Influence of Six Carbon-Based Nanomaterials on the Rheological Properties of Nanofluids
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Nanopowder Characterization
2.3. Methods
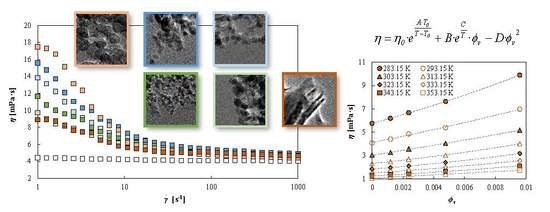
3. Results and Discussion
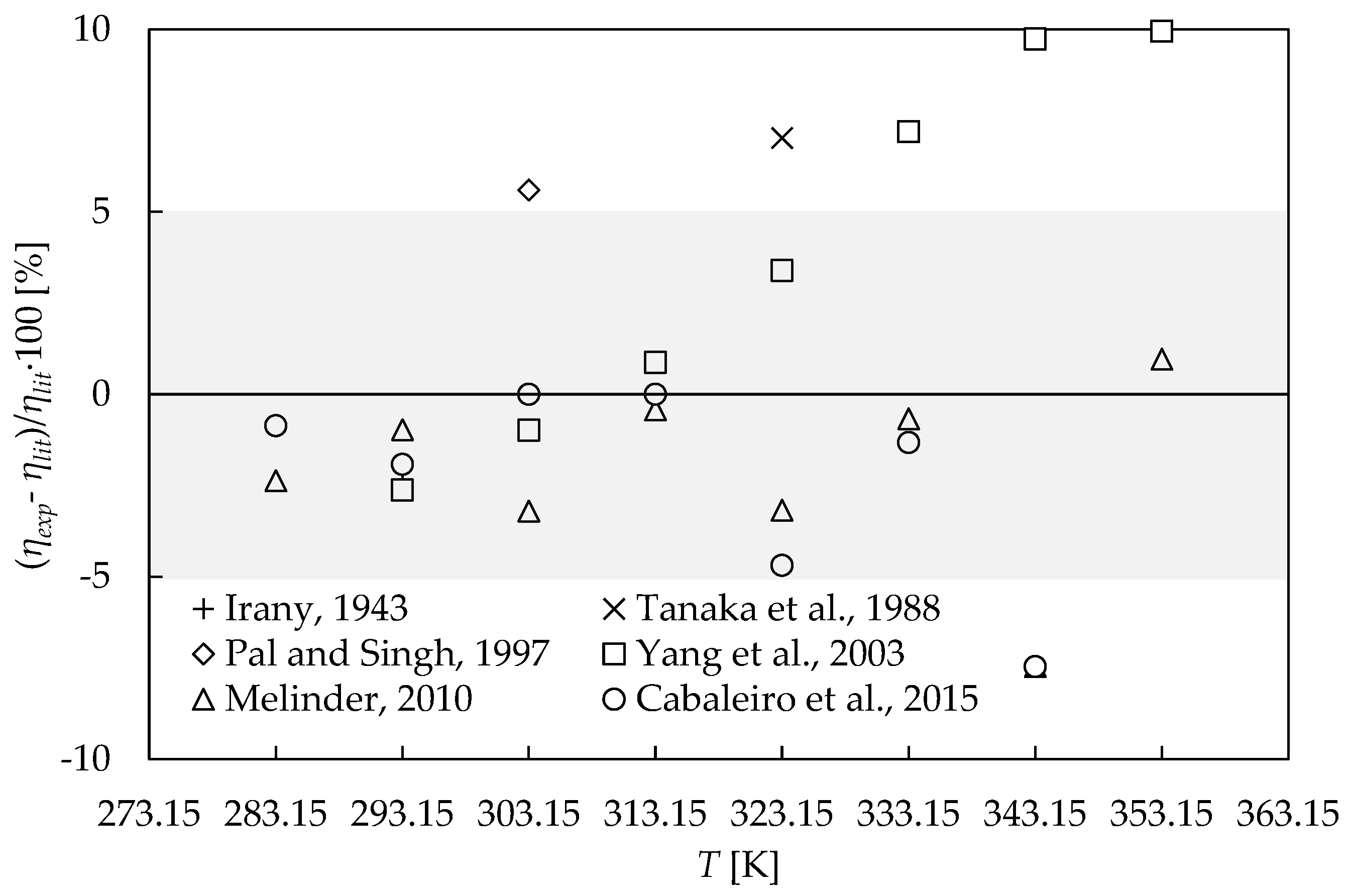
3.1. Experimental Validation
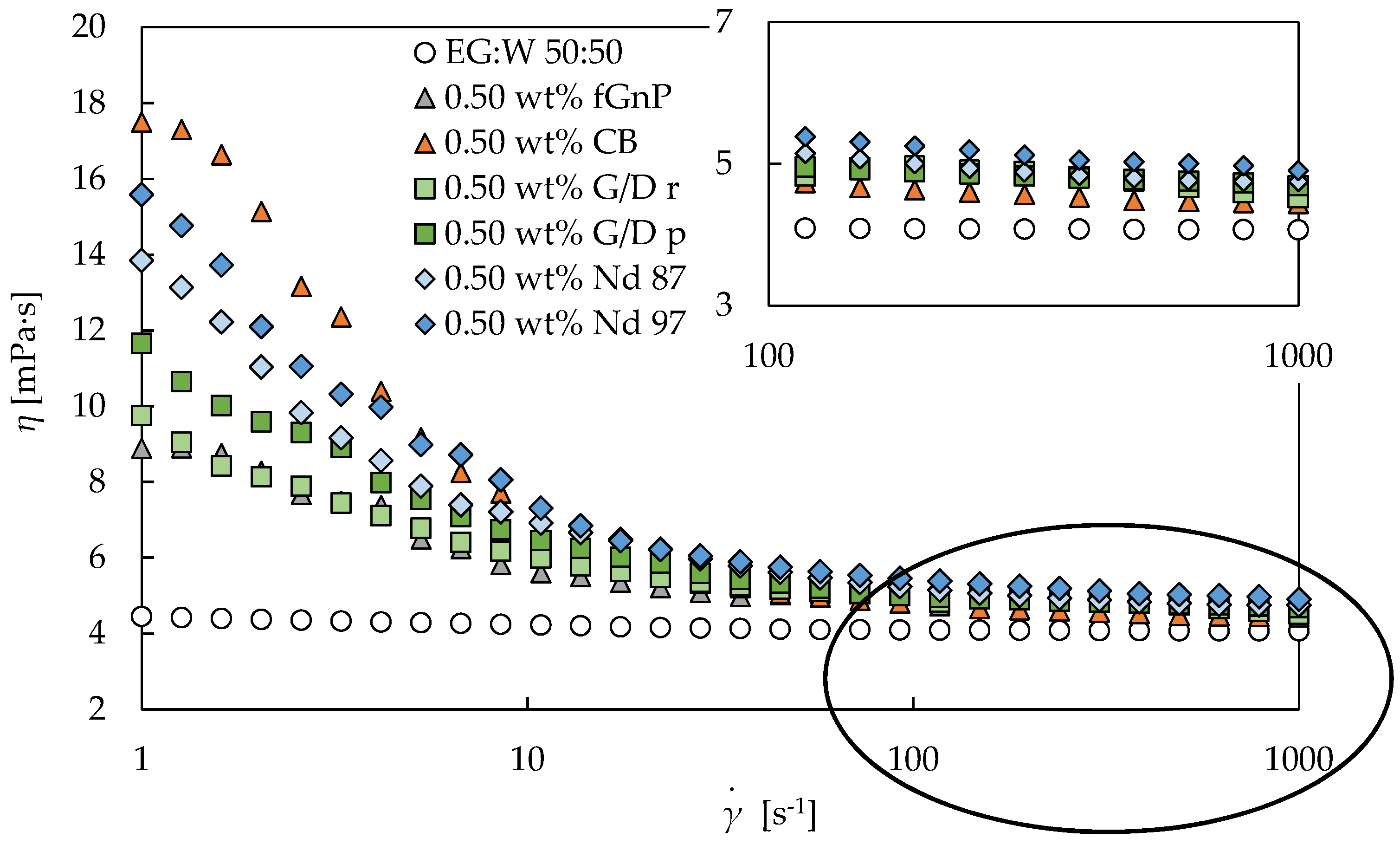
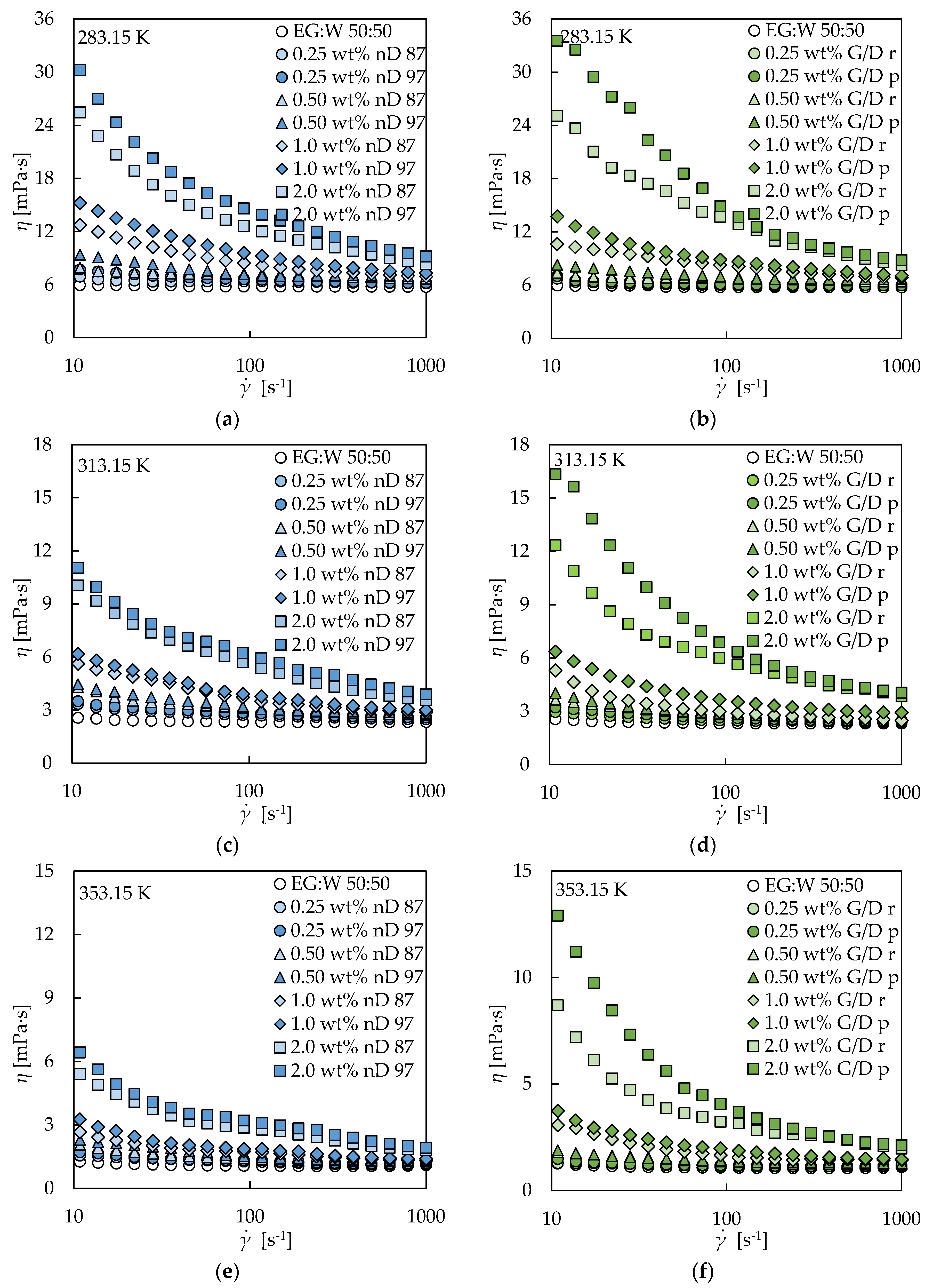
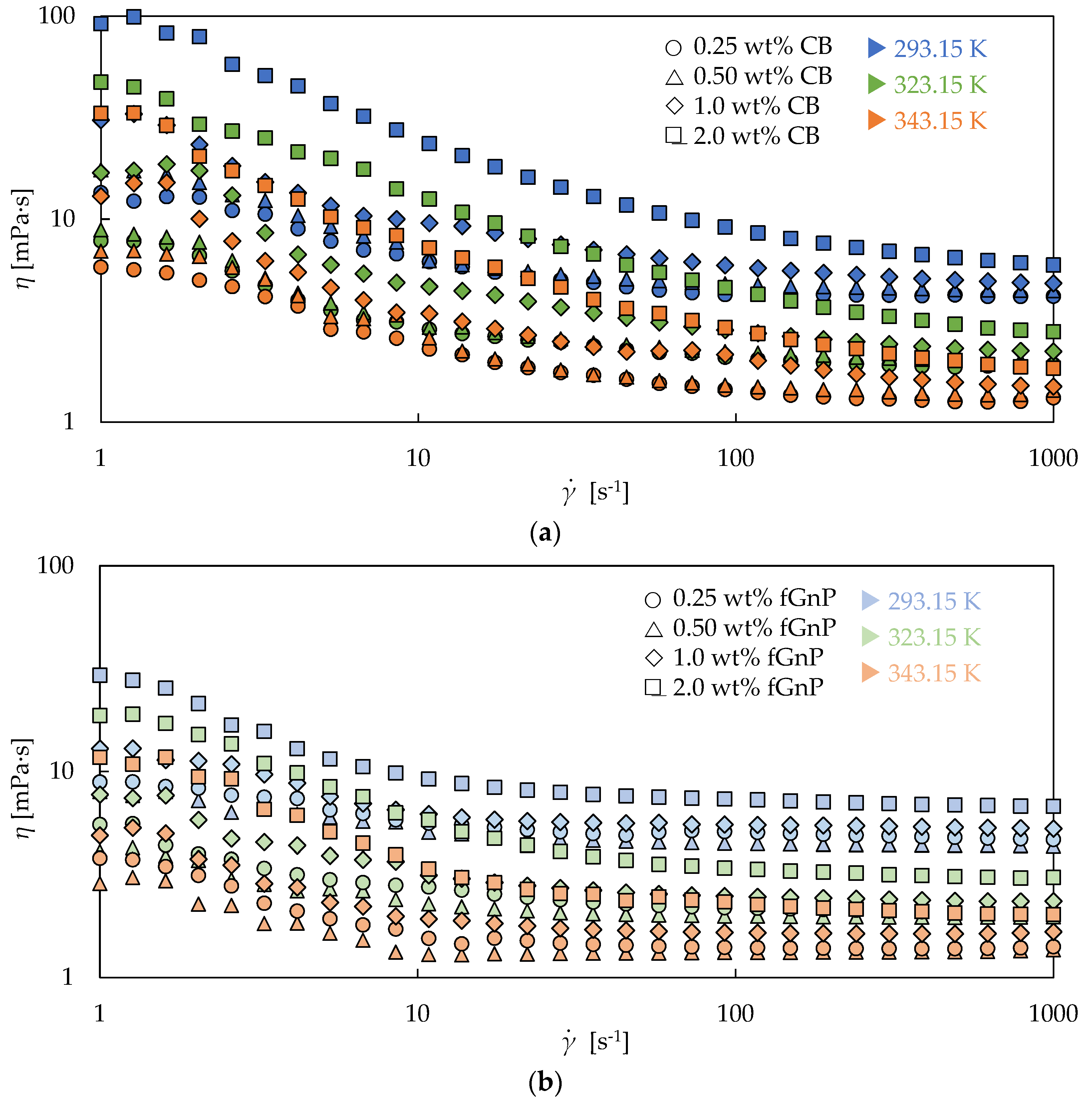
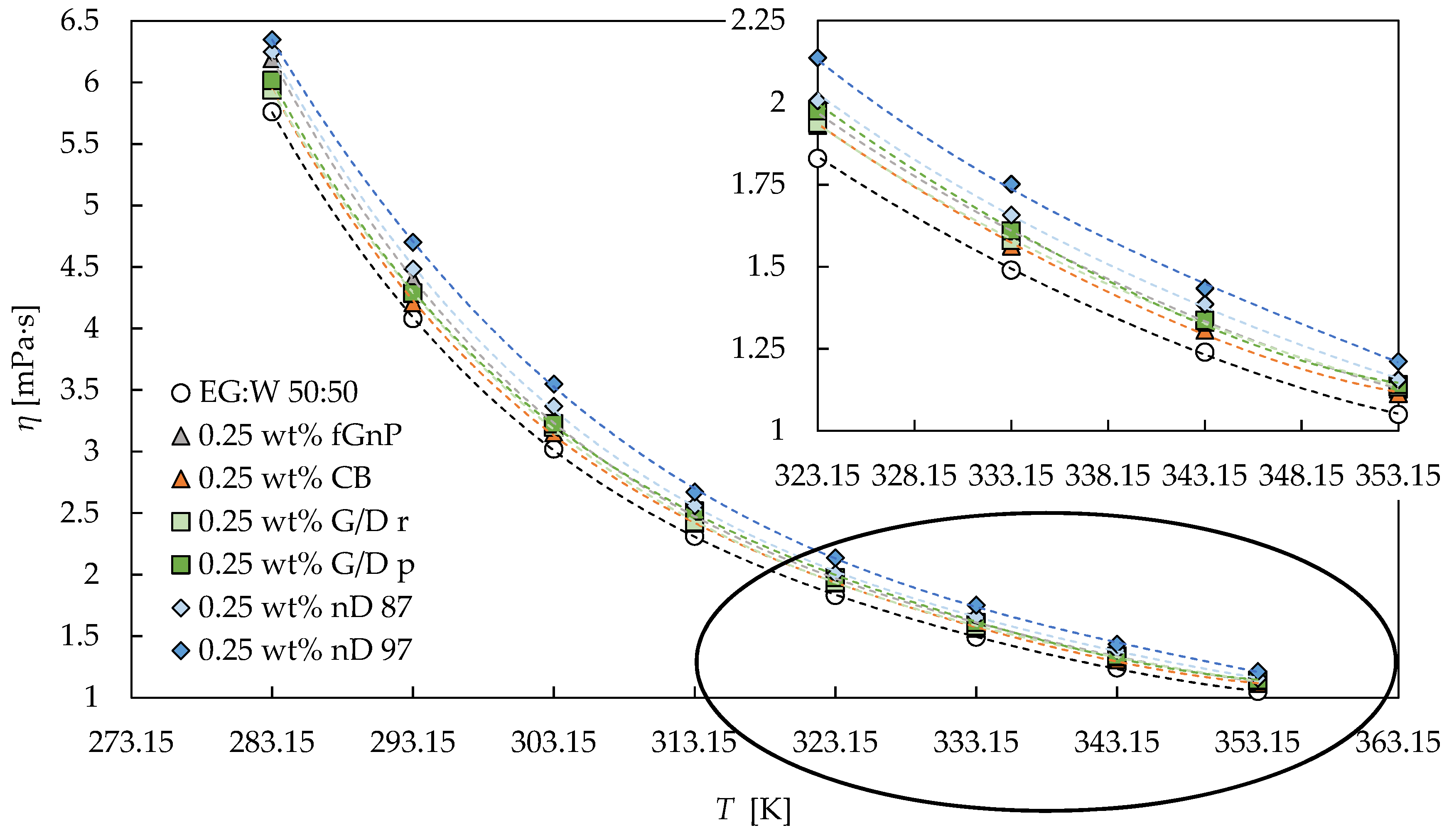
3.2. Rotational Rheology
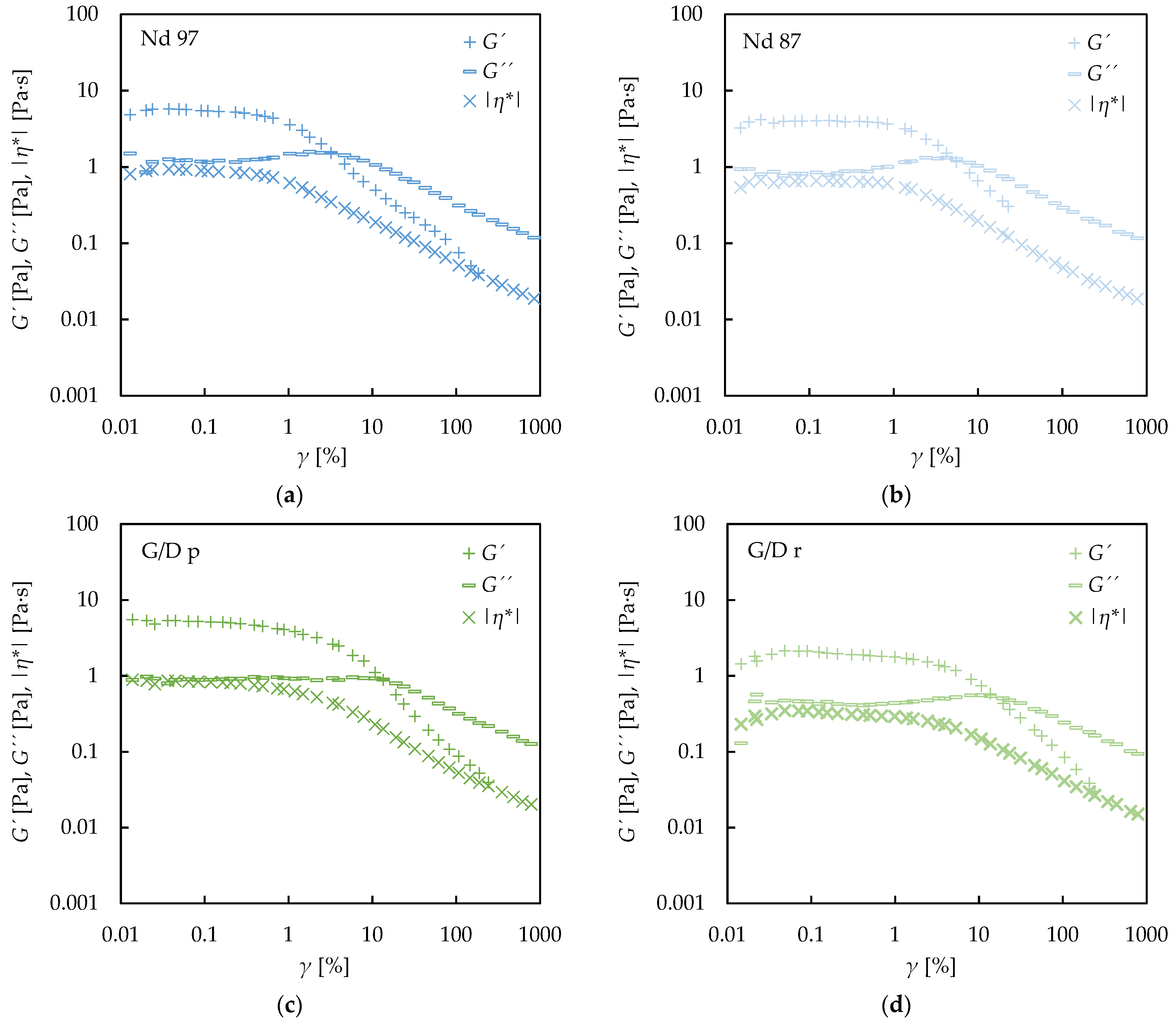
3.3. Oscillatory Rheology
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Nomenclature | |
| AAD | Absolute average deviation |
| ω | Angular frequency |
| CB | Carbon Black |
| η* | Complex viscosity |
| γ | Deformation |
| η | Dynamic viscosity |
| EG:W 50:50 | Ethylene glycol:water 50:50 vol% mixture |
| η0, η∞, k, m | Fitting parameters from Cross model, Equation (2) |
| K, n | Fitting parameters from Ostwald-de Waele model, Equation (1) |
| B, C, D | Fitting parameters from Vallejo et al.’s model, Equation (4) |
| η0, A, T0 | Fitting parameters from VFT model, Equation (3) |
| GnP | Graphene nanoplatelet |
| G/D | Graphite/diamond mixture |
| G/D p | Graphite/Diamond Nano-Mixture Purified |
| G/D r | Graphite/Diamond Nano-Mixture Raw |
| G″ | Loss modulus |
| wt% | Nanoadditive mass concentration, % |
| ϕm | Nanoadditive mass fraction |
| vol% | Nanoadditive volume concentration, % |
| ϕv | Nanoadditive volume fraction |
| Nd | Nanodiamond |
| nD 97 | NanoDiamnods Purified grade G01 |
| nD 87 | NanoDiamnods Purified grade G |
| Shear rate | |
| s | Standard deviation |
| G′ | Storage modulus |
| fGnP | Sulfonic acid-functionalized graphene nanoplatelets |
| T | Temperature |
| TEM | Transmission electron microscopy |
| VFT | Vogel-Fulcher-Tammann |
| Subscripts | |
| exp | Experimental value |
| lit | Literature value |
References
- Cheng, M.; Zhu, Y. The state of the art of wind energy conversion systems and technologies: A review. Energy Convers. Manag. 2014, 88, 332–347. [Google Scholar] [CrossRef]
- Ellabban, O.; Abu-Rub, H.; Blaabjerg, F. Renewable energy resources: Current status, future prospects and their enabling technology. Renew. Sustain. Energy Rev. 2014, 39, 748–764. [Google Scholar] [CrossRef]
- REN21. Renewables 2018 Global Status Report; REN21: Paris, France, 2018. [Google Scholar]
- Thulukkanam, K. Heat Exchanger Design Handbook; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Sheikholeslami, M.; Rashidi, M.; Hayat, T.; Ganji, D. Free convection of magnetic nanofluid considering mfd viscosity effect. J. Mol. Liq. 2016, 218, 393–399. [Google Scholar] [CrossRef]
- Choi, S. Enhancing thermal conductivity of fluids with nanoparticles. ASME-Publications-Fed 1995, 231, 99–106. [Google Scholar]
- Ganvir, R.; Walke, P.; Kriplani, V. Heat transfer characteristics in nanofluid-a review. Renew. Sustain. Energy Rev. 2017, 75, 451–460. [Google Scholar] [CrossRef]
- Esfahani, M.A.; Toghraie, D. Experimental investigation for developing a new model for the thermal conductivity of silica/water-ethylene glycol (40%–60%) nanofluid at different temperatures and solid volume fractions. J. Mol. Liq. 2017, 232, 105–112. [Google Scholar] [CrossRef]
- Machrafi, H.; Lebon, G. The role of several heat transfer mechanisms on the enhancement of thermal conductivity in nanofluids. Cont. Mech. Therm. 2016, 28, 1461–1475. [Google Scholar] [CrossRef] [Green Version]
- Devendiran, D.K.; Amirtham, V.A. A review on preparation, characterization, properties and applications of nanofluids. Renew. Sustain. Energy Rev. 2016, 60, 21–40. [Google Scholar] [CrossRef]
- Hedayatnasab, Z.; Abnisa, F.; Daud, W.M.A.W. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater. Des. 2017, 123, 174–196. [Google Scholar] [CrossRef]
- Khanafer, K.; Vafai, K. A review on the applications of nanofluids in solar energy field. Renew. Energy 2018, 123, 398–406. [Google Scholar] [CrossRef]
- Chamsa-ard, W.; Brundavanam, S.; Fung, C.; Fawcett, D.; Poinern, G. Nanofluid types, their synthesis, properties and incorporation in direct solar thermal collectors: A review. Nanomaterials 2017, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, A.; Sharshir, S.; Mostafa, M.E.; Essa, F.; Ali, M.K.A. Applications of nanofluids in solar energy: A review of recent advances. Renew. Sustain. Energy Rev. 2017, 82, 3483–3502. [Google Scholar] [CrossRef]
- Sani, E.; Vallejo, J.P.; Cabaleiro, D.; Lugo, L. Functionalized graphene nanoplatelet-nanofluids for solar thermal collectors. Sol. Energy Mater. Sol. Cells 2018, 185, 205–209. [Google Scholar] [CrossRef]
- Xu, G.; Chen, W.; Deng, S.; Zhang, X.; Zhao, S. Performance evaluation of a nanofluid-based direct absorption solar collector with parabolic trough concentrator. Nanomaterials 2015, 5, 2131–2147. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.R.; Safdari Shadloo, M.; Goodarzi, M.S.; Hadjadj, A.; Goshayeshi, H.R.; Afrand, M.; Kazi, S. A survey on experimental and numerical studies of convection heat transfer of nanofluids inside closed conduits. Adv. Mech. Eng. 2016, 8. [Google Scholar] [CrossRef] [Green Version]
- Ilyas, S.U.; Pendyala, R.; Narahari, M.; Susin, L. Stability, rheology and thermal analysis of functionalized alumina-thermal oil-based nanofluids for advanced cooling systems. Energy Convers. Manag. 2017, 142, 215–229. [Google Scholar] [CrossRef]
- Mahian, O.; Kolsi, L.; Amani, M.; Estellé, P.; Ahmadi, G.; Kleinstreuer, C.; Marshall, J.S.; Taylor, R.A.; Abu-Nada, E.; Rashidi, S.; et al. Recent advances in modeling and simulation of nanofluid flows—Part II: Applications. Phys. Rep. 2018. [Google Scholar] [CrossRef]
- Bashirnezhad, K.; Bazri, S.; Safaei, M.R.; Goodarzi, M.; Dahari, M.; Mahian, O.; Dalkılıça, A.S.; Wongwises, S. Viscosity of nanofluids: A review of recent experimental studies. Int. Commun. Heat Mass 2016, 73, 114–123. [Google Scholar] [CrossRef]
- Phuoc, T.X.; Massoudi, M.; Chen, R.-H. Viscosity and thermal conductivity of nanofluids containing multi-walled carbon nanotubes stabilized by chitosan. Int. J. Therm. Sci. 2011, 50, 12–18. [Google Scholar] [CrossRef]
- Halelfadl, S.; Estellé, P.; Aladag, B.; Doner, N.; Maré, T. Viscosity of carbon nanotubes water-based nanofluids: Influence of concentration and temperature. Int. J. Therm. Sci. 2013, 71, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Sadri, R.; Ahmadi, G.; Togun, H.; Dahari, M.; Kazi, S.N.; Sadeghinezhad, E.; Zubir, N. An experimental study on thermal conductivity and viscosity of nanofluids containing carbon nanotubes. Nanoscale Res. Lett. 2014, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-C.; Yang, J.-C.; Zhou, W.-W.; He, Y.-R.; Huang, Y.-M.; Jiang, B.-C. Experimental study on the characteristics of thermal conductivity and shear viscosity of viscoelastic-fluid-based nanofluids containing multiwalled carbon nanotubes. Thermochim. Acta 2013, 556, 47–53. [Google Scholar] [CrossRef]
- Baratpour, M.; Karimipour, A.; Afrand, M.; Wongwises, S. Effects of temperature and concentration on the viscosity of nanofluids made of single-wall carbon nanotubes in ethylene glycol. Int. Commun. Heat Mass 2016, 74, 108–113. [Google Scholar] [CrossRef]
- Alirezaie, A.; Saedodin, S.; Esfe, M.H.; Rostamian, S.H. Investigation of rheological behavior of mwcnt (cooh-functionalized)/mgo-engine oil hybrid nanofluids and modelling the results with artificial neural networks. J. Mol. Liq. 2017, 241, 173–181. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Shirazi, S.F.S.; Amiri, A.; Alehashem, M.S.; Dahari, M.; Kazi, S. Experimental investigation of thermo-physical properties, convective heat transfer and pressure drop of functionalized graphene nanoplatelets aqueous nanofluid in a square heated pipe. Energy Convers. Manag. 2016, 114, 38–49. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, S.-J. Influence of the nanoscaled hybrid based on nanodiamond@graphene oxide architecture on the rheological and thermo-physical performances of carboxylated-polymeric composites. Compos. Part A Appl. Sci. Manuf. 2018, 112, 356–364. [Google Scholar] [CrossRef]
- Esfe, M.H.; Hajmohammad, M.H. Thermal conductivity and viscosity optimization of nanodiamond-CO3O4/eg (40:60) aqueous nanofluid using nsga-ii coupled with rsm. J. Mol. Liq. 2017, 238, 545–552. [Google Scholar] [CrossRef]
- Marcos, M.A.; Cabaleiro, D.; Guimarey, M.J.; Comuñas, M.J.; Fedele, L.; Fernández, J.; Lugo, L. Peg 400-based phase change materials nano-enhanced with functionalized graphene nanoplatelets. Nanomaterials 2017, 8, 16. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, G.; Wang, N.; Zhang, X. Experimental study on the thermal start-up performance of the graphene/water nanofluid-enhanced solar gravity heat pipe. Nanomaterials 2018, 8, 72. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Szymczyk, A.; Pawlikowska, D.; Subocz, J.; Zenker, M.; Masztak, R. Electrically and thermally conductive low density polyethylene-based nanocomposites reinforced by mwcnt or hybrid mwcnt/graphene nanoplatelets with improved thermo-oxidative stability. Nanomaterials 2018, 8, 264. [Google Scholar] [CrossRef]
- Vallejo, J.P.; Gómez-Barreiro, S.; Cabaleiro, D.; Gracia-Fernández, C.; Fernández-Seara, J.; Lugo, L. Flow behaviour of suspensions of functionalized graphene nanoplatelets in propylene glycol–water mixtures. Int. Commun. Heat Mass 2018, 91, 150–157. [Google Scholar] [CrossRef]
- Vallejo, J.P.; Zyła, G.; Fernández-Seara, J.; Lugo, L. Rheological behaviour of functionalized graphene nanoplatelet nanofluids based on water and propylene glycol:Water mixtures. Int. Commun. Heat Mass 2018, 99, 43–53. [Google Scholar] [CrossRef]
- Minakov, A.; Rudyak, V.Y.; Pryazhnikov, M. Rheological behavior of water and ethylene glycol based nanofluids containing oxide nanoparticles. Colloids Surf. A 2018, 554, 279–285. [Google Scholar] [CrossRef]
- Żyła, G.; Vallejo, J.P.; Fal, J.; Lugo, L. Nanodiamonds-ethylene glycol nanofluids: Experimental investigation of fundamental physical properties. Int. J. Heat Mass Transf. 2018, 121, 1201–1213. [Google Scholar] [CrossRef]
- Żyła, G.; Fal, J.; Estellé, P. The influence of ash content on thermophysical properties of ethylene glycol based graphite/diamonds mixture nanofluids. Diam. Relat. Mater. 2017, 74, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Selvam, C.; Harish, S.; Lal, D.M. Effective thermal conductivity and rheological characteristics of ethylene glycol-based nanofluids with single-walled carbon nanohorn inclusions. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 86–93. [Google Scholar] [CrossRef]
- Agromayor, R.; Cabaleiro, D.; Pardiñas, A.A.; Vallejo, J.P.; Fernández-Seara, J.; Lugo, L. Heat transfer performance of functionalized graphene nanoplatelet aqueous nanofluids. Materials 2016, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Żyła, G.; Fal, J. Experimental studies on viscosity, thermal and electrical conductivity of aluminum nitride–ethylene glycol (aln-eg) nanofluids. Thermochim. Acta 2016, 637, 11–16. [Google Scholar] [CrossRef]
- Żyła, G.; Fal, J. Viscosity, thermal and electrical conductivity of silicon dioxide–ethylene glycol transparent nanofluids: An experimental studies. Thermochim. Acta 2017, 650, 106–113. [Google Scholar] [CrossRef]
- Irany, E.P. The viscosity function. Iv. Non-ideal systems. J. Am. Chem. Soc. 1943, 65, 1392–1398. [Google Scholar] [CrossRef]
- Pal, A.; Singh, W. Speeds of sound and viscosities in aqueous poly (ethylene glycol) solutions at 303.15 and 308.15 k. J. Chem. Eng. Data 1997, 42, 234–237. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ohta, K.; Kubota, H.; Makita, T. Viscosity of aqueous solutions of 1, 2-ethanediol and 1, 2-propanediol under high pressures. Int. J. Thermophys. 1988, 9, 511–523. [Google Scholar] [CrossRef]
- Yang, C.; Ma, P.; Jing, F.; Tang, D. Excess molar volumes, viscosities, and heat capacities for the mixtures of ethylene glycol + water from 273.15 k to 353.15 k. J. Chem. Eng. Data 2003, 48, 836–840. [Google Scholar] [CrossRef]
- Melinder, A. Properties of Secondary Working Fluids for Indirect Systems; International Institute of Refrigeration: Paris, France, 2010. [Google Scholar]
- Cabaleiro, D.; Colla, L.; Agresti, F.; Lugo, L.; Fedele, L. Transport properties and heat transfer coefficients of zno/(ethylene glycol+ water) nanofluids. Int. J. Heat Mass Transf. 2015, 89, 433–443. [Google Scholar] [CrossRef]
- Pastoriza-Gallego, M.J.; Lugo, L.; Legido, J.L.; Piñeiro, M.M. Rheological non-newtonian behaviour of ethylene glycol-based Fe2O3 nanofluids. Nanoscale Res. Lett. 2011, 6, 560. [Google Scholar] [CrossRef]
- Genc, S.; Derin, B. Synthesis and rheology of ferrofluids: A review. Curr. Opin. Chem. Eng. 2014, 3, 118–124. [Google Scholar] [CrossRef]
- Hermida-Merino, C.; Pérez-Rodríguez, M.; Pineiro, M.M.; Pastoriza-Gallego, M.J. Evidence of viscoplastic behavior of exfoliated graphite nanofluids. Soft Matter 2016, 12, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.; Gasiorek, J.; Swaffield, J.; Jack, L. Fluid Mechanics; Pearson Prentice Hall: Harlow, UK, 2005. [Google Scholar]
- Barnes, H.A.; Hutton, J.F.; Walters, K. An Introduction to Rheology; Elsevier: New York, NY, USA, 1989. [Google Scholar]
- Andersson, H.I.; Bech, K.H.; Dandapat, B.S. Magnetohydrodynamic flow of a power-law fluid over a stretching sheet. Int. J. Nonline Mech. 1992, 27, 929–936. [Google Scholar] [CrossRef]
- Cross, M.M. Rheology of non-newtonian fluids: A new flow equation for pseudoplastic systems. J. Colloid Interface Sci. 1965, 20, 417–437. [Google Scholar] [CrossRef]
- Kontopoulou, M. Applied Polymer Rheology: Polymeric Fluids with Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Tekalur, S.A.; Zavattieri, P.; Korach, C.S. Mechanics of Biological Systems and Materials, Volume 6; Springer: London, UK, 2016. [Google Scholar]
- Kringos, N.; Birgisson, B.; Frost, D.; Wang, L. Multi-Scale Modeling and Characterization of Infrastructure Materials; Proceedings of the International Rilem Symposium Stockholm, June 2013; Springer Science & Business Media: London, UK, 2013; Volume 8. [Google Scholar]
- Chen, H.-M.; Fu, X.; Luo, Z.-G. Properties and extraction of pectin-enriched materials from sugar beet pulp by ultrasonic-assisted treatment combined with subcritical water. Food Chem. 2015, 168, 302–310. [Google Scholar] [CrossRef]
- Rao, M.A. Rheology of Fluid, Semisolid, and Solid Foods: Principles and Applications; Springer Science & Business Media: London, UK, 2013. [Google Scholar]
- Sisko, A. The flow of lubricating greases. Ind. Eng. Chem. 1958, 50, 1789–1792. [Google Scholar] [CrossRef]
- Williamson, R.V. The flow of pseudoplastic materials. Ind. Eng. Chem. 1929, 21, 1108–1111. [Google Scholar] [CrossRef]
- Bansal, N.P.; Singh, J.P.; Ko, S.W.; Castro, R.H.R.; Pickrell, G.; Manjooran, N.J.; Nair, K.M.; Singh, G. Processing and Properties of Advanced Ceramics and Composites V; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Fulcher, G.S. Analysis of recent measurements of the viscosity of glasses. J. Am. Ceram. Soc. 1925, 8, 339–355. [Google Scholar] [CrossRef]
- Tammann, G.; Hesse, W. The dependence of viscosity upon the temperature of supercooled liquids. Z. Anorg. Allg. Chem. 1926, 156, 245–257. [Google Scholar] [CrossRef]
- Vogel, H. The law of the relation between the viscosity of liquids and the temperature. Phys. Z 1921, 22, 645–646. [Google Scholar]
- Vallejo, J.P.; Pérez-Tavernier, J.; Cabaleiro, D.; Fernández-Seara, J.; Lugo, L. Potential heat transfer enhancement of functionalized graphene nanoplatelet dispersions in a propylene glycol-water mixture. Thermophysical profile. J. Chem. Thermodyn. 2018, 123, 174–184. [Google Scholar] [CrossRef]
- Karimipour, A.; Bagherzadeh, S.A.; Goodarzi, M.; Alnaqi, A.A.; Bahiraei, M.; Safaei, M.R.; Shadloo, M.S. Synthesized CuFe2O4/SiO2 nanocomposites added to water/eg: Evaluation of the thermophysical properties beside sensitivity analysis & eann. Int. J. Heat Mass Transf. 2018, 127, 1169–1179. [Google Scholar]
- Afrand, M.; Nazari Najafabadi, K.; Sina, N.; Safaei, M.R.; Kherbeet, A.S.; Wongwises, S.; Dahari, M. Prediction of dynamic viscosity of a hybrid nano-lubricant by an optimal artificial neural network. Int. Commun. Heat Mass 2016, 76, 209–214. [Google Scholar] [CrossRef]
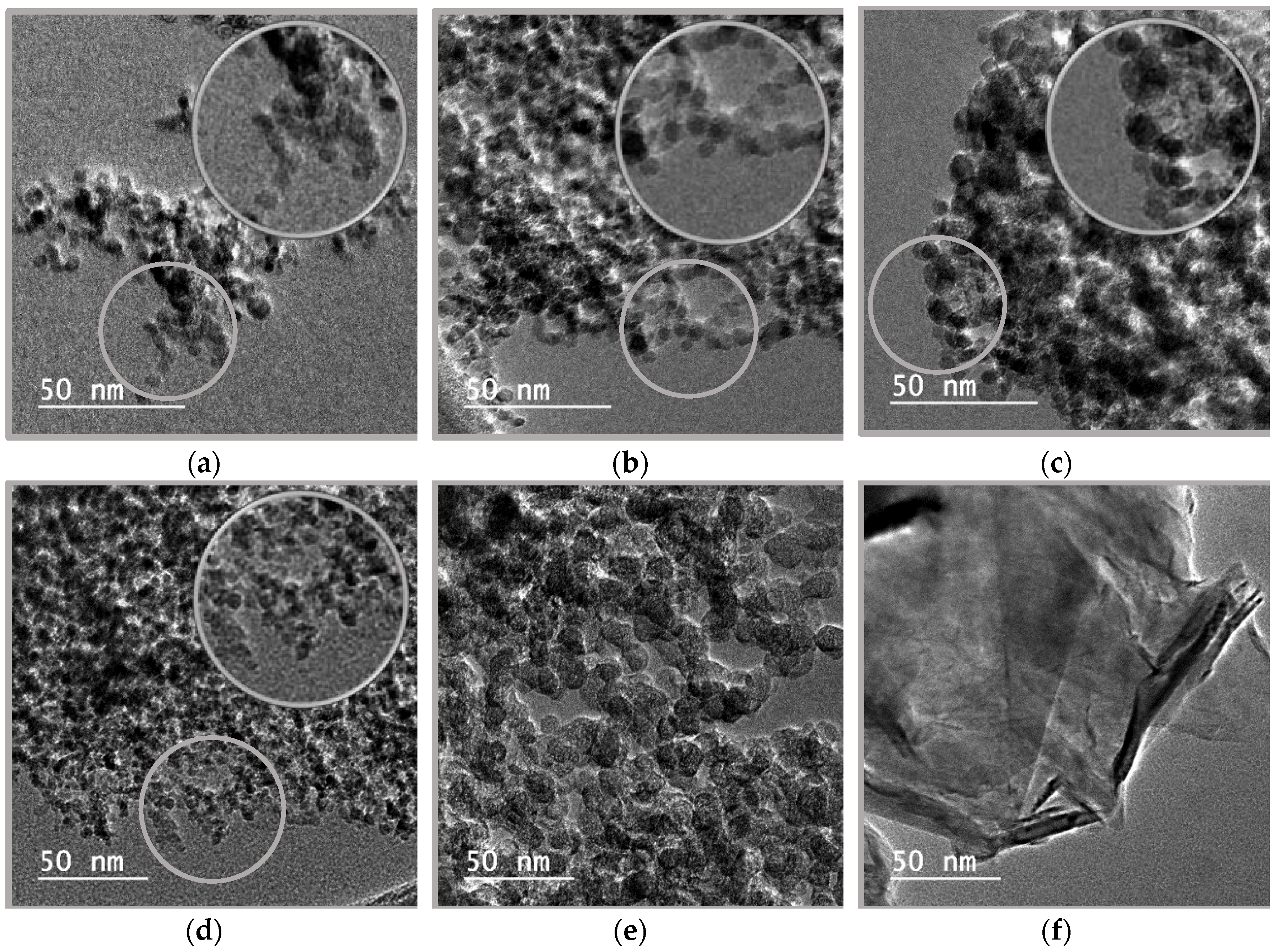


| Sample | Average Particle Size | Diamond Content | Non-Diamond Carbon Content | Ash Content | Specific Surface Area |
|---|---|---|---|---|---|
| nD 97 1 | 4 nm | >97 wt% | traces 3 | <1.4 wt% | 350 m2·g−1 |
| nD 87 1 | 4 nm | >87 wt% | <6 wt% 4 | <6 wt% | 290 m2·g−1 |
| G/D p 1 | 4 nm (diamond) | >20 wt% | - | <0.3 wt% | 470 m2·g−1 |
| G/D r 1 | 4 nm (diamond) | >20 wt% | - | <6 wt% | 370 m2·g−1 |
| CB 1 | 13 nm | - | - | <0.02 wt% | 550 m2·g−1 |
| fGnP 2 | - | - | - | - | - |
| Nanoadditive | ϕm | K (mPa·sn) | n | s (mPa·s) | AAD |
|---|---|---|---|---|---|
| nD 97 | 0.0025 | 9.39 | 0.831 | 0.31 | 4.0% |
| 0.0050 | 14.2 | 0.755 | 0.67 | 5.9% | |
| 0.010 | 22.3 | 0.695 | 1.4 | 8.4% | |
| 0.020 | 83.3 | 0.491 | 10.6 | 18% | |
| nD 87 | 0.0025 | 8.53 | 0.853 | 0.33 | 4.5% |
| 0.0050 | 11.9 | 0.798 | 0.80 | 6.1% | |
| 0.010 | 19.5 | 0.721 | 0.67 | 4.9% | |
| 0.020 | 54.9 | 0.564 | 8.2 | 15% | |
| G/D p | 0.0025 | 6.04 | 0.921 | 0.19 | 2.0% |
| 0.0050 | 11.0 | 0.802 | 0.33 | 3.9% | |
| 0.010 | 24.8 | 0.654 | 1.4 | 8.0% | |
| 0.020 | 108 | 0.437 | 6.4 | 14% | |
| G/D r | 0.0025 | 5.28 | 0.959 | 0.10 | 1.1% |
| 0.0050 | 9.06 | 0.848 | 0.30 | 3.6% | |
| 0.010 | 20.2 | 0.686 | 0.75 | 6.4% | |
| 0.020 | 68.3 | 0.538 | 4.1 | 8.3% |
| Set | ϕm | η0 (mPa·s) | η∞ (mPa·s) | k (s) | m | s (mPa·s) | AAD |
|---|---|---|---|---|---|---|---|
| CB | 0.0025 | 13.5 | 4.21 | 0.204 | 1.52 | 0.41 | 2.9% |
| 0.0050 | 17.5 | 4.56 | 0.250 | 1.58 | 0.57 | 3.5% | |
| fGnP | 0.0025 | 7.99 | 4.40 | 0.202 | 1.51 | 0.19 | 1.6% |
| 0.0050 | 8.89 | 4.85 | 0.212 | 1.77 | 0.16 | 2.2% | |
| 0.010 | 12.9 | 5.39 | 0.250 | 1.82 | 0.29 | 2.4% | |
| 0.020 | 25.2 | 6.97 | 0.259 | 1.91 | 0.46 | 3.0% |
| ϕm | Set | Temperature (K) | Fitting Parameters and Deviations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 283.15 | 293.15 | 303.15 | 313.15 | 323.15 | 333.15 | 343.15 | 353.15 | η0 (mPa·s) | A | T0 (K) | s (mPa·s) | AAD | ||
| 0 | - | 5.76 | 4.08 | 3.02 | 2.31 | 1.83 | 1.49 | 1.24 | 1.05 | 0.0413 | 4.37 | 150.25 | 0.006 | 0.17% |
| 0.0025 | nD 97 | 6.35 | 4.70 | 3.55 | 2.67 | 2.14 | 1.75 | 1.43 | 1.21 | 0.0323 | 6.03 | 132.61 | 0.082 | 0.85% |
| nD 87 | 6.25 | 4.48 | 3.37 | 2.56 | 2.01 | 1.66 | 1.39 | 1.16 | 0.0322 | 5.79 | 134.87 | 0.022 | 0.64% | |
| G/D p | 6.02 | 4.29 | 3.22 | 2.51 | 1.98 | 1.61 | 1.34 | 1.14 | 0.0395 | 4.97 | 142.42 | 0.014 | 0.27% | |
| G/D r | 5.94 | 4.28 | 3.20 | 2.43 | 1.94 | 1.58 | 1.33 | 1.13 | 0.0363 | 5.29 | 138.98 | 0.023 | 0.65% | |
| CB | 5.95 | 4.21 | 3.15 | 2.42 | 1.93 | 1.56 | 1.31 | 1.11 | 0.0371 | 5.06 | 141.69 | 0.024 | 0.43% | |
| fGnP | 6.19 | 4.40 | 3.24 | 2.47 | 1.97 | 1.61 | 1.33 | 1.13 | 0.0438 | 4.39 | 150.15 | 0.014 | 0.34% | |
| 0.0050 | nD 97 | 7.01 | 5.12 | 3.82 | 2.93 | 2.34 | 1.91 | 1.61 | 1.38 | 0.0350 | 6.38 | 128.42 | 0.030 | 0.87% |
| nD 87 | 6.92 | 4.89 | 3.68 | 2.87 | 2.20 | 1.84 | 1.51 | 1.31 | 0.0355 | 5.90 | 133.49 | 0.046 | 1.1% | |
| G/D p | 6.69 | 4.82 | 3.58 | 2.71 | 2.20 | 1.77 | 1.50 | 1.31 | 0.0409 | 5.25 | 139.48 | 0.040 | 1.1% | |
| G/D r | 6.58 | 4.79 | 3.50 | 2.70 | 2.13 | 1.76 | 1.45 | 1.26 | 0.0375 | 5.42 | 138.14 | 0.040 | 0.91% | |
| CB | 6.53 | 4.56 | 3.35 | 2.61 | 2.08 | 1.71 | 1.42 | 1.16 | 0.0394 | 5.08 | 141.68 | 0.072 | 1.1% | |
| fGnP | 6.67 | 4.85 | 3.55 | 2.68 | 2.10 | 1.70 | 1.39 | 1.23 | 0.0191 | 8.09 | 118.89 | 0.051 | 1.3% | |
| η0 (mPa·s) | A | T0 (K) | B (mPa·s) | C (K) | D (mPa·s) | s (mPa·s) | AAD |
|---|---|---|---|---|---|---|---|
| 0.0413 | 4.37 | 150.25 | 0.0131 | 2916.07 | 2821.67 | 0.040 | 0.89% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallejo, J.P.; Żyła, G.; Fernández-Seara, J.; Lugo, L. Influence of Six Carbon-Based Nanomaterials on the Rheological Properties of Nanofluids. Nanomaterials 2019, 9, 146. https://doi.org/10.3390/nano9020146
Vallejo JP, Żyła G, Fernández-Seara J, Lugo L. Influence of Six Carbon-Based Nanomaterials on the Rheological Properties of Nanofluids. Nanomaterials. 2019; 9(2):146. https://doi.org/10.3390/nano9020146
Chicago/Turabian StyleVallejo, Javier P., Gaweł Żyła, José Fernández-Seara, and Luis Lugo. 2019. "Influence of Six Carbon-Based Nanomaterials on the Rheological Properties of Nanofluids" Nanomaterials 9, no. 2: 146. https://doi.org/10.3390/nano9020146
APA StyleVallejo, J. P., Żyła, G., Fernández-Seara, J., & Lugo, L. (2019). Influence of Six Carbon-Based Nanomaterials on the Rheological Properties of Nanofluids. Nanomaterials, 9(2), 146. https://doi.org/10.3390/nano9020146