Recent Advances in Magnetite Nanoparticle Functionalization for Nanomedicine
Abstract
1. Introduction
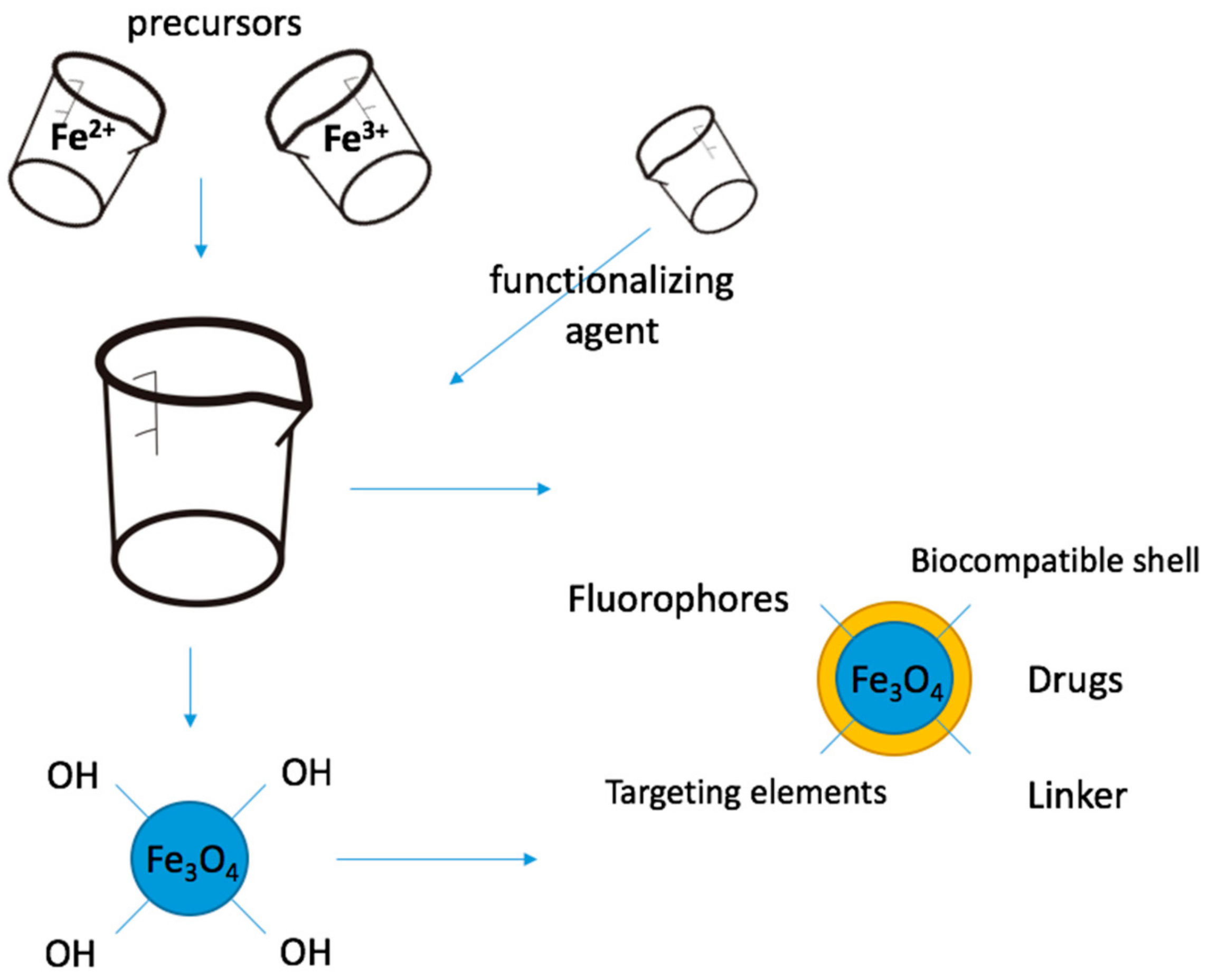
2. Functionalization of Magnetite Nanoparticles
3. Inorganic Functionalization of Magnetite Nanoparticles
3.1. Oxides
3.2. Metals
4. Carbon-Based Functionalization of Magnetite Nanoparticles
5. Organic Functionalization of Magnetite Nanoparticles
5.1. Small Molecules and Surfactants
5.2. Lipids
5.3. Polymers
5.4. Phytochemicals
5.5. Drug Molecules
6. Conclusions
- Multifunctionality of Fe3O4 nanoparticles is given by its properties (magnetism, biocompatibility);
- They have many applications in the medical field, among which a few have been approved by the FDA for clinical use (MRI contrast substance, magnetic hyperthermia, iron deficiency supplement);
- The route of synthesis also determines the surface functionality among other properties;
- Surface functionalization determines an alteration of the surface chemistry, leading to changes in the physical, chemical and biological properties;
- Classification of functionalization processes. Depending on: time of functionalization (in situ, respectively post synthesis), chemistry of functionalization (non-covalent and covalent), chemistry of the functionalizing agent (inorganic and organic);
- Non-specific physical sorption is preferred in applications such as drug delivery systems;
- Among the oxides, SiO2 coating of magnetite nanoparticles is the most common because it enhances the biocompatibility and stability of the nanoparticles; some common approaches to obtain this conjugation are the sol-gel method, respectively, microemulsion;
- The mesoporous silica coating is biocompatible and offers high controlled porosity; is good for drug delivery applications;
- Metal oxide (ZnO, TiO2) functionalization has photocatalytic applications;
- Surface functionalization of magnetite nanoparticles with metals induces an inert character; the most popular approach in this category is the conjugation of Fe3O4 with gold because of its biocompatibility and multifunctionality; approaches to obtain this type of nanoparticles are: reduction of gold ions on the surface of magnetite nanoparticles, respectively, the organic synthesis approach; the final applications are numerous: medical imaging (MRI, CT, PA), radiosensitiation, radiofrequency ablation, biosensing, cell sorting;
- Carbon-Fe3O4 nano-composites mostly have applications in electronics, but also in biosensing and drug delivery systems; in order to obtain these materials, the direct precipitation of magnetite nanoparticles on the surface of the carbon nanomaterial can be applied or a hydrothermal approach for in situ functionalization;
- The conjugation of magnetite nanoparticles with organic molecules has the advantage of improving the stability, biocompatibility and interaction with biological membranes of the Fe3O4; mostly has applications in the development of drug delivery systems;
- Surfactants have been used to improve the stability of the magnetite nano-constructs, but can have toxic effects;
- Lipid-encapsulated nanoparticles enhance the biocompatibility of the magnetite nanoparticles and improve their interaction with biological membranes, while preventing opsonisation;
- The functionalization of Fe3O4 with polymers is the type of surface modification most encountered for these nanoparticles and can be undertaken both in situ (through electrostatic interactions) or post-synthesis (through condensation); it increases the stability and biocompatibility of magnetite nanoparticles, leading to applications in medical imaging, hyperthermia treatment of cancer, drug delivery systems, tissue engineering;
- A polymer-coated Fe3O4 nanoparticle (MagForce) has been approved by the FDA for use in hyperthermia treatment of cancer;
- Drug-delivery systems based on magnetite nanoparticles can be developed for commercial medicines or phytochemicals; the therapeutic molecule can be directly conjugated on the Fe3O4 surface or can be attached through an intermediate layer;
- Phytochemicals-Fe3O4 are popular alternative medicines with antimicrobial, antitumor, anti-inflammatory or antiviral applications; conjugation with magnetite nanoparticles can be undertaken through both weak and strong interactions;
- Conventional drugs are mostly attached through strong interactions from the magnetite nanoparticles.
Author Contributions
Funding
Conflicts of Interest
References
- Amag Pharmaceuticals. Available online: http://www.amagpharma.com/our-products/ (accessed on 19 November 2019).
- Wáng, Y.X.J.; Idée, J.M. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant. Imaging Med. Surg. 2017, 7, 88–122. [Google Scholar] [CrossRef] [PubMed]
- MagForce. Fighting Cancer with Nanomedicine. Available online: http://www.magforce.de/en/home.html (accessed on 7 October 2019).
- Feraheme Ferumoxytol Injection. Available online: https://www.feraheme.com (accessed on 19 November 2019).
- Zhang, D.; Du, Y. The Biocompatibility Study of Fe3O4 Magnetic Nanoparticles Used in Tumor Hyperthermia. In Proceedings of the 2006 1st IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Zhuhai, China, 18–21 January 2006; pp. 339–342. [Google Scholar] [CrossRef]
- Chen, D.; Tang, Q.; Li, X.; Zhou, X.; Zhang, J.; Xue, W.-Q.; Xiang, J.-Y.; Guo, C.-Q. Biocompatibility of magnetic Fe3O4 nanoparticles and their cytotoxic effect on MCF-7 cells. Int. J. Nanomed. 2012, 7, 4973–4982. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, S.; Hou, P.; Yang, Y.; Weng, J.; Li, X.; Li, M. Synthesis and characterization of biocompatible Fe3O4 nanoparticles. J. Biomed. Mater. Res. 2007, 80A, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Ning, W.; Wang, W.; Yuan, X.; Bai, Z. Synthesis of size-controllable Fe3O4 magnetic submicroparticles and its biocompatible evaluation in vitro. J. Cent. South Univ. 2016, 23, 2784–2791. [Google Scholar] [CrossRef]
- Tseng, W.-K.; Chieh, J.-J.; Yang, Y.-F.; Chiang, C.-K.; Chen, Y.-L.; Yang, S.Y.; Horng, H.-E.; Yang, H.-C.; Wu, C.-C. A Noninvasive Method to Determine the Fate of Fe3O4 Nanoparticles following Intravenous Injection Using Scanning SQUID Biosusceptometry. PLoS ONE 2012, 7, e48510. [Google Scholar] [CrossRef]
- Gu, L.; Fang, R.H.; Sailor, M.J.; Park, J.H. In vivo clearance and toxicity of monodisperse iron oxide nanocrystals. ACS Nano 2012, 6, 4947–4954. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Naiki, T.; Lee, K.X. Green biosynthesis of superparamagnetic magnetite Fe3O4 nanoparticles and biomedical applications in targeted anticancer drug delivery system: A review. Arab. J. Chem. 2018, 1–22. [Google Scholar] [CrossRef]
- Patsula, V.; Moskvin, M.; Dutz, S.; Horák, D. Size-dependent magnetic properties of iron oxide nanoparticles. J. Phys. Chem. Solids 2016, 88, 24–30. [Google Scholar] [CrossRef]
- Li, Q.; Kartikowati, C.W.; Horie, S.; Ogi, T.; Iwaki, T.; Okuyama, K. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 9894. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Nguyen, N.H.A.; Ševců, A.; Stibor, I. Functionalized Magnetic Nanoparticles and Their Effect on Escherichia coli and Staphylococcus aureus. J. Nanomater. 2015, 2015, 416012–416022. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-X.; Luo, D.; Li, M.-M.; Xing, X.-F.; Ma, Z.-Z.; Xu, H. Recyclable Fe3O4 Nanoparticles Catalysts for Aza-Michael Addition of Acryl Amides by Magnetic Field. Catalysts 2017, 7, 219. [Google Scholar] [CrossRef]
- Alishiri, T.; Oskooei, H.A.; Heravi, M.M. Fe3O4 Nanoparticles as an Efficient and Magnetically Recoverable Catalyst for the Synthesis of α,β-Unsaturated Heterocyclic and Cyclic Ketones under Solvent-Free Conditions. Synth. Commun. 2013, 43, 3357–3362. [Google Scholar] [CrossRef]
- Araújo, R.; Castro, A.C.M.; Fiúza, A. The Use of Nanoparticles in Soil and Water Remediation Processes. Mater. Today Proc. 2015, 2, 315–320. [Google Scholar] [CrossRef]
- Jiang, B.; Lian, L.; Xing, Y.; Zhang, N.; Chen, Y.; Lu, P.; Zhang, D. Advances of magnetic nanoparticles in environmental application: Environmental remediation and (bio)sensors as case studies. Environ. Sci. Pollut. Res. 2018, 25, 30863–30879. [Google Scholar] [CrossRef]
- Gutierrez, A.M.; Dziubla, T.D.; Hilt, J.Z. Recent advances on iron oxide magnetic nanoparticles as sorbents of organic pollutants in water and wastewater treatment. Rev. Environ. Health 2017, 32, 111–117. [Google Scholar] [CrossRef]
- De Teresa, J.M.; Fernández-Pacheco, A.; Morellon, L.; Orna, J.; Pardo, J.A.; Serrate, D.; Algarabel, P.A.; Ibarra, M.R. Magnetotransport properties of Fe3O4 thin films for applications in spin electronics. Microelectron. Eng. 2007, 84, 1660–1664. [Google Scholar] [CrossRef]
- Guo, L.; Sun, H.; Qin, C.; Li, W.; Wang, F.; Song, W.; Du, J.; Zhong, F.; Ding, Y. Flexible Fe3O4 nanoparticles/N-doped carbon nanofibers hybrid film as binder-free anode materials for lithium-ion batteries. Appl. Sur. Sci. 2018, 459, 263–270. [Google Scholar] [CrossRef]
- Salimi, P.; Norouzi, O.; Pourhosseini, S.E.M. Two-step synthesis of nanohusk Fe3O4 embedded in 3D network pyrolytic marine biochar for a new generation of anode materials for Lithium-Ion batteries. J. Alloys Compd. 2019, 786, 930–937. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Kalantari, K.; Ahmad, M.B.; Shameli, K.; Bin Hussein, M.Z.; Khandanlou, R.; Khanehzaei, H. Size-Controlled Synthesis of Fe3O4 Magnetic Nanoparticles in the Layers of Montmorillonite. J. Nanomater. 2014, 2014, 739485–739494. [Google Scholar] [CrossRef]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room Temperature Co-Precipitation Synthesis of Magnetite Nanoparticles in a Large pH Window with Different Bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Zhang, C.; Guo, R.; Meng, S.; Zhang, J. Synthesis of Fe3O4 Nanoparticles Using Controlled Ammonia Vapor Diffusion under Ultrasonic Irradiation. Ind. Eng. Chem. Res. 2011, 50, 63534–63539. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Gestal, M.C.; Holban, A.M.; Grumezescu, V.; Vasile, B.S.; Mogoanta, L.; Iordache, F.; Bleotu, C.; Mogosanu, G.D. Biocompatible Fe3O4 increases the efficacy of amoxicillin delivery against Gram-positive and Gram-negative bacteria. Molecules 2014, 19, 5013–5027. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Lai, K.L.; Hu, H.; Zeng, X.-B.; Lan, F.; Liu, F.; Liu, K.-X.; Wu, Y.; Gu, Z.-W. The effect of [Fe3+]/[Fe2+] molar ratio and iron salts concentration on the properties of superparamagnetic iron oxide nanoparticles in the water/ethanol/toluene system. J. Nanoparticle Res. 2011, 13, 5135–5145. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; Ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Shi, L.; Huang, J.; He, Y. Recyclable purification-evaporation systems based on Fe3O4@TiO2 nanoparticles. Energy Procedia 2017, 142, 356–361. [Google Scholar] [CrossRef]
- Anghel, A.G.; Grumezescu, A.M.; Chirea, M.; Grumezescu, V.; Socol, G.; Iordache, F.; Oprea, A.E.; Anghel, I.; Holban, A.M. MAPLE Fabricated Fe3O4@Cinnamomum verum Antimicrobial Surfaces for Improved Gastrostomy Tubes. Molecules 2014, 19, 8981–8994. [Google Scholar] [CrossRef]
- Shen, L.; Qiao, Y.; Guo, Y.; Meng, S.; Yang, G.; Wu, M.; Zhao, J. Facile co-precipitation synthesis of shape-controlled magnetite nanoparticles. Ceram. Int. 2014, 40, 1519–1524. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, O.N.; Singh, K. Shape and Size-Dependent Magnetic Properties of Fe3O4 Nanoparticles Synthesized Using Piperidine. Nanoscale Res. Lett. 2017, 12, 298–305. [Google Scholar] [CrossRef]
- Shah, S.T.; Yehya, W.A.; Saad, O.; Simarani, K.; Chowdhury, Z.; Alhadi, A.A.; Al-Ani, L.A. Surface Functionalization of Iron Oxide Nanoparticles with Gallic Acid as Potential Antioxidant and Antimicrobial Agents. Nanomaterials 2017, 7, 306. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [PubMed]
- Lassoued, A.; Dkhil, B.; Gadri, A.; Ammar, S. Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Res. Phys. 2017, 7, 3007–3015. [Google Scholar] [CrossRef]
- Li, J.L.; Li, D.C.; Zhang, S.L.; Cui, H.C.; Wang, C. Analysis of the factors affecting the magnetic characteristics of nano-Fe3O4 particles. Chin. Sci. Bull. 2011, 8, 803–810. [Google Scholar] [CrossRef]
- Barbosa Salviano, L.; da Silva Cardoso, T.M.; Cordeiro Silva, G.; Silva Dantas, S.; de Mello Ferreira, A. Microstructural Assessment of Magnetite Nanoparticles (Fe3O4) Obtained by Chemical Precipitation Under Different Synthesis Conditions. Mater. Res. 2018, 21, e20170764. [Google Scholar] [CrossRef]
- Meng, H.; Zhang, Z.; Zhao, F.; Qiu, T.; Yang, J. Orthogonal optimization design for preparation of Fe3O4 nanoparticles via chemical coprecipitation. Appl. Surf. Sci. 2013, 280, 679–685. [Google Scholar] [CrossRef]
- Andrade, A.I.; Souza, D.M.; Pereira, M.C.; Fabris, J.D.; Domingues, R.Z. pH effect on the synthesis of magnetite nanoparticles by the chemical reduction-precipitation method. Quim. Nova 2010, 33, 524–527. [Google Scholar] [CrossRef]
- Ramadan, W.; Karim, M.; Hannoyer, B.; Saha, S. Effect of pH on the Structural and Magnetic Properties of Magnetite Nanoparticles Synthesized by Co-Precipitation. Adv. Mater. Res. 2012, 324, 129–132. [Google Scholar] [CrossRef]
- Kalska-Szostko, B.; Wykowska, U.; Satula, D.; Nordblad, P. Thermal treatment of magnetite nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 1385–1396. [Google Scholar] [CrossRef]
- Niu, J.M.; Zheng, Z.G. Effect of Temperature on Fe3O4 Nanoparticles prepared by Coprecipitation Method. Adv. Mater. Res. 2014, 900, 172–176. [Google Scholar] [CrossRef]
- Saragi, T.; Depi, B.L.; Butarbutar, S.; Permana, B. The impact of synthesis temperature on magnetite nanoparticles size synthesized by co-precipitation method. J. Phys. Conf. Ser. 2018, 1013, 012190. [Google Scholar] [CrossRef]
- Fayas, A.P.A.; Vinod, E.M.; Joseph, J.; Ganesan, R.; Pandey, R.K. Dependence of pH and surfactant effect in the synthesis of magneite (Fe3O4) nanopaticles and its properties. J. Magn. Magn. Mater. 2010, 322, 400–404. [Google Scholar] [CrossRef]
- Filippousi, M.; Angelakeris, M.; Katsikini, M.; Paloura, E.; Esthimiopoulos, I.; Wang, Y.; Zamboulis, D.; Van Tendeloo, G. Surfactant Effects on the Structural and Magnetic Properties of Iron Oxide Nanoparticles. J. Phys. Chem. C 2014, 118, 16209–16217. [Google Scholar] [CrossRef]
- Fatima, H.; Lee, D.-W.; Yun, H.J.; Kim, K.-S. Shape-controlled synthesis of magnetic Fe3O4 nanoparticles with different iron precursors and capping agents. RSC Adv. 2018, 8, 22917–22923. [Google Scholar] [CrossRef]
- Fotukian, S.M.; Barati, A.; Soleymani, M.; Alizadeh, A.M. Solvothermal synthesis of CuFe2O4 and Fe3O4 nanoparticles with high heating efficiency for magnetic hyperthermia application. J. Alloys Compd. 2019, 152548–152556. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, F.; Hong, R. Solvothermal synthesis of magnetic Fe3O4 microparticles via self-assembly of Fe3O4 nanoparticles. Particuology 2011, 9, 179–186. [Google Scholar] [CrossRef]
- Qi, M.; Zhang, K.; Li, S.; Wu, J.; Pham-Hui, C.; Diao, X.; Xiao, D.; He, H. Superparamagnetic Fe3O4 nanoparticles: Synthesis by a solvothermal process and functionalization for a magnetic targeted curcumin delivery system. New J. Chem. 2016, 40, 4480–4491. [Google Scholar] [CrossRef]
- Yan, J.; Mo, S.; Nie, J.; Chen, W.; Shen, X.; Hu, J.; Hao, G.; Tong, H. Hydrothermal synthesis of monodisperse Fe3O4 nanoparticles based on modulation of tartaric acid. Colloids Surf. A Physicochem. Eng. Asp. 2009, 340, 109–114. [Google Scholar] [CrossRef]
- Lu, T.; Wang, J.; Yin, J.; Wang, A.; Wang, X.; Zhang, T. Surfactant effects on the microstructures of Fe3O4 nanoparticles synthesized by microemulsion method. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 675–683. [Google Scholar] [CrossRef]
- Su, H.; Han, X.; He, L.; Deng, L.; Yu, K.; Jiang, H.; Wu, C.; Jia, Q.; Shan, S. Synthesis and characterization of magnetic dextran nanogel doped with iron oxide nanoparticles as magnetic resonance imaging probe. Int. J. Biol. Macromol. 2019, 128, 768–774. [Google Scholar] [CrossRef]
- Pham, X.N.; Nguyen, T.P.; Pham, T.N.; Tran, N.T.T.; Tran, T.V.T. Synthesis and characterization of chitosan-coated magnetite nanoparticles and their application in curcumin drug delivery. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 045010–045019. [Google Scholar] [CrossRef]
- Unni, M.; Uhl, A.M.; Savliwala, S.; Savitzky, B.H.; Dhavalikar, R.; Garraud, N.; Arnold, D.P.; Kourkoutis, L.F.; Andrew, J.S.; Rinaldi, C. Thermal Decomposition Synthesis of Iron Oxide Nanoparticles with Diminished Magnetic Dead Layer by Controlled Addition of Oxygen. ACS Nano 2017, 11, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Kumfer, B.M.; Shinoda, K.; Jeyadevan, B.; Kennedy, I.M. Gas-phase flame synthesis and properties of magnetic iron oxide nanoparticles with reduced oxidation state. J. Aerosol Sci. 2010, 41, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Lassenberger, A.; Gruenewald, T.A.; van Oostrum, P.D.J.; Rennhofer, H.; Amenitsch, H.; Zirbs, R.; Lichtenegger, H.C.; Reimhult, E. Monodisperse Iron Oxide Nanoparticles by Thermal Decomposition: Elucidating Particle Formation by Second-Resolved in Situ Small-Angle X-ray Scattering. Chem. Mater. 2017, 29, 4511–4522. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 Nanoparticles and their Magnetic Properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef]
- Xu, C.; Lu, X.; Dai, H. The Synthesis of Size-Adjustable Superparamagnetism Fe3O4 Hollow Microspheres. Nanoscale Res. Lett. 2017, 12, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Lykotrafitis, G.; Bao, G.; Suresh, S. Size-Dependent Endocytosis of Nanoparticles. Adv. Mater. 2009, 21, 419–424. [Google Scholar] [CrossRef]
- Bannunah, A.M.; Vllasaliu, D.; Lord, J.; Stolnik, S. Mechanisms of Nanoparticle Internalization and Transport Across an Intestinal Epithelial Cell Model: Effect of Size and Surface Charge. Mol. Pharm. 2014, 11, 4363–4373. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5–16. [Google Scholar] [CrossRef]
- Gratton, S.E.A.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of shape on Cellular Uptake of Gold Nanoparticles in the forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 3827–3836. [Google Scholar] [CrossRef] [PubMed]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiao, S.; Zhu, H.; Liang, H. Shape-dependent internalization kinetics of nanoparticles by membranes. Soft Matter 2016, 12, 2632–2641. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.N.; Xu, Q.X.; Davoodi, P.; Wang, D.P.; Wang, C.H. Enhanced intracellular delivery and controlled drug release of magnetic PLGA nanoparticles modified with transferrin. Acta Pharmacol. Sin. 2017, 38, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, J.V.; Kalicharan, D.; Couraud, P.-O.; Romero, I.A.; Weksler, B.; Hoekstra, D.; Zuhorn, I.S. Surface Characteristics of Nanoparticles Determine Their Intracellular Fate in and Processing by Human Blood–Brain Barrier Endothelial Cells In Vitro. Mol. Ther. 2011, 19, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- Ma, P.; Luo, Q.; Chen, J.; Gan, Y.; Du, J.; Ding, S.; Xi, Z.; Yang, X. Intraperitoneal injection of magnetic Fe3O4-nanoparticle induces hepatic and renal tissue injury via oxidative stress in mice. Int. J. Nanomed. 2012, 7, 4809–4818. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, N.; Chen, S.; Zhao, J.; Yang, X. Oxidative-damage effect of Fe3O4 nanoparticles on mouse hepatic and brain cells in vivo. Front. Biol. 2013, 8, 549–555. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Chen, B.; Ding, J.; Xia, G.; Gao, C.; Cheng, J.; Jin, N.; Zhou, Y.; Li, X.; et al. Pharmacokinetic parameters and tissue distribution of magnetic Fe3O4 nanoparticles in mice. Int. J. Nanomed. 2010, 5, 861–866. [Google Scholar] [CrossRef]
- Mejías, R.; Gutiérrez, L.; Salas, G.; Pérez-Yagüe, S.; Zotes, T.M.; Lázaro, F.J.; Morales, M.P.; Barber, D.F. Long term biotransformation and toxicity of dimercaptosuccinic acid-coated magnetic nanoparticles support their use in biomedical applications. J. Control. Release 2013, 171, 225–233. [Google Scholar] [CrossRef]
- De Tercero, M.D.; Bruns, M.; Martínez, I.G.; Türk, M.; Fehrenbacher, U.; Jennewein, S.; Barner, L. Continuous Hydrothermal Synthesis of In Situ Functionalized Iron Oxide Nanoparticles: A General Strategy to Produce Metal Oxide Nanoparticles With Clickable Anchors. Part. Part. Syst. Charact. 2013, 30, 229–234. [Google Scholar] [CrossRef]
- De Tercero, M.D.; Gonzáles Martínez, I.; Herrmann, M.; Bruns, M.; Kübel, C.; Jennewein, S.; Fehrenbacher, U.; Barner, L.; Türk, M. Synthesis of in situ functionalized iron oxide nanoparticles presenting alkyne groups via a continuous process using near-critical and supercritical water. J. Supercrit. Fluids 2013, 82, 83–95. [Google Scholar] [CrossRef]
- Karimzadeh, I.; Aghazadeh, M.; Doroudi, T.; Ganjali, M.R.; Kolivand, P.H. Superparamagnetic Iron Oxide (Fe3O4) Nanoparticles Coated with PEG/PEI for Biomedical Applications: A Facile and Scalable Preparation Route Based on the Cathodic Electrochemical Deposition Method. Adv. Phys. Chem. 2017, 2017, 9437487–9437494. [Google Scholar] [CrossRef]
- Bini, R.A.; Marques, R.F.C.; Santos, F.J.; Chaker, J.A.; Jafelicci, M. Synthesis and functionalization of magnetite nanoparticles with different amino-functional alkoxysilanes. J. Magn. Magn. Mater. 2012, 324, 534–539. [Google Scholar] [CrossRef]
- Rudakovskaya, P.G.; Gerasimov, V.M.; Metelkina, O.N.; Beloglazkina, E.K.; Savchenko, A.G.; Shchetinin, I.V.; Salikhov, S.V.; Abakumov, M.A.; Klyachko, N.L.; Golovin, Y.I.; et al. Synthesis and characterization of PEG-silane functionalized iron oxide(II, III) nanoparticles for biomedical application. Nanotechnol. Russ. 2015, 10, 896–903. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.R.; Li, J.; Zou, S.; Stach, E.A.; Takeuchi, K.J.; Takeuchi, E.S.; Marschilok, A.C.; Wong, S.S. Correlating Preparative Approaches with Electrochemical Performance of Fe3O4-MWNT Composites Used as Anodes in Li-Ion Batteries. J. Solid State Sci. Technol. 2017, 6, M3122–M3131. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef]
- Wei, W.; Bai, F.; Fan, H. Surfactant-Assisted Cooperative Self-Assembly of Nanoparticles into Active Nanostructures. iScience 2019, 11, 272–293. [Google Scholar] [CrossRef]
- Chen, L.; Wu, L.; Liu, F.; Qi, X.; Ge, Y.; Shen, S. Azo-functionalized Fe3O4 nanoparticles: A near-infrared light triggered drug delivery system for combined therapy of cancer with low toxicity. J. Mater. Chem. B 2016, 4, 3660–3669. [Google Scholar] [CrossRef]
- Gawali, S.L.; Barick, K.C.; Shetake, N.G.; Rajan, V.; Pandey, B.N.; Kumar, N.N.; Priyadarsini, N.K.I.; Hassan, P.A. pH-Labile Magnetic Nanocarriers for Intracellular Drug Delivery to Tumor Cells. ACS Omega 2019, 47, 11728–11736. [Google Scholar] [CrossRef]
- Sharma, K.S.; Ningthoujam, R.S.; Dubey, A.K.; Chattopadhyay, A.; Phapale, S.; Juluri, R.R.; Mukherjee, S.; Tewari, R.; Shetake, N.G.; Pandey, B.N.; et al. Synthesis and characterization of monodispersed water dispersible Fe3O4 nanoparticles and in vitro studies on human breast carcinoma cell line under hyperthermia condition. Sci. Rep. 2018, 8, 14766–14777. [Google Scholar] [CrossRef] [PubMed]
- Demin, A.M.; Krasnov, V.P.; Charushin, V.N. Covalent Surface Modification of Fe3O4 Magnetic Nanoparticles with Alkoxy Silanes and Amino Acids. Mendeleev Commun. 2013, 23, 14–16. [Google Scholar] [CrossRef]
- Arsalani, N.; Fattahi, H.; Nazarpoor, M. Synthesis and characterization of PVP-functionalized superparamagnetic Fe3O4 nanoparticles as an MRI contrast agent. eXPRESS Polym. Lett. 2010, 4, 329–338. [Google Scholar] [CrossRef]
- Han, C.W.; Choksi, T.; Milligan, C.A.; Majumdar, P.; Manto, M.J.; Cui, Y.; Sang, X.; Unocic, R.R.; Zemlyanov, D.Y.; Wang, C.; et al. A Discovery of Strong Metal-Support Bonding in Nano-engineered Au-Fe3O4 Dumbbell-like Nanoparticles by In-situ Transmission Electron Microscopy. Nano Lett. 2017, 17, 4576–4582. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Torati, S.R.; Iqbal, S.A.; Kim, C.G. A novel and rapid approach for the synthesis of biocompatible and highly stable Fe3O4/SiO2 and Fe3O4/C core/shell nanocubes and nanorods. New J. Chem. 2017, 41, 2724–2734. [Google Scholar] [CrossRef]
- Khosroshahi, M.E.; Ghazanfari, L.; Tahriri, M. Characterisation of binary (Fe3O4/SiO2) biocompatible nanocomposites as magnetic fluid. J. Exp. Nanosci. 2011, 6, 580–595. [Google Scholar] [CrossRef]
- Guo, X.; Mao, F.; Wang, W.; Yang, Y.; Bai, Z. Sulfhydryl-Modified Fe3O4@SiO2 Core/Shell Nanocomposite: Synthesis and Toxicity Assessment in Vitro. ACS Appl. Mater. Interfaces 2015, 7, 14983–14991. [Google Scholar] [CrossRef]
- Nikmah, A.; Taufiq, A.; Hidayat, A. Synthesis and Characterization of Fe3O4/SiO2 nanocomposites. IOP Conf. Ser. Earth Environ. Sci. 2019, 276, 012046. [Google Scholar]
- Ding, H.L.; Zhang, X.Y.; Wang, S.; Xu, J.M.; Xu, S.C.; Li, G.H. Fe3O4@SiO2 Core/Shell Nanoparticles: The Silica Coating Regulations with a Single Core for Different Core Sizes and Shell Thicknesses. Chem. Mater. 2012, 2423, 4572–4580. [Google Scholar] [CrossRef]
- Liu, C.Y.; Puig, T.; Obradors, X.; Ricart, S.; Ros, J. Ultra-fast microwave-assisted reverse microemulsion synthesis of Fe3O4@SiO2 core–shell nanoparticles as a highly recyclable silver nanoparticle catalytic platform in the reduction of 4-nitroaniline. RSC Adv. 2016, 6, 88762–88769. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Liu, S. Biocompatibility and biomedical applications of functionalized mesoporous silica nanoparticles. Biointerface Res. Appl. Chem. 2014, 4, 767–775. [Google Scholar]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis. Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Asefa, T.; Tao, Z. Biocompatibility of Mesoporous Silica Nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Isa, E.D.M.; Ahmad, H.; Rahman, M.B.A. Optimization of Synthesis Parameters of Mesoporous Silica Nanoparticles Based on Ionic Liquid by Experimental Design and Its Application as a Drug Delivery Agent. J. Nanomater. 2019, 2019, 4982054–4982062. [Google Scholar] [CrossRef]
- Jorge, J.; Verelst, M.; de Castro, G.R.; Martines, M.A.U. Synthesis parameters for control of mesoporous silica nanoparticles (MSNs). Biointerface Res. Appl. Chem. 2016, 6, 1520–1524. [Google Scholar]
- Sun, J.-G.; Jiang, Q.; Zhang, X.-P.; Shan, K.; Liu, B.-H.; Zhao, C.; Yan, B. Mesoporous silica nanoparticles as a delivery system for improving antiangiogenic therapy. Int. J. Nanomed. 2019, 14, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2017, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Laurent, S.; Fornara, A.; Astolfi, L.; Qin, J.; Roch, A.; Martini, A.; Toprak, M.S.; Muller, R.N.; Muhammed, M. Uniform mesoporous silica coated iron oxide nanoparticles as a highly efficient, nontoxic MRI T2 contrast agent with tunable proton relaxivities. Contrast Media Mol. Imaging 2012, 7, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, Z.; Bakhshi, B.; Javidi, J.; Adrangi, S. Synthesis of Silica-coated Iron Oxide Nanoparticles: Preventing Aggregation without Using Additives or Seed Pretreatment. Iran. J. Pharm. Res. 2018, 17, 386–395. [Google Scholar] [PubMed]
- Yin, N.Q.; Wu, P.; Yang, T.H.; Wang, M. Preparation and study of a mesoporous silica-coated Fe3O4 photothermal nanoprobe. RSC Adv. 2017, 7, 9123–9129. [Google Scholar] [CrossRef]
- Venkatathri, N. Synthesis of mesoporous silica nanosphere using different templates. Solid State Commun. 2007, 143, 493–497. [Google Scholar] [CrossRef]
- Kipkemboi, P.; Fogden, A.; Alfredsson, V.; Flostroem, K. Triblock Copolymers as Templates in Mesoporous Silica Formation: Structural Dependence on Polymer Chain Length and Synthesis Temperature. Langmuir 2001, 17, 5398–5402. [Google Scholar] [CrossRef]
- Peralta, M.E.; Jadhav, S.A.; Magnacca, G.; Scalarone, D.; Mártire, D.O.; Parolo, M.E.; Carlos, L. Synthesis and in vitro testing of thermoresponsive polymer-grafted core-shell magnetic mesoporous silica nanoparticles for efficient controlled and targeted drug delivery. J. Colloid Interface Sci. 2019, 544, 198–205. [Google Scholar] [CrossRef]
- Park, S.S.; Jung, M.H.; Lee, Y.-S.; Bae, J.-H.; Kim, S.-H.; Ha, C.-S. Functionalised mesoporous silica nanoparticles with excellent cytotoxicity against various cancer cells for pH-responsive and controlled drug delivery. Mater. Des. 2019, 184, 108187–108197. [Google Scholar] [CrossRef]
- Li, T.; Geng, T.; Md, A.; Banerjee, P.; Wang, B. Novel scheme for rapid synthesis of hollow mesoporous silica nanoparticles (HMSNs) and their application as an efficient delivery carrier for oral bioavailability improvement of poorly water-soluble BCS type II drugs. Colloids Surf. B Biointerfaces 2019, 176, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.; Abu-Zahra, N.H. Synthesis and characterization of polyethersulfone membranes impregnated with (3-aminopropyltriethoxysilane) APTES-Fe3O4 nanoparticles for As(V) removal from water. J. Environ. Chem. Eng. 2019, 7, 102875–102885. [Google Scholar] [CrossRef]
- Liang, X.X.; Ouyang, X.K.; Wang, S.; Yang, L.-Y.; Huang, F.; Ji, C.; Chen, X. Efficient adsorption of Pb(II) from aqueous solutions using aminopropyltriethoxysilane-modified magnetic attapulgite@chitosan (APTS-Fe3O4/APT@CS) composite hydrogel beads. Int. J. Biol. Macromol. 2019, 137, 741–750. [Google Scholar] [CrossRef]
- Langeroudi, M.P.; Binaeian, E. Tannin-APTES modified Fe3O4 nanoparticles as a carrier of Methotrexate drug: Kinetic, isotherm and thermodynamic studies. Mater. Chem. Phys. 2018, 218, 210–217. [Google Scholar] [CrossRef]
- Arum, Y.; Yun-Ok, O.; Kang, H.W.; Seok-Hwan, A.; Junghwan, O. Chitosan-Coated Fe3O4 Magnetic Nanoparticles as Carrier of Cisplatin for Drug Delivery. Fish. Aquat. Sci. 2015, 18, 89–98. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.; Zhang, Y.; Liu, J.; Xu, Q.; Xiao, H.; Wang, X.; Xu, H.; Zhou, J. Thiol modified Fe3O4@SiO2 as a robust, high effective, and recycling magnetic sorbent for mercury removal. Chem. Eng. J. 2013, 226, 30–38. [Google Scholar] [CrossRef]
- Badragheh, S.; Zeeb, M.; Olyai, M.R.T.B. Silica-coated magnetic iron oxide functionalized with hydrophobic polymeric ionic liquid:a promising nanoscale sorbent for simultaneous extraction of antidiabetic drugs from human plasma prior to their quantitation by HPLC. RSC Adv. 2018, 8, 30550–30561. [Google Scholar] [CrossRef]
- Rego, G.N.A.; Mamani, J.B.; Souza, T.K.F.; Nucci, M.P.; Silva, H.R.D.; Gamarra, L.F. Therapeutic evaluation of magnetic hyperthermia using Fe3O4-aminosilane-coated iron oxide nanoparticles in glioblastoma animal model. Einstein 2019, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shaleri Kardar, Z.S.; Beyki, M.H.; Shemirani, F. Bifunctional aminosilane-functionalized Fe3O4nanoparticles as efficient sorbent for preconcentration of cobalt ions from food and water samples. Res. Chem. Intermed. 2017, 43, 4079–4096. [Google Scholar] [CrossRef]
- Tian, F.; Chen, G.; Yi, P.; Zhang, J.; Li, A.; Zhang, J.; Zheng, L.; Deng, Z.; Shi, Q.; Peng, R.; et al. Fates of Fe3O4 and Fe3O4@SiO2 nanoparticles in human mesenchymal stem cells assessed by synchrotron radiation-based techniques. Biomaterials 2014, 35, 6412–6421. [Google Scholar] [CrossRef] [PubMed]
- Mostafaei, M.; Hosseini, S.N.; Khatami, M.; Javidanbardan, A.; Sepahy, A.A.; Asadi, E. Isolation of recombinant Hepatitis B surface antigen with antibody-conjugated superparamagnetic Fe3O4/SiO2 core-shell nanoparticles. Protein Expr. Purif. 2018, 145, 1–6. [Google Scholar] [CrossRef]
- Fan, Q.; Guan, Y.; Zhang, Z.; Xu, G.; Yang, Y.; Guo, C. A new method of synthesis well-dispersion and dense Fe3O4@SiO2 magnetic nanoparticles for DNA extraction. Chem. Phys. Lett. 2019, 715, 7–13. [Google Scholar] [CrossRef]
- Gan, Q.; Lu, X.; Yuan, Y.; Qian, J.; Zhou, H.; Lu, X.; Shi, J.; Liu, C. A magnetic, reversible pH-responsive nanogated ensemble based on Fe3O4 nanoparticles-capped mesoporous silica. Biomaterials 2011, 32, 1932–1942. [Google Scholar] [CrossRef]
- Cui, J.; Sun, B.; Lin, T.; Feng, Y.; Jia, S. Enzyme shielding by mesoporous organosilica shell on Fe3O4@silica yolk-shell nanospheres. Int. J. Biol. Macromol. 2018, 117, 673–682. [Google Scholar] [CrossRef]
- Asgari, M.; Soleymani, M.; Miri, T.; Barati, A. A robust method for fabrication of monodisperse magnetic mesoporous silica nanoparticles with core-shell structure as anticancer drug carriers. J. Mol. Liquids 2019, 292, 111367–111375. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Li, X.; Wang, D.; Wei, B.; Song, H.; Li, X.; Fu, S. Preparation and photocatalytic properties of magnetically reusable Fe3O4@ZnO core/shell nanoparticles. Phys. E 2016, 75, 66–71. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Z.; Chen, L.; Zhang, L.; Li, X.; Xu, H.; Wang, H.; Zhu, G. Preparation of magnetic Fe3O4/TiO2/Ag composite microspheres with enhanced photocatalytic activity. Solid State Sci. 2016, 52, 42–48. [Google Scholar] [CrossRef]
- Choi, K.H.; Min, J.; Park, S.Y.; Park, B.J.; Jung, J.S. Enhanced photocatalytic degradation of tri-chlorophenol by Fe3O4@TiO2@Au photocatalyst under visible-light. Ceram. Int. 2019, 45, 9477–9482. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhong, Y.; Zhang, J.; Wang, Z.; Wang, L.; An, Y.; Lin, M.; Gao, Z.; Zhang, D. Biocompatibility of Fe3O4@Au composite magnetic nanoparticles in vitro and in vivo. Int. J. Nanomed. 2011, 6, 2805–2819. [Google Scholar] [CrossRef]
- Nalluri, S.R.; Nagarjuna, R.; Patra, D.; Ganesan, R.; Balaji, G. Large Scale Solid-state Synthesis of Catalytically Active Fe3O4@M (M = Au, Ag and Au-Ag alloy) Core-shell Nanostructures. Sci. Rep. 2019, 9, 6603–6614. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Borwankar, A.U.; Willsey, B.W.; Yoon, K.Y.; Tam, J.O.; Sokolov, K.V.; Feldman, M.D.; Milner, T.E.; Johnston, K.P. Growth of textured thin Au coatings on iron oxide nanoparticles with near infrared absorbance. Nanotechnology 2013, 24, 025606–025620. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sanchez, L.M.; Alvarez, V.A. Advances in Magnetic Noble Metal/Iron-Based Oxide Hybrid Nanoparticles as Biomedical Devices. Bioengineering 2019, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Shao, H.; Zhan, S.; Hou, P.; Zhang, X.; Chai, Y.; Liu, H. Bi-phase dispersible Fe3O4@Au core–shell multifunctional nanoparticles: Synthesis, characterization and properties. Compos. Interfaces 2019, 26, 537–549. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, E.; Ou, G.; Gao, L.; Chen, J. Fe3O4-Au and Fe2O3-Au Hybrid Nanorods: Layer-by-Layer Assembly Synthesis and Their Magnetic and Optical Properties. Nanoscale Res. Lett. 2010, 5, 1755–1761. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, Q.; Dai, X.; Zhang, P.; Tan, X.; Zhong, Y.; Yao, C.; Song, M.; Song, G.; Zhang, G.; Peng, G.; et al. Fe3O4@Au composite magnetic nanoparticles modified with cetuximab for targeted magneto-photothermal therapy of glioma cells. Int. J. Nanomed. 2018, 13, 2491–2505. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Luo, J.; Fan, Q.; Suzuki, M.; Suzuki, I.S.; Engelhard, M.H.; Lin, Y.; Kim, N.; Jian, Q.; et al. Monodispersed Core−Shell Fe3O4@Au Nanoparticles. J. Phys. Chem. B 2005, 109, 21593–21601. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hou, Y.; Sun, S. Magnetic Core/Shell Fe3O4/Au and Fe3O4/Au/Ag Nanoparticles with Tunable Plasmonic Properties. J. Am. Chem. Soc. 2007, 129, 8698–8699. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, R.; Wang, S.; Ding, L.; Li, J.; Luo, Y.; Wang, X.; Shen, M.; Shi, X. Multifunctional Fe3O4 @ Au core/shell nanostars: A unique platform for multimode imaging and photothermal therapy of tumors. Sci. Rep. 2016, 6, 28325–28337. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhong, Y.; Ji, G.; Lu, Q.; Dai, X.; Guo, Z.; Zhang, P.; Peng, G.; Zhang, K.; Li, Y. Preparation and characterization of Fe3O4@Au-C225 composite targeted nanoparticles for MRI of human glioma. PLoS ONE 2018, 13, e0195703. [Google Scholar] [CrossRef]
- Kang, N.; Xu, D.; Han, Y.; Lv, X.; Chen, Z.; Zhou, T.; Ren, L.; Zhou, X. Magnetic targeting core/shell Fe3O4/Au nanoparticles for magnetic resonance/photoacoustic dual-modal imaging. Mater. Sci. Eng. C 2019, 98, 545–549. [Google Scholar] [CrossRef]
- Klein, S.; Hübner, J.; Menter, C.; Distel, L.V.R.; Neuhuber, W.; Kryschi, C. A Facile One-Pot Synthesis of Water-Soluble, Patchy Fe3O4-Au Nanoparticles for Application in Radiation Therapy. Appl. Sci. 2019, 9, 15. [Google Scholar] [CrossRef]
- Hu, R.; Zheng, M.; Wu, J.; Li, C.; Shen, D.; Yang, D.; Li, L.; Ge, M.; Chang, Z.; Dong, W. Core-Shell Magnetic Gold Nanoparticles for Magnetic Field-Enhanced Radio-Photothermal Therapy in Cervical Cancer. Nanomaterials 2017, 7, 111. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Yang, J.; Wei, P.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Hyaluronic acid-modified Fe3O4@Au core/shell nanostars for multimodal imaging and photothermal therapy of tumors. Biomaterials 2015, 38, 10–21. [Google Scholar] [CrossRef]
- Park, S.I.; Chung, S.H.; Kim, H.C.; Lee, S.G.; Lee, S.J.; Kim, H.; Kim, H.; Jeong, S.W. Prolonged heating of Fe3O4–Au hybrid nanoparticles in a radiofrequency solenoid coil. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 304–309. [Google Scholar] [CrossRef]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Yu, B.; Liu, T.; Du, X.; Luo, Z.; Zheng, W.; Chen, T. X-ray-responsive selenium nanoparticles for enhanced cancer chemo-radiotherapy. Colloids Surf. B Biointerfaces 2016, 139, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Mattea, F.; Vedelago, J.; Malano, F.; Gomez, C.; Strumia, M.C.; Valente, M. Silver nanoparticles in X-ray biomedical applications. Radiat. Phys. Chem. 2017, 130, 442–450. [Google Scholar] [CrossRef]
- Berbeco, R.I.; Ngwa, W.; Makrigiorgos, G.M. Localized Dose Enhancement to Tumor Blood Vessel Endothelial Cells via Megavoltage X-rays and Targeted Gold Nanoparticles: New Potential for External Beam Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Generalov, R.; Kuan, W.B.; Chen, W.; Kristensen, S.; Juzenas, P. Radiosensitizing effect of zinc oxide and silica nanocomposites on cancer cells. Colloids Surf. B Biointerfaces 2015, 129, 79–86. [Google Scholar] [CrossRef]
- Wolfe, T.; Chatterjee, D.; Lee, J.; Grant, J.D.; Bhattarai, S.; Tailor, R.; Goodrich, G.; Nicolucci, P.; Krishnan, S. Targeted gold nanoparticles enhance radiosensitization of prostate tumors to megavoltage radiation therapy in vivo. Nanomedicine 2015, 11, 1277–1283. [Google Scholar] [CrossRef]
- Miladi, I.; Aloy, M.T.; Armandy, E.; Mowat, P.; Kryza, D.; Magne, N.; Tillement, O.; Lux, F.; Billotey, C.; Janier, M.; et al. Combining ultrasmall gadolinium-based nanoparticles with photon irradiation overcomes radioresistance of head and neck squamous cell carcinoma. Nanomedicine 2015, 11, 247–257. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Jin, X.; He, P.; Zheng, X.; Dai, Z.; Ye, F.; Zhao, T.; Chen, W.; Li, Q. The dependence of radiation enhancement effect on the concentration of gold nanoparticles exposed to low- and high- LET radiations. Eur. J. Med. Phys. 2015, 31, 210–218. [Google Scholar] [CrossRef]
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef]
- Prado-Gotor, R.; Grueso, E. A kinetic study of the interaction of DNA with gold nanoparticles: Mechanistic aspects of the interaction. Phys. Chem. Chem. Phys. 2011, 13, 1479–1489. [Google Scholar] [CrossRef]
- Li, K.; Zhao, X.; Hammer, B.K.; Du, S.; Chen, Y. Nanoparticles Inhibit DNA Replication by Binding to DNA: Modeling and Experimental Validation. ACS Nano 2013, 7, 9664–9674. [Google Scholar] [CrossRef] [PubMed]
- Glazer, E.S.; Curley, S.A. Non-invasive radiofrequency ablation of malignancies mediated by quantum dots, gold nanoparticles and carbon nanotubes. Ther. Deliv. 2011, 2, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, Z.; Zhong, X.; Zhao, Z.; Li, J. Study on the Thermal Characteristics of Fe3O4 Nanoparticles and Gelatin Compound for Magnetic Fluid Hyperthermia in Radiofrequency Magnetic Field. IEEE Trans. Magn. 2014, 50, 1–4. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, J.; Zhang, B.; Qu, J. Synthesis of Ag@Fe3O4 Nanoparticles for Photothermal Treatment of Ovarian Cancer. J. Nanomater. 2019, 2019, 6457968–6457976. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Wang, L.; Zhu, X.; Zhang, H.; Song, D. Surface plasmon resonance biosensor based on Fe3O4/Au nanocomposites. Colloids Surf. B Biointerfaces 2010, 81, 600–606. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Lin, Y.; Du, D. The vital function of Fe3O4@Au nanocomposite for hydrolase biosensor design and its application in detection of methyl parathion. Nanoscale 2013, 5, 1121–1126. [Google Scholar] [CrossRef]
- Liu, F.M.; Nie, J.; Qin, Y.N.; Yin, W.; Hou, C.J.; Huo, D.Q.; He, B.; Xia, T.C. A biomimetic sensor based on specific receptor ETBD and Fe3O4@Au/MoS2/GN for signal enhancement shows highly selective electrochemical response to ultra-trace lead (II). J. Solid State Electrochem. 2017, 21, 3257–3268. [Google Scholar] [CrossRef]
- Li, S.; Liang, J.; Zhou, Z.; Li, G. An electrochemical immunosensor for AFP measurement based on the magnetic Fe3O4@Au@CS nanomaterials. IOP Conf. Ser. Mater. Sci. Eng. 2018, 382, 022017. [Google Scholar] [CrossRef]
- Cui, Y.R.; Hong, C.; Zhou, Y.L.; Li, Y.; Gao, X.M.; Zhang, X.X. Synthesis of orientedly bioconjugated core/shell Fe3O4@Au magnetic nanoparticles for cell separation. Talanta 2011, 85, 1246–1252. [Google Scholar] [CrossRef]
- Li, J.; Zou, S.; Gao, J.; Liang, J.; Zhou, H.; Liang, L.; Wu, W. Block copolymer conjugated Au-coated Fe3O4 nanoparticles as vectors for enhancing colloidal stability and cellular uptake. J. Nanobiotechnol. 2017, 15, 56–67. [Google Scholar] [CrossRef]
- Li, Y.; Yun, K.H.; Lee, H.; Goh, S.H.; Suh, Y.G.; Choi, Y. Porous platinum nanoparticles as a high-Z and oxygen generating nanozyme for enhanced radiotherapy in vivo. Biomaterials 2019, 197, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Klingberg, H.; Jauffred, L.; Kjaer, A.; Bendix, P.M.; Oddershede, L.B. Platinum nanoparticles: A non-toxic, effective and thermally stable alternative plasmonic material for cancer therapy and bioengineering. Nanoscale 2018, 10, 9097–9107. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Xie, J.; Zhang, Y.; Chen, Z.; Gu, N. Fe3O4@Pt nanoparticles with enhanced peroxidase-like catalytic activity. Mater. Lett. 2013, 105, 36–39. [Google Scholar] [CrossRef]
- Wu, D.; Ma, H.; Zhang, Y.; Jia, H.; Yan, T.; Wei, Q. Corallite-like Magnetic Fe3O4@MnO2@Pt Nanocomposited as Multiple Signal Amplifiers for the Detection of Carcinoembryonic Antigen. ACS Appl. Mater. Interfaces 2015, 7, 18786–18793. [Google Scholar] [CrossRef] [PubMed]
- Khaghani, S.; Ghanbari, D.J. Magnetic and photo-catalyst Fe3O4–Ag nanocomposite: Green preparation of silver and magnetite nanoparticles by garlic extract. Mater. Sci. Mater. Electron. 2017, 28, 2877–2886. [Google Scholar] [CrossRef]
- Gao, G.; Wang, K.; Huang, P.; Zhang, Y.; Bao, C.; Cui, D. Superparamagnetic Fe3O4–Ag hybrid nanocrystals as a potential contrast agent for CT imaging. Cryst. Eng. Commun. 2012, 14, 7556–7559. [Google Scholar] [CrossRef]
- Sadat, M.E.; Baghbador, M.K.; Dunn, A.W.; Wagner, H.P.; Ewing, R.C.; Zhang, J.; Xu, H.; Pauletti, G.M.; Mast, D.B.; Shi, D. Photoluminescence and photothermal effect of Fe3O4 nanoparticles for medical imaging and therapy. Appl. Phys. Lett. 2014, 105, 091903. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Lou, Z.; Chen, F.; Chang, S.; Miao, Y.; Zhou, Z.; Hu, X.; Feng, J.; Ding, Q.; et al. Radiosensitivity enhancement of Fe3O4@Ag nanoparticles on human glioblastoma cells. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S1), 975–984. [Google Scholar] [CrossRef]
- Nguyen, T.N.L.; Do, T.V.; Nguyen, T.V.; Dao, P.H.; Trinh, V.T.; Mac, V.P.; Nguyen, A.H.; Dinh, D.A.; Nguyen, T.A.; Vo, T.K.A.; et al. Antimicrobial activity of acrylic polyurethane/Fe3O4-Ag nanocomposite coating. Prog. Org. Coat. 2019, 132, 15–20. [Google Scholar] [CrossRef]
- Chang, M.; Lin, W.S.; Xiao, W.; Chen, Y.N. Antibacterial Effects of Magnetically-Controlled Ag/Fe3O4 Nanoparticles. Materials 2018, 11, 659. [Google Scholar] [CrossRef]
- Brollo, M.E.F.; Lopez-Ruiz, R.; Muraca, D.; Figueroa, S.J.A.; Pirota, K.R.; Knobel, M. Compact Ag@Fe3O4 Core-shell Nanoparticles by Means of Single-step Thermal Decomposition Reaction. Sci. Rep. 2014, 4, 6839–6845. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, J. Synergistic interaction between pseudocapacitive Fe3O4 nanoparticles and highly porous silicon carbide for high-performance electrodes as electrochemical supercapacitors. Nanotechnology 2017, 28, 195401–195414. [Google Scholar]
- Fan, H.; Niu, R.; Duan, J.; Liu, W.; Shen, W. Fe3O4@Carbon Nanosheets for All-Solid-State Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 19475–19483. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhao, H.; Wang, J.; Lv, P.; Zhang, T.; Xia, Q. Nanostructured Fe3O4@C as anode material for lithium-ion batteries. J. Power Sources 2014, 248, 15–21. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, J. Novel magnetic Fe3O4@C nanoparticles as adsorbents for removal of organic dyes from aqueous solution. J. Hazard. Mater. 2011, 193, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.Y.; Yang, W.J.; Bu, F.X.; Jiang, D.M.; Zhao, Z.J.; Zhang, Q.H.; Fang, Q.C.; Jiang, J.S. One-step hydrothermal synthesis of Fe3O4@C nanoparticles with great performance in biomedicine. J. Mater. Chem. B 2014, 2, 4481–4488. [Google Scholar] [CrossRef]
- Da Costa, T.R.; Baldi, E.; Figueiró, A.; Colpani, G.L.; Silva, L.L.; Zanetti, M.; de Mello, J.M.M.; Fiori, M.A. Fe3O4@C core-shell nanoparticles as adsorbent of ionic zinc: Evaluating of the adsorptive capacity. Mater. Res. 2019, 22 (Suppl. S1), e20180847. [Google Scholar] [CrossRef]
- Hein, C.D.; Liu, X.M.; Wang, D. Click chemistry, a powerful tool for pharmaceutical sciences. Pharm. Res. 2008, 25, 2216–2230. [Google Scholar] [CrossRef]
- Campidelli, S. Click Chemistry for Carbon Nanotubes Functionalization. Curr. Org. Chem. 2011, 15, 1151–1159. [Google Scholar] [CrossRef]
- Fan, X.; Jiao, G.; Zhao, W.; Jin, P.; Li, X. Magnetic Fe3O4-graphene composites as targeted drug nanocarriers for pH-activated release. Nanoscale 2013, 5, 1143–1152. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, R.; Campbell, E.; Naumov, A. Multifunctional graphene oxide/iron oxide nanoparticles for magnetic targeted drug delivery dual magnetic resonance/fluorescence imaging and cancer sensing. PLoS ONE 2019, 14, e0217072. [Google Scholar] [CrossRef]
- Namvari, M.; Namazi, H. Clicking graphene oxide and Fe3O4 nanoparticles together: An efficient adsorbent to remove dyes from aqueous solutions. Int. J. Environ. Sci. Technol. 2014, 11, 1527–1536. [Google Scholar] [CrossRef]
- Arukali Sammaiah, A.; Huang, W.; Wang, X. Synthesis of magnetic Fe3O4/graphene oxide nanocomposites and their tribological properties under magnetic field. Mater. Res. Express 2018, 5, 105006–105015. [Google Scholar] [CrossRef]
- Sadeghfar, F.; Ghaedi, M.; Asfaram, A.; Jannesar, R.; Javadian, H.; Pezeshkpour, V. Polyvinyl alcohol/Fe3O4@carbon nanotubes nanocomposite: Electrochemical-assisted synthesis, physicochemical characterization, optical properties, cytotoxicity effects and ultrasound-assisted treatment of aqueous based organic compound. J. Ind. Eng. Chem. 2018, 65, 349–362. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, X.; Ma, Q.; Tang, B.; Lu, Z.; Zhang, J.; Mo, G.; Ye, J.; Ye, J. A sensitive electrochemical sensor for simultaneous voltammetric sensing of cadmium and lead based on Fe3O4/multiwalled carbon nanotube/laser scribed graphene composites functionalized with chitosan modified electrode. Mater. Chem. Phys. 2019, 238, 121876–121877. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Cui, Y.; Chu, X.; Sun, B.; Zhou, N.; Shen, J. Magnetofluorescent Fe3O4/carbon quantum dots coated single-walled carbon nanotubes as dual-modal targeted imaging and chemo/photodynamic/photothermal triple-modal therapeutic agents. Chem. Eng. J. 2018, 338, 526–538. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Zhou, R.; Wu, H.; Shi, H.; Yu, S.; Liu, Y. Multifunctional glucose biosensors from Fe3O4 nanoparticles modified chitosan/ graphene nanocomposites. Sci. Rep. 2015, 5, 11129–11138. [Google Scholar] [CrossRef]
- Patsula, V.; Horák, D.; Kučka, J.; Mackova, H.; Lobaz, V.; Francova, P.; Herynek, V.; Heizer, T.; Paral, P.; Sefc, L. Synthesis and modification of uniform PEG-neridronate-modified magnetic nanoparticles determines prolonged blood circulation and biodistribution in a mouse preclinical model. Sci. Rep. 2019, 9, 10765–10777. [Google Scholar] [CrossRef]
- Yuan, G.; Yuan, Y.; Xu, K.; Luo, Q. Biocompatible PEGylated Fe3O4 nanoparticles as photothermal agents for near-infrared light modulated cancer therapy. Int. J. Mol. Sci. 2014, 15, 18776–18788. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 28–51. [Google Scholar] [CrossRef]
- Mostaghasi, E.; Zarepour, A.; Zarrabi, A. Folic acid armed Fe3O4-HPG nanoparticles as a safe nano vehicle for biomedical theranostics. J. Taiwan Inst. Chem. Eng. 2018, 82, 33–41. [Google Scholar] [CrossRef]
- Avedian, N.; Zaaeri, F.; Daryasari, M.P.; Javar, H.A.; Khoobi, M. pH-sensitive biocompatible mesoporous magnetic nanoparticles labeled with folic acid as an efficient carrier for controlled anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2018, 44, 323–332. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Zavareh, S.; Gazi, E.M.; Jamili, M. Assessment of novel core–shell Fe3O4@poly l-DOPA nanoparticles for targeted Taxol® delivery to breast tumor in a mouse model. Mater. Sci. Eng. C 2018, 93, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Chen, Y.C. Riboflavin immobilized Fe3O4 magnetic nanoparticles carried with n-butylidenephthalide as targeting-based anticancer agents, Artificial Cells. Nanomed. Biotechnol. 2019, 47, 210–220. [Google Scholar] [CrossRef]
- Arriortua, O.K.; Insausti, M.; Lezama, L.; de Muro, I.G.; Garaio, E.; de la Fuente, J.M.; Fratila, R.M.; Morales, M.P.; Costa, R.; Eceiza, M.; et al. RGD-Functionalized Fe3O4 nanoparticles for magnetic hyperthermia. Colloids Surf. B Biointerfaces 2018, 165, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Jiang, R.; Fan, Q.; Zhang, L.; Zhang, H.; Yang, M.; Ma, Y.; Wang, L.; Huang, W. Fluorescent-magnetic poly(poly(ethyleneglycol)monomethacrylate)-grafted Fe3O4 nanoparticles from post-atom-transfer-radical-polymerization modification: Synthesis, characterization, cellular uptake and imaging. J. Mater. Chem. 2012, 22, 6965–6973. [Google Scholar] [CrossRef]
- Stephen, Z.R.; Kievit, F.M.; Zhang, M. Magnetite Nanoparticles for Medical MR Imaging. Mater. Today 2011, 14, 330–338. [Google Scholar] [CrossRef]
- Wang, Z.; Lam, A.; Acosta, E. Suspensions of Iron Oxide Nanoparticles Stabilized by Anionic Surfactants. J. Surfactants Deterg. 2013, 16, 397–407. [Google Scholar] [CrossRef]
- Choi, Y.W.; Lee, H.; Song, Y.; Sohn, D. Colloidal stability of iron oxide nanoparticles with multivalent polymer surfactants. J. Colloid Interface Sci. 2015, 443, 8–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Newton, B.; Lewis, E.; Fu, P.P.; Kafoury, R.; Ray, P.C.; Yu, H. Cytotoxicity of organic surface coating agents used for nanoparticles synthesis and stability. Toxicol. Vitr. 2015, 29, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.I.P.; Lochte, F.; Echeverria, C.; Pereira, L.C.J.; Coutinho, J.T.; Ferreira, I.M.M.; Novo, C.M.M.; Borges, J.P.M.R. Thermal and magnetic properties of iron oxide colloids: Influence of surfactants. Nanotechnology 2015, 26, 425704–425715. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.; Mitsumori, L.M.; Kushleika, J.V.; Rosenfeld, M.E.; Krishnan, K.M. Cytotoxicity of iron oxide nanoparticles made from the thermal decomposition of organometallics and aqueous phase transfer with Pluronic F127. Contrast Media Mol. Imaging 2010, 5, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shen, H.; Li, X.; Ruan, J.; Yan, W.Q. Magnetic fluids’ stability improved by oleic acid bilayer-coated structure via one-pot synthesis. Chem. Pap. 2016, 70, 1642–1648. [Google Scholar] [CrossRef]
- Coricovac, D.E.; Moacă, E.A.; Pinzaru, I.; Citu, C.; Soica, C.; Mihail, C.V.; Pacurariu, C.; Tutelyan, V.A.; Tsatsakis, A.; Dehelean, C.A. Biocompatible Colloidal Suspensions Based on Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Toxicological Profile. Front. Pharmacol. 2017, 8, 154–172. [Google Scholar] [CrossRef] [PubMed]
- Mulder, W.J.M.; Strijkers, G.J.; van Tilborg, G.A.F.; Cormode, D.P.; Fayad, Z.A.; Nicolay, K. Nanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imaging. Acc. Chem. Res. 2009, 42, 904–914. [Google Scholar] [CrossRef]
- Yang, J.; Pinar, A. Understanding the role of grafted polystyrene chain conformation in assembly of magnetic nanoparticles. Phys. Rev. E 2014, 90, 042601. [Google Scholar]
- Nagesha, D.K.; Plouffe, B.D.; Phan, M.; Lewis, L.H.; Sridhar, S.; Murthy, S.K. Functionalization-induced improvement in magnetic properties of Fe3O4 nanoparticles for biomedical applications. J. Appl. Phys. 2009, 105, 07B317. [Google Scholar] [CrossRef]
- Zhang, G.; Qie, F.; Hou, J.; Luo, S.; Luo, L.; Sun, X.; Tan, T. One-pot solvothermal method to prepare functionalized Fe3O4 nanoparticles for bioseparation. J. Mater. Res. 2012, 27, 1006–1013. [Google Scholar] [CrossRef]
- Ooi, F.; DuChene, J.S.; Qiu, J.; Graham, J.O.; Engelhard, M.H.; Cao, G.; Gai, Z.; Wei, W.D. A Facile Solvothermal Synthesis of Octahedral Fe3O4 Nanoparticles. Small 2015, 11, 2649–2653. [Google Scholar] [CrossRef]
- Kekalo, K.; Koo, K.; Zeitchick, E.; Baker, I. Microemulsion Synthesis of Iron Core/Iron Oxide Shell Magnetic Nanoparticles and Their Physicochemical Properties. Mater. Res. Soc. Symp. Proc. 2012, 1416, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Baharuddin, A.A.; Ang, B.C.; Hussein, N.A.A.; Andriyana, A.; Wong, Y.H. Mechanisms of highly stabilized ex-situ oleic acid-modified iron oxide nanoparticles functionalized with 4-pentynoic acid. Mater. Chem. Phys. 2018, 203, 212–222. [Google Scholar] [CrossRef]
- Justin, C.; Samrot, A.V.; Sruthi, D.P.; Sahithya, C.S.; Bhavya, K.S.; Saipriya, C. Preparation, characterization and utilization of coreshell super paramagnetic iron oxide nanoparticles for curcumin delivery. PLoS ONE 2018, 13, e0200440. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Namvar, F.; Nadi, B.; Rahman, M.Z.A.; Amin, J. Synthesis, Surface Modification and Characterisation of Biocompatible Magnetic Iron Oxide Nanoparticles for Biomedical Applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [PubMed]
- Luchini, A.; Vitiello, G. Understanding the Nano-bio Interfaces: Lipid-Coatings for Inorganic Nanoparticles as Promising Strategy for Biomedical Applications. Front. Chem. 2019, 7, 343–359. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Szebeni, J. Stealth liposomes and long circulating nanoparticles: Critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog. Lipid Res. 2003, 42, 463–478. [Google Scholar] [CrossRef]
- Gogoi, M.; Jaiswal, M.K.; Dev Sarma, H.; Bahadur, D.; Banerjee, R. Biocompatibility and therapeutic evaluation of magnetic liposomes designed for self-controlled cancer hyperthermia and chemotherapy. Integr. Biol. 2017, 9, 555–565. [Google Scholar] [CrossRef]
- Ramishetti, S.; Huang, L. Intelligent design of multifunctional lipid-coated nanoparticle platforms for cancer therapy. Ther. Deliv. 2012, 3, 1429–1445. [Google Scholar] [CrossRef]
- Wijaya, A.; Hamad-Schifferli, K. High-Density Encapsulation of Fe3O4 Nanoparticles in Lipid Vesicles. Langmuir 2007, 23, 9546–9550. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, X.; Miao, Y.; Li, J.; Gan, Y. Lipid-coated iron oxide nanoparticles for dual-modal imaging of hepatocellular carcinoma. Int. J. Nanomed. 2017, 12, 2033–2044. [Google Scholar] [CrossRef]
- Radoń, A.; Drygała, A.; Hawełek, L.; Łukowiec, D. Structure and optical properties of Fe3O4 nanoparticles synthesized by co-precipitation method with different organic modifiers. Mater. Charact. 2017, 131, 148–156. [Google Scholar] [CrossRef]
- Anbarasu, M.; Anandan, M.; Chinnasamy, E.; Gopinath, V.; Balamurugan, K. Synthesis and characterization of polyethylene glycol (PEG) coated Fe3O4 nanoparticles by chemical co-precipitation method for biomedical applications. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015, 135, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zou, P.; Yang, L.; Cao, J.; Sun, Y.; Han, D.; Yang, S.; Wang, Z.; Chen, G.; Wang, B.; et al. A comprehensive study on the synthesis and paramagnetic properties of PEG-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2014, 303, 425–432. [Google Scholar] [CrossRef]
- Gao, G.; Qiu, P.; Qian, Q.; Zhou, N.; Wang, K.; Song, H.; Fu, H.; Cui, D. PEG-200-assisted hydrothermal method for the controlled-synthesis of highly dispersed hollow Fe3O4 nanoparticles. J. Alloys Compd. 2013, 574, 340–344. [Google Scholar] [CrossRef]
- Wang, R.; Degirmenci, V.; Xin, H.; Li, Y.; Wang, L.; Chen, J.; Hu, X.; Zhang, D. PEI-Coated Fe3O4 Nanoparticles Enable Efficient Delivery of Therapeutic siRNA Targeting REST into Glioblastoma Cells. Int. J. Mol. Sci. 2018, 19, 2230. [Google Scholar] [CrossRef]
- Ping, T.; Wang, Q.; Zhou, Y.; Nie, J. Reducing oxygen inhibition by Fe3O4@PEI nanoparticles co-initiator. J. Photochem. Photobiol. Chem. 2019, 373, 171–175. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, C.; Zhang, F.; Yang, Y.; Wu, G.; Yu, A.; Guan, N. Size-controlled synthesis of magnetite (Fe3O4) nanoparticles coated with Glucose and Gluconic Acid from a Single Fe(III) Precursor by a Sucrose Bifunctional Hydrothermal Method. J. Phys. Chem. C 2009, 113, 16002–16008. [Google Scholar] [CrossRef]
- Sari, A.Y.; Eko, A.S.; Candra, K.; Hasibuan, D.P.; Ginting, M.; Sebayang, P.; Simamora, P. Synthesis, Properties and Application of Glucose Coated Fe3O4 Nanoparticles Prepared by Co-precipitation Method. IOP Conf. Ser. Mater. Sci. Eng. 2017, 214, 012021. [Google Scholar] [CrossRef]
- Barbaro, D.; Di Bari, L.; Gandin, V.; Evangelisti, C.; Vitulli, G.; Schiavi, E.; Marzano, C.; Ferretti, A.M.; Salvadori, P. Glucose-coated superparamagnetic iron oxide nanoparticles prepared by metal vapour synthesis are electively internalized in a pancreatic adenocarcinoma cell line expressing GLUT1 transporter. PLoS ONE 2015, 10, e0123159. [Google Scholar] [CrossRef]
- Predescu, A.M.; Matei, E.; Berbecaru, A.C.; Pantilimon, C.; Dragan, C.; Vidu, R.; Predescu, C.; Kuncser, V. Synthesis and characterization of dextran-coated iron oxide nanoparticles. R. Soc. Open Sci. 2018, 5, 171525–171536. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Q.; Du, M.; Vermorken, A.; Cui, Y.; Zhang, L.; Guo, L.; Ma, L.; Chen, M. Cetuximab and Doxorubicin loaded dextran-coated Fe3O4 magnetic nanoparticles as novel targeted nanocarriers for non-small cell lung cancer. J. Magn. Magn. Mater. 2019, 481, 122–128. [Google Scholar] [CrossRef]
- Shen, M.; Yu, Y.; Fan, G.; Jin, Y.M.; Tang, W.; Jia, W. The synthesis and characterization of monodispersed chitosan-coated Fe3O4 nanoparticles via a facile one-step solvothermal process for adsorption of bovine serum albumin. Nanoscale Res. Lett. 2014, 9, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Veisi, H.; Sajjadifar, S.; Biabri, P.M.; Hemmati, S. Oxo-vanadium complex immobilized on chitosan coated-magnetic nanoparticles (Fe3O4): A heterogeneous and recyclable nanocatalyst for the chemoselective oxidation of sulfides to sulfoxides with H2O2. Polyhedron 2018, 153, 240–247. [Google Scholar] [CrossRef]
- Lotfi, S.; Bahari, A.; Mahjoub, S. In vitro biological evaluations of Fe3O4compared with core–shell structures of chitosan-coated Fe3O4 and polyacrylic acid-coated Fe3O4 nanoparticles. Res. Chem. Intermed. 2019, 45, 3497–3512. [Google Scholar] [CrossRef]
- Illés, E.; Szekeres, M.; Tóth, I.Y.; Farkas, K.; Foeldesi, I.; Szabo, A.; Ivan, B.; Tombacz, E. PEGylation of Superparamagnetic Iron Oxide Nanoparticles with Self-Organizing Polyacrylate-PEG Brushes for Contrast Enhancement in MRI Diagnosis. Nanomaterials 2018, 8, 776. [Google Scholar] [CrossRef]
- Illés, E.; Szekeres, M.; Tóth, I.Y.; Szabó, Á.; Iván, B.; Turcu, R.; Vékás, L.; Zupkó, I.; Jaics, G.; Tombácz, E. Multifunctional PEG-carboxylate copolymer coated superparamagnetic iron oxide nanoparticles for biomedical application. J. Magn. Magn. Mater. 2018, 451, 710–720. [Google Scholar] [CrossRef]
- Iglesias, G.R.; Reyes-Ortega, F.; Checa Fernandez, B.L.; Delgado, Á.V. Hyperthermia-Triggered Gemcitabine Release from Polymer-Coated Magnetite Nanoparticles. Polymers 2018, 10, 269. [Google Scholar] [CrossRef]
- Dutta, B.; Shetake, N.G.; Gawali, S.L.; Barick, B.K.; Barick, K.C.; Babu, P.D.; Pandey, B.N.; Priyadarsini, K.I.; Hassan, P.A. PEG mediated shape-selective synthesis of cubic Fe3O4 nanoparticles for cancer therapeutics. J. Alloys Compd. 2018, 737, 347–355. [Google Scholar] [CrossRef]
- You, L.; Liu, X.; Fang, Z.; Xu, Q.; Zhang, Q. Synthesis of multifunctional Fe3O4@PLGA-PEG nano-niosomes as a targeting carrier for treatment of cervical cancer. Mater. Sci. Eng. 2019, 94, 291–302. [Google Scholar] [CrossRef]
- Sun, X.; Shen, J.; Yu, D.; Ouyang, X. Preparation of pH-sensitive Fe3O4@C/carboxymethyl cellulose/chitosan composite beads for diclofenac sodium delivery. Int. J. Biol. Macromol. 2019, 127, 594–605. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Makino, M.; Ohura, T.; Yamamoto, K.; Enomoto, Y.; Takase, H. Surface modification of Fe3O4 nanoparticles with dextran via a coupling reaction between naked Fe3O4 mechano-cation and naked dextran mechano-anion: A new mechanism of covalent bond formation. Adv. Powder Technol. 2019, 30, 795–806. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.; Li, W.; Bai, H.; Gao, Y.; Ma, J.; Liu, W.; Xi, G. Dextran coated Fe3O4 nanoparticles as a near-infrared laser-driven photothermal agent for efficient ablation of cancer cells in vitro and in vivo. Mater. Sci. Eng. 2018, 90, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Banobre-Lopez, M.; Pineiro-Redondo, Y.; Sandri, M.; Tampieri, A.; De Santis, R.; Dediu, V.A.; Rivas, J. Hyperthermia Induced in Magnetic Scaffolds for Bone Tissue Engineering. IEEE Trans. Magn. 2014, 50, 1–7. [Google Scholar] [CrossRef]
- Lai, W.Y.; Feng, S.W.; Chan, Y.H.; Chang, W.J.; Wang, H.T.; Huang, H.M. In Vivo Investigation into Effectiveness of Fe3O4/PLLA Nanofibers for Bone Tissue Engineering Applications. Polymers 2018, 10, 804. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; von Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. J. Neurooncol. 2007, 81, 53–60. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Grauer, O.; Jaber, M.; Hess, K.; Weckesser, M.; Schwindt, W.; Maring, S.; Woelfer, J.; Stummer, W. Combined intracavitary thermotherapy with iron oxide nanoparticles and radiotherapy as local treatment modality in recurrent glioblastoma patients. J. Neurooncol. 2019, 141, 83–84. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Diagaradjane, P.; Krishnan, S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther. Deliv. 2011, 2, 1001–1014. [Google Scholar] [CrossRef]
- Goya, G.F.; Lima, E., Jr.; Arelaro, A.D.; Torres, T.E.; Rechenberg, H.R.; Rossi, L.; Marquina, C.; Ibarra, M.R. Magnetic Hyperthermia with Fe3O4 nanoparticles: The Influence of Particle Size on Energy Absorption. IEEE Trans. Magn. 2008, 44, 4444–4447. [Google Scholar] [CrossRef]
- Kaur, P.; Aliru, M.L.; Chadha, A.S.; Asea, A.; Krishnan, S. Hyperthermia using nanoparticles—Promises and pitfalls. Int. J. Hyperth. 2016, 32, 76–88. [Google Scholar] [CrossRef]
- Temelie, M.; Popescu, R.C.; Cocioaba, D.; Vasile, B.S.; Savu, D. Biocompatibility study of magnetite nanoparticles synthesized using a green method. Rom. J. Phys. 2018, 63, 703–716. [Google Scholar]
- Rasouli, E.; Basirun, W.J.; Rezayi, M.; Shameli, K.; Nourmohammadi, E.; Khandanlou, R.; Izadiyan, Z.; Sarkarizi, H.K. Ultrasmall superparamagnetic Fe3O4 nanoparticles: Honey-based green and facile synthesis and in vitro viability assay. Int. J. Nanomed. 2018, 2018, 6903–6911. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, G.; Logeshwaran, V.; Sarathbabu, S.; Jha, P.K.; Jeyaraj, M.; Rajkuberan, C.; Senthilkumar, N.; Sivaramakrishnan, S. Green synthesis of magnetic Fe3O4 nanoparticles using Couroupitaguianensis Aubl. fruit extract for their antibacterial and cytotoxicity activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, C.; Grumezescu, V.; Grumezescu, A.M.; Saviuc, C.; Lazăr, V.; Andronescu, E. Hybrid magnetite nanoparticles/Rosmarinus officinalis essential oil nanobiosystem with antibiofilm activity. Nanoscale Res. Lett. 2012, 7, 209–216. [Google Scholar] [CrossRef]
- Rădulescu, M.; Andronescu, E.; Holban, A.M.; Vasile, B.S.; Iordache, F.; Mogoantă, L.; Mogoșanu, G.D.; Grumezescu, A.M.; Georgescu, M.; Chifiriuc, M.C. Antimicrobial Nanostructured Bioactive Coating Based on Fe3O4 and Patchouli Oil for Wound Dressing. Metals 2016, 6, 103. [Google Scholar] [CrossRef]
- Sadeghzadeh, H.; Pilehvar-Soltanahmadi, Y.; Akbarzadeh, A.; Dariushnejad, H.; Sanjarian, F.; Zarghami, N. The Effects of Nanoencapsulated Curcumin-Fe3O4 on Proliferation and hTERT Gene Expression in Lung Cancer Cells. Anti-Cancer Agents Med. Chem. 2017, 17, 1363–1373. [Google Scholar] [CrossRef]
- Ruíz-Baltazar, A.J.; Reyes-López, S.Y.; Mondragón-Sánchez, M.L.; Robles-Cortés, A.I.; Pérez, R. Eco-friendly synthesis of Fe3O4 nanoparticles: Evaluation of their catalytic activity in methylene blue degradation by kinetic adsorption models. Res. Phys. 2019, 12, 989–995. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Kuwano, N.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Lee, K.X. Green Synthesis of Magnetite (Fe3O4) Nanoparticles Using Seaweed (Kappaphycus alvarezii) Extract. Nanoscale Res. Lett. 2016, 11, 276–283. [Google Scholar] [CrossRef]
- Hosseini, A.; Ghorbani, A. Cancer therapy with phytochemicals: Evidence from clinical studies. Avicenna J. Phytomed. 2015, 5, 84–97. [Google Scholar]
- Shivani Thoidingjam, S.; Tiku, A.B. Therapeutic efficacy of Phyllanthus emblica-coated iron oxide nanoparticles in A549 lung cancer cell line. Nanomedicine 2019, 14, 2355–2371. [Google Scholar] [CrossRef]
- Ramirez-Nuñez, A.L.; Jimenez-Garcia, L.F.; Goya, G.F.; Sanz, B.; Santoyo-Salazar, J. In vitro magnetic hyperthermia using polyphenol-coated Fe3O4@γFe2O3 nanoparticles from Cinnamomun verumand Vanilla planifolia: The concert of green synthesis and therapeutic possibilities. Nanotechnology 2018, 29, 074001. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Adamczyk-Grochala, J.; Bloniarz, D.; Olszowka, J.; Kulpa-Greszta, M.; Litwinienko, G.; Tomaszewska, A.; Wnuk, M.; Pazik, R. AMPK-mediated senolytic and senostatic activity of quercetin surface functionalized Fe3O4 nanoparticles during oxidant-induced senescence in human fibroblasts. Redox Biol. 2020, 28, 101337. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimpour, S.; Esmaeili, A.; Beheshti, S. Effect of quercetin-conjugated superparamagnetic iron oxide nanoparticles on diabetes-induced learning and memory impairment in rats. Int. J. Nanomed. 2018, 2018, 6311–6324. [Google Scholar] [CrossRef] [PubMed]
- Dorniani, D.; Hussein, M.Z.; Kura, A.U.; Fakurazi, S.; Shaari, A.H.; Ahmad, Z. Preparation of Fe3O4 magnetic nanoparticles coated with gallic acid for drug delivery. Int. J. Nanomed. 2012, 7, 5745–5756. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, F.; Jiang, W.; Wu, X.; Cai, Y.; Tang, J.; Gao, X.; Gao, F. Folic acid-conjugated superparamagnetic iron oxide nanoparticles for tumor-targeting MR imaging. Drug Deliv. 2016, 23, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Nam, K.C.; Cho, G.; Jung, J.S.; Park, B.J. Enhanced Photodynamic Anticancer Activities of Multifunctional Magnetic Nanoparticles (Fe3O4) Conjugated with Chlorin e6 and Folic Acid in Prostate and Breast Cancer Cells. Nanomaterials 2018, 8, 722. [Google Scholar] [CrossRef]
- Popescu, R.C.; Andronescu, E.; Vasile, B.S.; Trusca, R.; Boldeiu, A.; Mogoanta, L.; Mogosanu, G.D.; Temelie, M.; Radu, M.; Grumezescu, A.M.; et al. Fabrication and Cytotoxicity of Gemcitabine-Functionalized Magnetite Nanoparticles. Molecules 2017, 22, 1080. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Yuan, K.; Zhang, Z.; Zhang, X.; Fang, X. A multi-controlled drug delivery system based on magnetic mesoporous Fe3O4 nanopaticles and a phase change material for cancer thermo-chemotherapy. Nanotechnology 2017, 28, 405101–405131. [Google Scholar] [CrossRef]
- Xia, K.; Lyu, Y.; Yuan, W.; Wang, G.; Stratton, H.; Zhang, S.; Wu, J. Nanocarriers of Fe3O4as a Novel Method for Delivery of the Antineoplastic Agent Doxorubicin into HeLa Cells in vitro. Front. Oncol. 2019, 9, 250–257. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M.; Zhang, J.; Dong, W. Dual-pH/Magnetic-Field-Controlled Drug Delivery Systems Based on Fe3O4@SiO2-Incorporated Salecan Graft Copolymer Composite Hydrogels. ChemMedChem 2017, 12, 1600–1609. [Google Scholar] [CrossRef]



| No. | Reaction Parameter | Property | Measure | Reference |
|---|---|---|---|---|
| 1 | Fe3+/Fe2+ ratio | Iron oxide phase | Directly proportional | [37] |
| Magnetism | Inversely proportional | [38,39] | ||
| Dimension | Directly proportional | [39,40] | ||
| 2 | pH value | Iron oxide phase | Inversely proportional | [41] |
| Magnetism | Inversely proportional | [38,42] | ||
| Dimension | Insignificant | [42] | ||
| 3 | Type of base | Iron oxide phase | Depending on the type of base | [26] |
| Magnetism | Depending on the type of base | [26] | ||
| Dimension | Depending on the type of base | [26] | ||
| 4 | Temperature | Iron oxide phase | Directly proportional | [43] |
| Magnetism | Inversely proportional | [44] | ||
| Dimension | Inversely proportional | [40,45] | ||
| 5 | Concentration of precursors | Dimension | Directly proportional | [40] |
| 6 | pH of the precursor solution | Iron oxide phase | [40] | |
| Magnetism | [40] | |||
| Dimension | Directly proportional | [40] | ||
| 7 | Addition of surfactants | Dimension | Directly proportional | [38,46,47] |
| Surface charge | Dependent on the surfactant | [47] | ||
| Composition | Dependent on the surfactant | [47] | ||
| Shape | Dependent on the surfactant | [33] | ||
| Magnetisation | Dependent of the surfactant | [47] |
| No. | System Description | Application | Type of Conjugation | Evaluation | Reference |
|---|---|---|---|---|---|
| 1 | Fe3O4@SiO2 | Magnetic resonance imaging contrast substance as in vivo stem cell tracker | Negatively charged Fe3O4@citrate act as seeds for Si precursor; encapsulation using sol gel method; | Determination of distribution and chemical changes dynamics of Fe3O4@SiO2; high chemical stability; distribution in cytoplasm; | [119] |
| 2 | Fe3O4@SiO2/anti-rHBsAg (Hepatitis B surface antigen) | Purification of recombinant Hepatitis B for vaccine production; | In situ functionalization; encapsulation using sol gel method; | In vitro isolation of rHBsAg antigen from Pichia pastoris yeast | [120] |
| 3 | Fe3O4@SiO2 | Plasmid DNA purification | SiCl4 cross-linker between Fe3O4@NH3 and (3-aminopropyl)triethoxysilane (APTES); encapsulation using sol gel method; | Efficient in vitro plasmid DNA purification from E. Coli DH5a cells | [121] |
| 4 | Fe3O4@boronic acid/mesoporous (m) SiO2 | Magnetic and pH triggered drug release; | − | Biocompatibility and high uptake in MC3T3-E1 cells; Controlled drug release and good magnetic properties; | [122] |
| 5 | Fe3O4@mSiO2/catalase (CAT) | Enzyme protection in catalysis; | Encapsulation in SiO2 using TMOS (tetramethoxysilane) functionalization with APTES for CAT conjugation and growth of mSiO2 using CTAB as template and TMOS; | Good stability and catalytic activity | [123] |
| 6 | Fe3O4@oleic acid@mSiO2/5-Fluorouracil | Drug delivery for cancer therapy; | In situ Fe3O4@oleic acid were functionalized with CTAB through weak interaction (Van der Waals); hydrolisation of tetraethoxysilane (TEOS) on Fe3O4/CTAB; encapsulation in mSiO2 using the inversed microemulsion method; | In vitro biocompatibility for MCF-7 cells; efficient drug loading; | [124] |
| No. | System Description | Application | Type of Conjugation | Evaluation | Reference |
|---|---|---|---|---|---|
| 1. | Fe3O4 @APS–graphene/5-Fluorouracil | Drug-delivery systems for cancer treatment; | Amide bonding using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide | In vitro drug release at acidic pH; efficient in vitro internalizing in hepatocarcinoma HepG2 cells; biocompatibility of the carrier nanoparticles; | [184] |
| 2. | Fe3O4@ APTES/graphene oxide (GO)/doxorubicin | Drug-delivery systems and imaging diagnosis in cancer management; | Amide bonding using N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) | In vitro low cytotoxicity compared to GO; superparamegnetic properties and 10.7 r2/r1 relaxivity; fluorescence in VIS; high doxorubicin loading and 2.5 fold higher efficiency; (Figure 3) | [185] |
| 3. | Fe3O4@azide-sodium ascorbate-GO@ alkyne | Efficient absorbent and removal of dyes; | Click chemistry approach between the azide functional groups on the Fe3O4, sodium L-ascorbate and alkyne functional groups on GO; | Superparamagnetic properties; efficient absorbent and removal of dyes; | [186] |
| 4. | Fe3O4@GO | Magnetic fluids; | Absorption; | Improvement of friction and wear performances with magnetic field; | [187] |
| 5. | Polyvinyl alcohol (PVA)/ Fe3O4@ carbon nanotubes (CNTs) | Absorbent and dye removal; Anti-bacterial effects; | − | Optimal dye removal and anti-bacterial properties; | [188] |
| 6. | Fe3O4/multi walled CNTs/laser scribed graphene/chitosan/glassy carbon electrode | Detection of heavy metals | − | Electrode for the determination of Cd2+ and Pb2+ using square wave anodic stripping voltammetry; wide linear range; ultralow detection limit; excellent repeatability, reproducibility, stability; | [189] |
| 7. | Single-walled CNTs-PEG-Fe3O4@ carbon quantum dots (CQD)/doxorubicin/sgc8c aptamer | Targeted photodynamic and photothermal ablation of tumor cells; controlled drug delivery; targeted imaging using fluorescence and magnetic resonance imaging (MRI) | Through polyethylene glycol (PEG) linker using amide bonding; | Near infrared triggered production of reactive oxygen species and heat; good imaging properties; good biocompatibility of the carrier and cellular internalization; high drug loading ability; selective accumulation at tumor site in human adenocarcinoma (HeLa) tumor-bearing mice intravenously injected with the system; | [190] |
| 8. | GO-Chitosan/Fe3O4/glucose oxidase | Glucose biosensor and magnetic resonance imaging; | − | Good glucose biosensing ability; | [191] |
| No. | System Description | Application | Type of Conjugation | Evaluation | Reference |
|---|---|---|---|---|---|
| 1. | Fe3O4@ poly(polyethylene glycol methacrylate-co-acrylic acid) (P(PEGMA-AA)) | Hyperthermia and MRI contrast substance; | Electrostatic interactions between the acrylic acid and positively-charged Fe3O4; | Improved stability and salt tolerance; excellent blood compatibility; formation of blood protein corona; resistance to cell internalization; improvement of contrast in MRI; | [240,241] |
| 2. | Fe3O4/methyl methacrylate/ethylene glycol dimethacrylate/hydroxyl ethyl methacrylate/gemcitabine | Hyperthermia and drug delivery for cancer therapy | − | Good incorporation of drug; temperature triggered release; (Figure 5) | [242] |
| 3. | Fe3O4@PEG/Doxorubicin | Drug delivery and hyperthermia in cancer treatment; | In situ conjugation | pH responsive release of drug; no cytotoxicity of Fe3O4@PEG for human fibroblasts; Fe3O4@PEG/Doxorubicin showed good internalization and cytotoxicity for mouse skin fibrosarcoma; good magnetic properties; | [243] |
| 4. | Fe3O4@ poly(lactic-co-glycolicacid) (PLGA)-PEG@ folic acid/curcumin | Targeted drug delivery for cancer treatment; | Encapsulation; | High drug loading and delivery; high in vitro targeting efficiency for cervical carcinoma; in vitro induction of apoptosis and reduction of tumor cell proliferation; | [244] |
| 5. | Fe3O4@ C/carboxymethyl cellulose/chitosan/diclofenac sodium | Controlled drug delivery; | In situ conjugation and subsequent electrostatic conjugation; | High drug-loading efficiency; pH sensitive drug delivery; | [245] |
| 6. | Fe3O4@ dextran | − | Covalent binding via electron pairing; | − | [246] |
| 7. | Fe3O4@dextran | Near-infrared (NIR) photothermal ablation of tumor cells; | In situ encapsulation; | In vitro biocompatibility; in vitro and in vivo tumor growth inhibition after NIR activation; | [247] |
| 8. | Fe3O4@ poly ε acrylic acid-gelatin/hydroxyapatite/polycaprolactone | Bone tissue engineering scaffolds for hyperthermia cancer treatment; | Electrostatic interactions between the acrylic acid and positively-charged Fe3O4; | Characterisation of the magnetic behaviour for hyperthermia applications; | [248] |
| 9. | Fe3O4/poly-L-lactide (PLLA) nanofibers | Bone tissue engineering; | − | In vivo evaluation on tibia defect rabbit model; computer tomography and histological investigations revealed higher bone-healing potential than conventional PLLA | [249] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, R.C.; Andronescu, E.; Vasile, B.S. Recent Advances in Magnetite Nanoparticle Functionalization for Nanomedicine. Nanomaterials 2019, 9, 1791. https://doi.org/10.3390/nano9121791
Popescu RC, Andronescu E, Vasile BS. Recent Advances in Magnetite Nanoparticle Functionalization for Nanomedicine. Nanomaterials. 2019; 9(12):1791. https://doi.org/10.3390/nano9121791
Chicago/Turabian StylePopescu, Roxana Cristina, Ecaterina Andronescu, and Bogdan Stefan Vasile. 2019. "Recent Advances in Magnetite Nanoparticle Functionalization for Nanomedicine" Nanomaterials 9, no. 12: 1791. https://doi.org/10.3390/nano9121791
APA StylePopescu, R. C., Andronescu, E., & Vasile, B. S. (2019). Recent Advances in Magnetite Nanoparticle Functionalization for Nanomedicine. Nanomaterials, 9(12), 1791. https://doi.org/10.3390/nano9121791






