Graphenic Materials for Biomedical Applications
Abstract
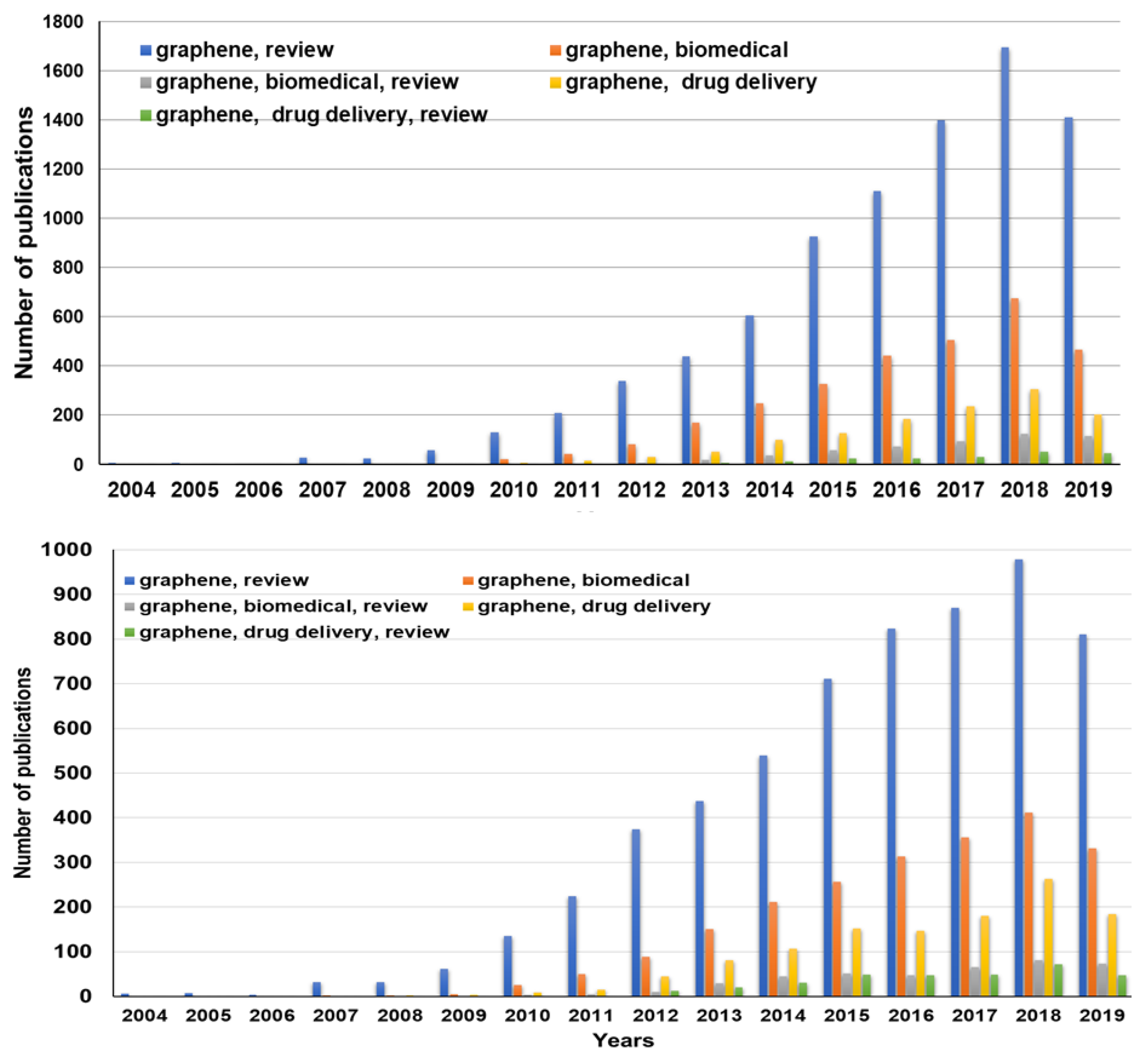
1. Introduction
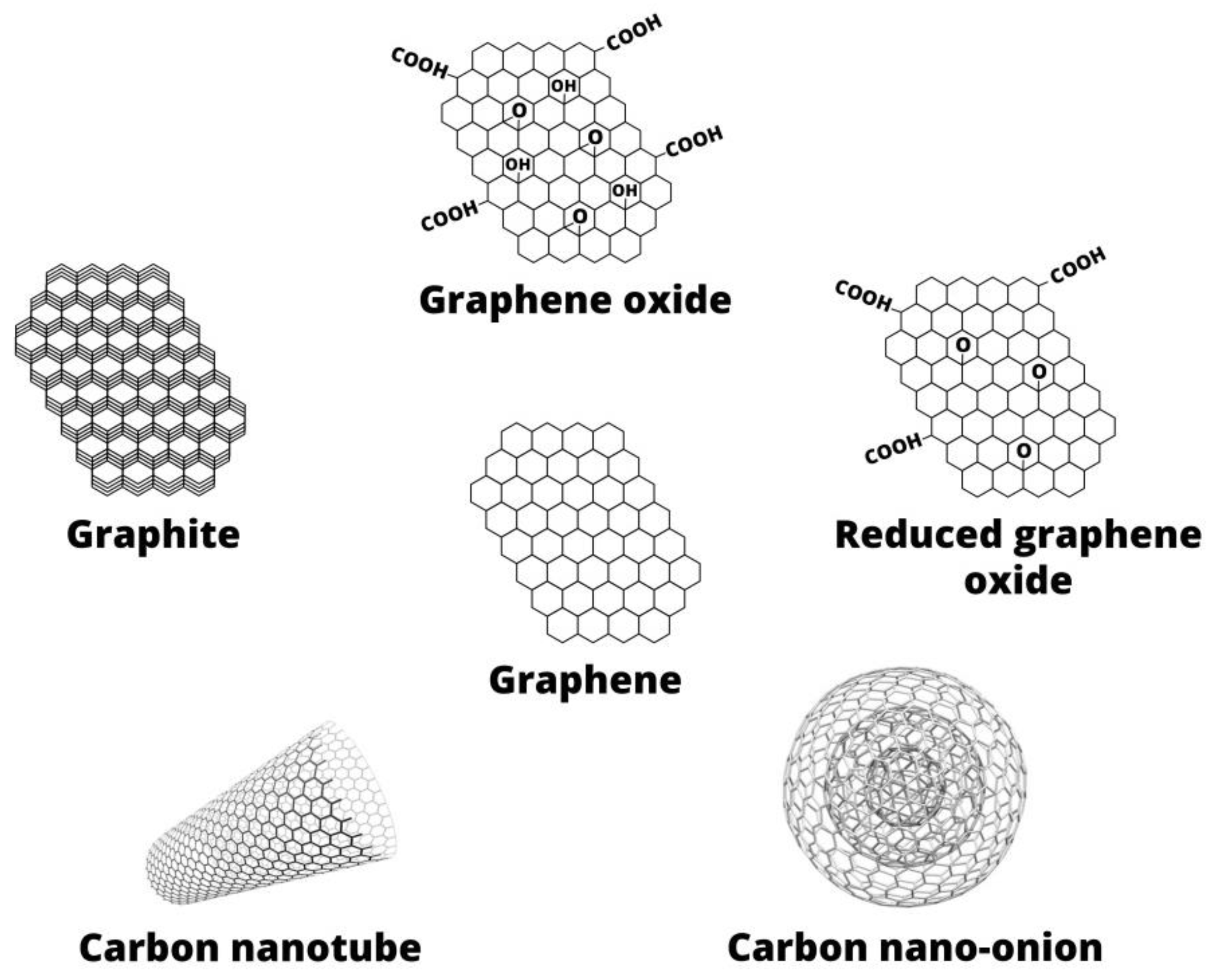
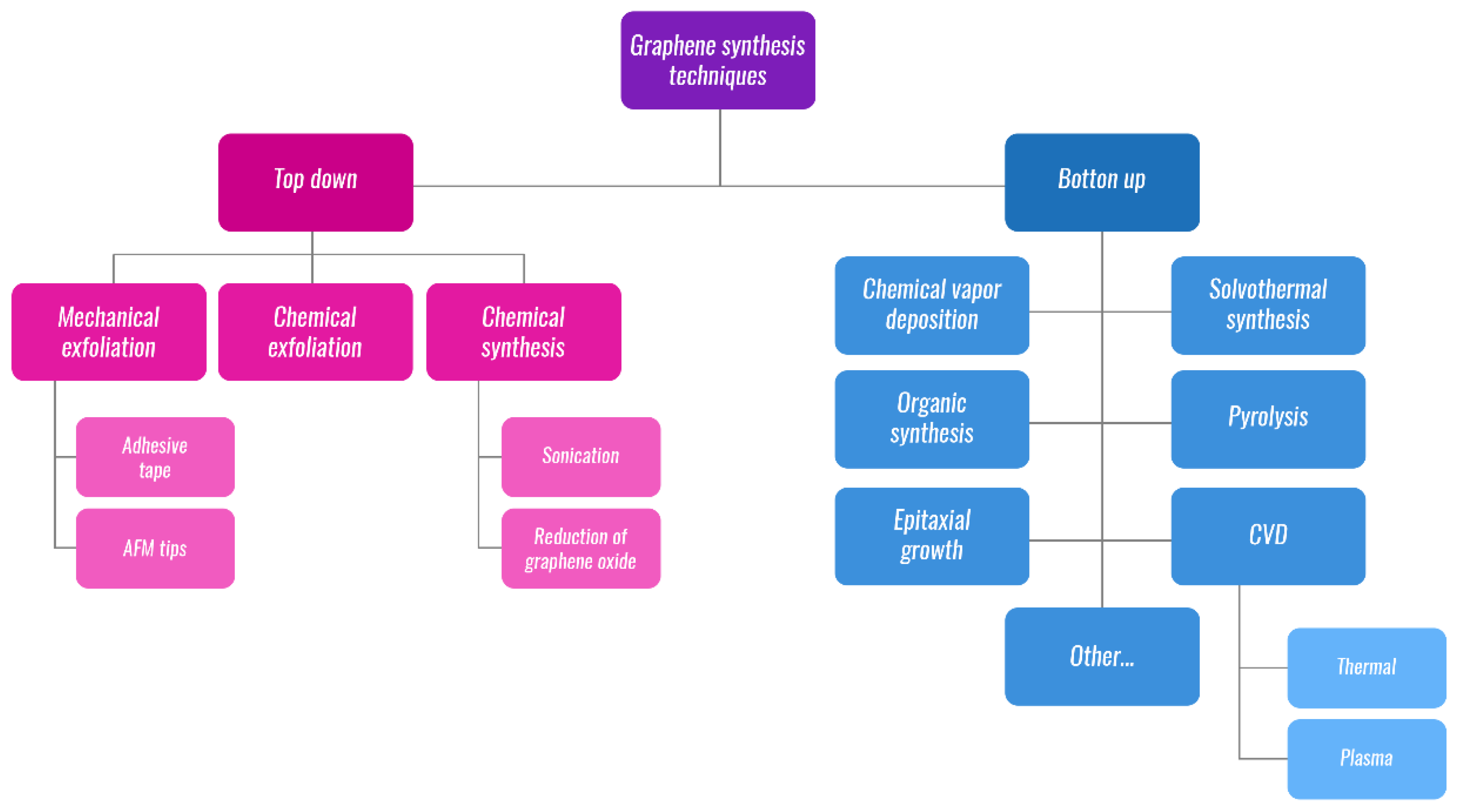
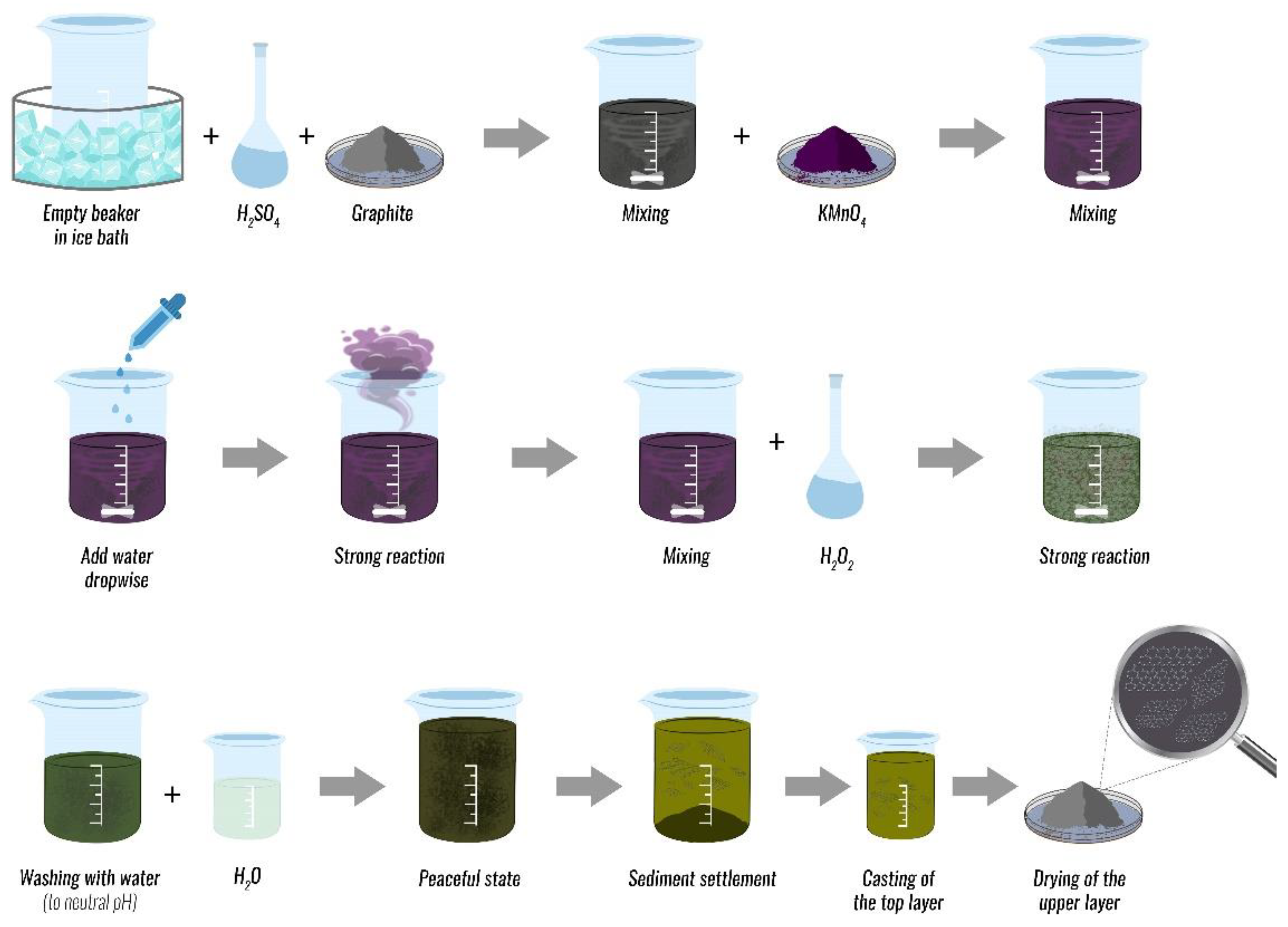
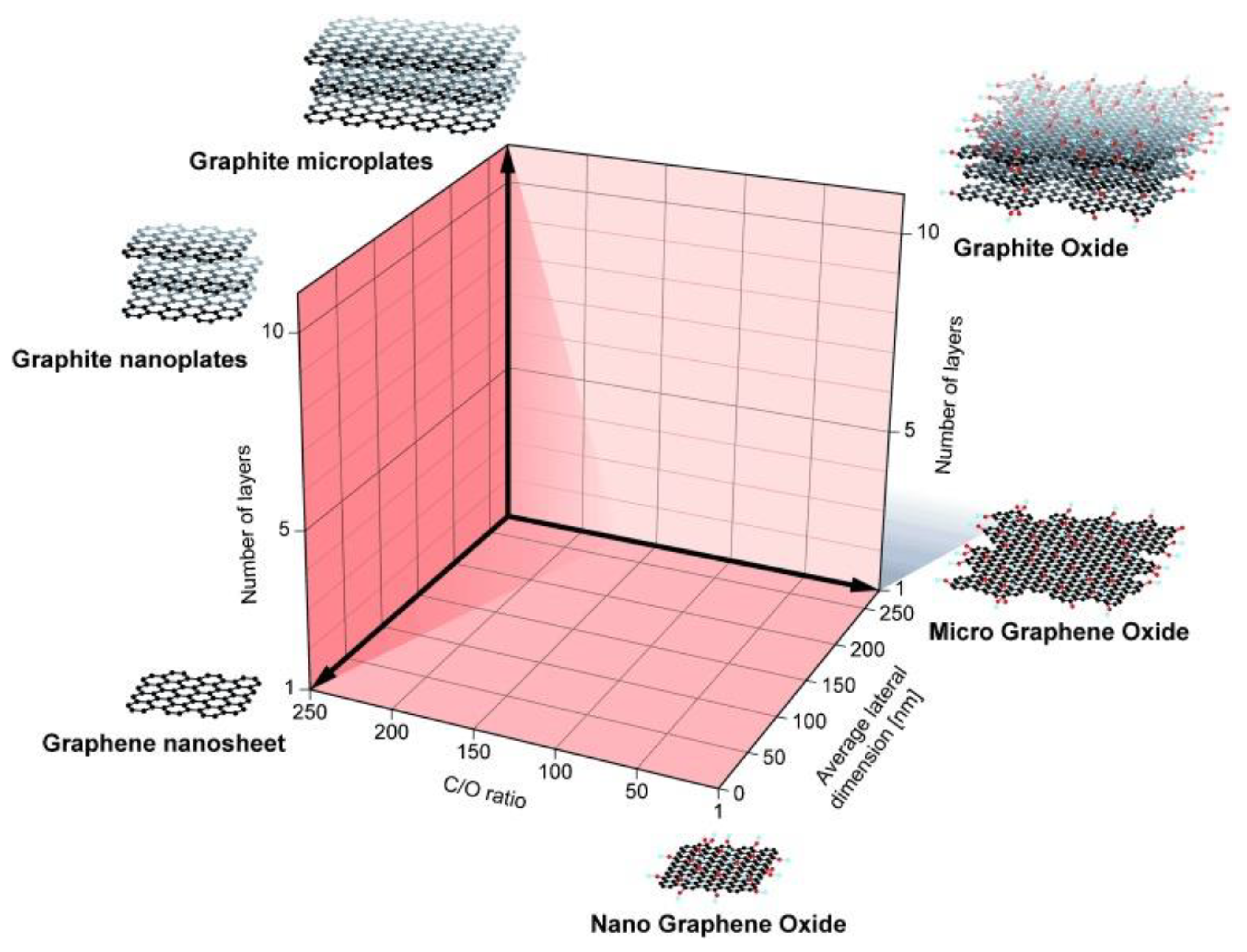
2. Graphene-Based Materials
3. Functionalization
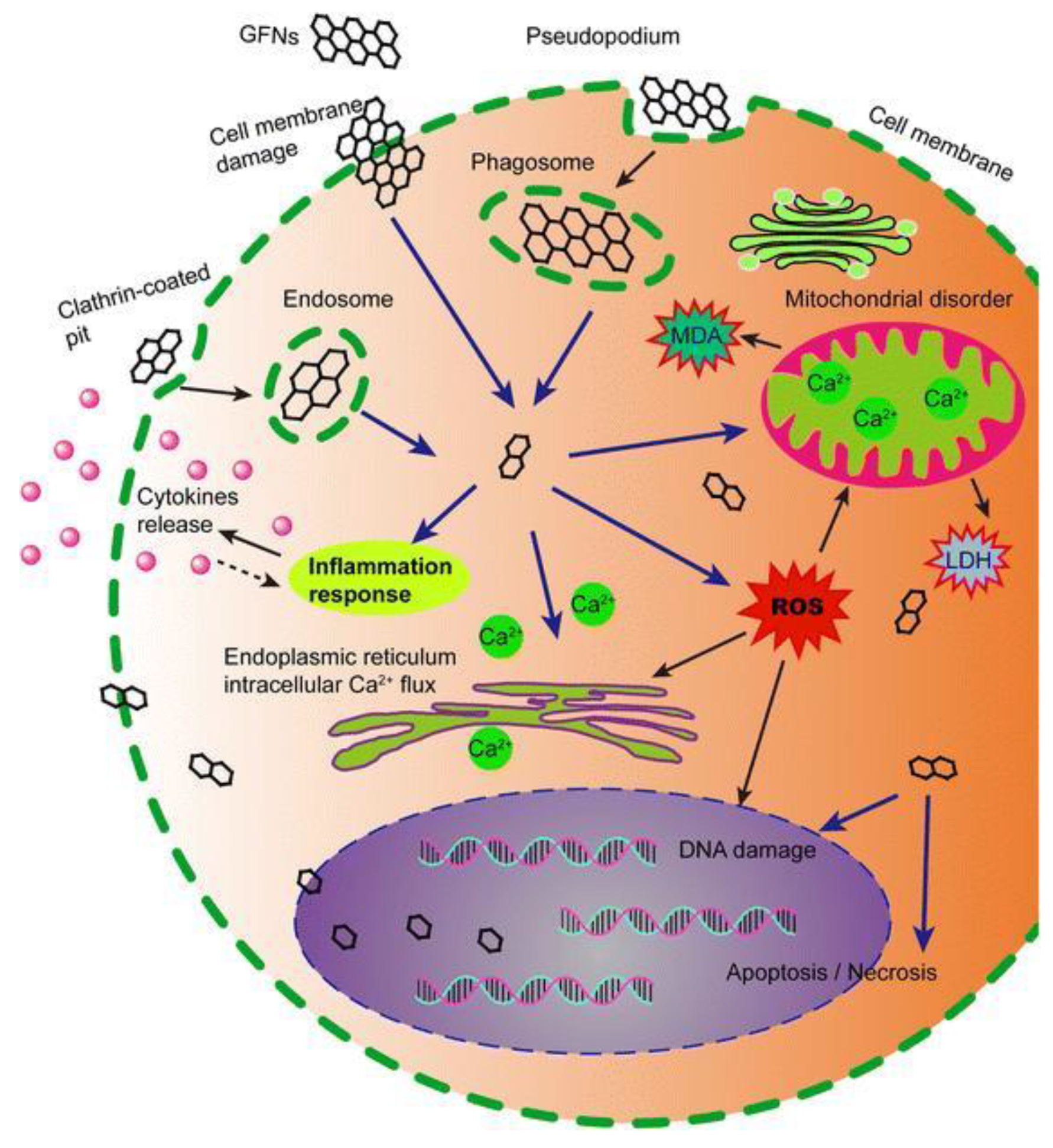
4. Cytotoxicity and Biocompatibility
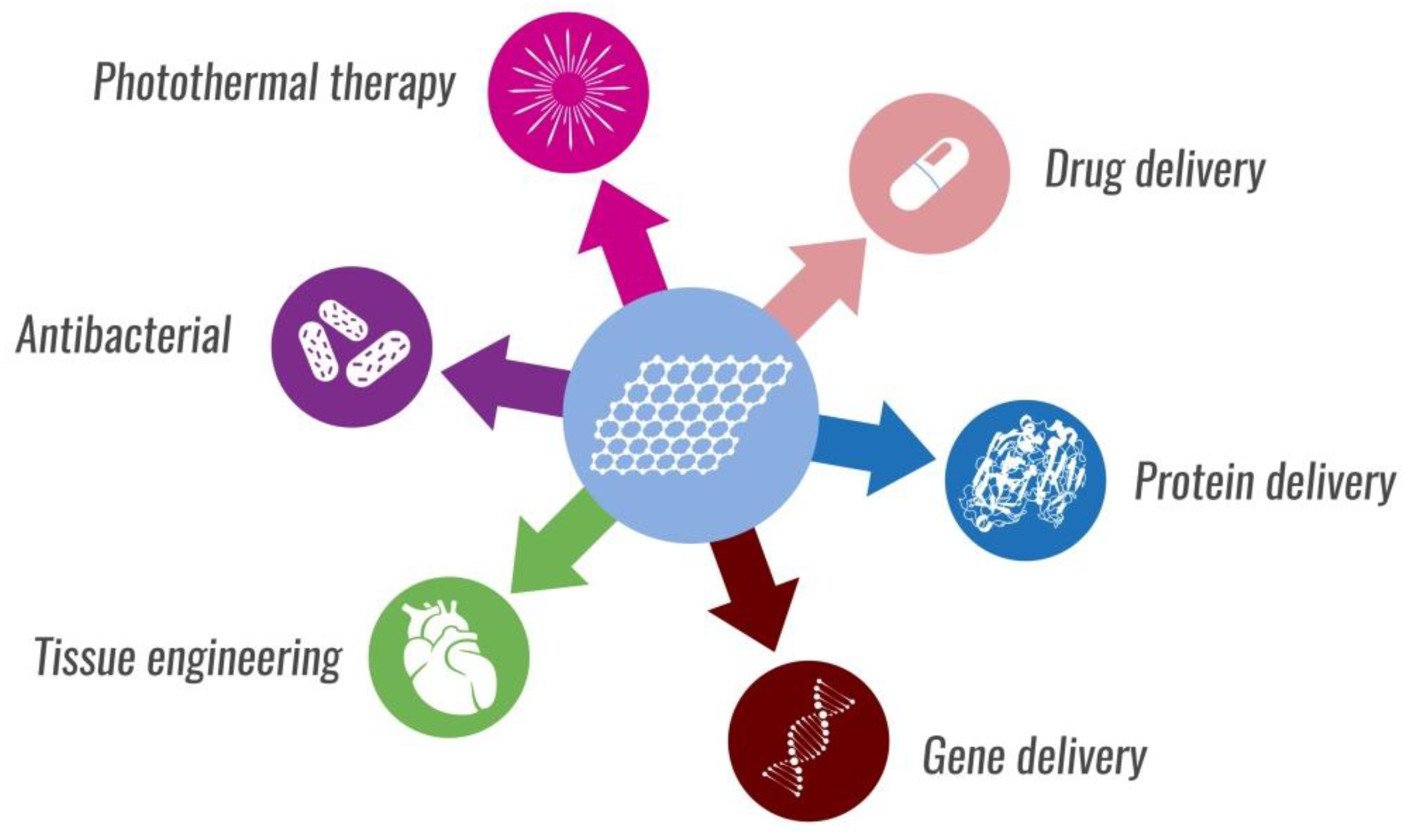
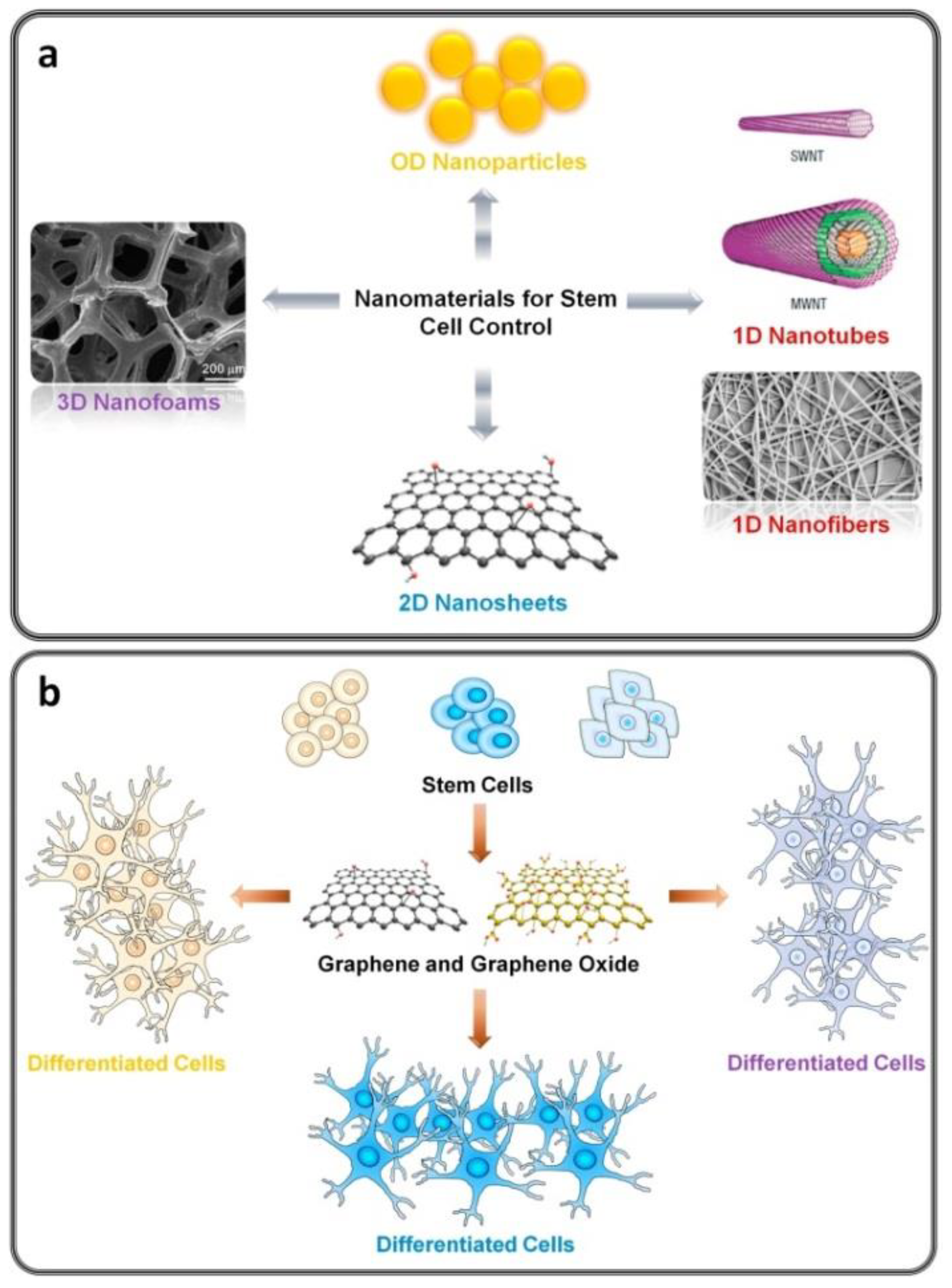
5. Nanotheranostics and Tissue Engineering
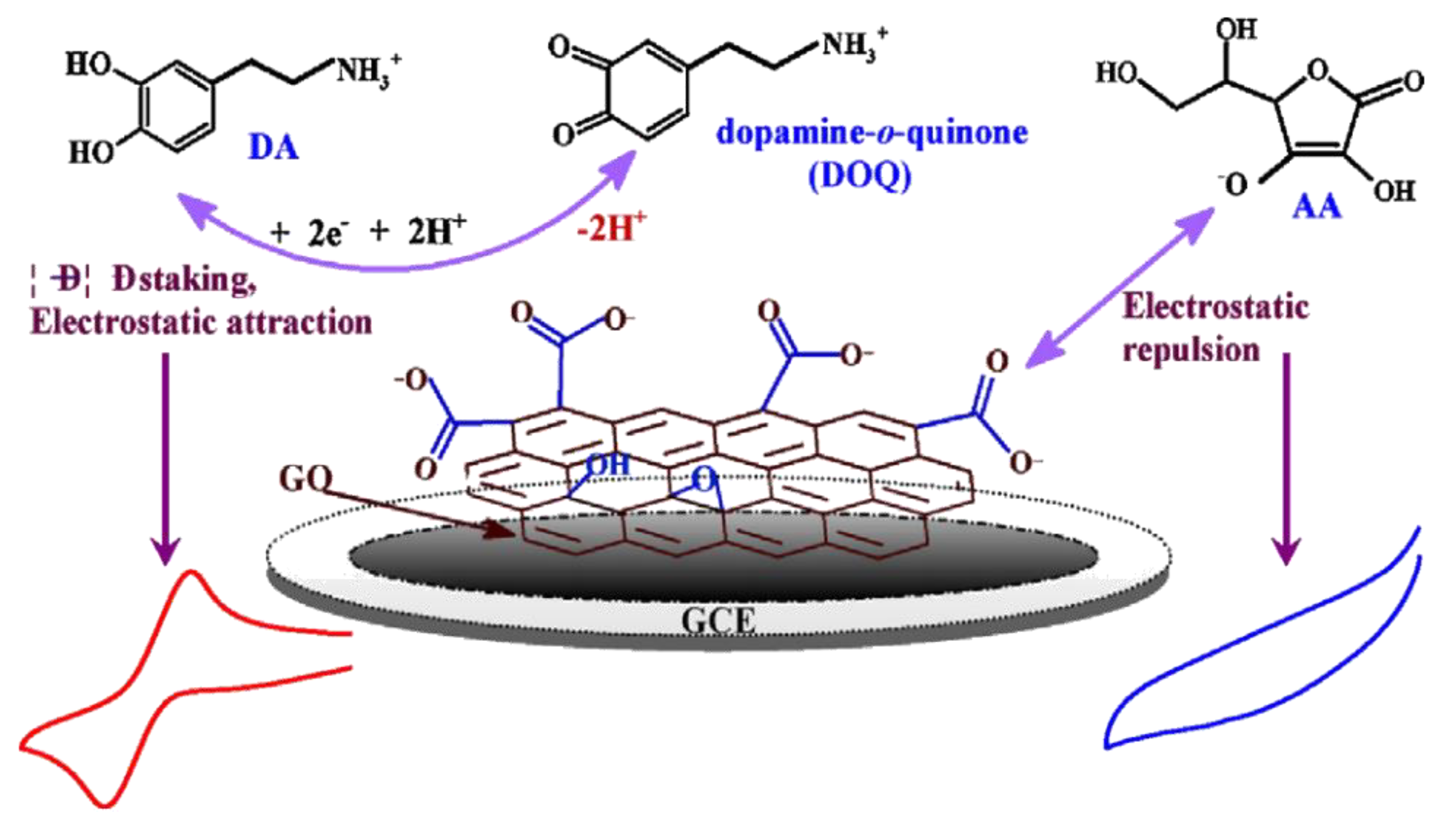
6. Biosensors
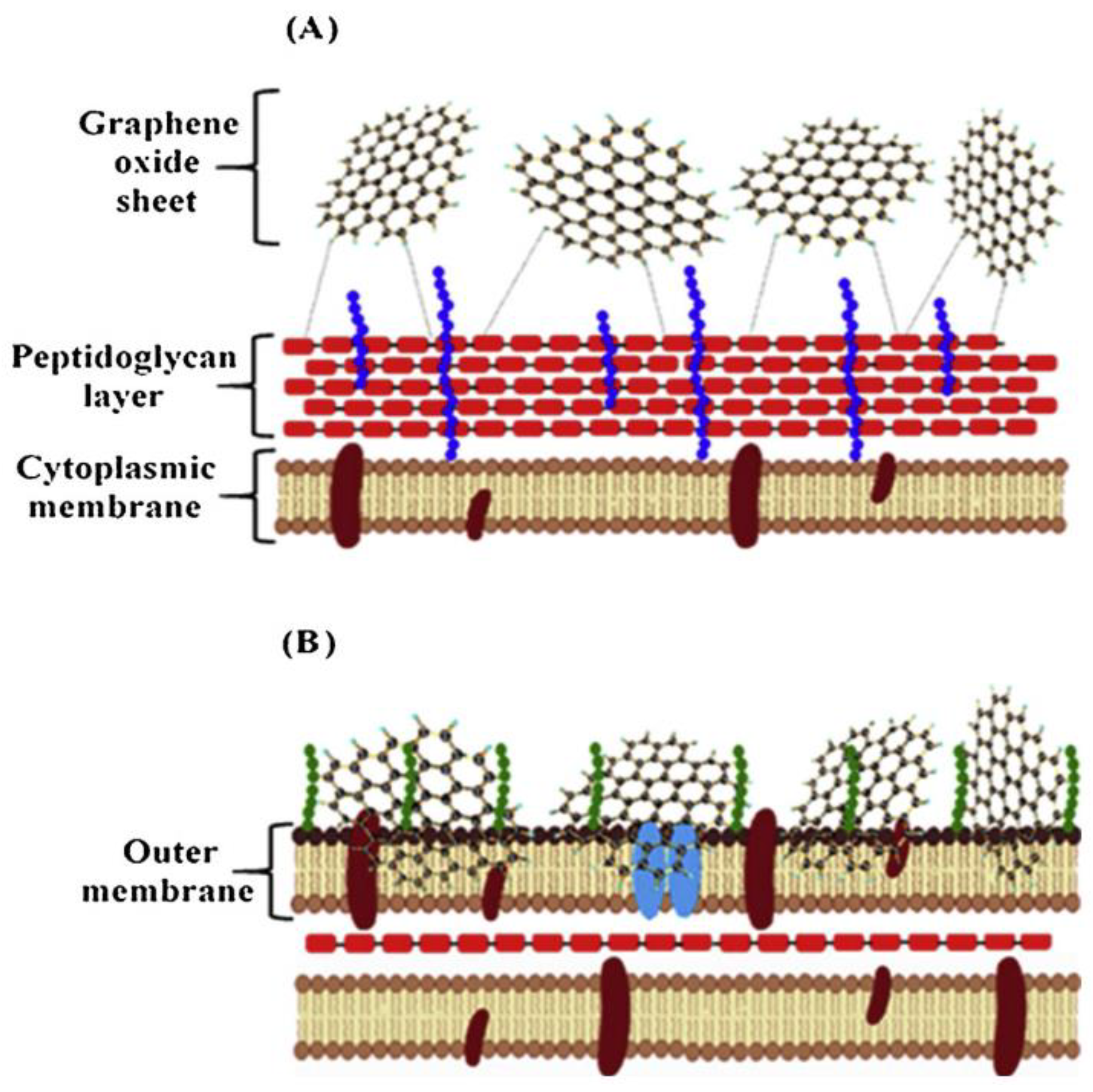
7. Antibacterial Properties
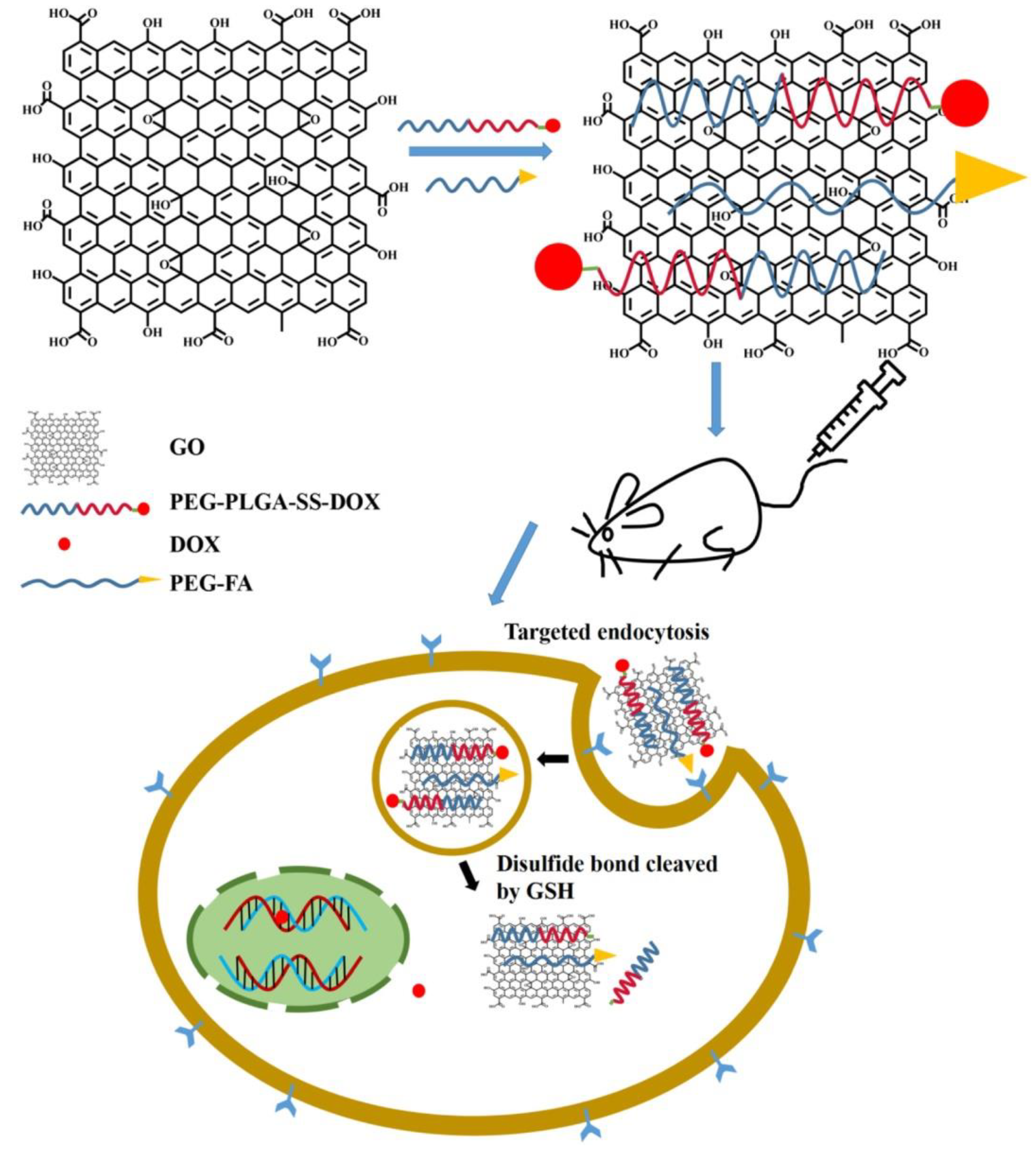
8. Drug Delivery Systems
9. Conclusions
Funding
Conflicts of Interest
References
- Erol, O.; Uyan, I.; Hatip, M.; Yilmaz, C. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomed. Nanotechnol. 2018, 14, 2433–2454. [Google Scholar] [CrossRef]
- Patel, D.K.; Seo, Y.R.; Lim, K.T. Stimuli-responsive graphene nanohybrids for biomedical applications. Stem Cells Int. 2019, 2019, 9831853. [Google Scholar] [CrossRef]
- Gong, X.; Liu, G.; Li, Y.; Yu, D.Y.W.; Teoh, W.Y. Functionalized-graphene composites: Fabrication and applications in sustainable energy and environment. Chem. Mater. 2016, 28, 8082–8118. [Google Scholar] [CrossRef]
- Dhas, N.; Parekh, K.; Pandey, A.; Kudarha, R.; Mutalik, S.; Mehta, T. Two dimensional carbon based nanocomposites as multimodal therapeutic and diagnostic platform: A biomedical and toxicological perspective. J. Control. Release 2019, 308, 130–161. [Google Scholar] [CrossRef]
- Chakraborty, M.; Hashmi, M.S.J. Wonder material graphene: Properties, synthesis and practical applications. Adv. Mater. Process. Technol. 2018, 4, 573–602. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef]
- Kiew, S.F.; Kiew, L.V.; Lee, H.B.; Imae, T.; Chung, L.Y. Assessing biocompatibility of graphene oxide-based nanocarriers: A review. J. Control. Release 2016, 226, 217–228. [Google Scholar] [CrossRef]
- Yang, Y.; Asiri, A.M.; Tang, Z.; Du, D.; Lin, Y. Graphene based materials for biomedical applications. Mater. Today 2013, 16, 365–373. [Google Scholar] [CrossRef]
- Pattnaik, S.; Swain, K.; Lin, Z. Graphene and graphene-based nanocomposites: Biomedical applications and biosafety. J. Mater. Chem. B 2016, 4, 7813–7831. [Google Scholar] [CrossRef]
- Muthoosamy, K.; Abubakar, I.B.; Bai, R.G.; Loh, H. Exceedingly higher co-loading of curcumin and paclitaxel onto polymer-functionalized reduced graphene oxide for highly potent synergistic anticancer treatment. Sci. Rep. 2016, 6, 32808. [Google Scholar] [CrossRef]
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Tavakkoli Yaraki, M.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and its derivatives: Opportunities and challenges in dentistry. Mater. Sci. Eng. C 2019, 102, 171–185. [Google Scholar] [CrossRef]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.T.; Chen, X. Nanocarbons for biology and medicine: Sensing, imaging, and drug delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef]
- Ioniţă, M.; Vlăsceanu, G.M.; Watzlawek, A.A.; Voicu, S.I.; Burns, J.S.; Iovu, H. Graphene and functionalized graphene: Extraordinary prospects for nanobiocomposite materials. Compos. Part B Eng. 2017, 121, 34–57. [Google Scholar]
- Phiri, J.; Gane, P.; Maloney, T.C. General overview of graphene: Production, properties and application in polymer composites. Mater. Sci. Eng. B 2017, 215, 9–28. [Google Scholar] [CrossRef]
- Yang, G.; Li, L.; Lee, W.B.; Ng, M.C. Structure of graphene and its disorders: A review. Sci. Technol. Adv. Mater. 2018, 19, 613–648. [Google Scholar] [CrossRef]
- Walter, J.; Nacken, T.J.; Damm, C.; Thajudeen, T.; Eigler, S.; Peukert, W. Determination of the lateral dimension of graphene oxide nanosheets using analytical ultracentrifugation. Small 2015, 11, 814–825. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Yop, K.; Park, S.; Ro, W. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Lawal, A.T. Graphene-based nano composites and their applications. A review. Biosens. Bioelectron. 2019, 141, 111384. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Luo, G.; Xing, M. Two-dimensional graphene family material. Sensors 2019, 19, 1–34. [Google Scholar]
- Girao, A.F.; Serrano, M.C.; Completo, A.; Marques, P.A.A.P. Do biomedical engineers dream of graphene sheets? Biomater. Sci. 2019, 7, 1228–1239. [Google Scholar] [CrossRef]
- Kitko, K.E.; Zhang, Q. Graphene-based nanomaterials: From production to integration with modern tools in neuroscience. Front. Syst. Neurosci. 2019, 13, 1–17. [Google Scholar] [CrossRef]
- Xia, M.Y.; Xie, Y.; Yu, C.H.; Chen, G.Y.; Li, Y.H.; Zhang, T.; Peng, Q. Graphene-based nanomaterials: The promising active agents for antibiotics-independent antibacterial applications. J. Control. Release 2019, 307, 16–31. [Google Scholar] [CrossRef]
- De Melo-Diogo, D.; Lima-Sousa, R.; Alves, C.G.; Correia, I.J. Graphene family nanomaterials for application in cancer combination photothermal therapy. Biomater. Sci. 2019, 7, 3534–3551. [Google Scholar] [CrossRef]
- Bullo, S.; Buskaran, K.; Baby, R.; Dorniani, D.; Fakurazi, S.; Hussein, M.Z. Dual drugs anticancer nanoformulation using graphene oxide-PEG as nanocarrier for protocatechuic acid and chlorogenic acid. Pharm. Res. 2019, 36, 91. [Google Scholar] [CrossRef]
- Tiwari, H.; Karki, N.; Pal, M.; Basak, S.; Verma, R.K.; Bal, R.; Kandpal, N.D.; Bisht, G.; Sahoo, N.G. Functionalized graphene oxide as a nanocarrier for dual drug delivery applications: The synergistic effect of quercetin and gefitinib against ovarian cancer cells. Colloids Surf. B Biointerfaces 2019, 178, 452–459. [Google Scholar] [CrossRef]
- Charmi, J.; Nosrati, H.; Amjad, J.M.; Mohammadkhani, R.; Danafar, H. Polyethylene glycol (PEG) decorated graphene oxide nanosheets for controlled release curcumin delivery. Heliyon 2019, 5, e01466. [Google Scholar] [CrossRef]
- Assali, A.; Akhavan, O.; Mottaghitalab, F.; Adeli, M.; Dinarvand, R.; Razzazan, S.; Arefian, E.; Soleimani, M.; Atyabi, F. Cationic graphene oxide nanoplatform mediates miR-101 delivery to promote apoptosis by regulating autophagy and stress. Int. J. Nanomedicine 2018, 13, 5865–5886. [Google Scholar] [CrossRef]
- Taniselass, S.; Arshad, M.K.M.; Gopinath, S.C.B. Graphene-based electrochemical biosensors for monitoring noncommunicable disease biomarkers. Biosens. Bioelectron. 2019, 130, 276–292. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Madannejad, R.; Shoaie, N.; Jahanpeyma, F.; Darvishi, M.H.; Azimzadeh, M.; Javadi, H. Toxicity of carbon-based nanomaterials: Reviewing recent reports in medical and biological systems. Chem. Biol. Interact. 2019, 307, 206–222. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Maleki, A.; de la Guardia, M.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Baradaran, B.; Mokhtarzadeh, A.; Hamblin, M.R. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Li, Y.; Feng, Y.; Feng, W. Carbon-based functional nanomaterials: Preparation, properties and applications. Compos. Sci. Technol. 2019, 179, 10–40. [Google Scholar] [CrossRef]
- Setaro, A. Advanced carbon nanotubes functionalization. J. Phys. Condens. Matter 2017, 29, 11–14. [Google Scholar] [CrossRef]
- Guo, X.; Mei, N. Assessment of the toxic potential of graphene family nanomaterials. J. Food Drug Anal. 2014, 22, 105–115. [Google Scholar] [CrossRef]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vazquez, E.; Ballerini, L.; et al. Classification framework for grapheme-based materials. Angew. Chem. 2014, 53, 7714–7718. [Google Scholar] [CrossRef]
- Bottari, G.; Herranz, M.A.; Wibmer, L.; Volland, M.; Rodriguez-Perez, L.; Guldi, D.M.; Hirsch, A.; Martin, N.; D’Souza, F.; Torres, T. Chemical functionalization and characterization of graphene-based materials. Chem. Soc. Rev. 2017, 46, 4464–4500. [Google Scholar] [CrossRef]
- Lin, L.; Bin-Tay, W.; Aslam, Z.; Westwood, A.V.K. Determination of the lateral size and thickness of solution-processed graphene flakes. J. Phys. Conf. Ser. 2017, 902, 012026. [Google Scholar] [CrossRef]
- Wei, P.; Shen, J.; Wu, K.; Yang, N. Defect-dependent electrochemistry of exfoliated graphene layers. Carbon 2019, 154, 125–131. [Google Scholar] [CrossRef]
- Mohandoss, M.; Sen Gupta, S.; Kumar, R.; Islam, M.R.; Som, A.; Mohd, A.G.; Pradeep, T.; Maliyekkal, S.M. Self-propagated combustion synthesis of few-layered graphene: An optical properties perspective. Nanoscale 2018, 10, 7581–7588. [Google Scholar] [CrossRef]
- Aliyev, E.; Filiz, V.; Khan, M.M.; Lee, Y.J.; Abetz, C.; Abetz, V. Structural characterization of graphene oxide: Surface functional groups and fractionated oxidative debris. Nanomaterials 2019, 9, 1180. [Google Scholar] [CrossRef]
- Cho, J.H.; Na, S.R.; Park, S.; Akinwande, D.; Liechti, K.M.; Cullinan, M.A. Controlling the number of layers in graphene using the growth pressure. Nanotechnology 2019, 30, 22. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Lin, M.L.; Cong, X.; Liu, H.N.; Tan, P.H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef]
- Shearer, C.J.; Slattery, A.D.; Stapleton, A.J.; Shapter, J.G.; Gibson, C.T. Accurate thickness measurement of graphene. Nanotechnology 2016, 27, 125704. [Google Scholar] [CrossRef] [PubMed]
- Mellado, C.; Figueroa, T.; Baez, R.; Melendrez, M.; Fernandez, K. Effects of probe and bath ultrasonic treatments on graphene oxide structure. Mater. Today Chem. 2019, 13, 1–7. [Google Scholar] [CrossRef]
- Amaro-Gahete, J.; Benitez, A.; Otero, R.; Esquivel, D.; Jimenez-Sanchidrian, C.; Morales, J.; Caballero, A.; Romero-Salguero, F.J. A comparative study of particle size distribution of graphene nanosheets synthesized by an ultrasound-assisted method. Nanomaterials 2019, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Borode, A.O.; Ahmed, N.A.; Olubambi, P.A. Surfactant-aided dispersion of carbon nanomaterials in aqueous solution. Phys. Fluids 2019, 31, 071301. [Google Scholar] [CrossRef]
- Qu, Y.; He, F.; Yu, C.; Liang, X.; Liang, D.; Ma, L.; Zhang, Q.; Lv, J.; Wu, J. Advances on graphene-based nanomaterials for biomedical applications. Mater. Sci. Eng. C 2018, 90, 764–780. [Google Scholar] [CrossRef]
- Karki, N.; Tiwari, H.; Pal, M.; Chaurasia, A.; Bal, R.; Joshi, P.; Sahoo, N.G. Functionalized graphene oxides for drug loading, release and delivery of poorly water soluble anticancer drug: A comparative study. Colloids Surf. B Biointerfaces 2018, 169, 265–272. [Google Scholar] [CrossRef]
- Nanda, S.S.; Papaefthymiou, G.C.; Yi, D.K.; Nanda, S.S.; Papaefthymiou, G.C.; Yi, D.K. Functionalization of graphene oxide and its biomedical applications functionalization of graphene oxide and its biomedical applications. Crit. Rev. Solid State Mater. Sci. 2015, 40, 291–315. [Google Scholar] [CrossRef]
- Vacchi, I.A.; Raya, J.; Bianco, A.; Menard-Moyon, C. Controlled derivatization of hydroxyl groups of graphene oxide in mild conditions. 2D Mater. 2018, 5, 035037. [Google Scholar] [CrossRef]
- Cha, J.; Kim, J.; Ryu, S.; Hong, S.H. Comparison to mechanical properties of epoxy nanocomposites reinforced by functionalized carbon nanotubes and graphene nanoplatelets. Compos. Part B Eng. 2019, 162, 283–288. [Google Scholar] [CrossRef]
- Punetha, V.D.; Rana, S.; Yoo, H.J.; Chaurasia, A.; McLeskey, J.T.; Sekkarapatti Ramasamy, M.; Sahoo, N.G.; Cho, J.W. Functionalization of carbon nanomaterials for advanced polymer nanocomposites: A comparison study between CNT and graphene. Prog. Polym. Sci. 2017, 67, 1–47. [Google Scholar] [CrossRef]
- De Sousa, M.; Martins, C.H.Z.; Franqui, L.S.; Fonseca, L.C.; Delite, F.S.; Lanzoni, E.M.; Martinez, D.S.T.; Alves, O.L. Covalent functionalization of graphene oxide with d-mannose: Evaluating the hemolytic effect and protein corona formation. J. Mater. Chem. B 2018, 6, 2803–2812. [Google Scholar] [CrossRef]
- Nandanapalli, K.R.; Mudusu, D.; Lee, S. Functionalization of graphene layers and advancements in device applications. Carbon 2019, 152, 954–985. [Google Scholar] [CrossRef]
- Ji, X.; Xu, Y.; Zhang, W.; Cui, L.; Liu, J. Review of functionalization, structure and properties of graphene/polymer composite fibers. Comp. A Appl. Sci. Manufact. 2016, 87, 29–45. [Google Scholar] [CrossRef]
- Eckhart, K.E.; Holt, B.D.; Sydlik, S.A.; Laurencin, M.G. Covalent conjugation of bioactive peptides to graphene oxide for biomedical applications. Biomater. Sci. 2019, 7, 3876–3885. [Google Scholar] [CrossRef] [PubMed]
- Cherian, R.S.; Sandeman, S.; Ray, S.; Savina, I.N.; Ashtami, J.; Mohanan, P.V. Green synthesis of Pluronic stabilized reduced graphene oxide: Chemical and biological characterization. Colloids Surf. B Biointerfaces 2019, 179, 94–106. [Google Scholar] [CrossRef]
- Khawaja, H.; Zahir, E.; Asghar, M.A.; Asghar, M.A. Graphene oxide, chitosan and silver nanocomposite as a highly effective antibacterial agent against pathogenic strains. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 246–255. [Google Scholar] [CrossRef]
- Martin, C.; Kostarelos, K.; Prato, M.; Bianco, A. Biocompatibility and biodegradability of 2D materials: Graphene and beyond. Chem. Commun. 2019, 55, 5540–5546. [Google Scholar] [CrossRef]
- Jia, P.P.; Sun, T.; Junaid, M.; Yang, L.; Ma, Y.B.; Cui, Z.S.; Wei, D.P.; Shi, H.F.; Pei, D.S. Nanotoxicity of different sizes of graphene (G) and graphene oxide (GO) in vitro and in vivo. Environ. Pollut. 2019, 247, 595–606. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene nanomaterials: Synthesis, biocompatibility, and cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef] [PubMed]
- Frontinan-Rubio, J.; Gomez, M.V.; Martin, C.; Gonzalez-Dominguez, J.M.; Duran-Prado, M.; Vazquez, E. Differential effects of graphene materials on the metabolism and function of human skin cells. Nanoscale 2018, 10, 11604–11615. [Google Scholar] [CrossRef] [PubMed]
- Keremidarska-Markova, M.; Hristova-Panusheva, K.; Andreeva, T.; Speranza, G.; Wang, D.; Krasteva, N. Cytotoxicity evaluation of ammonia-modified graphene oxide particles in lung cancer cells and embryonic stem cells. Adv. Condens. Matter Phys. 2018, 2018, 9571828. [Google Scholar] [CrossRef]
- Syama, S.; Mohanan, P.V. Safety and biocompatibility of graphene: A new generation nanomaterial for biomedical application. Int. J. Biol. Macromol. 2016, 86, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Nasirzadeh, N.; Azari, M.R.; Rasoulzadeh, Y.; Mohammadian, Y. An assessment of the cytotoxic effects of graphene nanoparticles on the epithelial cells of the human lung. Toxicol. Ind. Health 2019, 35, 79–87. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F. From nanoengineering to nanomedicine: A facile route to enhance biocompatibility of graphene as a potential nano-carrier for targeted drug delivery using natural deep eutectic solvents. Chem. Eng. Sci. 2019, 195, 95–106. [Google Scholar] [CrossRef]
- Dallavalle, M.; Calvaresi, M.; Bottoni, A.; Melle-Franco, M.; Zerbetto, F. Graphene can wreak havoc with cell membranes. ACS Appl. Mater. 2015, 7, 4406–4414. [Google Scholar] [CrossRef]
- Bondar, O.V.; Saifullina, D.V.; Shakhmaeva, I.I.; Mavlyutova, I.I.; Abdullin, T.I. Monitoring of the zeta potential of human cells upon reduction in their viability and interaction with polymers. Acta Nat. 2012, 1, 78–81. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, M.K.; Kulkarni, P.P.; Sonkar, V.K.; Gracio, J.J.; Dash, D. Amine-modified graphene: Thrombo-protective safer alternative to graphene oxide for biomedical applications. ACS Nano 2012, 6, 2731–2740. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Bae, S.C.; Granick, S. Nanoparticle-induced surface reconstruction of phospholipid membranes. Proc. Natl. Acad. Sci. USA 2008, 105, 18171–18175. [Google Scholar] [CrossRef]
- Majidi, H.J.; Babaei, A.; Bafrani, Z.A.; Shahrampour, D.; Zabihi, E.; Jafari, S.M. Investigating the best strategy to diminish the toxicity and enhance the antibacterial activity of graphene oxide by chitosan addition. Carbohydr. Polym. 2019, 225, 115–220. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, F.; Wang, S.; Ma, J.; Xu, M.; Gao, M.; Liu, R.; Chen, W.; Liu, S. Reduction of graphene oxide alters its cyto-compatibility towards primary and immortalized macrophages. Nanoscale 2018, 10, 14637–14650. [Google Scholar] [CrossRef]
- Thomas, S.; Grohens, Y.; Ninan, N. Nanotechnology Applications for Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Olad, A.; Hagh, H.B.K. Graphene oxide and amin-modified graphene oxide incorporated chitosan-gelatin scaffolds as promising materials for tissue engineering. Compos. Part B Eng. 2019, 162, 692–702. [Google Scholar] [CrossRef]
- Kenry, L.W.; Loh, K.P.; Lim, C.T. When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials 2018, 155, 236–250. [Google Scholar] [CrossRef]
- Teradal, N.L.; Jelinek, R. Carbon nanomaterials in biological studies and biomedicine. Adv. Healthc. Mater. 2017, 6, 1–36. [Google Scholar] [CrossRef]
- Lasocka, I.; Szulc-Dabrowska, L.; Skibniewski, M.; Skibniewska, E.; Strupinski, W.; Pasternak, I.; Kmiec, H.; Kowalczyk, P. Biocompatibility of pristine graphene monolayer: Scaffold for fibroblasts. Toxicol. Vitr. 2018, 48, 276–285. [Google Scholar] [CrossRef]
- Aval, N.A.; Emadi, R.; Valiani, A.; Kharaziha, M.; Karimipour, M.; Rahbarghazi, R. Nano-featured poly (lactide-co-glycolide)-graphene microribbons as a promising substrate for nerve tissue engineering. Compos. Part B Eng. 2019, 173, 106863. [Google Scholar] [CrossRef]
- Olad, A.; Hagh, H.B.K.; Mirmohseni, A.; Azhar, F.F. Graphene oxide and montmorillonite enriched natural polymeric scaffold for bone tissue engineering. Ceram. Int. 2019, 45, 15609–15619. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, H. Nanocomposites for bone repair and osteointegration with soft tissues. In Nanocomposites for Musculoskeletal Tissue Regeneration; Liu, H., Ed.; Woodhead Publishing & Elsevier: Kidlington, UK, 2016; pp. 241–257. [Google Scholar]
- Purohit, S.D.; Bhaskar, R.; Singh, H.; Yadav, I.; Gupta, M.K.; Mishra, N.C. Development of a nanocomposite scaffold of gelatin–alginate–graphene oxide for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 592–602. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Y.; Chen, G.; Wei, C.; Zhang, X.; Zeng, B.; Yi, C.; Wang, C.; Yu, D. Concentration-dependent cellular behavior and osteogenic differentiation effect induced in bone marrow mesenchymal stem cells treated with magnetic graphene oxide. J. Biomed. Mater. Res. Part A 2020, 108, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Jafarkhani, M.; Salehi, Z.; Bagheri, Z.; Aayanifard, Z.; Rezvan, A.; Doosthosseini, H.; Shokrgozar, M.A. Graphene functionalized decellularized scaffold promotes skin cell proliferation. Can. J. Chem. Eng. 2020, 98. in press. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, P.; Shi, X.; Ling, T.; Zeng, W.; Chen, A.; Yang, W.; Zhou, Z. Hierarchically porous hydroxyapatite hybrid scaffold incorporated with reduced graphene oxide for rapid bone ingrowth and repair. ACS Nano 2019, 13, 9595–9606. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. RSC Adv. 2019, 9, 8778–8781. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Zhou, S. Carbon-based hybrid nanogels: A synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem. Soc. Rev. 2018, 47, 4198–4232. [Google Scholar] [CrossRef]
- Singh, D.P.; Herrera, C.E.; Singh, B.; Singh, S.; Singh, R.K.; Kumar, R. Graphene oxide: An efficient material and recent approach for biotechnological and biomedical applications. Mater. Sci. Eng. C 2018, 86, 173–197. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lee, J.J. Electrochemical dopamine sensors based on graphene. J. Electrochem. Sci. Technol. 2019, 10, 185–195. [Google Scholar]
- Wang, W.; Su, H.; Wu, Y.; Zhou, T.; Li, T. Review-biosensing and biomedical applications of graphene: A review of current progress and future prospect. J. Electrochem. Soc. 2019, 166, B505–B520. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Nano-antimicrobials: Activity, benefits and weaknesses. In Nanostructures in Therapeutic Medicine–Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–54. [Google Scholar]
- Gao, F.; Cai, X.; Wang, X.; Gao, C.; Liu, S.; Gao, F.; Wang, Q. Highly sensitive and selective detection of dopamine in the presence of ascorbic acid at graphene oxide modified electrode. Sens. Actuators B Chem. 2013, 186, 380–387. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Nanocomposites: Synergistic nanotools for management mycotoxigenic fungi. In Nanomycotoxicology–Treating Mycotoxins in the Nano Way; Rai, M., Abd-Elsalam, K.A., Eds.; Academic Press & Elsevier: London, UK, 2019; pp. 349–383. [Google Scholar]
- Jampilek, J.; Kralova, K. Impact of nanoparticles on toxigenic fungi. In Nanomycotoxicology–Treating Mycotoxins in the Nano Way; Rai, M., Abd-Elsalam, K.A., Eds.; Academic Press & Elsevier: London, UK, 2019; pp. 309–348. [Google Scholar]
- Jampilek, J.; Kralova, K. Nanomaterials applicable in food protection. In Nanotechnology Applications in the Food Industry; Rai, R.V., Bai, J.A., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 75–96. [Google Scholar]
- Gomes, R.N.; Borges, I.; Pereira, A.T.; Maia, A.F.; Pestana, M.; Magalhaes, F.D.; Pinto, A.M.; Goncalves, I.C. Antimicrobial graphene nanoplatelets coatings for silicone catheters. Carbon 2018, 139, 635–647. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Lopresti, F. Effect of graphene and fabrication technique on the release kinetics of carvacrol from polylactic acid. Compos. Sci. Technol. 2019, 169, 60–69. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, J.; Gao, Y.; Li, T.; Wang, H.; Yan, H.; Niu, B.; Guo, R. Antibacterial graphene oxide coatings on polymer substrate. Appl. Surf. Sci. 2018, 436, 624–630. [Google Scholar] [CrossRef]
- Palmieri, V.; Lauriola, M.C.; Ciasca, G.; Conti, C.; De Spirito, M.; Papi, M. The graphene oxide contradictory effects against human pathogens. Nanotechnology 2017, 28, 152001–152018. [Google Scholar] [CrossRef]
- Skrlova, K.; Malachova, K.; Munoz-Bonilla, A.; Merinska, D.; Rybkova, Z.; Fernandez-Garcia, M.; Placha, D. Biocompatible polymer materials with antimicrobial properties for preparation of stents. Nanomaterials 2019, 9, 1548. [Google Scholar] [CrossRef]
- Pulingam, T.; Thong, K.L.; Ali, M.E.; Appaturi, J.N.; Dinshaw, I.J.; Ong, Z.Y.; Leo, B.F. Graphene oxide exhibits differential mechanistic action towards Gram-positive and Gram-negative bacteria. Colloids Surf. B Biointerfaces 2019, 181, 6–15. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K.; Campos, E.V.R.; Fraceto, L.F. Bio-based nanoemulsion formulations applicable in agriculture, medicine and food industry. In Nanobiotechnology in Bioformulations; Prasad, R., Kumar, V., Kumar, M., Choudhary, D.K., Eds.; Springer: Cham, Germany, 2019; pp. 33–84. [Google Scholar]
- Jampilek, J.; Kralova, K. Application of nanobioformulations for controlled release and targeted biodistribution of drugs. In Nanobiomaterials: Applications in Drug Delivery; Sharma, A.K., Keservani, R.K., Kesharwani, R.K., Eds.; CRC Press: Warentown, NJ, USA, 2018; pp. 131–208. [Google Scholar]
- Jampilek, J.; Kralova, K. Natural biopolymeric nanoformulations for brain drug delivery. In Nanocarriers for Brain Targetting: Principles and Applications; Keservani, R.K., Sharma, A.K., Kesharwani, R.K., Eds.; Apple Academic Press & CRC Press: Warentown, NJ, USA, 2019; pp. 131–203. [Google Scholar]
- Cheng, W.; Chen, Y.; Teng, L.; Lu, B.; Ren, L.; Wang, Y. Antimicrobial colloidal hydrogels assembled by graphene oxide and thermo-sensitive nanogels for cell encapsulation. J. Colloid Interface Sci. 2018, 513, 314–323. [Google Scholar] [CrossRef]
- Rasoulzadehzali, M.; Namazi, H. Facile preparation of antibacterial chitosan/graphene oxide-Ag bio-nanocomposite hydrogel beads for controlled release of doxorubicin. Int. J. Biol. Macromol. 2018, 116, 54–63. [Google Scholar] [CrossRef]
- Zhang, Q.; Tu, Q.; Hickey, M.E.; Xiao, J.; Gao, B.; Tian, C.; Heng, P.; Jiao, Y.; Peng, T.; Wang, J. Preparation and study of the antibacterial ability of graphene oxide-catechol hybrid polylactic acid nanofiber mats. Colloids Surf. B Biointerfaces 2018, 172, 496–505. [Google Scholar] [CrossRef]
- Diez-Orejas, R.; Feito, M.J.; Cicuendez, M.; Casarrubios, L.; Rojo, J.M.; Portoles, M.T. Graphene oxide nanosheets increase Candida albicans killing by pro-inflammatory and reparative peritoneal macrophages. Colloids Surf. B Biointerfaces 2018, 171, 250–259. [Google Scholar] [CrossRef]
- Sandhya, P.K.; Jose, J.; Sreekala, M.S.; Padmanabhan, M.; Kalarikkal, N.; Thomas, S. Reduced graphene oxide and ZnO decorated graphene for biomedical applications. Ceram. Int. 2018, 44, 15092–15098. [Google Scholar] [CrossRef]
- Jampilek, J. How can we bolster the antifungal drug discovery pipeline? Future Med. Chem. 2016, 8, 1393–1397. [Google Scholar] [CrossRef]
- Tran, D.L.; Thi, P.L.; Hoang-Thi, T.T.; Park, K.D. Graphene oxide immobilized surfaces facilitate the sustained release of doxycycline for the prevention of implant related infection. Colloids Surf. B Biointerfaces 2019, 181, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Si, Y.; Wang, F.; Su, L.; Xia, C.C.; Yao, J.; Chen, H.; Liu, X. Interaction processes of ciprofloxacin with graphene oxide and reduced graphene oxide in the presence of montmorillonite in simulated gastrointestinal fluids. Sci. Rep. 2017, 7, 2588. [Google Scholar] [CrossRef]
- Pi, J.; Shen, L.; Shen, H.; Yang, E.; Wang, W.; Wang, R.; Huang, D.; Lee, B.S.; Hu, C.; Chen, C.; et al. Mannosylated graphene oxide as macrophage-targeted delivery system for enhanced intracellular M. tuberculosis killing efficiency. Mater. Sci. Eng. C 2019, 103, 109777. [Google Scholar] [CrossRef]
- Liu, P.; Wang, S.; Liu, X.; Ding, J.; Zhou, W. Platinated graphene oxide: A nanoplatform for efficient gene-chemo combination cancer therapy. Eur. J. Pharm. Sci. 2018, 121, 319–329. [Google Scholar] [CrossRef]
- Magrez, A.; Kasas, S.; Salicio, V.; Pasquier, N.; Seo, J.W.; Celio, M.; Catsicas, S.; Schwaller, B.; Forro, L. Cellular Toxicity of Carbon-Based Nanomaterials. Nano Lett. 2006, 6, 1121–1125. [Google Scholar] [CrossRef]
- Pulskamp, K.; Diabate, S.; Krug, H.F. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol. Lett. 2007, 168, 58–74. [Google Scholar] [CrossRef]
- Yu, X.; Cai, H.; Zhang, W.; Li, X.; Pan, N.; Luo, Y.; Wang, X.; Hou, J.G. Tuning chemical enhancement of SERS by controlling the chemical reduction of graphene oxide nanosheets. ACS Nano 2011, 5, 952–958. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef]
- Valentini, F.; Calcaterra, A.; Ruggiero, V.; Pichichero, E.; Martino, A.; Iosi, F.; Bertuccini, L.; Antonaroli, S.; Mardente, S.; Zicari, A.; et al. Functionalized graphene derivatives: Antibacterial properties and cytotoxicity. J. Nanomat. 2019, 2019, 2752539. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial properties of graphene-based nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Jampílek, J.; Kos, J.; Kralova, K. Potential of Nanomaterial applications in dietary supplements and foods for special medical purposes. Nanomaterials 2019, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J.; Kralova, K. Recent advances in lipid nanocarriers applicable in the fight against cancer. In Nanoarchitectonics in Biomedicine; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 219–294. [Google Scholar]
- Jampilek, J.; Kralova, K. Nano-biopesticides in agriculture: State of art and future opportunities. In Nano-Biopesticides Today and Future Perspectives; Koul, O., Ed.; Academic Press & Elsevier: Amsterdam, The Netherlands, 2019; pp. 397–447. [Google Scholar]
- Jampilek, J.; Kralova, K. Nanotechnology based formulations for drug targeting to central nervous system. In Nanoparticulate Drug Delivery Systems; Keservani, R.K., Sharma, A.K., Eds.; Apple Academic Press & CRC Press: Warentown, NJ, USA, 2019; pp. 151–220. [Google Scholar]
- Jampilek, J.; Kralova, K. Nanomaterials for delivery of nutrients and growth-promoting compounds to plants. In Nanotechnology: An Agricultural Paradigm; Prasad, R., Kumar, M., Kumar, V., Eds.; Springer: Singapore, 2017; pp. 177–226. [Google Scholar]
- Kozik, V.; Bak, A.; Pentak, D.; Hachula, B.; Pytlakowska, K.; Rojkiewicz, M.; Jampilek, J.; Sieron, K.; Jazowiecka-Rakus, J.; Sochanik, A. Derivatives of graphene oxide as potential drug carriers. J. Nanosci. Nanotechnol. 2019, 19, 2489–2492. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Nanopesticides: Preparation, targeting and controlled release. In Nanotechnology in the Agri-Food Industry–New Pesticides and Soil Sensors; Grumezescu, A.M., Ed.; Elsevier: London, UK, 2017; pp. 81–127. [Google Scholar]
- Abdollahi, Z.; Taheri-Kafrani, A.; Bahrani, S.A.; Kajani, A.A. PEGAylated graphene oxide/superparamagnetic nanocomposite as a high-efficiency loading nanocarrier for controlled delivery of methotrexate. J. Biotechnol. 2019, 298, 88–97. [Google Scholar] [CrossRef]
- Yuen, W.W.; Du, N.R.; Chan, C.H.; Silva, E.A.; Mooney, D.J. Mimicking nature by codelivery of stimulant and inhibitor to create temporally stable and spatially restricted angiogenic zones. Proc. Natl. Acad. Sci. USA 2010, 107, 17933–17938. [Google Scholar] [CrossRef]
- Dube, A.; Reynolds, J.L.; Law, W.C.; Maponga, C.C.; Prasad, P.N.; Morse, G.D. Multimodal nanoparticles that provide immunomodulation and intracellular drug delivery for infectious diseases. Nanomed. Nanotechnol. 2014, 10, 831–838. [Google Scholar] [CrossRef]
- Sharma, M.; Chatterjee, M.; Singh, K.; Satapathia, S. Design of a multimodal colloidal polymeric drug delivery vesicle: A detailed pharmaceutical study. NANOSO 2019, 18, 100245. [Google Scholar] [CrossRef]
- Browne, S.; Pandit, A. Multi-modal delivery of therapeutics using biomaterial scaffolds. J. Mater. Chem. B 2014, 2, 6692–6707. [Google Scholar] [CrossRef]
- Feng, L.; Wu, L.; Qu, X. New horizons for diagnostics and therapeutic applications of graphene and graphene oxide. Adv. Mater. 2013, 25, 168–186. [Google Scholar] [CrossRef]
- Tabish, T.A. Graphene-based materials: The missing piece in nanomedicine? Biochem. Biophys. Res. Commun. 2018, 504, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.; Naik, R.R.; Dai, L. Carbon Nanomaterials for Biomedical Applications; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Chee, W.K.; Lim, H.N.; Huang, N.M.; Harrison, I. Nanocomposites of graphene/polymers: A review. RSC Adv. 2015, 5, 68014–68051. [Google Scholar] [CrossRef]
- Gao, Y.; Zhong, S.; Xu, L.; He, S.; Dou, Y.; Zhao, S.; Chen, P.; Cui, X. Mesoporous silica nanoparticles capped with graphene quantum dots as multifunctional drug carriers for photo-thermal and redox-responsive release. Microporous Mesoporous Mater. 2019, 278, 130–137. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, L.; Li, L.; He, Y.; Cao, W.; Liu, H.; Niu, K.; Gao, D. Trimodal synergistic antitumor drug delivery system based on graphene oxide. Nanomedicine 2019, 15, 142–152. [Google Scholar] [CrossRef]
- Campbell, E.; Hasan, M.T.; Pho, C.; Callaghan, K.; Akkaraju, G.R.; Naumov, A.V. Graphene oxide as a multifunctional platform for intracellular delivery, imaging, and cancer sensing. Sci. Rep. 2019, 9, 416. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Gong, J.; Mu, R.; Li, Y.; Wu, C.; Pang, J. Fabrication of chitosan-coated konjac glucomannan/sodium alginate/graphene oxide microspheres with enhanced colon-targeted delivery. Int. J. Biol. Macromol. 2019, 131, 209–217. [Google Scholar] [CrossRef]
- Huang, C.; Wu, J.; Jiang, W.; Liu, R.; Li, Z.; Luan, Y. Amphiphilic prodrug-decorated graphene oxide as a multi-functional drug delivery system for efficient cancer therapy. Mater. Sci. Eng. C 2018, 89, 15–24. [Google Scholar] [CrossRef]
- Dong, J.; Wang, K.; Sun, L.; Sun, B.; Yang, M.; Chen, H.; Wang, Y.; Sun, J.; Dong, L. Application of graphene quantum dots for simultaneous fluorescence imaging and tumor-targeted drug delivery. Sens. Actua. B Chem. 2018, 256, 616–623. [Google Scholar] [CrossRef]
- Zare-Zardini, H.; Taheri-Kafrani, A.; Amiri, A.; Bordbar, A.K. New generation of drug delivery systems based on ginsenoside Rh2-, Lysine- and Arginine-treated highly porous graphene for improving anticancer activity. Sci. Rep. 2018, 8, 586. [Google Scholar] [CrossRef]
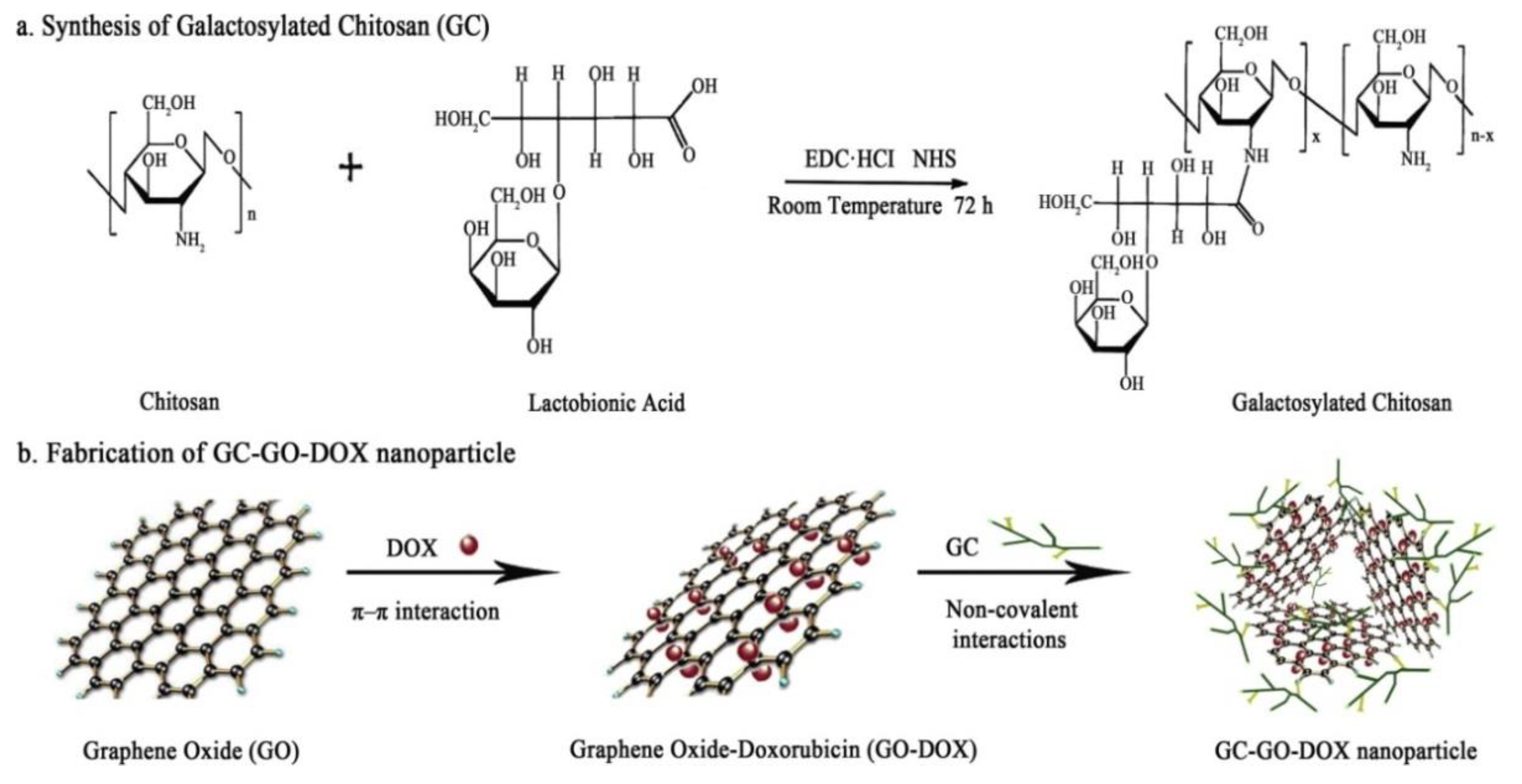
- Wang, C.; Zhang, Z.; Chen, B.; Gu, L.; Li, Y.; Yu, S. Design and evaluation of galactosylated chitosan/graphene oxide nanoparticles as a drug delivery system. J. Colloid Interface Sci. 2018, 516, 332–341. [Google Scholar] [CrossRef]
- Javanbakht, S.; Namazi, H. Doxorubicin loaded carboxymethyl cellulose/graphene quantum dot nanocomposite hydrogel films as a potential anticancer drug delivery system. Mater. Sci. Eng. C 2018, 87, 50–59. [Google Scholar] [CrossRef]
- Javanbakht, S.; Pooresmaeil, M.; Namazi, H. Green one-pot synthesis of carboxymethylcellulose/Zn-based metal-organic framework/graphene oxide bio-nanocomposite as a nanocarrier for drug delivery system. Carbohydr. Polym. 2019, 208, 294–301. [Google Scholar] [CrossRef]
- Borandeh, S.; Abdolmaleki, A.; Abolmaali, S.S.; Tamaddon, A.M. Synthesis, structural and in-vitro characterization of β-cyclodextrin grafted L-phenylalanine functionalized graphene oxide nanocomposite: A versatile nanocarrier for pH-sensitive doxorubicin delivery. Carbohydr. Polym. 2018, 201, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, M.; Fan, Y.; Zhang, Q.; Wang, Y.; Yuan, W.; Zhou, N.; Che, J. Novel controlled drug release system engineered with inclusion complexes based on carboxylic graphene. Colloids Surf. B Biointerfaces 2019, 175, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Kaya, D.; Kucukada, K.; Alemdar, N. Modeling the drug release from reduced graphene oxide-reinforced hyaluronic acid/gelatin/poly (ethylene oxide) polymeric films. Carbohydr. Polym. 2019, 215, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, Z.; Sahraei, R.; Ghaemy, M. Preparation of spherical porous hydrogel beads based on ion-crosslinked gum tragacanth and graphene oxide: Study of drug delivery behavior. Carbohydr. Polym. 2018, 194, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, J.; Wang, Y.; Zhao, L.; Li, Y.; Liu, C. Formation of graphene oxide-hybridized nanogels for combinative anticancer therapy. Nanomedicine 2018, 14, 2387–2395. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, F.; Liu, L.; Zhang, Y.; Li, Y.; Li, H.; Xie, J. Surface modification of graphene oxide nanosheets by protamine sulfate/sodium alginate for anti-cancer drug delivery application. Appl. Surf. Sci. 2018, 440, 853–860. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Shen, B.; Chen, L.; Mo, J.; Feng, J. Dual-sensitive graphene oxide loaded with proapoptotic peptides and anticancer drugs for cancer synergetic therapy. Langmuir 2019, 35, 6120–6128. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, H.T.; Phung, C.D.; Pathak, S.; Regmi, S.; Ha, D.H.; Kim, J.O.; Yong, C.S.; Kim, S.K.; Choi, J.E.; et al. Targeted delivery of doxorubicin for the treatment of bone metastasis from breast cancer using alendronate-functionalized graphene oxide nanosheets. J. Ind. Eng. Chem. 2019, 76, 310–317. [Google Scholar] [CrossRef]
- Abbasian, M.; Roudi, M.M.; Mahmoodzadeh, F.; Eskandani, M.; Jaymand, M. Chitosan-grafted-poly(methacrylic acid)/graphene oxide nanocomposite as a pH-responsive de novo cancer chemotherapy nanosystem. Int. J. Biol. Macromol. 2018, 118, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Pooresmaeil, M.; Namazi, H. Surface modification of graphene oxide with stimuli-responsive polymer brush containing β-cyclodextrin as a pendant group: Preparation, characterization, and evaluation as controlled drug delivery agent. Colloids Surf. B Biointerfaces 2018, 172, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Namazi, H. Blue fluorescent graphene oxide hybrid: Synthesis, characterization, and application as a drug delivery system. J. Drug Deliv. Sci. Technol. 2018, 48, 355–362. [Google Scholar] [CrossRef]
- Vinothini, K.; Rajendran, N.K.; Munusamy, M.A.; Alarfaj, A.A.; Rajan, M. Development of biotin molecule targeted cancer cell drug delivery of doxorubicin loaded κ-carrageenan grafted graphene oxide nanocarrier. Mater. Sci. Eng. C 2019, 100, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Deb, A.; Vimala, R. Natural and synthetic polymer for graphene oxide mediated anticancer drug delivery—A comparative study. Int. J. Biol. Macromol. 2018, 107, 2320–2333. [Google Scholar] [CrossRef]
- Thapa, R.K.; Soe, Z.C.; Ou, W.; Poudel, K.; Jeong, J.H.; Jin, S.G.; Ku, S.K.; Choi, H.G.; Lee, Y.M.; Yong, C.S.; et al. Palladium nanoparticle-decorated 2-D graphene oxide for effective photodynamic and photothermal therapy of prostate solid tumors. Colloids Surf. B Biointerfaces 2018, 169, 429–437. [Google Scholar] [CrossRef]
- Li, G.; Yang, Y.; Zhou, R.; Meng, F.; Li, X. Functionalized graphene oxide as a nanocarrier of new copper (II) complexes for targeted therapy on nasopharyngeal carcinoma. Eur. J. Pharm. Sci. 2018, 123, 249–259. [Google Scholar] [CrossRef]
- Wang, X.; Cao, F.; Yan, M.; Liu, Y.; Zhu, X.; Sun, H.; Ma, G. Alum-functionalized graphene oxide nanocomplexes for effective anticancer vaccination. Acta Biomater. 2019, 83, 390–399. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. β-Cyclodextrin grafted magnetic graphene oxide applicable as cancer drug delivery agent: Synthesis and characterization. Mater. Chem. Phys. 2018, 218, 62–69. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, F.; Peng, H.; Zhang, Y.; Li, Y.; Xu, Y.; Xie, J. Layer-by-layer modification of magnetic graphene oxide by chitosan and sodium alginate with enhanced dispersibility for targeted drug delivery and photothermal therapy. Colloids Surf. B Biointerfaces 2019, 176, 462–470. [Google Scholar] [CrossRef]
- Xie, P.; Du, P.; Li, J.; Liu, P. Stimuli-responsive hybrid cluster bombs of PEGylated chitosan encapsulated DOX-loaded superparamagnetic nanoparticles enabling tumor-specific disassembly for on-demand drug delivery and enhanced MR imaging. Carbohydr. Polym. 2019, 205, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.D.; Ganganboina, A.B.; Tsai, Y.C.; Chiu, H.C.; Doong, R. Multifunctional GQDs-concanavalin A@Fe3O4 nanocomposites for cancer cells detection and targeted drug delivery. Anal. Chim. Acta 2018, 1027, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.O.; Baldi, G.; Doumett, S.; Garcia-Hevia, L.; Gallo, J.; Banobre-Lopez, M.; Drazic, G.; Calhelha, R.C.; Ferreira, I.C.F.R.; Lima, R.; et al. Multifunctional graphene-based magnetic nanocarriers for combined hyperthermia and dual stimuli-responsive drug delivery. Mater. Sci. Eng. C 2018, 93, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Shirvalilou, S.; Khoei, S.; Khoee, S.; Jamali, N. Chemico-biological interactions development of a magnetic nano-graphene oxide carrier for improved glioma-targeted drug delivery and imaging: In vitro and in vivo evaluations. Chem. Biol. Interact. 2018, 295, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.V.; Shim, K.H.; Vo Thi, T.T.; Kook, J.K.; An, S.S.A.; Lee, S.W. Targeted and controlled drug delivery by multifunctional mesoporous silica nanoparticles with internal fluorescent conjugates and external polydopamine and graphene oxide layers. Acta Biomater. 2018, 74, 397–413. [Google Scholar] [CrossRef]
- Moldovan, M.; Prodan, D.; Sarosi, C.; Carpa, R.; Socaci, C.; Rosu, M.C.; Pruneanu, S. Synthesis, morpho-structural properties and antibacterial effect of silicate-based composites containing graphene oxide/hydroxyapatite. Mater. Chem. Phys. 2018, 217, 48–53. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Zhang, F.; Wang, X.; Liu, X.; Zhang, Y. Polydopamine doped reduced graphene oxide/mesoporous silica nanosheets for chemo-photothermal and enhanced photothermal therapy. Mater. Sci. Eng. C 2019, 96, 138–145. [Google Scholar] [CrossRef]
- Yuan, Y.G.; Gurunathan, S. Combination of graphene oxide-silver nanoparticle nanocomposites and cisplatin enhances apoptosis and autophagy in human cervical cancer cells. Int. J. Nanomedicine 2017, 12, 6537–6558. [Google Scholar] [CrossRef]
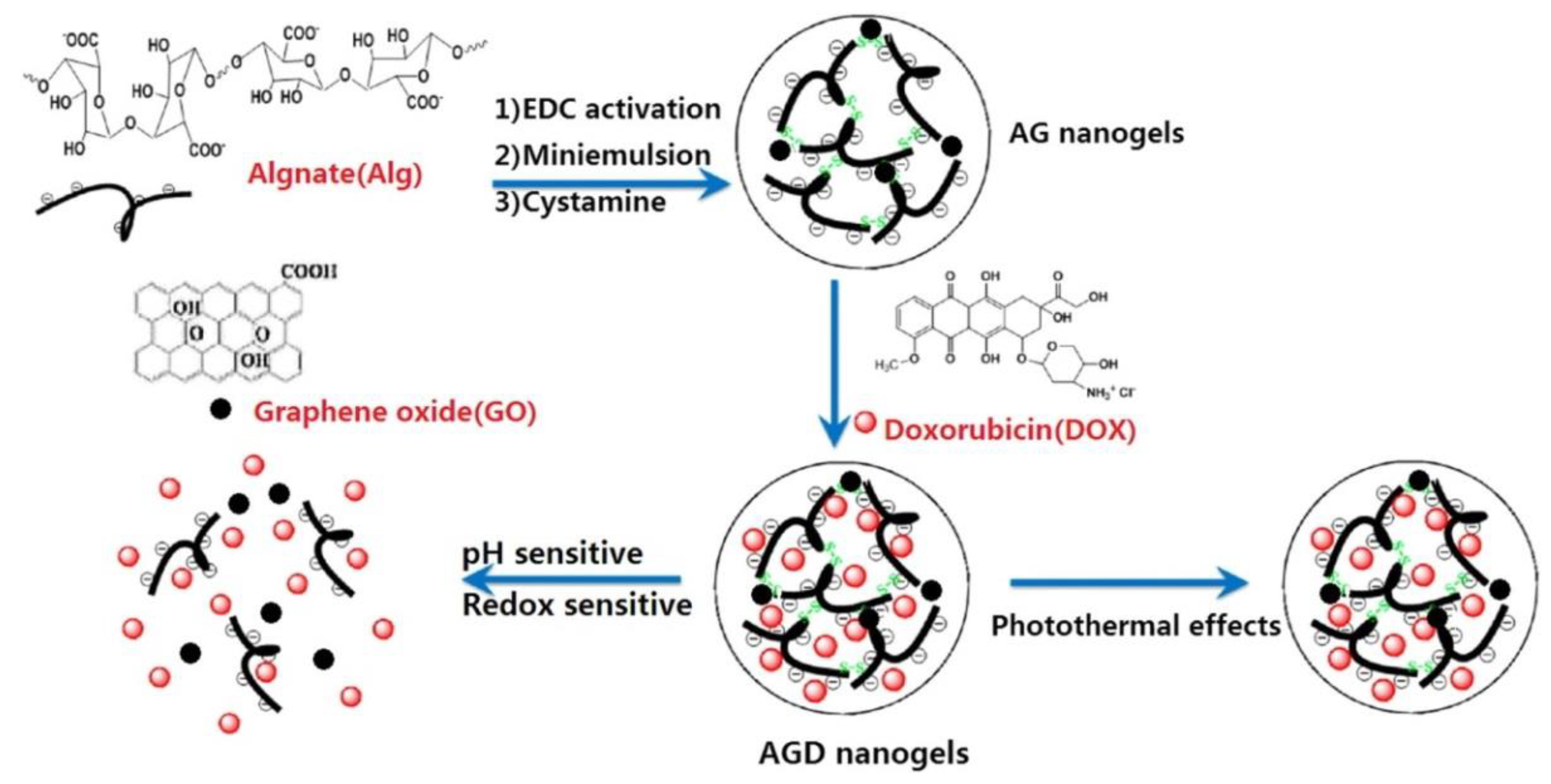
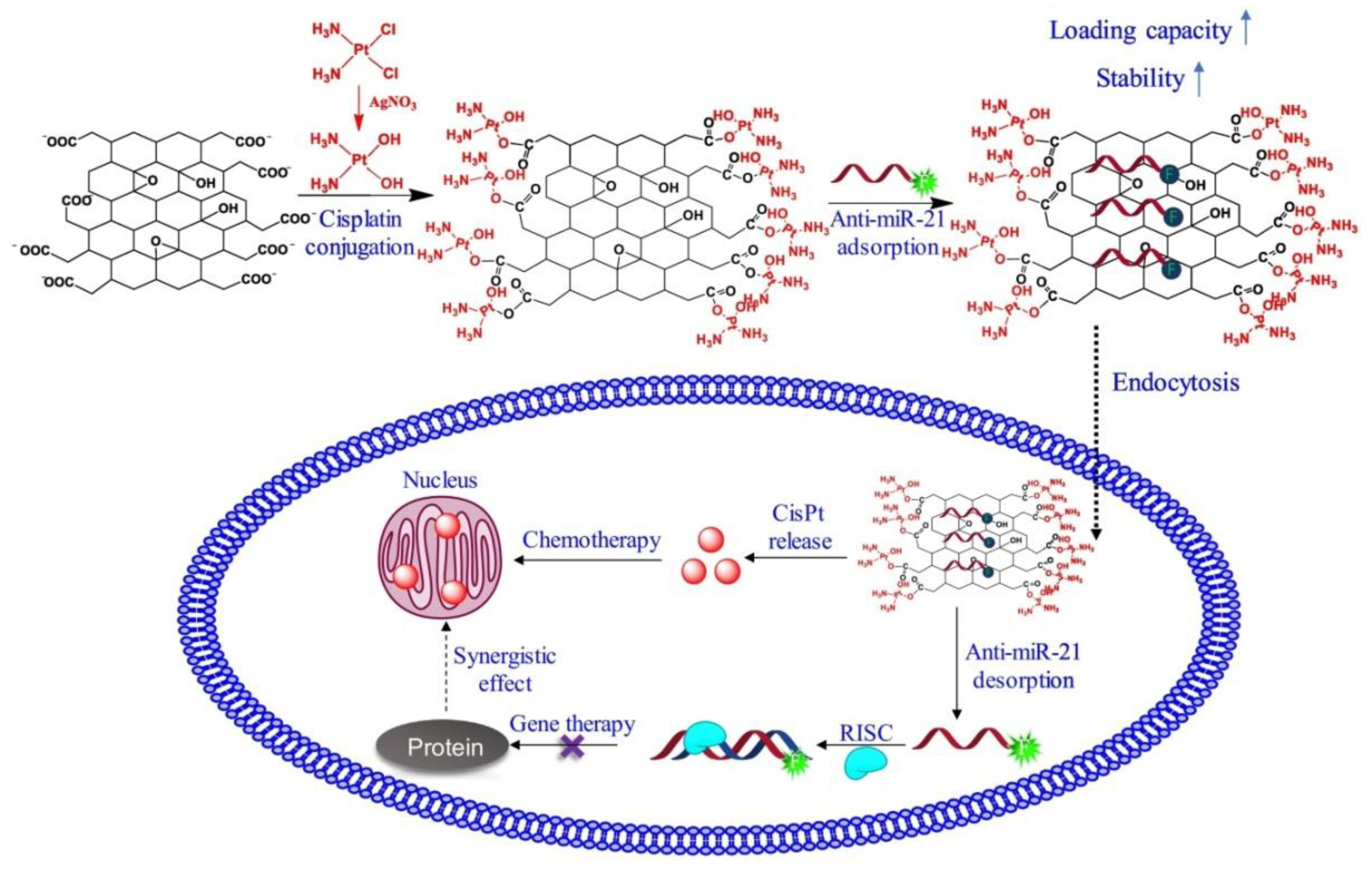
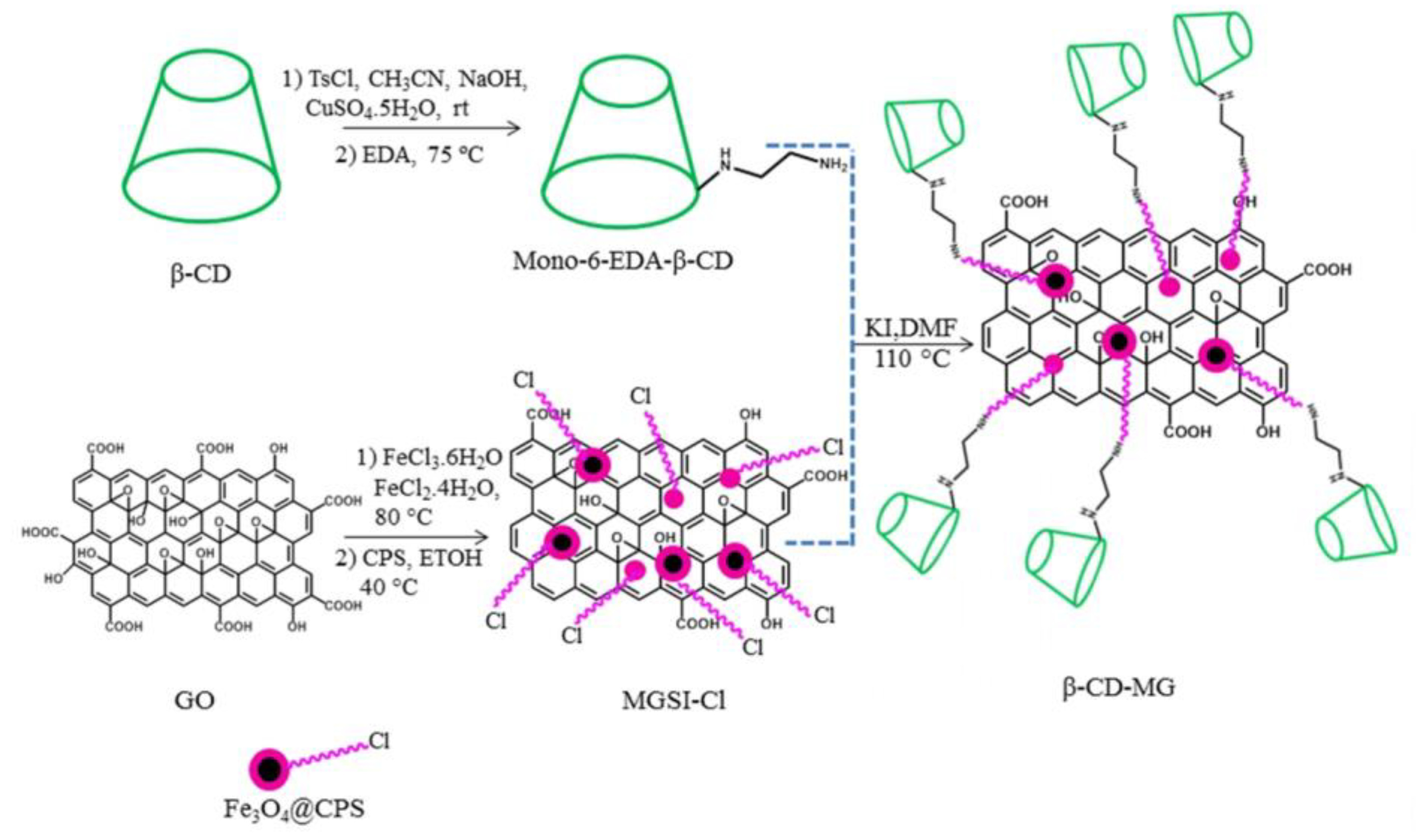
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plachá, D.; Jampilek, J. Graphenic Materials for Biomedical Applications. Nanomaterials 2019, 9, 1758. https://doi.org/10.3390/nano9121758
Plachá D, Jampilek J. Graphenic Materials for Biomedical Applications. Nanomaterials. 2019; 9(12):1758. https://doi.org/10.3390/nano9121758
Chicago/Turabian StylePlachá, Daniela, and Josef Jampilek. 2019. "Graphenic Materials for Biomedical Applications" Nanomaterials 9, no. 12: 1758. https://doi.org/10.3390/nano9121758
APA StylePlachá, D., & Jampilek, J. (2019). Graphenic Materials for Biomedical Applications. Nanomaterials, 9(12), 1758. https://doi.org/10.3390/nano9121758





