Quantification of Carbon Nanotube Doses in Adherent Cell Culture Assays Using UV-VIS-NIR Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
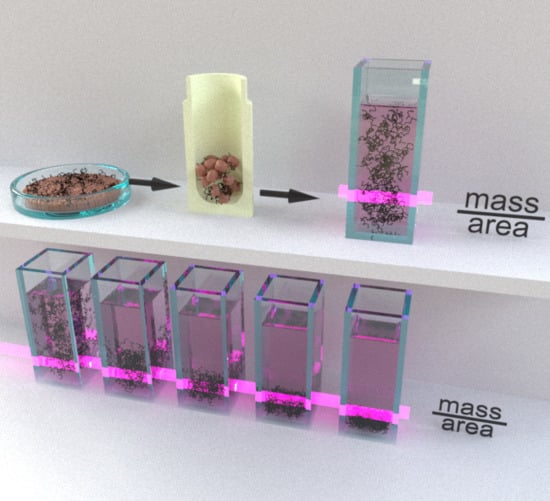
2.1. Sample Preparation
2.2. Characterization Methods
2.3. Fluorescence-Enhanced Dark Field Microscopy
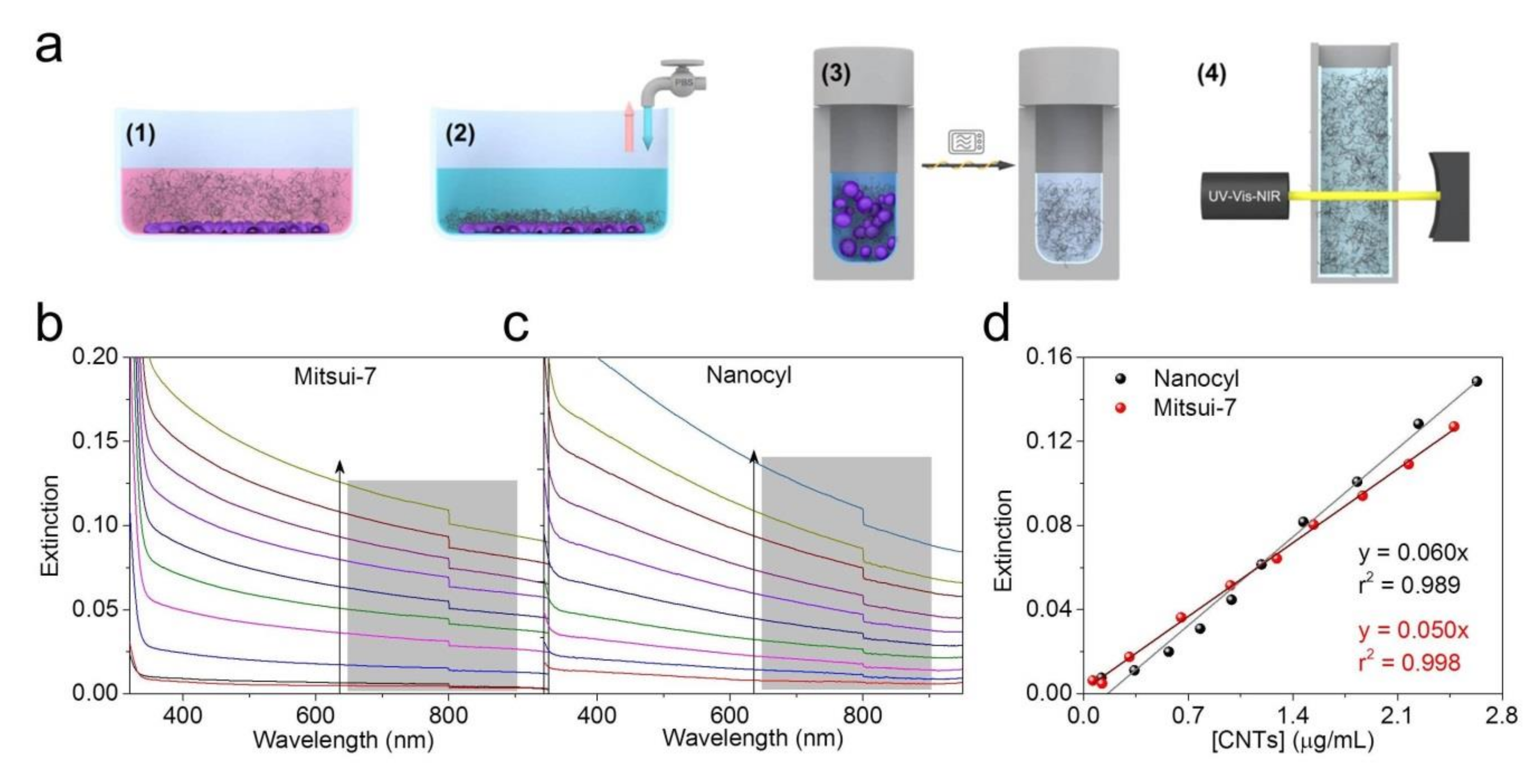
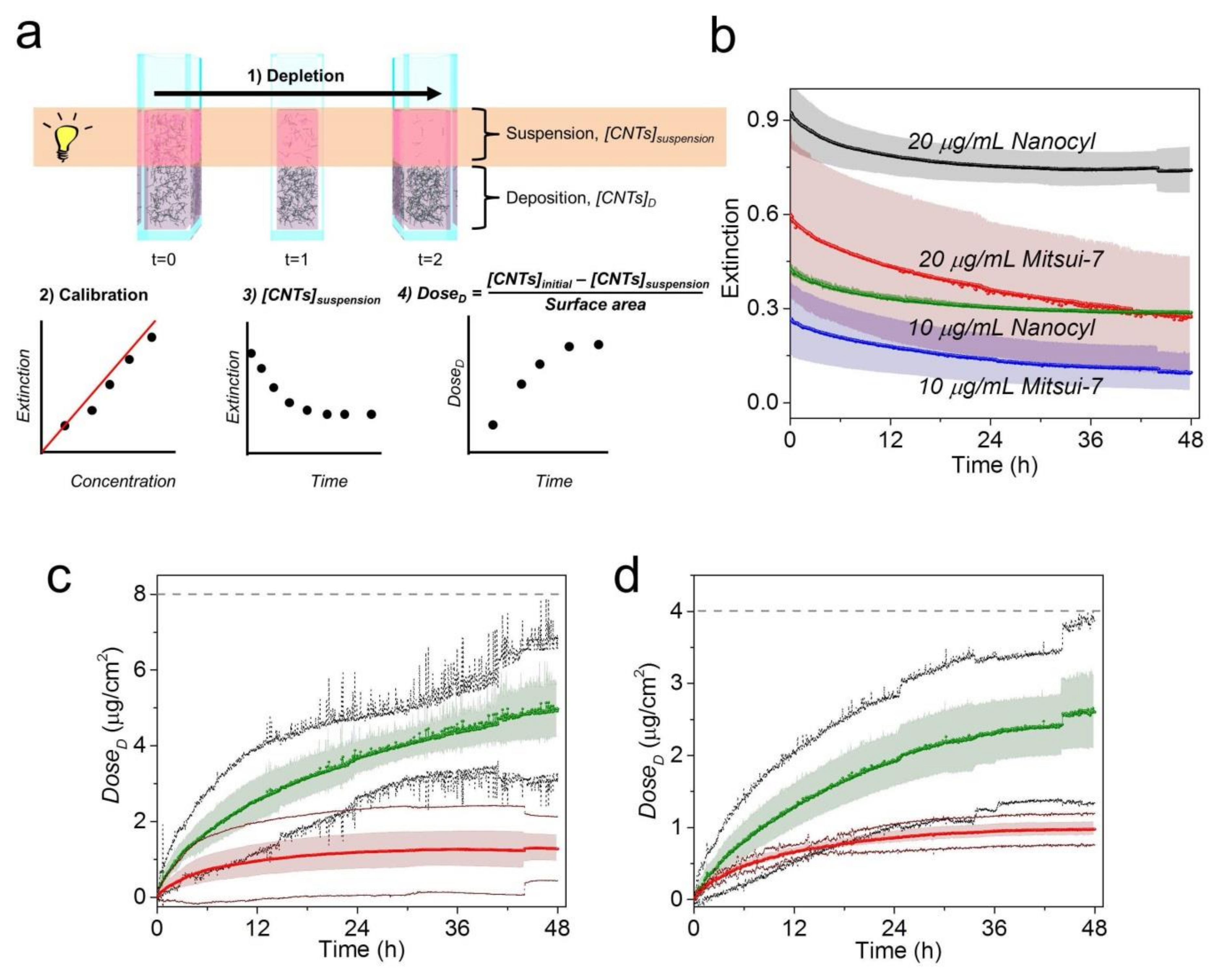
2.4. CNT Cellular Uptake/Association Quantification by UV-VIS-NIR Spectroscopy
2.5. Monitoring CNT Sedimentation Profile by UV-VIS-NIR Spectroscopy
3. Results and Discussion
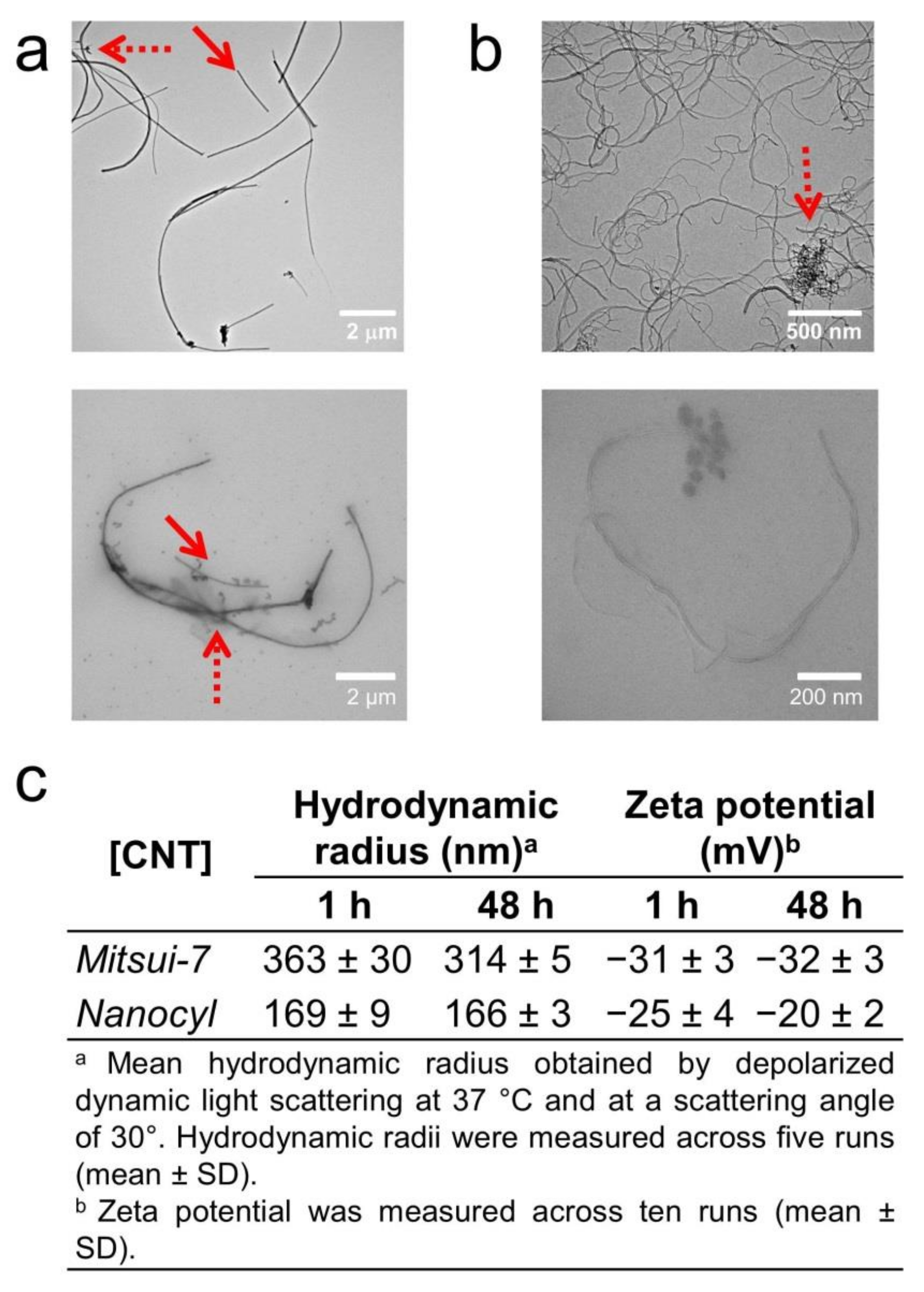
3.1. Characterization of CNTs
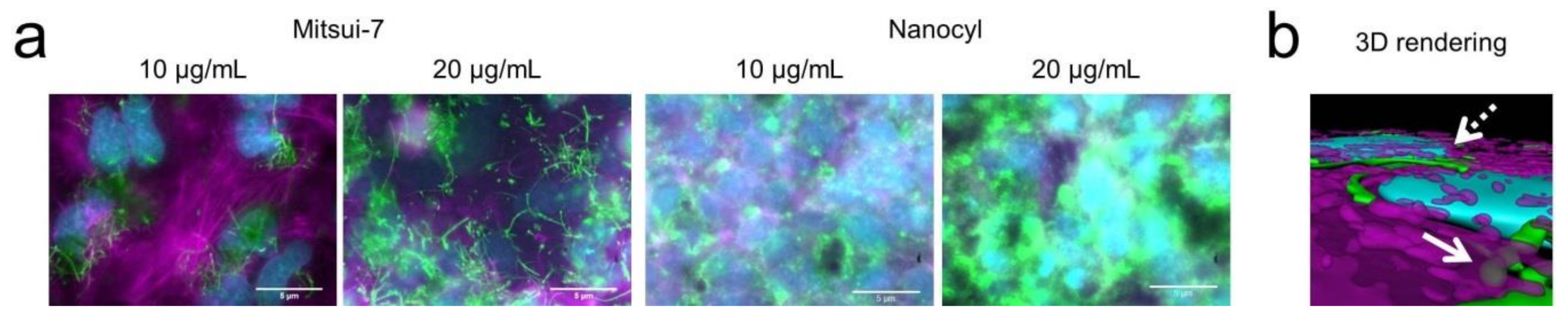
3.2. Interaction of CNTs with Cells
3.3. Sample Preparation by Extraction of Cell-Associated CNTs and Determination of Concentration by UV-VIS-NIR Spectroscopy
3.4. Complementary Study Using Sedimentation Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Endo, M.; Strano, M.S.; Ajayan, P.M. Potential Applications of Carbon Nanotubes. In Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties and Applications; Jorio, A., Dresselhaus, G., Dresselhaus, M.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 13–62. [Google Scholar]
- Nel, A.E.; Nasser, E.; Godwin, H.; Avery, D.; Bahadori, T.; Bergeson, L.; Beryt, E.; Bonner, J.C.; Boverhof, D.; Carter, J.; et al. A multi-stakeholder perspective on the use of alternative test strategies for nanomaterial safety assessment. ACS Nano 2013, 7, 6422–6433. [Google Scholar] [CrossRef] [PubMed]
- Schmid, O.; Cassee, F.R. On the pivotal role of dose for particle toxicology and risk assessment: Exposure is a poor surrogate for delivered dose. Part. Fibre Toxicol. 2017, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Limbach, L.K.; Li, Y.; Grass, R.N.; Brunner, T.J.; Hintermann, M.A.; Muller, M.; Gunther, D.; Stark, W.J. Oxide nanoparticle uptake in human lung fibroblasts: Effects of particle size, agglomeration, and diffusion at low concentrations. Environ. Sci. Technol. 2005, 39, 9370–9376. [Google Scholar] [CrossRef] [PubMed]
- DeLoid, G.; Cohen, J.M.; Darrah, T.; Derk, R.; Rojanasakul, L.; Pyrgiotakis, G.; Wohlleben, W.; Demokritou, P. Estimating the effective density of engineered nanomaterials for in vitro dosimetry. Nat. Commun. 2014, 5, 3514. [Google Scholar] [CrossRef] [PubMed]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Pirela, S.V.; Pal, A.; Liu, J.; Srebric, J.; Demokritou, P. Advanced computational modeling for in vitro nanomaterial dosimetry. Part. Fibre Toxicol. 2015, 12, 32. [Google Scholar] [CrossRef]
- Böhmert, L.; König, L.; Sieg, H.; Lichtenstein, D.; Paul, N.; Braeuning, A.; Voigt, A.; Lampen, A. In vitro nanoparticle dosimetry for adherent growing cell monolayers covering bottom and lateral walls. Part. Fibre Toxicol. 2018, 15, 42. [Google Scholar] [CrossRef]
- Thomas, D.G.; Smith, J.N.; Thrall, B.D.; Baer, D.R.; Jolley, H.; Munusamy, P.; Kodali, V.; Demokritou, P.; Cohen, J.; Teeguarden, J.G. ISD3: A particokinetic model for predicting the combined effects of particle sedimentation, diffusion and dissolution on cellular dosimetry for in vitro systems. Part. Fibre Toxicol. 2018, 15, 6. [Google Scholar] [CrossRef]
- Booth, A.; Jensen, K.A. Protocol for Producing Reproducible Dispersions of Manufactured Nanomaterials in Environmental Exposure Media. Available online: http://safenano.re.kr/download.do?SEQ=177 (accessed on 17 October 2019).
- Fong, W.K.; Moore, T.L.; Balog, S.; Vanhecke, D.; Rodriguez-Lorenzo, L.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle Behaviour in Complex Media: Methods for Characterizing Physicochemical Properties, Evaluating Protein Corona Formation, and Implications for Biological Studies. In Biological Responses to Nanoscale Particles: Molecular and Cellular Aspects and Methodological Approachesp; Gehr, P., Zellner, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 101–150. [Google Scholar]
- Geers, C.; Rodriguez-Lorenzo, L.; Andreas Urban, D.; Kinnear, C.; Petri-Fink, A.; Balog, S. A new angle on dynamic depolarized light scattering: Number-averaged size distribution of nanoparticles in focus. Nanoscale 2016, 8, 15813–15821. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Chortarea, S.; Zerimariam, F.; Barosova, H.; Septiadi, D.; Clift Martin, J.D.; Petri-Fink, A.; Rothen-Rutishauser, B. Profibrotic Activity of Multiwalled Carbon Nanotubes Upon Prolonged Exposures in Different Human Lung Cell Types. Appl. Vitr. Toxicol. 2019, 5, 47–61. [Google Scholar] [CrossRef]
- Laura, M.; Franco, T.; Camillo La, M.; Adalberto, B.; Alberto, B.; Gianfranco, R. Interactions and effects of BSA-functionalized single-walled carbon nanotubes on different cell lines. Nanotechnology 2016, 27, 155704. [Google Scholar]
- Holt, B.D.; Dahl, K.N.; Islam, M.F. Quantification of uptake and localization of bovine serum albumin-stabilized single-wall carbon nanotubes in different human cell types. Small 2011, 7, 2348–2355. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Du, J.; Zhao, L.; Wang, L.; Liu, Y.; Li, D.; Yang, Y.; Zhou, R.; Zhao, Y.; Chai, Z.; et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc. Natl. Acad. Sci. USA 2011, 108, 16968–16973. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, K.; Reddy, R.; Reddy, N. A review on protein functionalized carbon nanotubes. J. Appl. Biomater. Funct. Mater. 2015, 13, e301–e312. [Google Scholar] [CrossRef] [PubMed]
- Elgrabli, D.; Abella-Gallart, S.; Aguerre-Chariol, O.; Robidel, F.; Rogerieux, F.; Boczkowski, J.; Lacroix, G. Effect of BSA on carbon nanotube dispersion for in vivo and in vitro studies. Nanotoxicology 2007, 1, 266–278. [Google Scholar] [CrossRef]
- Wang, X.; Xia, T.A.; Ntim, S.A.; Ji, Z.X.; George, S.; Meng, H.A.; Zhang, H.Y.; Castranova, V.; Mitra, S.; Nel, A.E. Quantitative Techniques for Assessing and Controlling the Dispersion and Biological Effects of Multiwalled Carbon Nanotubes in Mammalian Tissue Culture Cells. ACS Nano 2010, 4, 7241–7252. [Google Scholar] [CrossRef] [PubMed]
- Vietti, G.; Lison, D.; van den Brule, S. Mechanisms of lung fibrosis induced by carbon nanotubes: Towards an Adverse Outcome Pathway (AOP). Part. Fibre Toxicol. 2016, 13, 11. [Google Scholar] [CrossRef]
- Mercer, R.R.; Hubbs, A.F.; Scabilloni, J.F.; Wang, L.; Battelli, L.A.; Friend, S.; Castranova, V.; Porter, D.W. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Part. Fibre Toxicol. 2011, 8, 21. [Google Scholar] [CrossRef]
- Porter, D.W.; Hubbs, A.F.; Mercer, R.R.; Wu, N.; Wolfarth, M.G.; Sriram, K.; Leonard, S.; Battelli, L.; Schwegler-Berry, D.; Friend, S.; et al. Mouse pulmonary dose-and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology 2010, 269, 136–147. [Google Scholar] [CrossRef]
- Mercer, R.R.; Hubbs, A.F.; Scabilloni, J.F.; Wang, L.; Battelli, L.A.; Schwegler-Berry, D.; Castranova, V.; Porter, D.W. Distribution and persistence of pleural penetrations by multi-walled carbon nanotubes. Part. Fibre Toxicol. 2010, 7, 28. [Google Scholar] [CrossRef]
- Septiadi, D.; Abdussalam, W.; Rodriguez-Lorenzo, L.; Spuch-Calvar, M.; Bourquin, J.; Petri-Fink, A.; Rothen-Rutishauser, B. Revealing the Role of Epithelial Mechanics and Macrophage Clearance during Pulmonary Epithelial Injury Recovery in the Presence of Carbon Nanotubes. Adv. Mater. 2018, 30, e1806181. [Google Scholar] [CrossRef] [PubMed]
- Snyder-Talkington, B.N.; Pacurari, M.; Dong, C.; Leonard, S.S.; Schwegler-Berry, D.; Castranova, V.; Qian, Y.; Guo, N.L. Systematic analysis of multiwalled carbon nanotube-induced cellular signaling and gene expression in human small airway epithelial cells. Toxicol. Sci. 2013, 133, 79–89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maruyama, K.; Haniu, H.; Saito, N.; Matsuda, Y.; Tsukahara, T.; Kobayashi, S.; Tanaka, M.; Aoki, K.; Takanashi, S.; Okamoto, M.; et al. Endocytosis of Multiwalled Carbon Nanotubes in Bronchial Epithelial and Mesothelial Cells. Biomed. Res. Int. 2015, 2015, 793186. [Google Scholar] [CrossRef] [PubMed]
- Visalli, G.; Bertuccio, M.P.; Iannazzo, D.; Piperno, A.; Pistone, A.; Di Pietro, A. Toxicological assessment of multi-walled carbon nanotubes on A549 human lung epithelial cells. Toxicol. Vitr. 2015, 29, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Ryabenko, A.G.; Dorofeeva, T.V.; Zvereva, G.I. UV–VIS–NIR spectroscopy study of sensitivity of single-wall carbon nanotubes to chemical processing and Van-der-Waals SWNT/SWNT interaction. Verification of the SWNT content measurements by absorption spectroscopy. Carbon 2004, 42, 1523–1535. [Google Scholar] [CrossRef]
- Lauret, J.S.; Voisin, C.; Cassabois, G.; Delalande, C.; Roussignol, P.; Jost, O.; Capes, L. Ultrafast carrier dynamics in single-wall carbon nanotubes. Phys. Rev. Lett. 2003, 90, 057404. [Google Scholar] [CrossRef]
- Angelikopoulos, P.; Gromov, A.; Leen, A.; Nerushev, O.; Bock, H.; Campbell, E.E.B. Dispersing Individual Single-Wall Carbon Nanotubes in Aqueous Surfactant Solutions below the cmc. J. Phys. Chem. C 2010, 114, 2–9. [Google Scholar] [CrossRef]
- Gonzalez, M.H.; Souza, G.B.; Oliveira, R.V.; Forato, L.A.; Nobrega, J.A.; Nogueira, A.R. Microwave-assisted digestion procedures for biological samples with diluted nitric acid: Identification of reaction products. Talanta 2009, 79, 396–401. [Google Scholar] [CrossRef]
- Ivanova, M.V.; Lamprecht, C.; Loureiro, M.J.; Huzil, J.T.; Foldvari, M. Pharmaceutical characterization of solid and dispersed carbon nanotubes as nanoexcipients. Int. J. Nanomed. 2012, 7, 403–415. [Google Scholar]
- Cho, E.C.; Liu, Y.; Xia, Y. A Simple Spectroscopic Method for Differentiating Cellular Uptakes of Gold Nanospheres and Nanorods from Their Mixtures. Angew. Chem. 2010, 49, 1976–1980. [Google Scholar] [CrossRef]
- Rischitor, G.; Parracino, M.; La Spina, R.; Urbán, P.; Ojea-Jiménez, I.; Bellido, E.; Valsesia, A.; Gioria, S.; Capomaccio, R.; Kinsner-Ovaskainen, A.; et al. Quantification of the cellular dose and characterization of nanoparticle transport during in vitro testing. Part. Fibre Toxicol. 2016, 13, 47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spyrogianni, A.; Herrmann, I.K.; Lucas, M.S.; Leroux, J.C.; Sotiriou, G.A. Quantitative analysis of the deposited nanoparticle dose on cell cultures by optical absorption spectroscopy. Nanomedicine 2016, 11, 2483–2496. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, H.J.; Kim, K.H.; Jang, S.P. Extinction coefficient of water-based multi-walled carbon nanotube nanofluids for application in direct-absorption solar collectors. Micro Nano Lett. 2014, 9, 635–638. [Google Scholar] [CrossRef]
- Gan, Y.; Qiao, L. Optical Properties and Radiation-Enhanced Evaporation of Nanofluid Fuels Containing Carbon-Based Nanostructures. Energy Fuels 2012, 26, 4224–4230. [Google Scholar] [CrossRef]
- Reed, G.F.; Lynn, F.; Meade, B.D. Use of coefficient of variation in assessing variability of quantitative assays. Clin. Diagn. Lab. Immunol. 2002, 9, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Halamoda-Kenzaoui, B.; Ceridono, M.; Colpo, P.; Valsesia, A.; Urbán, P.; Ojea-Jiménez, I.; Gioria, S.; Gilliland, D.; Rossi, F.; Kinsner-Ovaskainen, A. Dispersion Behaviour of Silica Nanoparticles in Biological Media and Its Influence on Cellular Uptake. PLoS ONE 2015, 10, e0141593. [Google Scholar] [CrossRef] [PubMed]
- Drasler, B.; Sayre, P.; Steinhäuser, K.G.; Petri-Fink, A.; Rothen-Rutishauser, B. In vitro approaches to assess the hazard of nanomaterials. NanoImpact 2017, 8, 99–116. [Google Scholar] [CrossRef]
- Poulsen, S.S.; Saber, A.T.; Williams, A.; Andersen, O.; Købler, C.; Atluri, R.; Pozzebon, M.E.; Mucelli, S.P.; Simion, M.; Rickerby, D.; et al. MWCNTs of different physicochemical properties cause similar inflammatory responses, but differences in transcriptional and histological markers of fibrosis in mouse lungs. Toxicol. Appl. Pharmacol. 2015, 284, 16–32. [Google Scholar] [CrossRef]
| CNT Type; [CNTs]Initial [µg/cm2] | Estimated DoseD [µg/cm2] a | Delivered Fraction (%) | Measured DoseD [µg/cm2] a | Delivered Fraction (%) |
|---|---|---|---|---|
| Mitsui-7; 3.9 | 2.60 ± 0.51 | 66 ± 11 | 3.33 ± 1.38 | 85 ± 35 |
| Nanocyl; 3.9 | 0.98 ± 0.09 | 25 ± 2 | 1.88 ± 0.70 | 48 ± 18 |
| Mitsui-7; 7.9 | 4.98 ± 0.76 | 63 ± 8 | 6.44 ± 2.03 | 82 ± 26 |
| Nanocyl; 7.9 | 1.28 ± 0.34 | 16 ± 4 | 2.41 ± 0.94 | 31 ± 12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Septiadi, D.; Rodriguez-Lorenzo, L.; Balog, S.; Spuch-Calvar, M.; Spiaggia, G.; Taladriz-Blanco, P.; Barosova, H.; Chortarea, S.; Clift, M.J.D.; Teeguarden, J.; et al. Quantification of Carbon Nanotube Doses in Adherent Cell Culture Assays Using UV-VIS-NIR Spectroscopy. Nanomaterials 2019, 9, 1765. https://doi.org/10.3390/nano9121765
Septiadi D, Rodriguez-Lorenzo L, Balog S, Spuch-Calvar M, Spiaggia G, Taladriz-Blanco P, Barosova H, Chortarea S, Clift MJD, Teeguarden J, et al. Quantification of Carbon Nanotube Doses in Adherent Cell Culture Assays Using UV-VIS-NIR Spectroscopy. Nanomaterials. 2019; 9(12):1765. https://doi.org/10.3390/nano9121765
Chicago/Turabian StyleSeptiadi, Dedy, Laura Rodriguez-Lorenzo, Sandor Balog, Miguel Spuch-Calvar, Giovanni Spiaggia, Patricia Taladriz-Blanco, Hana Barosova, Savvina Chortarea, Martin J. D. Clift, Justin Teeguarden, and et al. 2019. "Quantification of Carbon Nanotube Doses in Adherent Cell Culture Assays Using UV-VIS-NIR Spectroscopy" Nanomaterials 9, no. 12: 1765. https://doi.org/10.3390/nano9121765
APA StyleSeptiadi, D., Rodriguez-Lorenzo, L., Balog, S., Spuch-Calvar, M., Spiaggia, G., Taladriz-Blanco, P., Barosova, H., Chortarea, S., Clift, M. J. D., Teeguarden, J., Sharma, M., Petri-Fink, A., & Rothen-Rutishauser, B. (2019). Quantification of Carbon Nanotube Doses in Adherent Cell Culture Assays Using UV-VIS-NIR Spectroscopy. Nanomaterials, 9(12), 1765. https://doi.org/10.3390/nano9121765