon Nanoparticles on the Crystallization of Calcium Carbonate in Aqueous Solution. Nanomaterials 2019, 9, 179. https://doi.org/10.3390/nano9020179
Wan C, Wang L-T, Sha J-Y, Ge H-H. Effect of Carbon Nanoparticles on the Crystallization of Calcium Carbonate in Aqueous Solution. Nanomaterials. 2019; 9(2):179. https://doi.org/10.3390/nano9020179
Chicago/Turabian StyleWan, Chuan, Le-Tian Wang, Jun-Yi Sha, and Hong-Hua Ge. 2019. "Effect of Carbon Nanoparticles on the Crystallization of Calcium Carbonate in Aqueous Solution" Nanomaterials 9, no. 2: 179. https://doi.org/10.3390/nano9020179
APA StyleWan, C., Wang, L.-T., Sha, J.-Y., & Ge, H.-H. (2019). Effect of Carbon Nanoparticles on the Crystallization of Calcium Carbonate in Aqueous Solution. Nanomaterials, 9(2), 179. https://doi.org/10.3390/nano9020179




