1. Introduction
The design and synthesis of precise monodispersed iron oxide magnetic nanoparticles (MNP) is a growing research field due to their applicability in nanotechnologies, particularly in the biomedical sector [
1]. In nanomedicine, MNPs comprised of the magnetic iron oxides magnetite (Fe
3O
4) or maghemite (γ-Fe
2O
3) are considered very attractive due to their low toxicity and cheap precursors. They can act as contrast agents in MRI and can also be heated by an alternating magnetic field or laser light to provide hyperthermia as a remote, switchable therapy for cancer treatment [
2,
3,
4]. MNP can be functionalised with probes (like fluorescent tags for imaging), drugs and targeting or a combination of all of these to form smart theranostic that can be targeted to a specific region of the body by a magnetic field.
However, “green” (ambient, non-toxic conditions) synthesis of iron oxides is notoriously difficult to control. Minor changes to any number of reaction conditions (iron precursors, choice of base, ratio of iron oxidation states of the precursors etc.) will lead to the production of a range of iron oxides [
1]. For example, the partial oxidation of ferrous hydroxide using an excess of NaOH under N
2 at 90 °C will precipitate octahedral magnetite particles approximately 20–80 nm in size. Changing the base used can result in needle-shaped FeOOH by-products [
5]. Furthermore, if the excess of NaOH is reduced to stoichiometric base:ferrous ions concentrations the particle size increases dramatically up to approximately 1 µm, as the excess of ferrous ions increases the particle products reduce to 400 nm diameter as the excess of ferrous ions increases [
6]. A co-precipitation of ferric and ferrous ions at room temperature under N
2 with KOH base results in mostly small MNP of poor crystallinity, with a heterogeneous shape and size population ranging from <5 nm up to micrometre scales [
2,
5]. Although this does allow scope to synthesise various morphologies and sizes, the overwhelming drawback is that it is near impossible to synthesise a mono-dispersed and reproducible product with respect to consistent and monodispersed size and shape. Furthermore, ferric oxide impurities are common (which represent a large proportion of the heterogeneity observed in the co-precipitation synthesis).
A key challenge for the green synthesis of magnetite is the reproducibility of batch nanoparticle synthesis. Very subtle changes in conditions can have huge effects on the final MNP product, which is more exaggerated and difficult to address in batch synthesis, with batch to batch variations being inevitable.
Micro and macro fluidic systems offer a unilaminar controlled fluid environment, where the fluid flow and dynamics can be quantified, modelled, controlled, and reproduced with higher accuracy, allowing a more reproducible synthesis compared to batch synthesis [
7]. Furthermore, defining and achieving precise reaction environments enables a more detailed analysis of the synthesis process, such as the possibility of adding probes for analysis or reagents at precise reactions points. An additional benefit of a continuous fluidic system is that the reaction time points equate to channel position, allowing screening and monitoring of the synthesis at every stage in situ. Such systems can be readily modified to incorporate characterisation instrumentation such as spectroscopy, microscopy and filming to analyse reactions as they occur in real-time at the microscale [
8].
Microfluidics have been investigated for the synthesis of inorganic nanoparticles for over a decade [
9], but this research has concentrated on the synthesis of quantum dots and noble metal (Ag, Au) nanoparticles [
7,
8,
9,
10,
11]. To date there have only been two studies that use a fluidics system to fabricate iron-oxide MNP [
12,
13], which may be surprising considering the obvious benefits controlling the reaction environment has on fastidious iron-oxide synthesis. Abou-Hassen et al. reported some preliminary results for a co-precipitation of magnetite [
12]. They reported issues with clogging so utilised a millimetric system, but could not obtain magnetite even when coated with a surfactant to prevent oxidation, instead producing 7 nm sized maghemite coated nanoparticles. In the same year Frenz et al. reported a more sophisticated microfluidic synthesis of iron oxide [
13]. Again, the aim was to produce magnetite but they noted it readily oxides to maghemite on contact with air. They utilised aqueous microdroplets of reagents in an organic solvent, where an electric voltage initiates reagent mixing of the droplets to nucleate the formation of 4 nm sized nanoparticles.
While this method is elegant, yields and overall outputs were found to be low. In general, while the small volumes of microfluidic synthesis offer excellent control and reproducibility over batch synthesis, the drawbacks are that these smaller scale syntheses offer smaller quantities of product and issues with clogging of the tubing requires elaborate solutions leading to more complex fabrication of the devices.
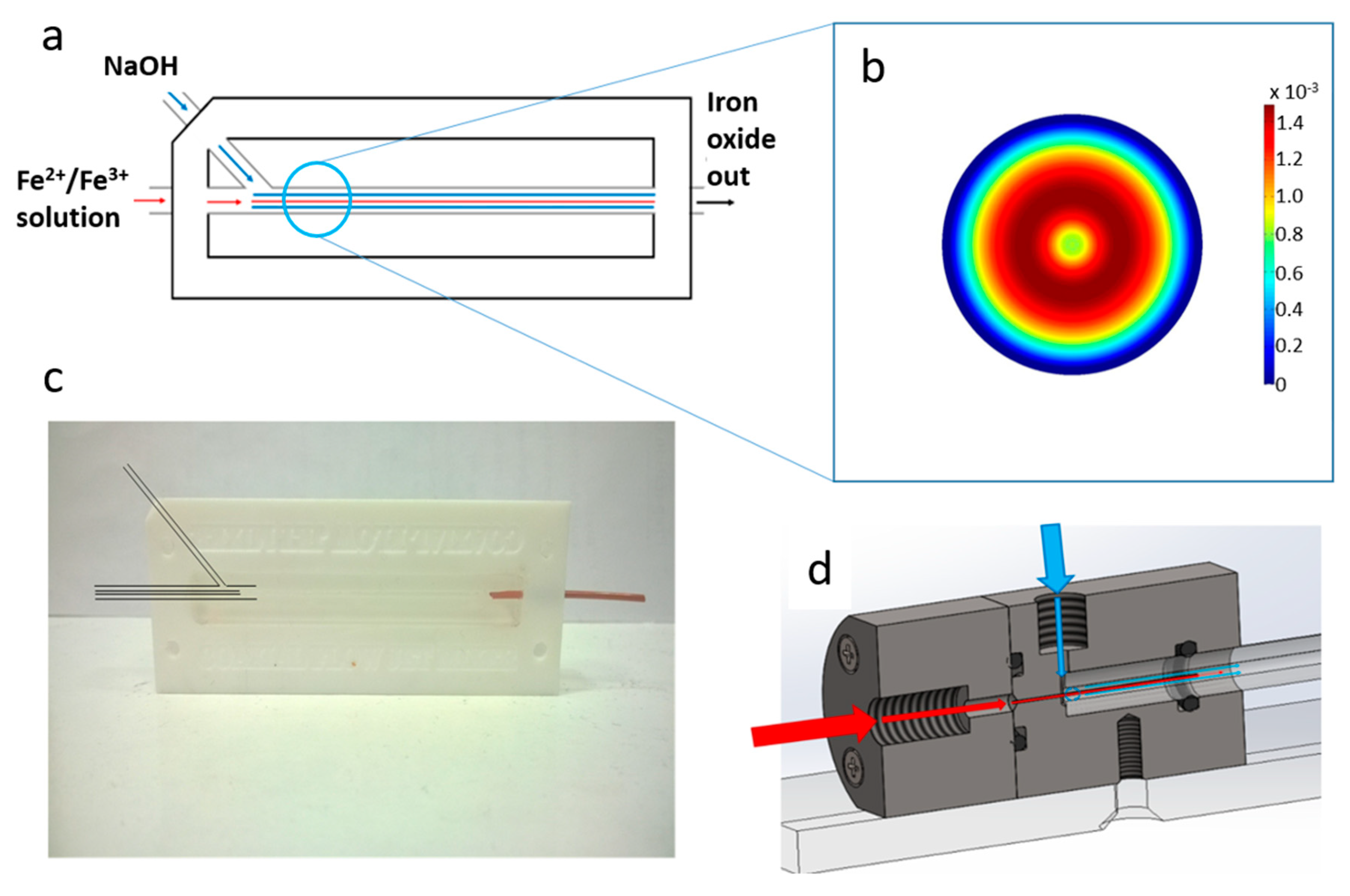
In this work we present a simple (easily castable from PDMS) millimetric macro fluidics device for the synthesis of magnetite MNP’s, and improve on this with a more sophisticated fluidic device machined out of polyether ethyl ketone (PEEK) utilising a glass capillary as the reaction vessel, but of the same simple design (
Figure 1a). With the aim of attaining simple green reaction conditions we do not use droplets, organic solvents or surfactants. We explore tuning the particle formation by varying the ratio of ferrous ions to ferric ions in the fluidic synthesis. This demonstrates an excellent simple platform technology for the synthesis of a vast range of nanoparticles with the ability to tune their properties with further development.
Control over the formation of magnetite MNPs have also been achieved in the natural world through biomineralisation using biological additives. In nature many inorganic minerals are formed with exceptional precision over the mineral composition, size and morphology, utilising a range of biomineralisation proteins. One such example is the biomineralisation of magnetite MNP by Magnetotactic bacteria within liposomes in their cells known as magnetosomes [
14,
15]. Magnetosome membrane specific (Mms) biomineralisation proteins embedded in this liposome are responsible for nucleation of the iron oxide mineral and crystallisation to a precise size and shape [
14,
15]. Previously, Mms6 has been shown to control the formation of magnetite MNP in vitro when added to green co-precipitation chemical synthesis, showing a promising new methodology of controlling nanoparticle synthesis with biological additives [
16,
17,
18]. It is thought that Mms6 is a nucleating protein for magnetite MNPs [
19,
20].
In this work we also demonstrate how the fluidics device we produced can be used in conjunction with a biomineralisation protein additive to control the particle formation further. The addition of an Mms protein is a proof-of-concept to demonstrate how such a simple fluidics system could be utilised as a platform to help to understand how proteins (or indeed any other nanomaterial additive) function in vitro in a controlled fluidic environment.
2. Materials and Methods
2.1. Reagents
Iron (II) sulphate, iron (III) sulphate and sodium hydroxide were purchased from Sigma-Aldrich (Gillingham, United Kingdom) and used without further purification. Iron contents were confirmed via ICP-MS prior to experimentation. PDMS was prepared from Dow Corning (Midland, MI, United States) kit sylgard 184 in the standard ratio 1:10 curing agent:polymer.
2.2. Macrofluidics Device Fabrication
2.2.1. PDMS Device
The device was constructed from a PFTE hollowed out block with a long channel milled from the centre. Initially a stiff metal wire with a diameter of 1.6 mm was pushed through the two holes along the axis of the device. A second input hole, intersected the main channel at an angle of 45° as shown in
Figure 1a,c. The blunt end of a needle was shaped using a drill bit to meet and fit the shape of the central wire. Triton X-100 was wiped over the wires/needle so they could be easily removed from the device after casting. The space was then filled with liquid PDMS and cured at 60 °C for 24 h. When cured, the central wire and needle was carefully removed, leaving behind a central channel and side inlet in PDMS. The materials and equipment required to create this device are cheap and readily available, and the PTFE block is reusable for many castings. The block was fastened to a heavy object to prevent lateral movement when connected to stiff fluidic tubes. The fluid from the central channel was fed into a PEEK tube with an inner diameter of 0.02”. The internal hole was expanded using a shaped drill bit to ensure a smooth transition in diameter.
2.2.2. PEEK Device
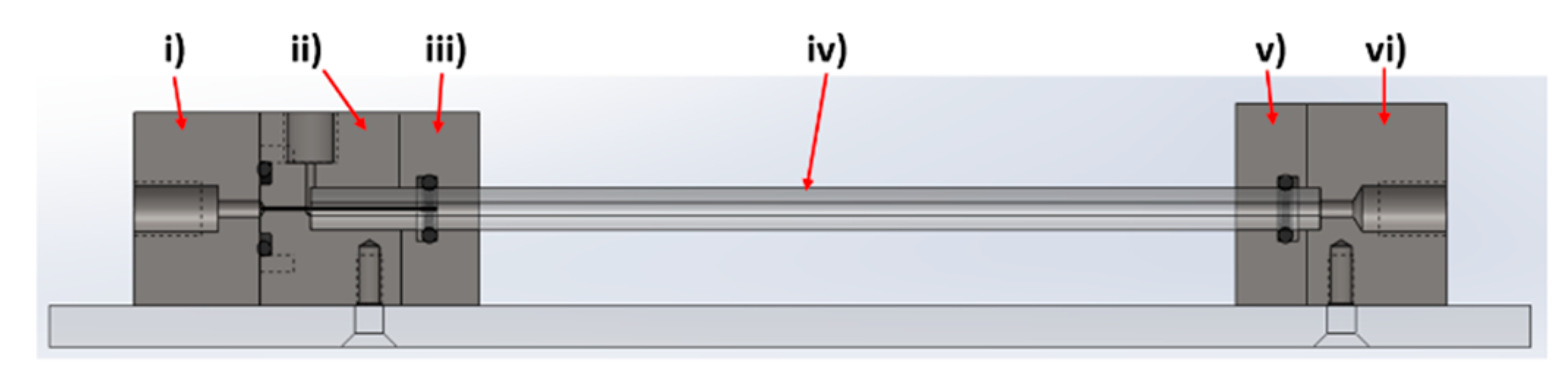
The device was designed and modelled in SolidWorks, comprising of 6 individual components; (i) Fe inlet, (ii) NaOH inlet, (iii) inlet faceplate, (iv) capillary, (v) outlet faceplate, (vi) outlet (
Figure 2).
The rig was machined from PEEK, with o-ring seals between components i–ii, ii–iii, and v–vi to ensure no leakage of solution, a problem often observed in PDMS cast systems. A 27-gauge blunt-end needle (0.41 mm OD, 0.016 ID) was set through component i) with the use of epoxy resin. This needle was sprayed with Teflon spray to aid flow. The capillaries used were 0.5 m in length, with a 5 mm outer diameter and 1.5 mm internal diameter. The capillary tube was inserted between components ii/iii (inlet) and components v/vi (outlet). The system was locked in position with clamp stands to prevent movement when connected to the fluidic tubing.
Fluidic connections for both devices (Upchurch Scientific, purchased from Kinesis (Cambridge, UK) or Fischer Scientific (Loughborough, United Kingdom)) and PEEK tubing with an outer diameter 1/16” and inner diameter of 0.02” were used. Glass capillaries were initially used but were replaced, to reduce problems with clogging, with PEEK capillaries. All tubes were cut with tube cutters (Upchurch Scientific A-327, A-350) to ensure clean and perpendicular cuts. Two syringe drivers (Harvard Apparatus, Cambridge, United Kindrom) were used to control the flow rate of iron and base into the mixer. Glass syringes (SGE Europe Ltd., Milton Keynes, United Kingdom) with volumes of 1 mL and 10 mL were used for the inner and outer flows respectively. Luer fittings were used to connect PEEK tubing to syringes.
2.3. Protein Expression and Purification
The mms6 sequence from Magnetospirillum magneticum AMB-1 was introduced into a pTTQ8 based expression vector by cohesive end cloning with the resulting plasmid, pHis8mms6, encoding N-terminally octahistidine tagged Mms6. The protein was produced in E. coli BL21 star (DE3) cells (Invitrogen, Waltham, MA, United States) harbouring a pRARE (Merck, Nottingham, United Kingdom) plasmid to compensate for codon bias in the mms6 sequence. Cells were cultured in autoinducing Superbroth (Formedium, Hunstanton, United Kingdom) at 37 °C with shaking for 24 h in the presence of carbenicillin and chloramphenicol to select for the pHis8mms6 and pRARE plasmids respectively. Cells were lysed by sonication in 25 mM Tris pH 7.4, 100 mM NaCl. The insoluble material, containing the His8-Mms6 inclusion bodies, was collected by centrifugation at 16,000× g and resuspended in 6 M Guanidine Hydrochloride, 25 mM Tris pH 7.4 to solubilise the proteins. Further centrifugation at 16,000× g was performed to remove any material not solubilised by the Guanidine treatment. The supernatant was mixed with nickel charged nitrilotriacetic acid (NTA) resin (Amintra resin, Expedeon, Cambridge, United Kingdom) to allow binding of the histidine tagged Mms6. The resin was subsequently packed into a gravity flow column and washed extensively with Wash Buffer (6 M Guanidine hydrochloride, 25 mM Tris pH 7.4, 10 mM Imidazole) before elution of the bound protein in 300 mM Imidazole supplemented Wash Buffer. The eluted protein was refolded by rapidly diluting into a large volume of Refolding Buffer (500 mM NaCl, 25 mM tris pH 7.4) before being concentrated using a 10 kDa molecular weight cut off centrifugal concentrator (Sartorius, Binbrook, United Kingdom). The concentrated material was subjected to centrifugation to remove any small amounts of precipitated protein before dialysis against 500 mM NaCl using a 3.5 kDa molecular weight cut off slide-a-lyser (Thermo Scientific, Waltham, MA, United States). The refolded His8-Mms6 was quantified by absorbance at 280 nm and stored at 193 K.
2.4. Continous Flow MNP Synthesis
The device was cleaned with ultrapure water, then dilute hydrochloric acid (1 M) followed by ultrapure water again by pumping 10 mL through both ports.
All reagents were prepared with ultrapure water, and deoxygenated by sparging with N2 for 30 min. The outer flow syringe driver was loaded with a 10 mL luer lock syringe of NaOH (1 M) and connected to the co-axial fluidic device via capillary tubing. This was set at a continuous rate of 360 µL/min. The inner flow syringe driver was loaded with a 1 mL luer lock syringe of a mixed ratio of Fe2+ and Fe3+ salts (ferrous sulphate pentahydrate and ferric sulphate heptahydrate) varied from a 4:1 (0.2 ferric) to 1:2 (0.7 ferric) Fe2+:Fe3+ ratio with a total iron concentration of (0.05 M) and connected to the co-axial fluidic device via capillary tubing. This was set at a continuous rate of 90 µL/min (although these rates were varied (see results) these were found to be optimum). The solutions were prepared immediately prior to the experiment.
The iron oxide material formed and flowed to the end of the device where it reached the exit port and dripped into a round bottom flask which was kept under an atmosphere of nitrogen. The iron oxide product was magnetically separated and washed 3× in deoxygenated ultrapure water and subsequently dried in a vacuum oven overnight.
Further improvements to the PDMS device included magnetically collecting the iron oxide as it exited the device using a magnetic trap. This was also washed 3× with deoxygenated ultrapure water and dried under vacuum.
2.5. Continous Flow MNP Synthesis Modified with Mms6
The iron oxide synthesis was further modified with the addition of Mms proteins, where 50 µg of protein was added to the 1 mL Fe salt solution before the reaction. The experiment then proceeded as before.
2.6. Characterisation
2.6.1. Magnetic Susceptibility
Magnetic susceptibility was a measured on a known amount of dry iron oxide nanoparticles using a bench-top Bartington MS2G magnetic susceptometer (Bartington Instruments, Witney, United Kingdom)) at room temperature. The sample was loaded into the instrument in an eppendorf (a blank was subtracted of an empty eppendorf tube). A reading in emu was recorded. Each sample was analysed in triplicate.
2.6.2. Magnetometry
Magnetic susceptibility was performed on a known quantity (1–5 mg) of dry iron oxide nanoparticles on a MPMS 3 SQUID magnetometer (Quantum Design, Surrey, United Kingdom) in vibrating sample mode, with the samples packed in size 3 gelatine capsules. The samples were run at 300 K between −7 and 7 T with a sweep rate of 0.01 T/s. Preliminary magnetic susceptibility measurements were performed on a known quantity (approx. 5 mg) of dry iron oxide nanoparticles using a bench-top Bartington MS2G magnetic susceptometer at room temperature. The sample was loaded into the instrument in an eppendorf (a blank was subtracted of an empty eppendorf tube). A reading in emu was recorded. Each sample was analysed in triplicate.
2.6.3. Transmission Electron Microscopy
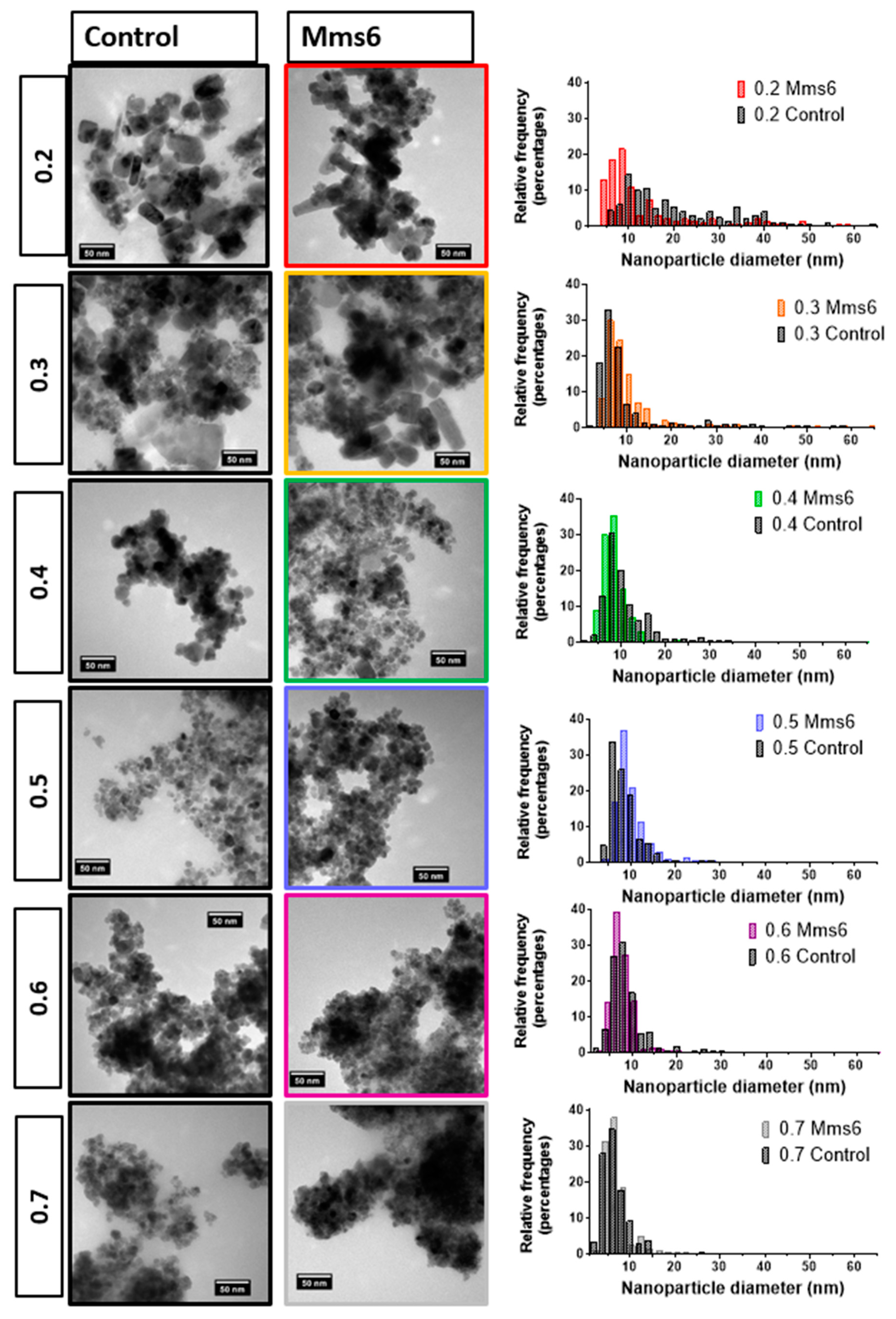
10 µL of a 1 mg/mL suspension of nanoparticles in hexane was dropped onto a carbon coating copper TEM grid and allowed to dry down. Grids were imaged using a FEI Tecnai G2 Spirit electron microscope (Thermo Scientific, Waltham, MA, United States) and the TEM images were analysed using Image-J software (v1.52 a, public domain, National Institute of Health, Md, USA). >200 particles per samples were randomly selected and measured.
2.6.4. X-Ray Diffraction (XRD)
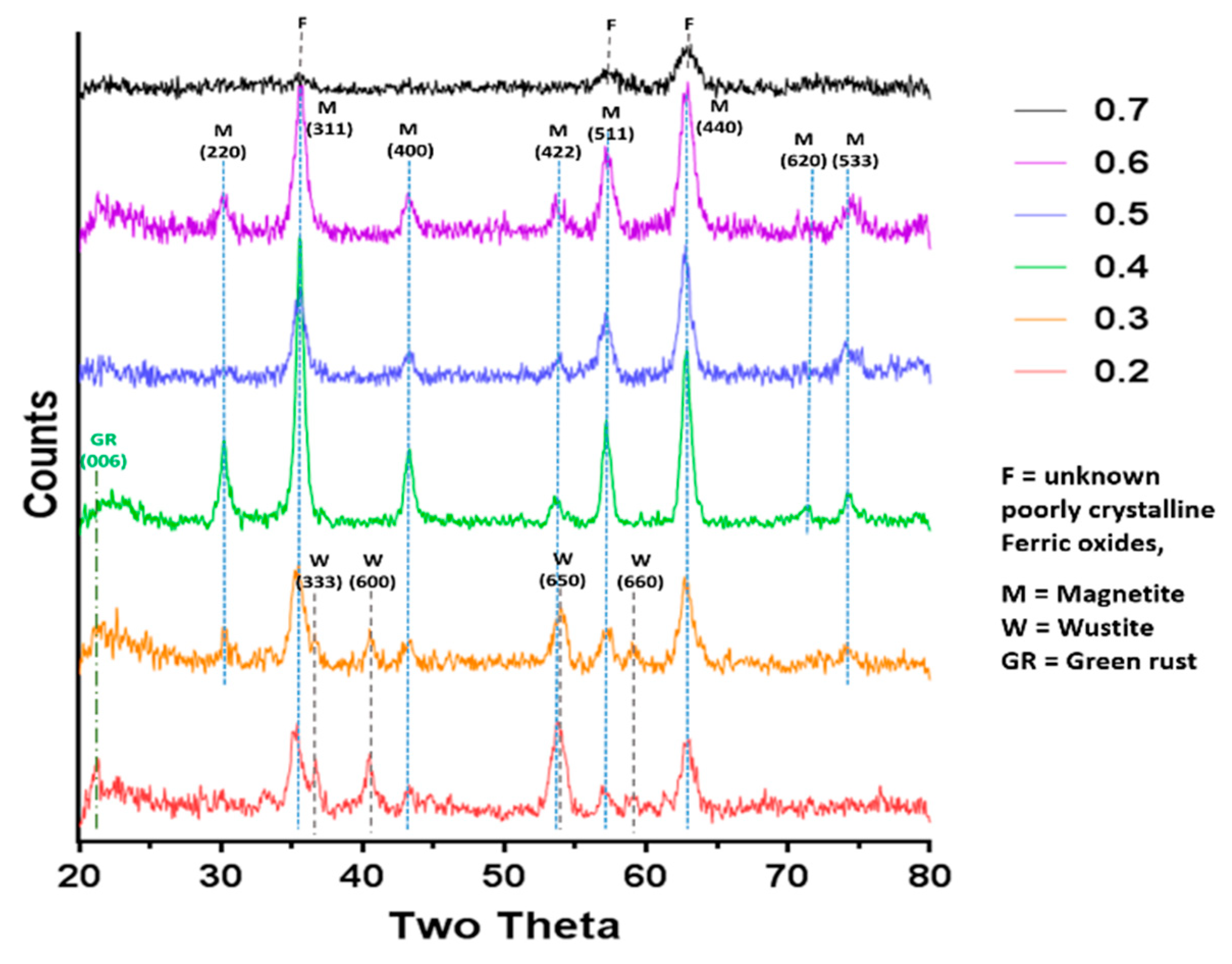
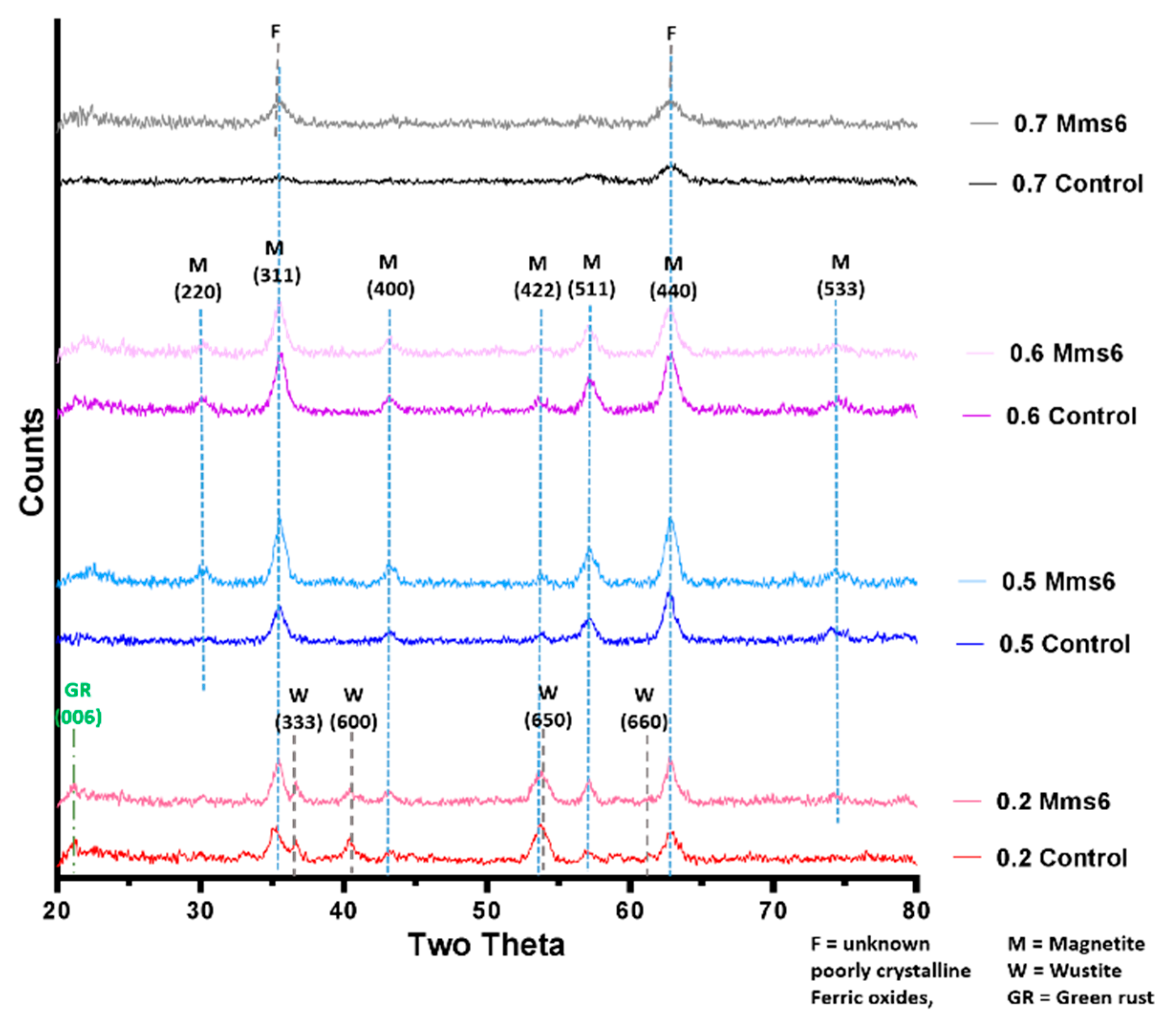
XRD data collected by analysis of dry iron oxide nanoparticles in a Bruker D8 powder diffractometer (Bruker, Coventry, United Kingdom). Diffraction images were collected at 0.022 degree increments from 20–80 degrees, with a fixed wavelength at λ = 1.54178 Å from a Cu Kα X-ray source.
4. Discussion
A key issue that occurs in many microfluidic systems is clogging and fouling, and in the case of PDMS systems, leakage. The problem of clogging is exacerbated by the magnetic nature of the formed iron oxide products, resulting in magnetic aggregation which leads to clogging and obstruction of laminar flow. These issues were addressed in PDMS systems by increasing the diameter of tubing, ensuring the fluidic channel is as straight as possible, and by shaping the needle used to cast the device for a smoother co-axial junction. However, this system was still prone to leaking so the design of a second PEEK system addressed this issue by utilising a capillary as the reaction vessel joined to the inlets by specifically machined connectors, sealed with o-rings. The transparent nature of the material of the PEEK system also allows the reaction lifetime to be potentially monitored with optical microscopic or spectroscopic techniques with greater ease. Both devices showed consistent results from run to run within the same device and also across the two devices (once the flow rates were optimised) (see
Supplementary Table S1), showing the formation of magnetite in this simple fluidic system has good reaction control.
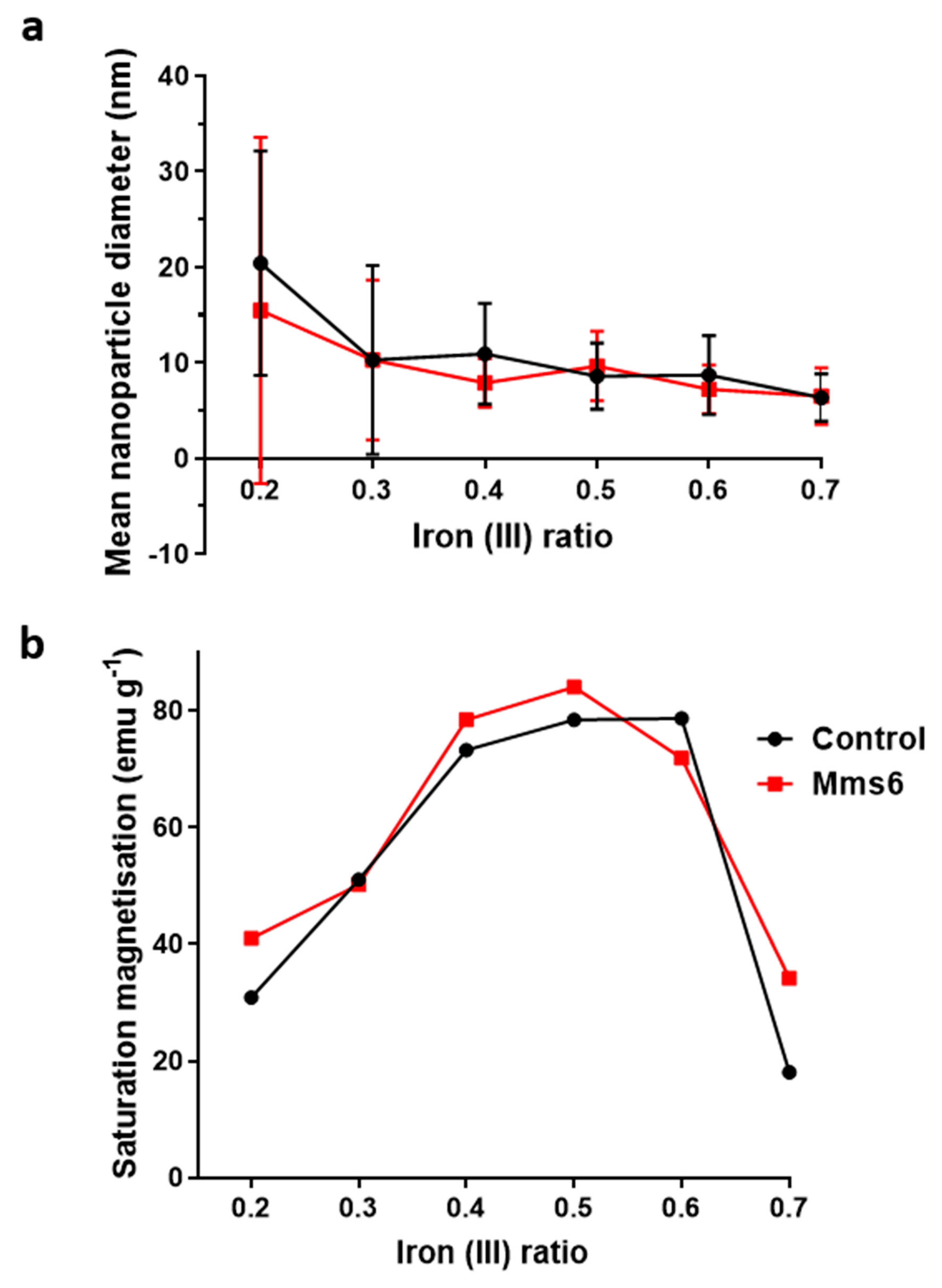
Magnetite is stoichiometrically formed of 2 Fe
3+:1 Fe
2+ giving an X ratio of 0.667. Oxidation is known to occur when performing this reaction so initial precursor ratios of 0.5 and 0.6 are commonly used. It is clear from this study that X = 0.4–0.6 give a good yield of magnetite, demonstrated by the magnetism, TEM and XRD, with lower X-values contaminated with ferrous iron oxides such as wüstite and green rust (0.2 and 0.3). It is also worth acknowledging that XRD only detects crystalline materials so amorphous phases could also be present. There has been detailed theoretic and experimental research into the iron oxides that form at different ferrous:ferric precursor ratios [
19,
22]. The results in this paper tally well with previous findings where mixtures of ferrous hydroxides, ferrous oxides, green rust and a small amount of magnetite can be formed at lower ratios while mixtures of magnetite and ferric-oxides such as schwertmannite and goethite are seen in higher ratios (above the stoichiometric ratio). It is excellent to see the fluidic system reproduces the results from previous batch studies and tally well with theoretical work. However, what has not been seen before is the ability to tune the size with varying X. It is not clear from searching the literature if this has not been explored before in all synthetic forms of precipitating magnetite, batch, fluidic or otherwise, or whether this effect is something only seen in a flow synthesis. In the flow the iron solution is been fed the base at the interface at a continuous rate at the interface were the crystals will be seeded. Perhaps at ratios closer to stoichiometric, the nucleation is so rapid that the particles do not have the capacity to grow quite as much before all the iron solution is used up, forming smaller particles. However, in the lower ratios, fewer particles can be nucleated giving more iron feedstock to grow the smaller number of particles more.
We developed the experiment further by adding the biomineralisation protein Mms6 to the flow synthesis. In agreement with our previous study this aided the formation of magnetite at ratios where the control showed less magnetite [
19]. This is thought to be possible as the acidic rich DEEVE amino acid motif on the C-terminus of the protein is exposed and is capable of binding iron ions [
19,
20,
24,
25]. The N-terminus is hydrophobic and as such causes the protein to self-assemble into rafts within the native environment or micelles in aqueous solution, displaying the acidic residues as a negative charged nucleation surface for iron binding. Importantly it has been show to bind ferrous ions in some sites and ferric ions less specifically but in abundance, encouraging the nucleation of magnetite specifically [
20]. Previous work has also shown that Mms6 controls the size of the particles to be 20 nm when formed in solution. However, we did not see this control in the flow synthesis. We hypothesis that the synthesis in flow increases the effect of the chemical kinetics of particle formation over slower additive modified crystal growth giving little room for Mms6 to be effective, especially with respect to particle size control. However interestingly, it has been previously reported that the curvature of the charged surface may be key to size controlling aspect of Mms6. When Mms6 is displayed on a flat surface the particles formed are larger (90 nm) compared to 20 nm in solution, so it could also be the case that Mms6 in the flow system cannot form the correct size-controlling surface [
26].
Finally, this device, experiments and results demonstrates how this system could be a beneficial platform for the study of nanoparticle formation more generally. The reaction vessel is long so time points can be equated to distance. This can be useful in both experiment design (introducing additive such as Mms6 at different reaction points) and analysis (studying the reaction intermediates in situ). Similarly, this could be universally extended to study the action and effect of a full range of different additive and at different stages in a wet chemistry synthesis of any nanoparticles.