Synthesis of ZnxCd1-xSe@ZnO Hollow Spheres in Different Sizes for Quantum Dots Sensitized Solar Cells Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
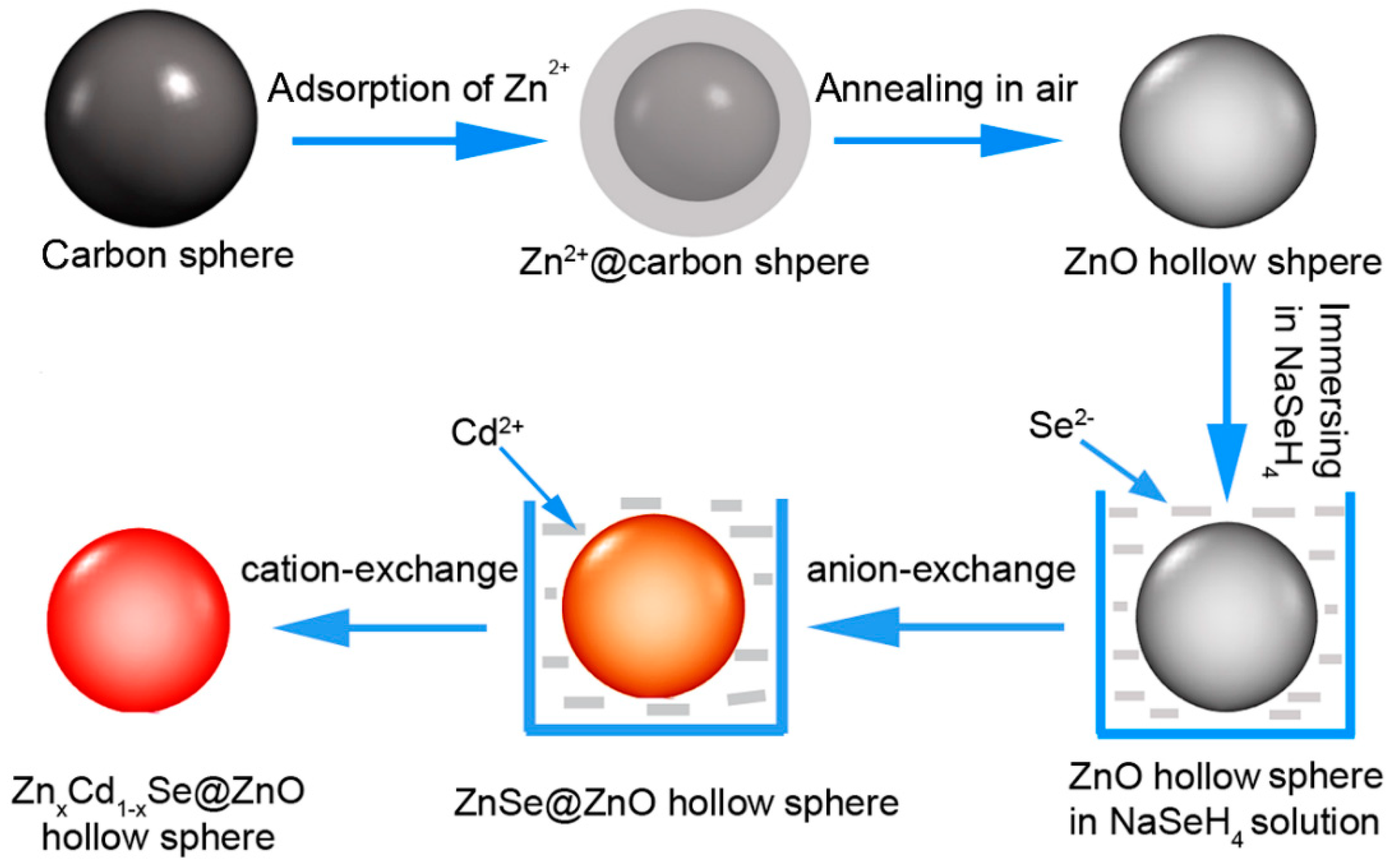
2.2. Preparation of ZnO Hollow Microspheres
2.3. Construction of ZnxCd1-xSe@ZnO HS Photoanodes and QDSSCs
2.4. Characterization
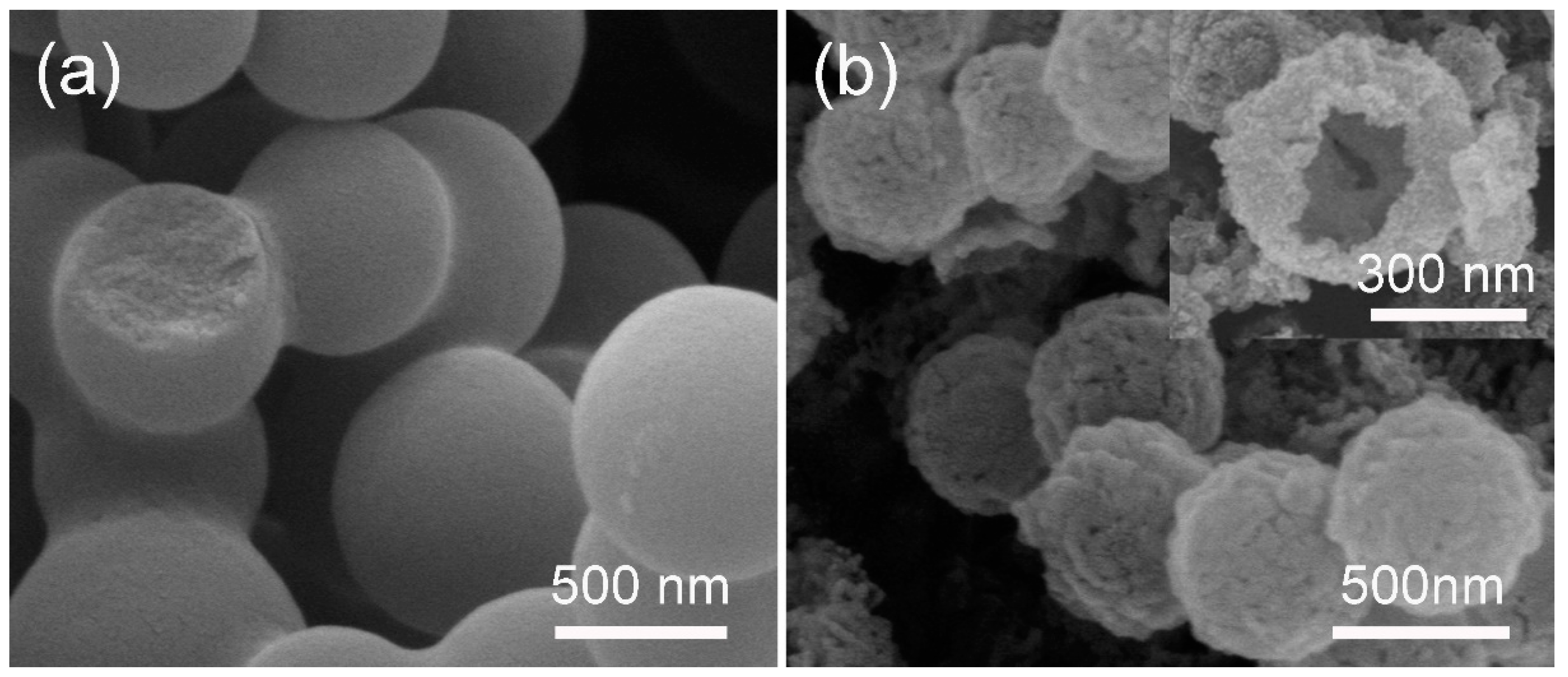
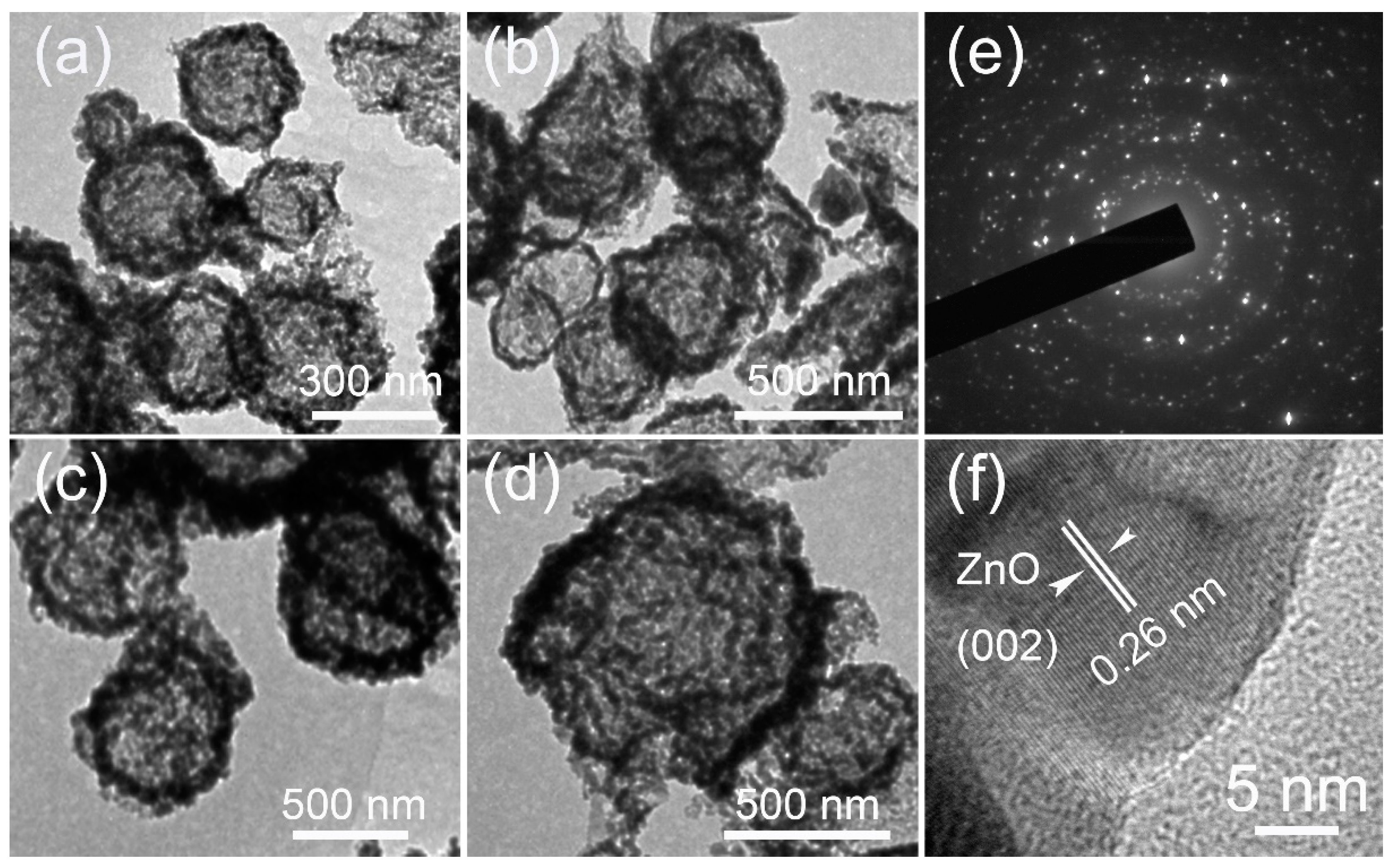
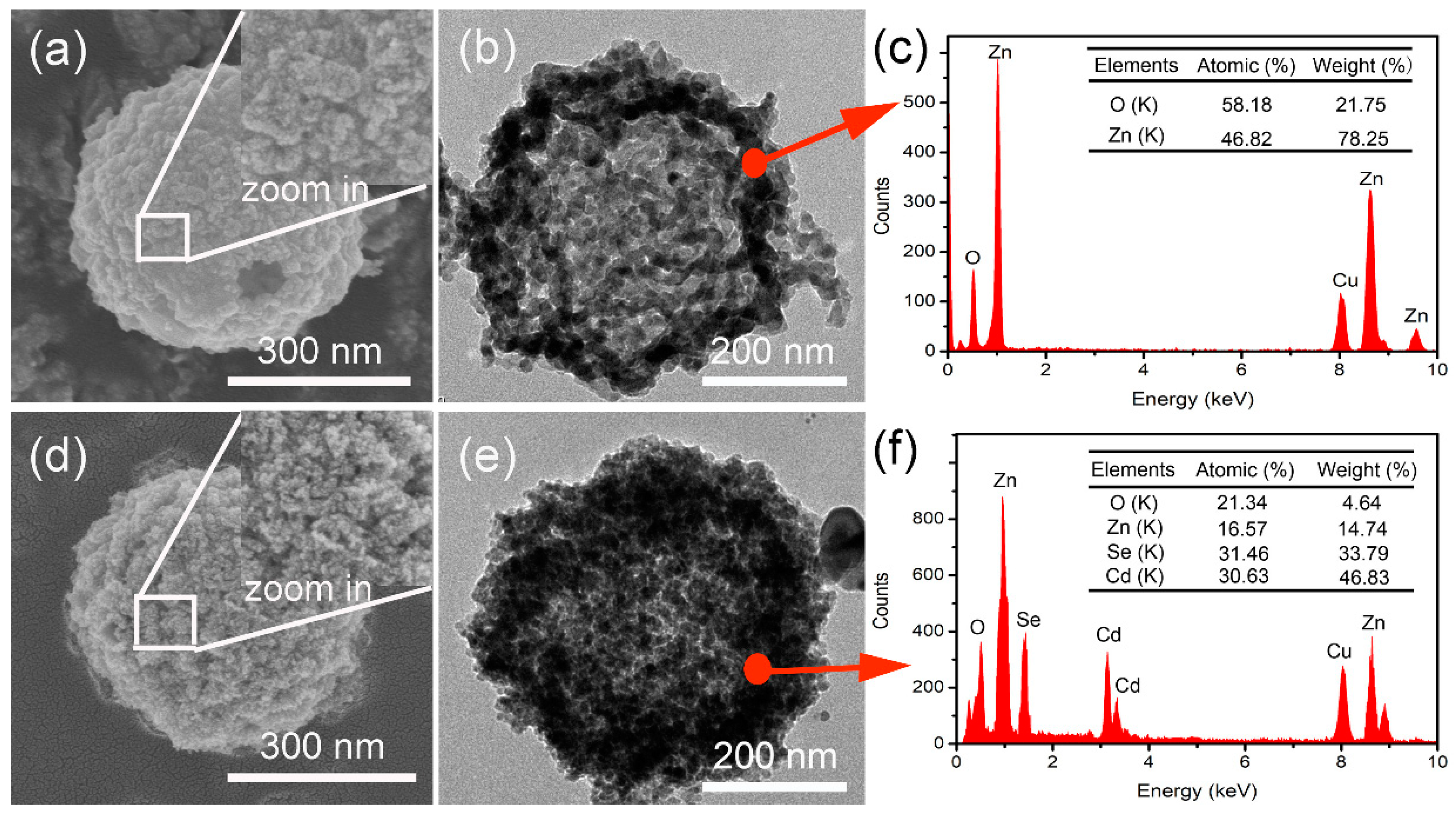
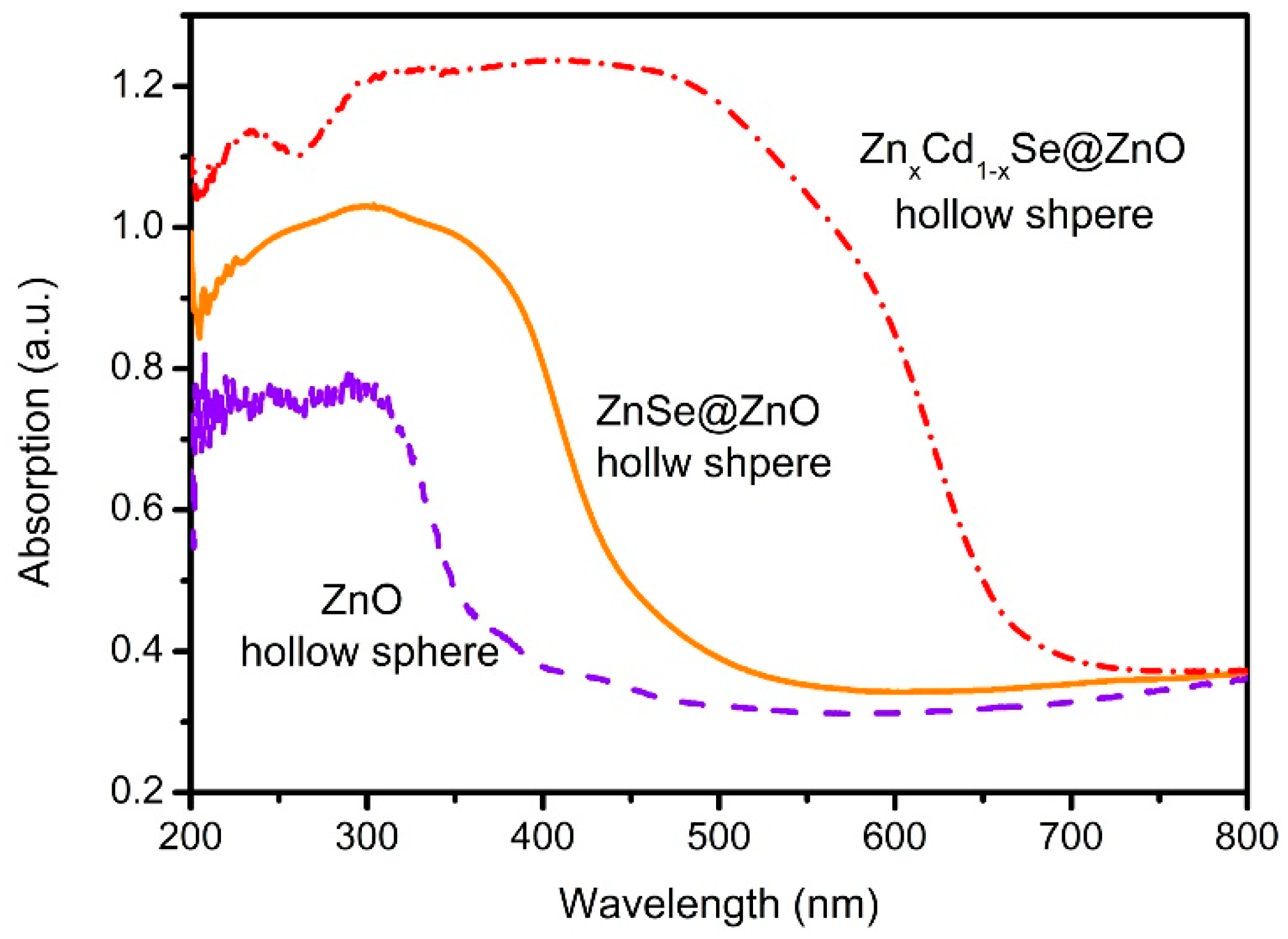
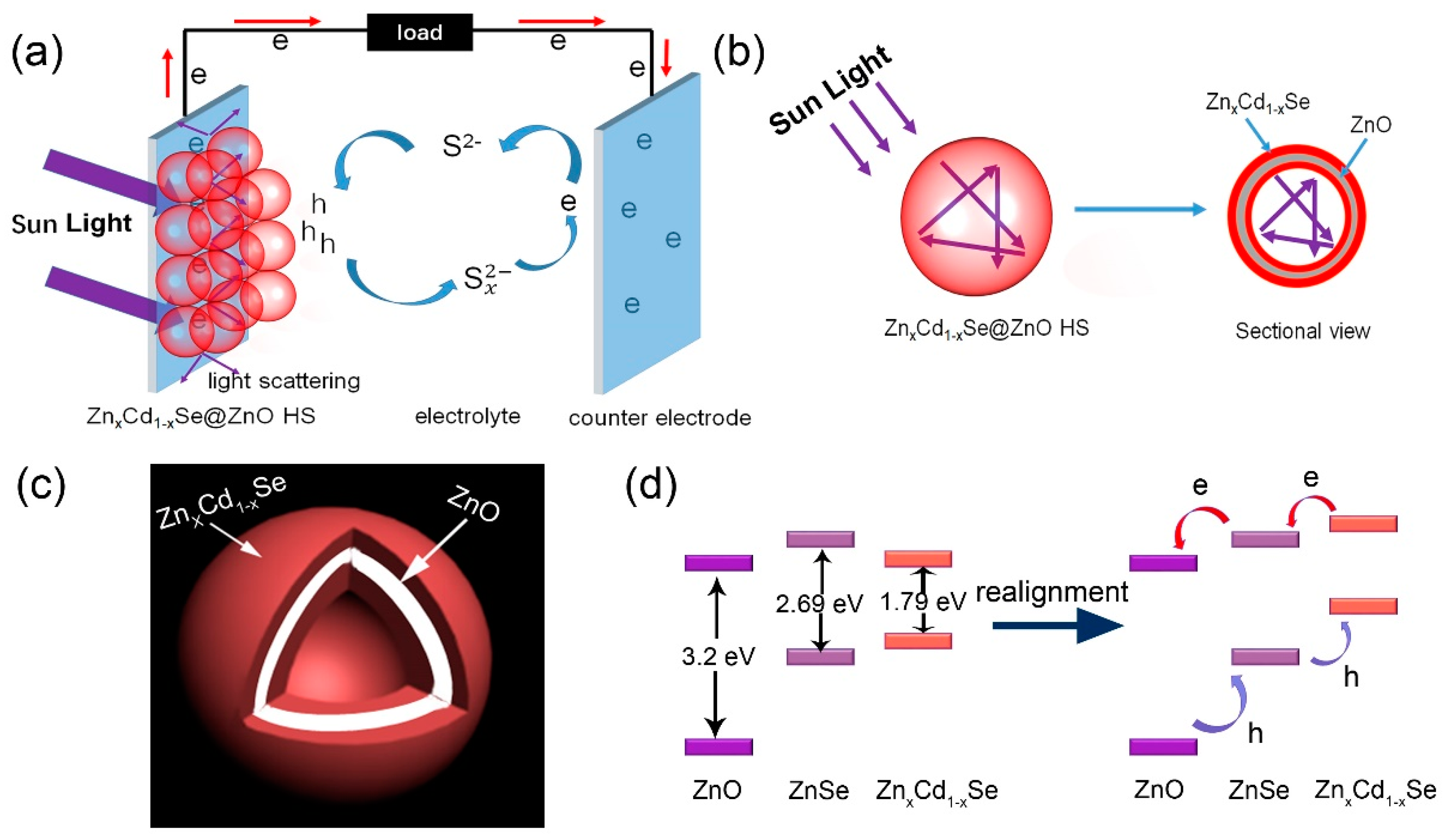
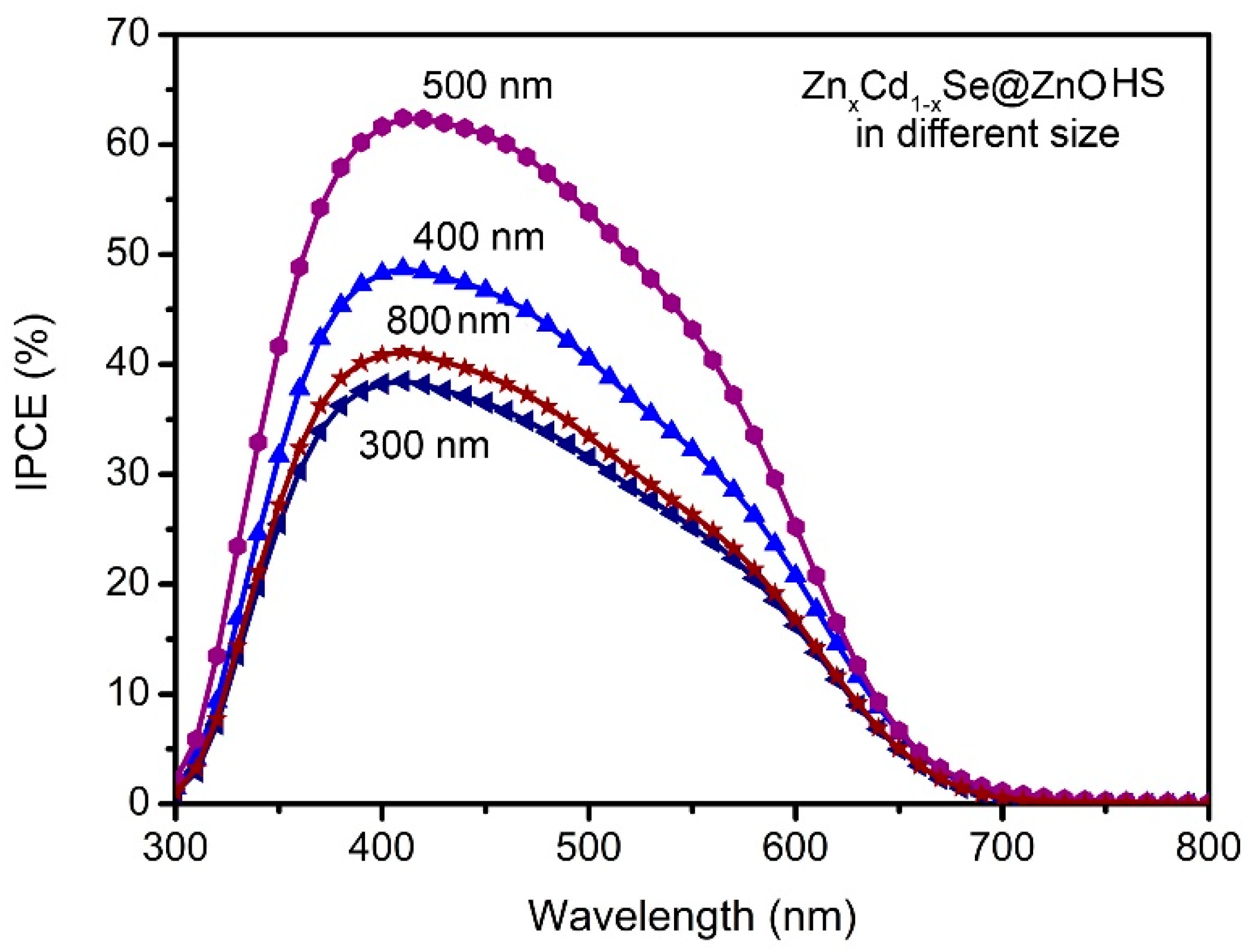
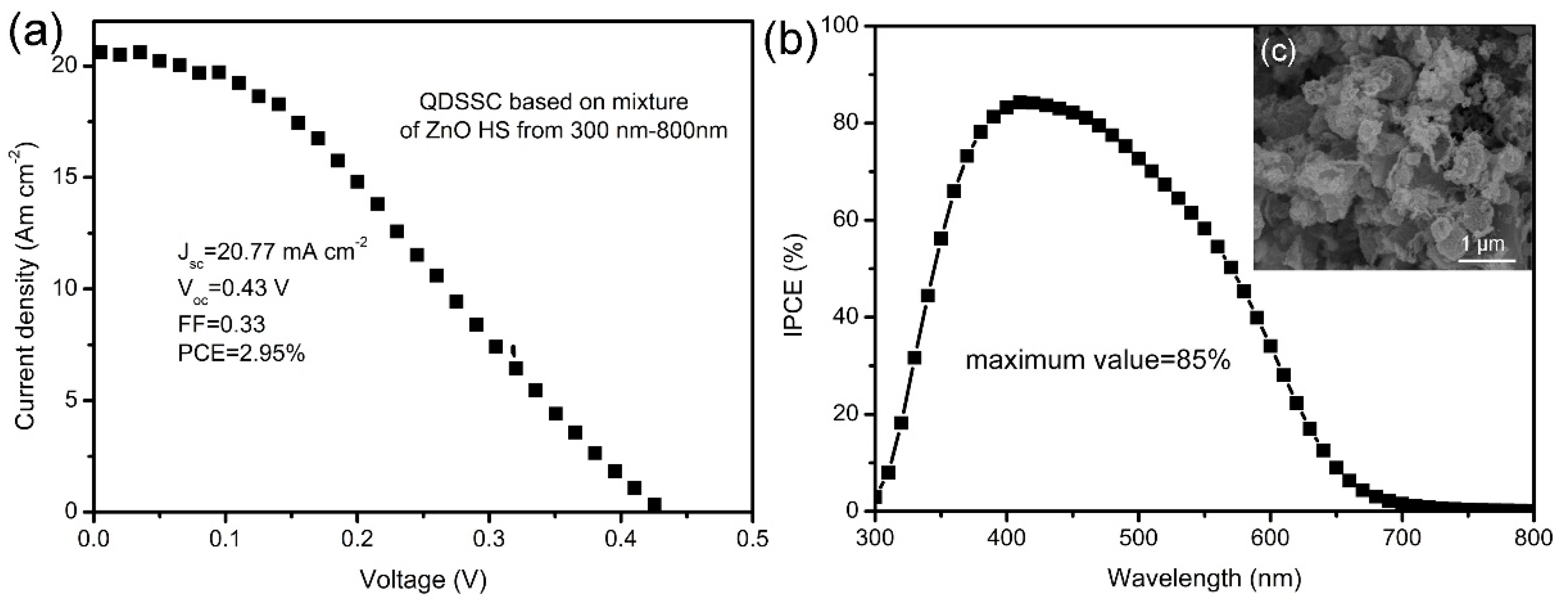
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lu, Y.B.; Li, L.; Su, S.C.; Chen, Y.L.; Song, Y.L.; Jiao, S.J. A novel TiO2 nanostructure as photoanode for highly efficient CdSe quantum dot-sensitized solar cells. RSC Adv. 2017, 7, 9795–9802. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L.; Liu, Y.; Sun, S. Efficient quantum dot-sensitized solar cell based on CdSxSe1−x/Mn-CdS/TiO2 nanotube array electrode. Electrochim. Acta 2015, 153, 200–209. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, X.; Liu, Y.; Chen, J.; Chen, J. Balance on the charge generation, separation and transfer performance of different TiO2 nanostructures in quantum dot sensitized solar cells. Mater. Res. Bull. 2017, 94, 463–471. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Tian, J.; Cao, G. Semiconductor quantum dot-sensitized solar cells. Nano Rev. 2013, 4, 22578. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Zhang, Y.; Tusamalee, P.; Nakazawa, N.; Yoshihara, Y.; Wang, R.; Toyoda, T.; Hayase, S.; Shen, Q. Interface passivation effects on photovoltaic performance of quantum dot sensitized inverse opal TiO2 solar cells. Nanomaterials 2018, 8, 460. [Google Scholar] [CrossRef]
- Ruhle, S.; Shalom, M.; Zaban, A. Quantum-dot-sensitized solar cells. Chemphyschem 2010, 11, 2290–2304. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Nahm, C.; Kim, J.; Kim, C.; Kang, S.; Hwang, T.; Park, B. Review paper: Toward highly efficient quantum-dot- and dye-sensitized solar cells. Curr. Appl. Phys. 2013, 13, S2–S13. [Google Scholar] [CrossRef]
- Li, G.; Sheng, L.; Hu, J.; Li, P.; Wang, K. Engineering flexible dye-sensitized solar cells for portable electronics. Sol. Energy 2019, 177, 80–98. [Google Scholar] [CrossRef]
- Sacco, A.; Bella, F.; Pierre, S.D.L.; Catellino, M.; Bainco, S.; Bongiovanni, R.; Pirri, C.F. Electrodes/electrolyte interfaces in the presence of a surface-modified photopolymer electrolyte: Application in dye-sensitized solar cells. ChemPhysChem 2015, 16, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Bella, F.; Sacco, A.; Salvador, G.P.; Bianco, S.; Tresso, E.; Pirri, C.F.; Bongiovanni, R. First Pseudohalogen polymer electrolyte for dye-sensitized solar cells promising for in situ photopolymerization. J. Phys. Chem. C 2013, 117, 20421–20430. [Google Scholar] [CrossRef]
- Bella, F.; Imperiyka, M.; Ahmad, A. Photochemically produced quasi-linear copolymers for stable and efficient electrolytes in dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2014, 289, 73–80. [Google Scholar] [CrossRef]
- Wang, W.; Feng, W.; Du, J.; Xue, W.; Zhang, L.; Zhao, L.; Li, Y.; Zhong, X. Cosensitized quantum dot solar cells with conversion efficiency over 12%. Adv. Mater. 2018, 30, 1705746. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Du, Z.; Hu, J.S.; Pan, Z.; Shen, Q.; Sun, J.; Long, D.; Dong, H.; Sun, L.; Zhong, X.; et al. Zn-Cu-In-Se quantum dot solar cells with a certified power conversion efficiency of 11.6%. J. Am. Chem. Soc. 2016, 138, 4201–4209. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Du, Z.; Pan, Z.; Du, J.; Zhong, X. Solar paint from TiO2 particles supported quantum dots for photoanodes in quantum dot–sensitized solar cells. ACS Omega 2018, 3, 1102–1109. [Google Scholar] [CrossRef]
- Sahu, A.; Chaurashiya, R.; Hiremath, K.; Dixit, A. Nanostructured zinc titanate wide band gap semiconductor as a photoelectrode material for quantum dot sensitized solar cells. Sol. Energy 2018, 163, 338–346. [Google Scholar] [CrossRef]
- Wen, S.; Li, M.; Yang, J.; Mei, X.; Wu, B.; Liu, X.; Heng, J.; Qin, D.; Hou, L.; Wang, D. Rationally controlled synthesis of CdSexTe1-x alloy nanocrystals and their application in efficient graded band gap solar cells. Nanomaterials 2017, 7, 380. [Google Scholar] [CrossRef]
- Xi, J.; Zhang, Q.; Park, K.; Sun, Y.; Cao, G. Enhanced power conversion efficiency in dye-sensitized solar cells with TiO2 aggregates/nanocrystallites mixed photoelectrodes. Electrochim. Acta 2011, 56, 1960–1966. [Google Scholar] [CrossRef]
- Zhang, Q.; Chou, T.P.; Russo, B.; Jenekhe, S.A.; Cao, G. Aggregation of ZnO nanocrystallites for high conversion efficiency in dye-sensitized solar cells. Angew. Chem. Int. Ed. Engl. 2008, 47, 2402–2406. [Google Scholar] [CrossRef]
- Titirici, M.M.; Antonietti, M.; Thomas, A. A Generalized synthesis of metal oxide hollow spheres using a hydrothermal approach. Chem. Mater. 2006, 18, 3808–3812. [Google Scholar] [CrossRef]
- Li, H.; Cheng, C.; Li, X.; Liu, J.; Guan, C.; Tay, Y.Y.; Fan, H.J. Composition-graded ZnxCd1−xSe@ZnO core-shell nanowire array electrodes for photoelectrochemical hydrogen generation. J. Phys. Chem. C 2012, 116, 3802–3807. [Google Scholar] [CrossRef]
- Gertman, R.; Osherov, A.; Golan, Y.; Visoly-Fisher, I. Chemical bath deposited PbS thin films on ZnO nanowires for photovoltaic applications. Thin Solid Films 2014, 550, 149–155. [Google Scholar] [CrossRef]
- Justin Raj, C.; Karthick, S.N.; Park, S.; Hemalatha, K.V.; Kim, S.K.; Prabakar, K.; Kim, H.J. Improved photovoltaic performance of CdSe/CdS/PbS quantum dot sensitized ZnO nanorod array solar cell. J. Power Sources 2014, 248, 439–446. [Google Scholar] [CrossRef]
- Hou, J.; Zhao, H.; Huang, F.; Jing, Q.; Cao, H.; Wu, Q.; Peng, S.; Cao, G. High performance of Mn-doped CdSe quantum dot sensitized solar cells based on the vertical ZnO nanorod arrays. J. Power Sources 2016, 325, 438–445. [Google Scholar] [CrossRef]
- Zervos, M.; Vasile, E.; Vasile, E.; Othonos, A. Core-shell PbS/Sn:In2O3 and branched PbIn2S4/Sn:In2O3 nanowires in quantum dot sensitized solar cells. Nanotechnology 2017, 28, 054004. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, G.; Yin, J.; Liang, J.; Sun, W.; Shen, Z. Fabrication of ZnO nanostructures sensitized with CdS quantum dots for photovoltaic application using a convenient solution method. Mater. Res. Bull. 2015, 61, 492–498. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L.; Liu, Y.; Sun, S. Arrays of ZnxCd1−xSe/TiO2 nanotubes: Fabrication by ion-exchange and photovoltaic applications. J. Mater. Sci. Mater. Electron. 2015, 26, 1625–1633. [Google Scholar] [CrossRef]
- Zhou, R.; Wan, L.; Niu, H.; Yang, L.; Mao, X.; Zhang, Q.; Miao, S.; Xu, J.; Cao, G. Tailoring band structure of ternary CdSxSe1−x quantum dots for highly efficient sensitized solar cells. Sol. Energy Mater. Sol. Cells 2016, 155, 20–29. [Google Scholar] [CrossRef]
- Jiang, T.; Shen, M.; Dai, P.; Wu, M.; Yu, X.; Li, G.; Xu, X.; Zeng, H. Cd-free Cu-Zn-In-S/ZnS quantum dots@SiO2 multiple cores nanostructure: Preparation and application for white LEDs. Nanotechnology 2017, 28, 435702. [Google Scholar] [CrossRef]
- Xu, J.; Yang, X.; Yang, Q.D.; Wong, T.L.; Lee, S.T.; Zhang, W.J.; Lee, C.S. Arrays of CdSe sensitized ZnO/ZnSe nanocables for efficient solar cells with high open-circuit voltage. J. Mater. Chem. 2012, 22, 13374. [Google Scholar] [CrossRef]
- Myung, Y.; Kang, J.H.; Choi, J.W.; Jang, D.M.; Park, J. Polytypic ZnCdSe shell layer on a ZnO nanowire array for enhanced solar cell efficiency. J. Mater. Chem. 2012, 22, 2157–2165. [Google Scholar] [CrossRef]
- Xu, J.; Yang, X.; Wang, H.; Chen, X.; Luan, C.; Xu, Z.; Lu, Z.; Roy, V.A.; Zhang, W.; Lee, C.S. Arrays of ZnO/ZnxCd1−xSe nanocables: Band gap engineering and photovoltaic applications. Nano Lett. 2011, 11, 4138–4143. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Lai, X.; Halpert, J.E.; Yang, N.; Yi, L.; Zhai, J.; Wang, D.; Tang, Z.; Jiang, L. Accurate control of multishelled ZnO hollow microspheres for dye-sensitized solar cells with high efficiency. Adv. Mater. 2012, 24, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Kalanur, S.S.; Chae, S.Y.; Joo, O.S. Transparent Cu1.8S and CuS thin films on FTO as efficient counter electrode for quantum dot solar cells. Electrochim. Acta 2013, 103, 91–95. [Google Scholar] [CrossRef]
- Wang, X.; Hu, P.; Fangli, Y.; Yu, L. Preparation and characterization of ZnO hollow spheres and ZnO-carbon composite materials using colloidal carbon spheres as templates. J. Phys. Chem. C 2007, 111, 6706–6712. [Google Scholar] [CrossRef]
- Lai, X.; Li, J.; Korgel, B.A.; Dong, Z.; Li, Z.; Su, F.; Du, J.; Wang, D. General synthesis and gas-sensing properties of multiple-shell metal oxide hollow microspheres. Angew. Chem. Int. Ed. Engl. 2011, 50, 2738–2741. [Google Scholar] [CrossRef]
- Lai, X.; Halpert, J.E.; Wang, D. Recent advances in micro-/nano-structured hollow spheres for energy applications: From simple to complex systems. Energy Environ. Sci. 2012, 5, 5604–5618. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, Q.; Uchaker, E.; Lan, J.; Yin, M.; Cao, G. Mesoporous TiO2 beads for high efficiency CdS/CdSe quantum dot co-sensitized solar cells. J. Mater. Chem. A 2014, 2, 2517–2525. [Google Scholar] [CrossRef]

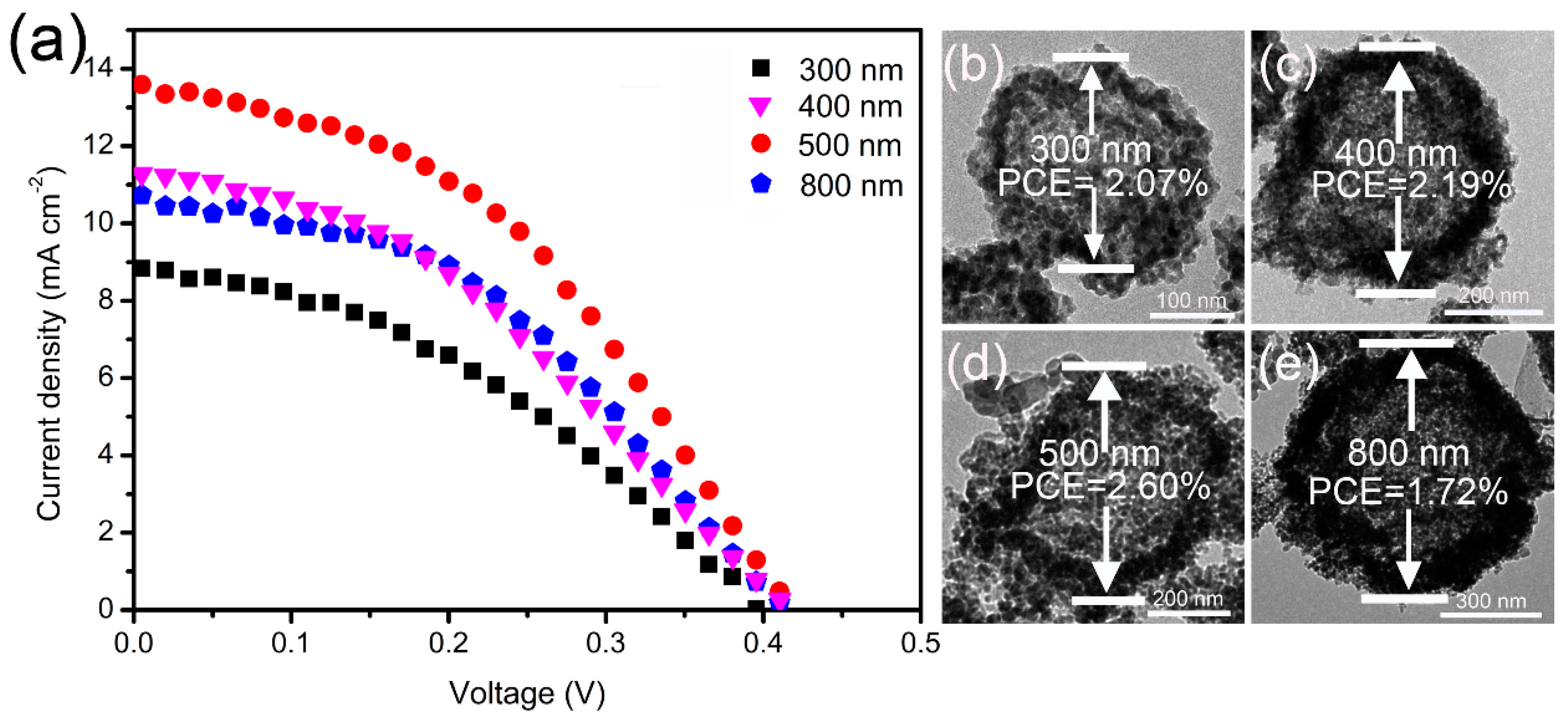
| Size (nm) | Jsc (mA·cm−2) | Voc (V) | FF | PCE (%) |
|---|---|---|---|---|
| ~300 | 11.30 | 0.42 | 0.44 | 2.07 |
| ~400 | 11.61 | 0.41 | 0.46 | 2.19 |
| ~500 | 13.46 | 0.42 | 0.46 | 2.60 |
| ~800 | 8.79 | 0.40 | 0.49 | 1.72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Li, Z. Synthesis of ZnxCd1-xSe@ZnO Hollow Spheres in Different Sizes for Quantum Dots Sensitized Solar Cells Application. Nanomaterials 2019, 9, 132. https://doi.org/10.3390/nano9020132
Yu L, Li Z. Synthesis of ZnxCd1-xSe@ZnO Hollow Spheres in Different Sizes for Quantum Dots Sensitized Solar Cells Application. Nanomaterials. 2019; 9(2):132. https://doi.org/10.3390/nano9020132
Chicago/Turabian StyleYu, Libo, and Zhen Li. 2019. "Synthesis of ZnxCd1-xSe@ZnO Hollow Spheres in Different Sizes for Quantum Dots Sensitized Solar Cells Application" Nanomaterials 9, no. 2: 132. https://doi.org/10.3390/nano9020132
APA StyleYu, L., & Li, Z. (2019). Synthesis of ZnxCd1-xSe@ZnO Hollow Spheres in Different Sizes for Quantum Dots Sensitized Solar Cells Application. Nanomaterials, 9(2), 132. https://doi.org/10.3390/nano9020132





