Reinforcing Mechanism of Reduced Graphene Oxide on Flexural Strength of Geopolymers: A Synergetic Analysis of Hydration and Chemical Composition
Abstract
1. Introduction
2. Materials and Methods
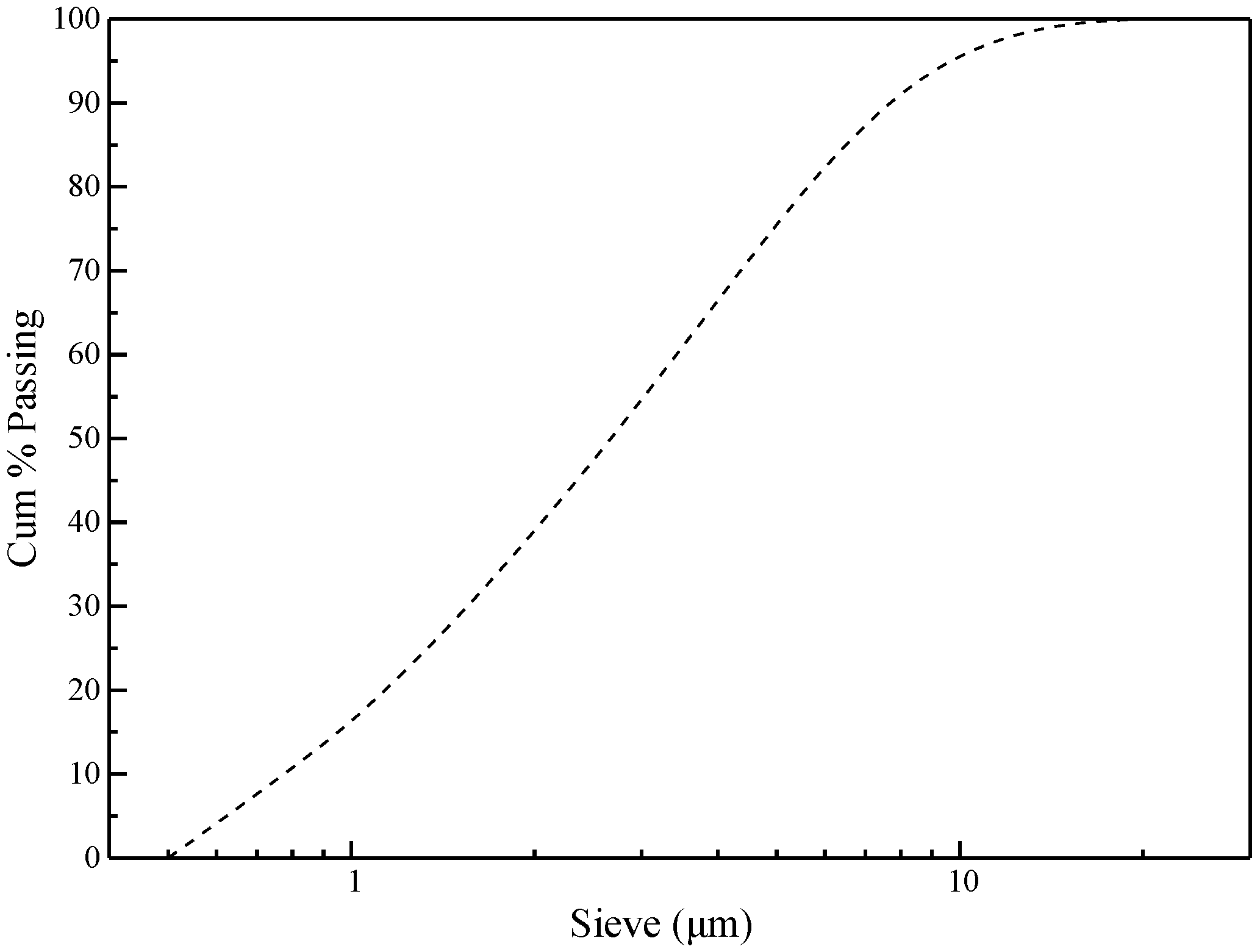
2.1. Raw Materials
2.2. Test Methods
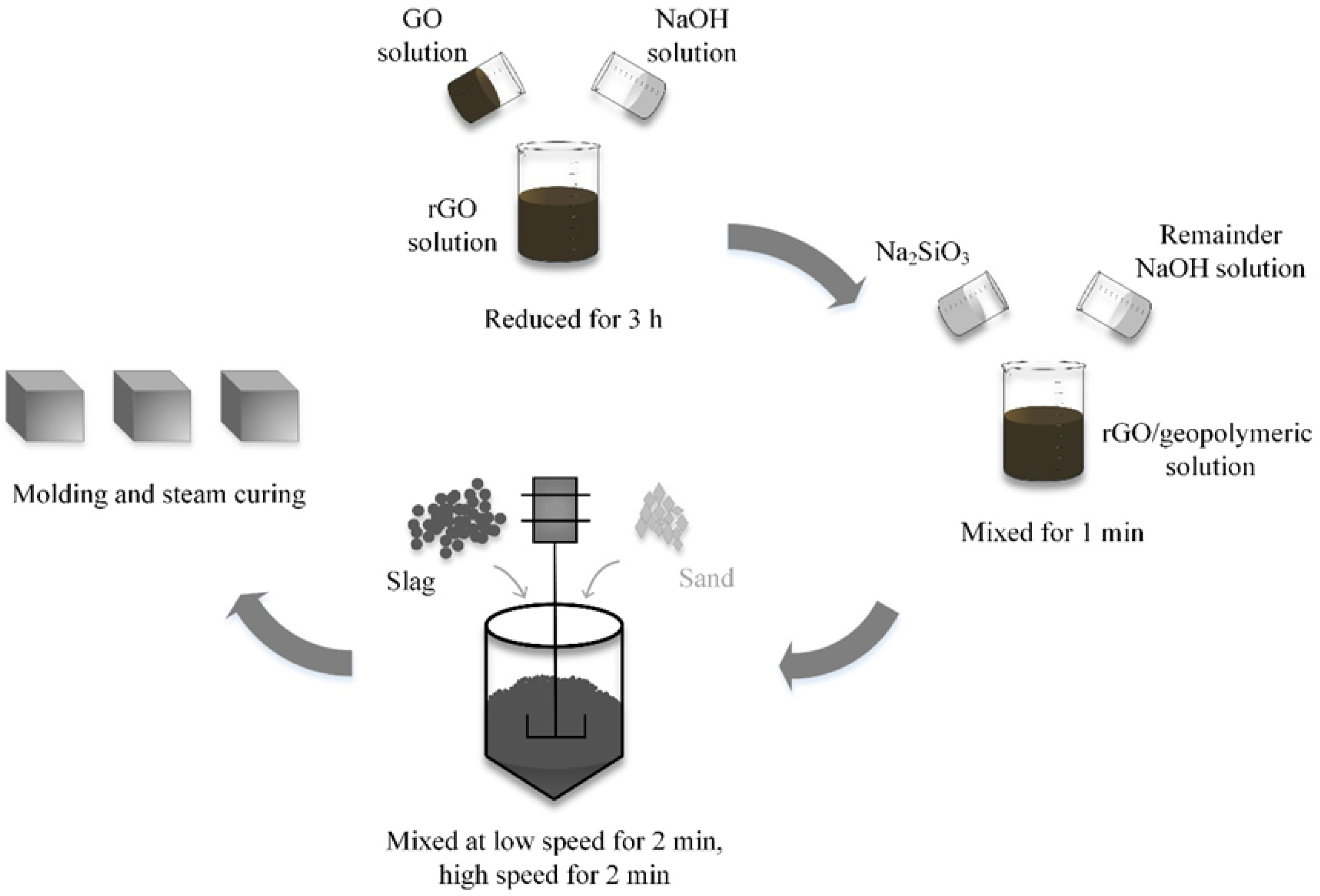
2.2.1. Preparation of GO Solution
2.2.2. Sample Preparation
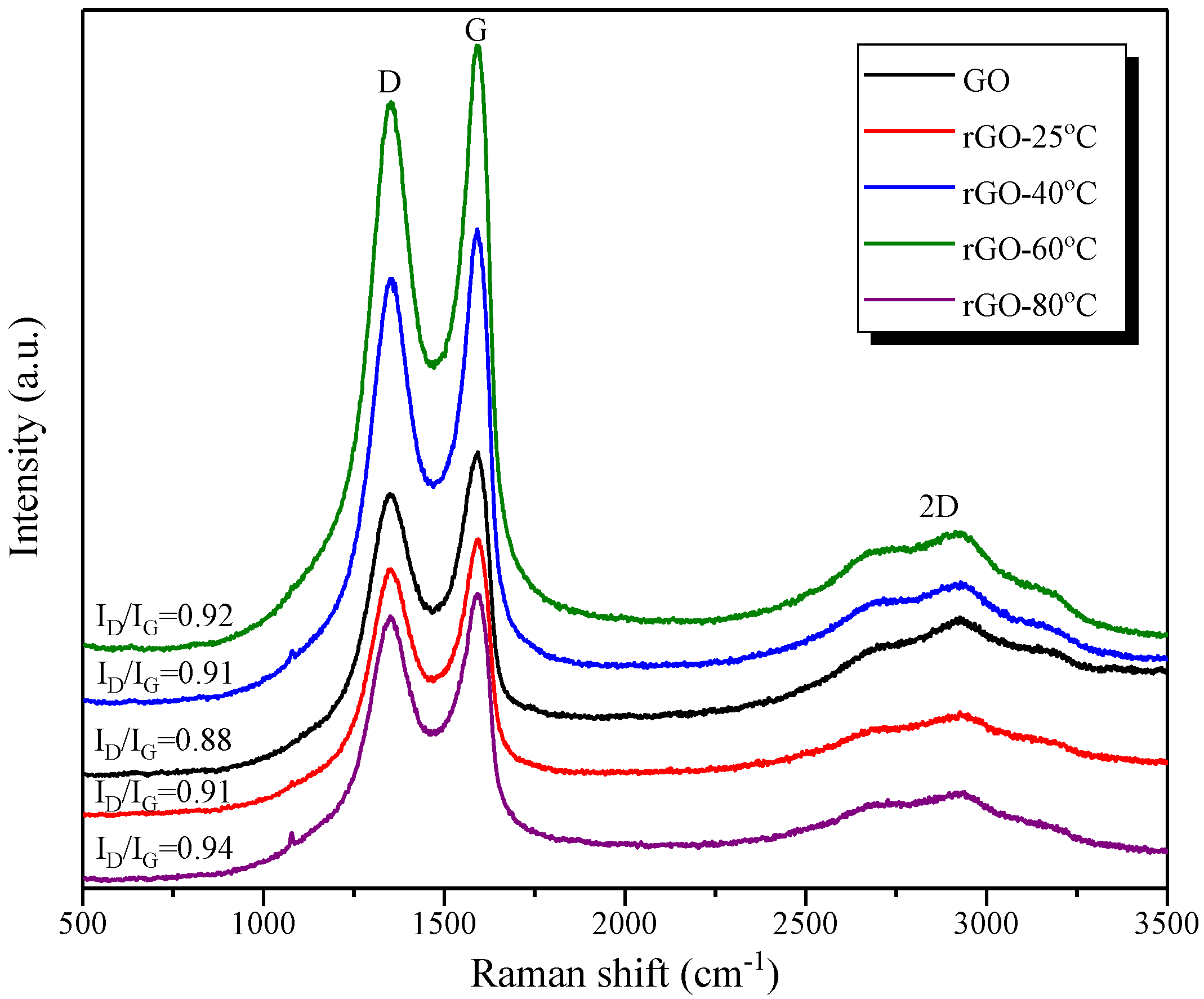
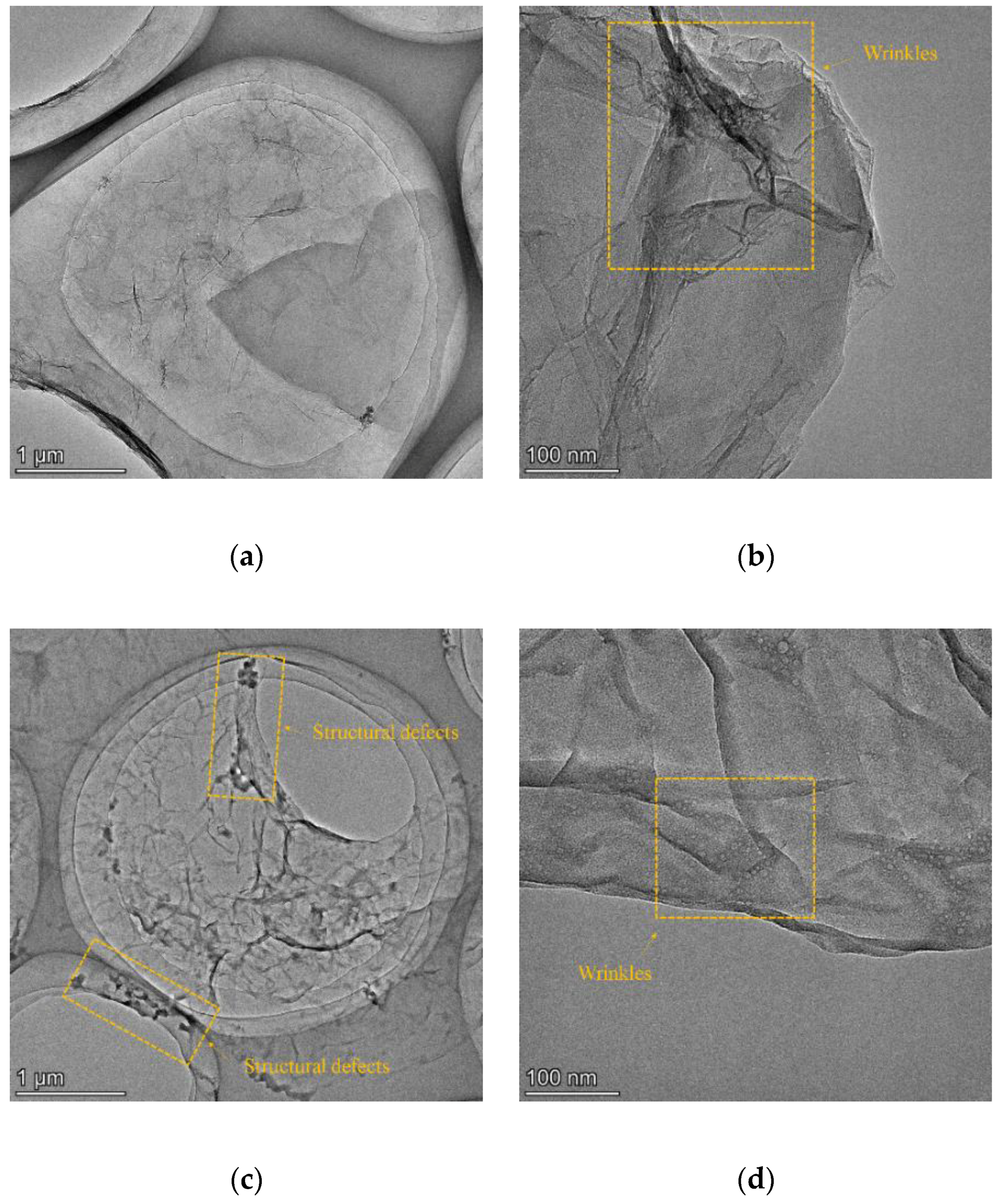
2.2.3. Characterization of rGO Nanosheets
2.2.4. Heat of Hydration
2.2.5. X-Ray Diffraction (XRD) Test
2.2.6. Nuclear Magnetic Resonance (NMR) Test
2.2.7. Flexural Strength Test
3. Results and Discussion
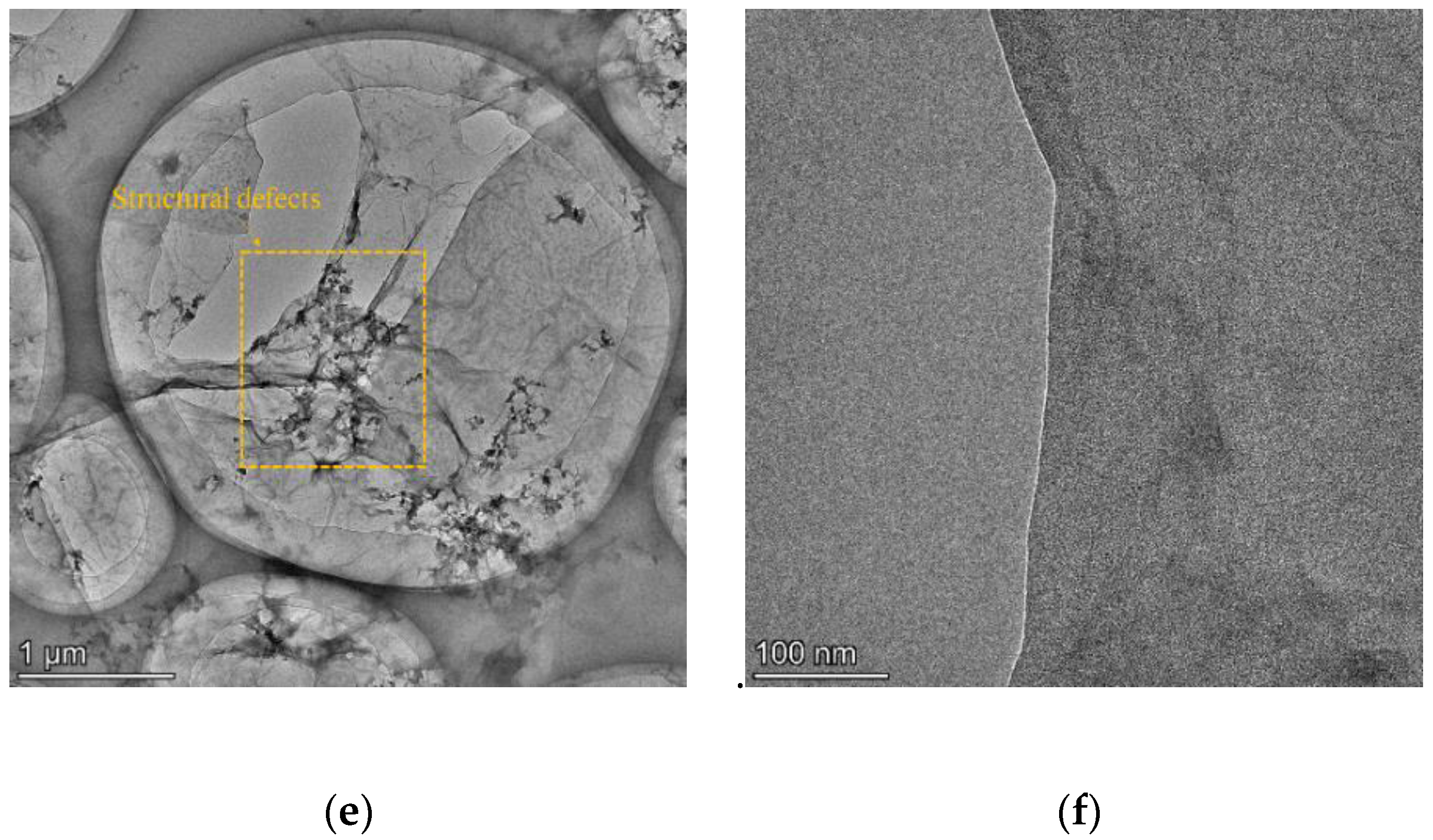
3.1. Characterization of rGO Nanosheets
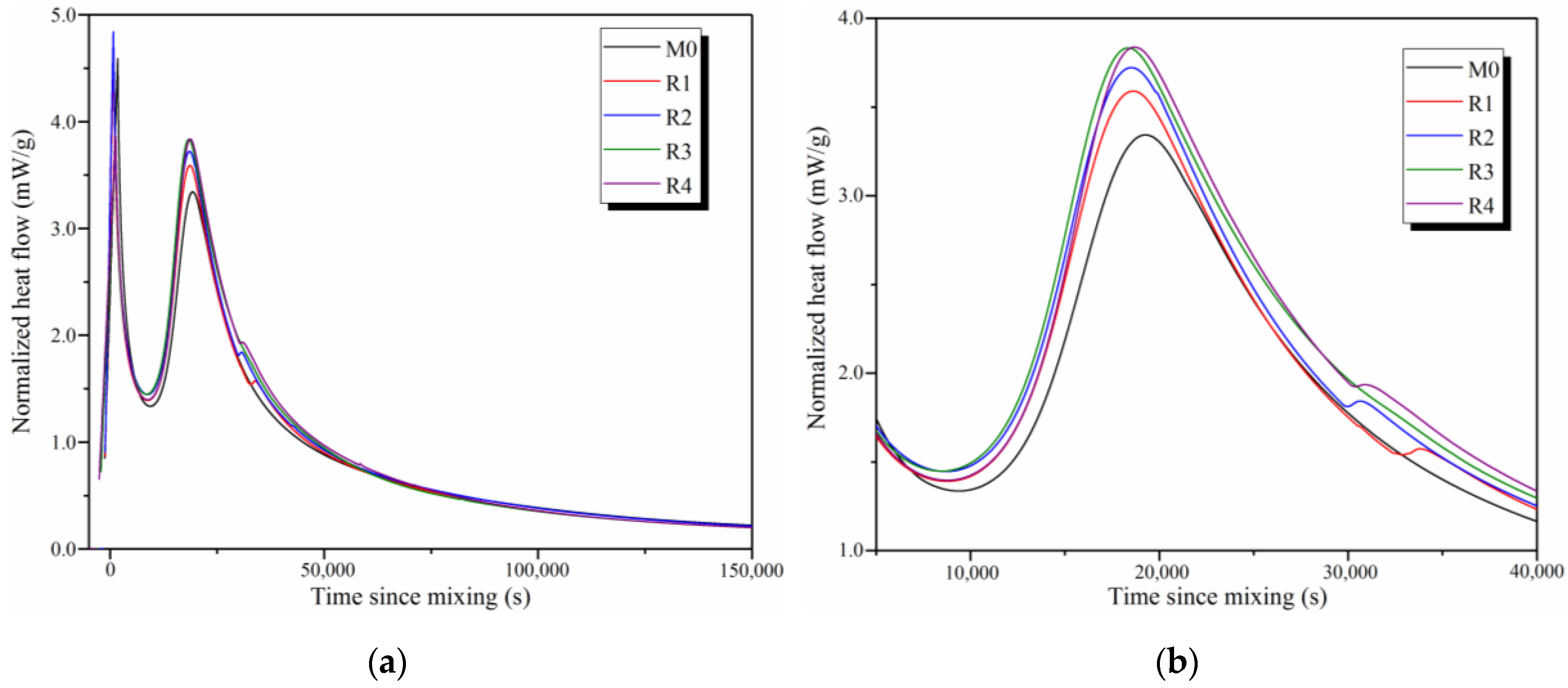
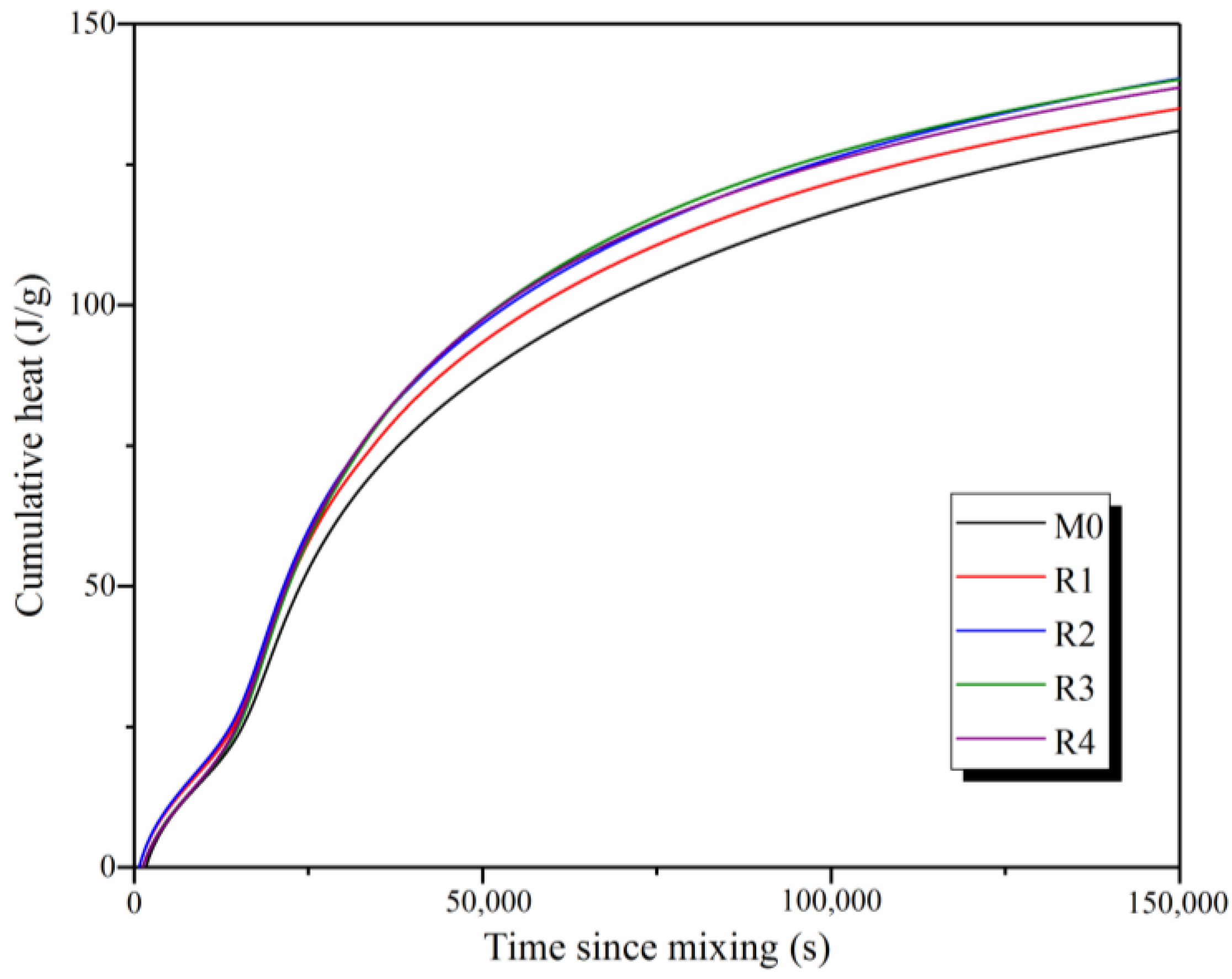
3.2. Hydration Process
3.3. Chemical Compositions
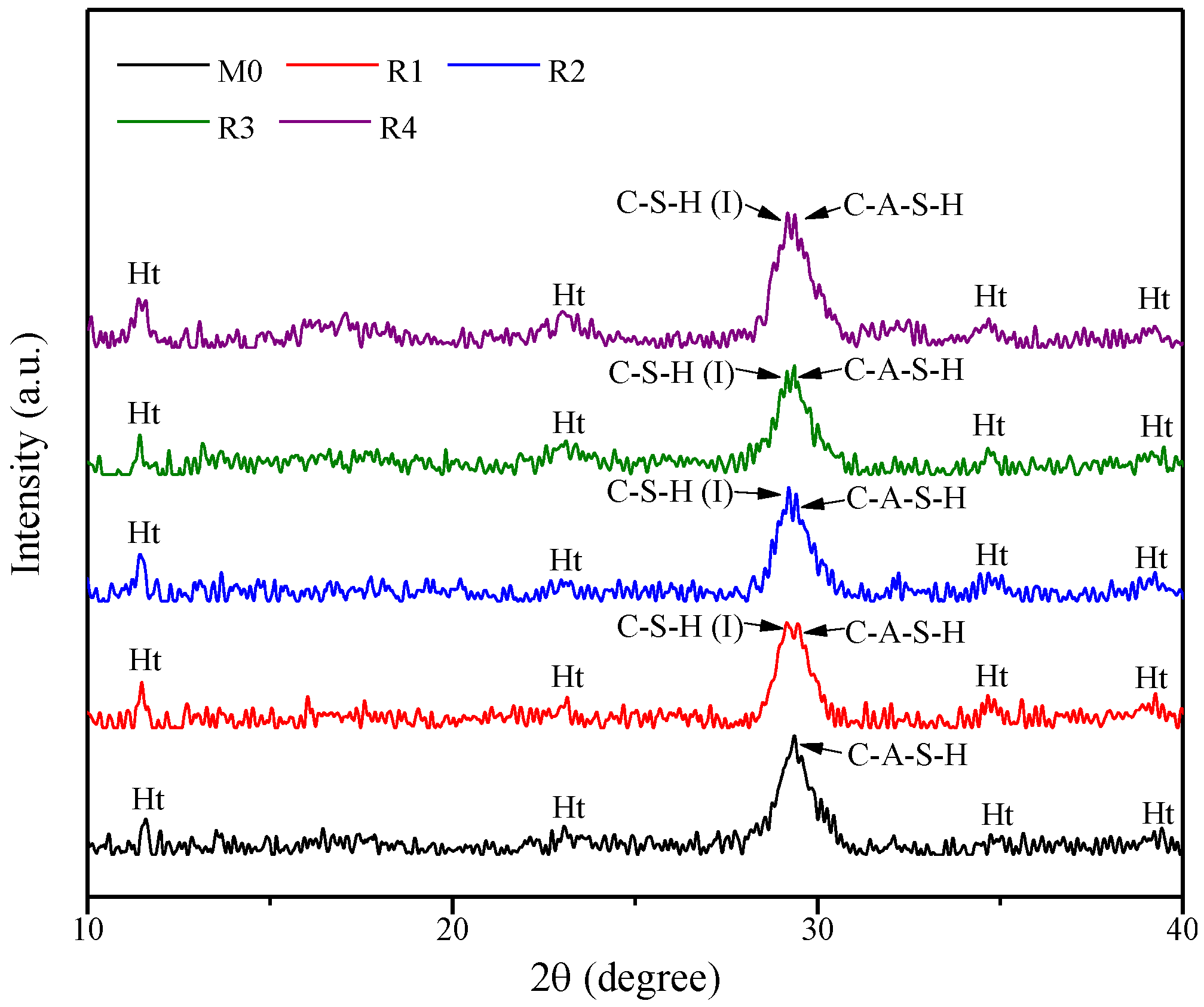
3.3.1. XRD Analysis
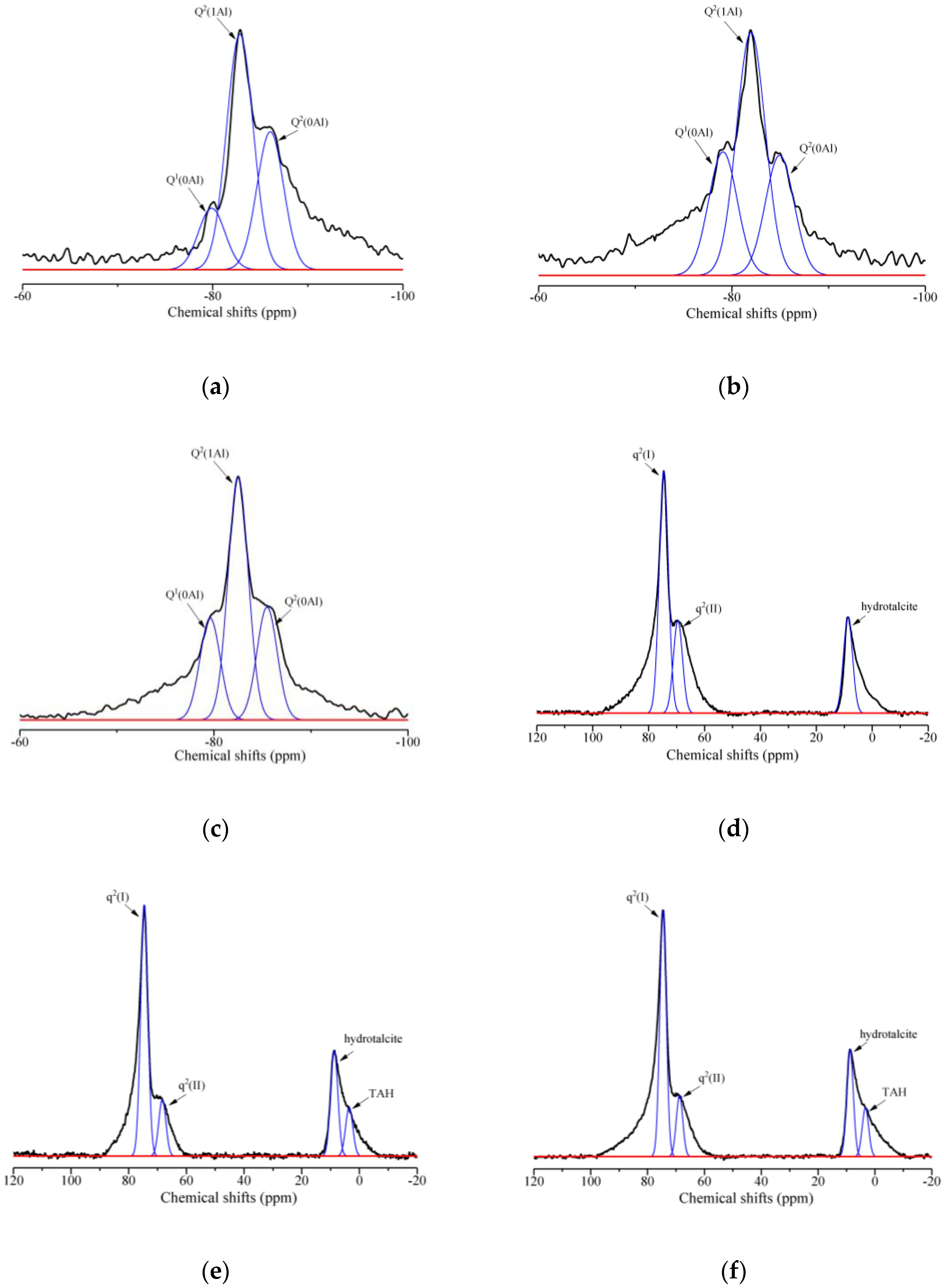
3.3.2. NMR analysis
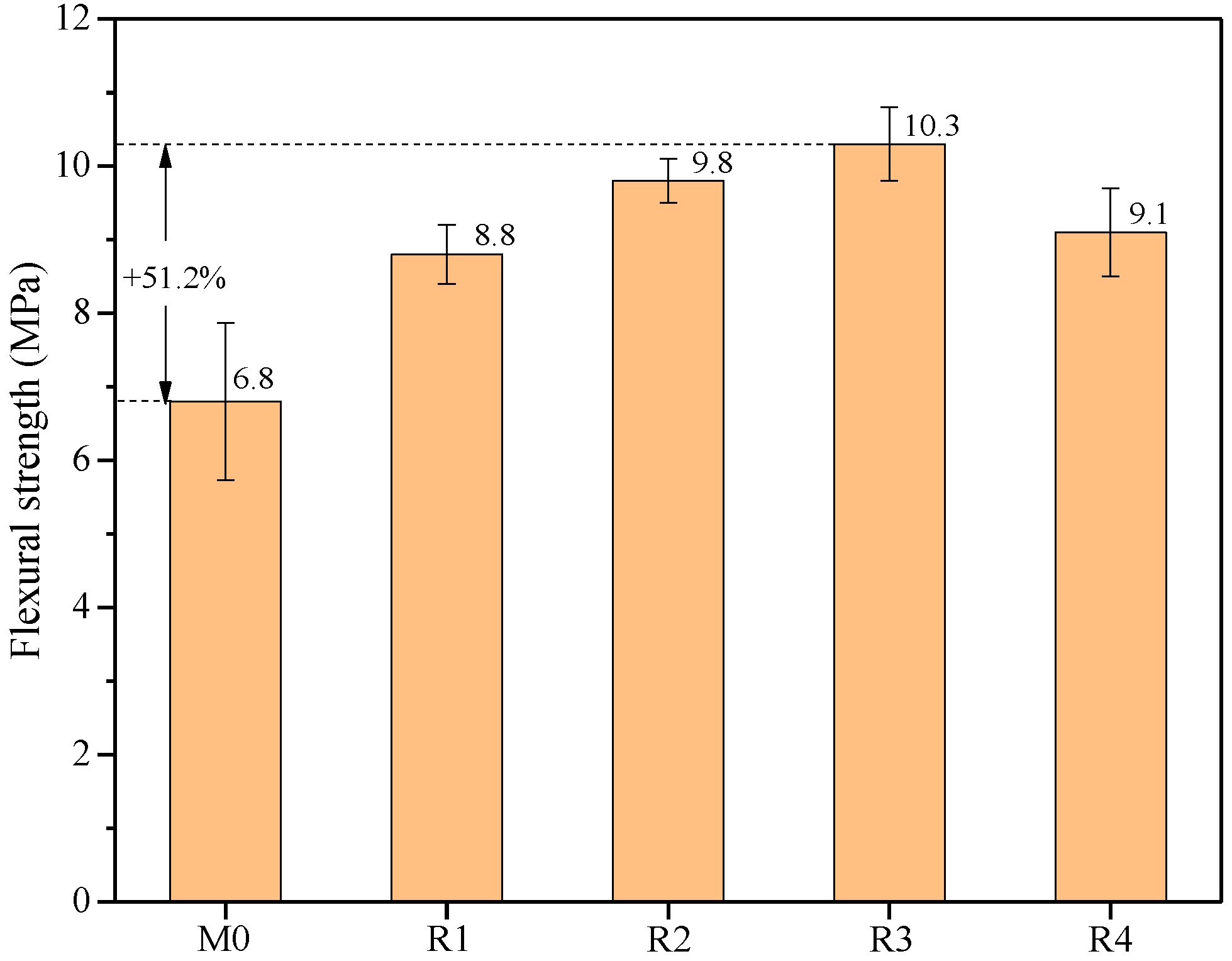
3.4. Flexural Strength
4. Conclusions
- With the addition of rGO nanosheets, the hydration degree of the samples significantly increases, since rGO nanosheets increase the relative concentration of OH− ions and provide many nucleation sites for the formation of C-S-H and C-A-S-H gels. When the reduction temperature is 60 °C, the hydration degree of rGO-reinforced geopolymers is the highest with a cumulative heat of approximately 140 J/g at 150,000 s.
- With the addition of rGO nanosheets, the samples prefer to generate C-S-H(I) and C-A-S-H phases simultaneously due to the repellency of rGO nanosheets with negative changes toward Al species. In addition, the reduction degree has no obvious effect on the chemical composition of rGO-reinforced geopolymers.
- With the addition of rGO nanosheets, the integrated area percentage of Q1(0Al) increases from 4.44% to 11.14%, whereas that of Q2(0Al) has no obvious change, indicating that rGO nanosheets largely restrain the substitution of Al on the end-of-chain silicates of C-(A)-S-H gels rather than the middle-of-chain silicates.
- With the addition of rGO nanosheets, the flexural strength of the samples remarkably increases. The flexural strength of R3 is 51.2% higher than that of M0, owing to the promotion of slag hydration and the simultaneous formation of C-S-H(I) and C-A-S-H gels. In addition, the flexural strength of R4 is lower than that of R3 due to the significant structural defects in rGO nanosheets under alkali reduction at 80 °C.
Author Contributions
Funding
Conflicts of Interest
References
- Rovnaník, P.; Bayer, P.; Rovnaníková, P. Characterization of alkali activated slag paste after exposure to high temperatures. Constr. Build. Mater. 2013, 47, 1479–1487. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Phoo-Ngernkham, T.; Li, L.Y.; Damrongwiriyanupap, N.; Chindaprasirt, P.J.C.; Materials, B. Strength development and durability of alkali-activated fly ash mortar with calcium carbide residue as additive. Constr. Build. Mater. 2018, 162, 714–723. [Google Scholar] [CrossRef]
- Bouaissi, A.; Li, L.Y.; Al Bakri Abdullah, M.M.; Bui, Q.-B. Mechanical properties and microstructure analysis of FA-GGBS-HMNS based geopolymer concrete. Constr. Build. Mater. 2019, 210, 198–209. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Mehrali, M.; Alengaram, U.J.; Jumaat, M.Z. Graphene nanoplatelet-fly ash based geopolymer composites. Cem. Concr. Res. 2015, 76, 222–231. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Marconcini, P.; Macucci, M. The k.p method and its application to graphene, carbon nanotubes and graphene nanoribbons: The Dirac equation. Riv. Nuovo Cim. 2011, 34, 489–584. [Google Scholar]
- Liming, D. Functionalization of graphene for efficient energy conversion and storage. Acc. Chem. Res. 2012, 46, 31–42. [Google Scholar]
- Changgu, L.; Xiaoding, W.; Kysar, J.W.; James, H. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902. [Google Scholar] [CrossRef]
- Xu, D.; Ivan, S.; Anthony, B.; Andrei, E.Y. Approaching ballistic transport in suspended graphene. Nat. Nanotechnol. 2008, 3, 491–495. [Google Scholar]
- Paszkiewicz, S.; Pawlikowska, D.; Kurcz, M.; Szymczyk, A.; Irska, I.; Stanik, R.; Gude, M.; Linares, A.; Ezquerra, T.A.; Lipińska, L.; et al. Functional Properties of poly(trimethylene terephthalate)-block-poly(caprolactone) based nanocomposites containing graphene oxide (GO) and reduced graphene oxide (rGO). Nanomaterials 2019, 9, 1459. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, S.; Wang, Y.; Sun, X.; Sun, K. Reduced graphene oxide/carbon nanotubes reinforced calcium phosphate cement. Ceram. Int. 2017, 43, 13083–13088. [Google Scholar] [CrossRef]
- Shu, Y.; He, P.; Jia, D.; Yang, Z.; Duan, X.; Wang, S.; Yu, Z. Effect of reduced graphene oxide content on the microstructure and mechanical properties of graphene–geopolymer nanocomposites. Ceram. Int. 2016, 42, 752–758. [Google Scholar]
- Saafi, M.; Tang, L.; Fung, J.; Rahman, M.; Liggat, J. Enhanced properties of graphene/fly ash geopolymeric composite cement. Cem. Concr. Res. 2015, 67, 292–299. [Google Scholar] [CrossRef]
- Shu, Y.; He, P.; Jia, D.; Duan, X.; Yang, Z.; Wang, S.; Yu, Z. In situ processing of graphene/leucite nanocomposite through graphene oxide/geopolymer. J. Am. Ceram. Soc. 2016, 99, 1164–1173. [Google Scholar]
- Liu, M.; Zhao, Y.; Xiao, Y.; Yu, Z. Performance of cement pastes containing sewage sludge ash at elevated temperatures. Constr. Build. Mater. 2019, 211, 785–795. [Google Scholar] [CrossRef]
- Jia, Z.; Chen, C.; Shi, J.; Zhang, Y.; Sun, Z.; Zhang, P. The microstructural change of C-S-H at elevated temperature in Portland cement/GGBFS blended system. Cem. Concr. Res. 2019, 123, 105773. [Google Scholar] [CrossRef]
- Ground Granulated Blast Furnace Slag Used for Cement, Mortar and Concrete; GB/T 18046; Chinese National Standard: Taipei, Taiwan, 2017.
- Long, W.J.; Li, H.D.; Fang, C.L.; Xing, F. Uniformly dispersed and re-agglomerated graphene oxide-based cement pastes: A comparison of rheological properties, mechanical properties and microstructure. Nanomaterials 2018, 8, 31. [Google Scholar] [CrossRef]
- Long, W.J.; Wei, J.J.; Ma, H.; Xing, F.J.N. Dynamic mechanical properties and microstructure of graphene oxide nanosheets reinforced cement composites. Nanomaterials 2017, 7, 407. [Google Scholar] [CrossRef]
- Shi, Z.; Shi, C.; Wan, S.; Li, N.; Zhang, Z. Effect of alkali dosage and silicate modulus on carbonation of alkali-activated slag mortars. Cem. Concr. Res. 2018, 113, 55–64. [Google Scholar] [CrossRef]
- Method of Testing Cements-Determination of Strength; GB/T 17671; Chinese National Standard: Taipei, Taiwan, 1999.
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Long, W.J.; Ye, T.H.; Li, L.X.; Feng, G.L. Electrochemical characterization and inhibiting mechanism on calcium leaching of graphene oxide reinforced cement composites. Nanomaterials 2019, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Long, W.J.; Gu, Y.C.; Xing, F.; Khayat, K.H. Microstructure development and mechanism of hardened cement paste incorporating graphene oxide during carbonation. Cem. Concr. Compos. 2018, 94, 72–84. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, T.; Xu, D.; Zhou, Z.; Du, P.; Xie, N.; Cheng, X.; Liu, Y. Effect of nano-silica on the efflorescence of waste based alkali-activated inorganic binder. Constr. Build. Mater. 2018, 167, 381–390. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. Isothermal calorimetry and in-situ XRD study of the NaOH activated fly ash, metakaolin and slag. Cem. Concr. Res. 2018, 103, 110–122. [Google Scholar] [CrossRef]
- Lasic, D.D.; Pintar, M.M.; Blinc, R. Are proton NMR observations supportive of the osmotic model of cement hydration? Philos. Mag. Lett. 1988, 58, 227–232. [Google Scholar] [CrossRef]
- Zhou, H.; Xuequan, W.U.; Zhongzi, X.U.; Tang, M. Kinetic study on hydration of alkali-activated slag. Cem. Concr. Res. 1993, 23, 1253–1258. [Google Scholar]
- Li, N.; Shi, C.; Zhang, Z. Understanding the roles of activators towards setting and hardening control of alkali-activated slag cement. Compos. Part B Eng. 2019, 171, 34–45. [Google Scholar] [CrossRef]
- Deng, Z.; Zhou, W.; Yue, H.; Guo, X. Study on the hydration mechanism of a hardened slag-based plugging agent activated by alkalis. Constr. Build. Mater. 2019, 203, 343–355. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Jiang, Q.; Cheng, G.; Li, L.; Kang, Y.; Wang, D. A green route to sustainable alkali-activated materials by heat and chemical activation of lithium slag. J. Clean. Prod. 2019, 225, 1184–1193. [Google Scholar] [CrossRef]
- Xu, G.; Du, S.; He, J.; Shi, X. The role of admixed graphene oxide in a cement hydration system. Carbon 2019, 148, 141–150. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.S.; Rico, A.; Rodríguez, J. A model for the C-A-S-H gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, X.; Vollpracht, A. Pervious concrete made of alkali activated slag and geopolymers. Constr. Build. Mater. 2018, 189, 797–803. [Google Scholar] [CrossRef]
- Li, Z.; Nedeljković, M.; Chen, B.; Ye, G. Mitigating the autogenous shrinkage of alkali-activated slag by metakaolin. Cem. Concr. Res. 2019, 122, 30–41. [Google Scholar] [CrossRef]
- Wang, J.; Du, P.; Zhou, Z.; Xu, D.; Xie, N.; Cheng, X. Effect of nano-silica on hydration, microstructure of alkali-activated slag. Constr. Build. Mater. 2019, 220, 110–118. [Google Scholar] [CrossRef]
- Wang, S.D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Zuo, Y.; Nedeljković, M.; Ye, G. Pore solution composition of alkali-activated slag/fly ash pastes. Cem. Concr. Res. 2019, 115, 230–250. [Google Scholar] [CrossRef]
- Walkley, B.; Nicolas, R.S.; Sani, M.A.; Rees, G.J.; Hanna, J.V.; Deventer, J.S.J.V.; Provis, J.L. Phase evolution of C-(N)-A-S-H/N-A-S-H gel blends investigated via alkali-activation of synthetic calcium aluminosilicate precursors. Cem. Concr. Res. 2016, 89, 120–135. [Google Scholar] [CrossRef]
- L’Hôpital, E.; Lothenbach, B.; Saout, G.L.; Kulik, D.; Scrivener, K. Incorporation of aluminium in calcium-silicate-hydrates. Cem. Concr. Res. 2015, 75, 91–103. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Assessing the porosity and shrinkage of alkali activated slag-fly ash composites designed applying a packing model. Constr. Build. Mater. 2016, 119, 175–184. [Google Scholar] [CrossRef]
- Tan, H.; Deng, X.; He, X.; Zhang, J.; Zhang, X.; Su, Y.; Yang, J. Compressive strength and hydration process of wet-grinded granulated blast-furnace slag activated by sodium sulfate and sodium carbonate. Cem. Concr. Compos. 2019, 97, 387–398. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Apply 29Si, 27Al MAS NMR and selective dissolution in identifying the reaction degree of alkali activated slag-fly ash composites. Ceram. Int. 2017, 43, 12408–12419. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D.; Meinhold, R.H.; Sherriff, B.L.; Xu, Z. 27Al and 25Mg solid-state magic-angle spinning nuclear magnetic resonance study of hydrotalcite and its thermal decomposition sequence. J. Mater. Chem. 1993, 3, 1263–1269. [Google Scholar] [CrossRef]
- Myers, R.J.; Bernal, S.A.; Gehman, J.D.; Van Deventer, J.S.J.; Provis, J.L.; Struble, L. The Role of Al in cross-linking of alkali-activated slag cements. J. Am. Ceram. Soc. 2015, 98, 996–1004. [Google Scholar] [CrossRef]
- Richardson, I.G.; Girão, A.V.; Taylor, R.; Jia, S. Hydration of water- and alkali-activated white Portland cement pastes and blends with low-calcium pulverized fuel ash. Cem. Concr. Res. 2016, 83, 1–18. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; Deventer, J.S.J.V. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
| Component (wt %) | Slag | |
|---|---|---|
| Chemical compositions | CaO | 39.08 |
| SiO2 | 35.12 | |
| Al2O3 | 14.20 | |
| Fe2O3 | 0.62 | |
| MgO | 8.47 | |
| TiO2 | 0.71 | |
| MnO | 0.69 | |
| Na2O | 0.50 | |
| K2O | 0.48 | |
| Loss on ignition (%) | 0.10 | |
| Physical properties | Bulk density (g/cm3) | 1.20 |
| Density (g/cm3) | 2.90 | |
| Specific surface area (m2/kg) | 430.00 |
| Appearance | Solid Content (mass %) | pH | Carbon (mass %) | Oxygen (mass %) | Hydrogen (mass %) |
|---|---|---|---|---|---|
| Black paste | 54.22 | 2.40 | 46.03 | 46.68 | 2.84 |
| Chemical Shifts (±1 ppm) | Site Type | Sample ID | ||
|---|---|---|---|---|
| M0 | R3 | R4 | ||
| −85 | Q2(0Al) | 40.09 | 43.06 | 42.22 |
| −82 | Q2(1Al) | 55.47 | 45.80 | 47.76 |
| −79 | Q1(0Al) | 4.44 | 11.14 | 10.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, W.-J.; Ye, T.-H.; Luo, Q.-L.; Wang, Y.; Mei, L. Reinforcing Mechanism of Reduced Graphene Oxide on Flexural Strength of Geopolymers: A Synergetic Analysis of Hydration and Chemical Composition. Nanomaterials 2019, 9, 1723. https://doi.org/10.3390/nano9121723
Long W-J, Ye T-H, Luo Q-L, Wang Y, Mei L. Reinforcing Mechanism of Reduced Graphene Oxide on Flexural Strength of Geopolymers: A Synergetic Analysis of Hydration and Chemical Composition. Nanomaterials. 2019; 9(12):1723. https://doi.org/10.3390/nano9121723
Chicago/Turabian StyleLong, Wu-Jian, Tao-Hua Ye, Qi-Ling Luo, Yaocheng Wang, and Liu Mei. 2019. "Reinforcing Mechanism of Reduced Graphene Oxide on Flexural Strength of Geopolymers: A Synergetic Analysis of Hydration and Chemical Composition" Nanomaterials 9, no. 12: 1723. https://doi.org/10.3390/nano9121723
APA StyleLong, W.-J., Ye, T.-H., Luo, Q.-L., Wang, Y., & Mei, L. (2019). Reinforcing Mechanism of Reduced Graphene Oxide on Flexural Strength of Geopolymers: A Synergetic Analysis of Hydration and Chemical Composition. Nanomaterials, 9(12), 1723. https://doi.org/10.3390/nano9121723





