RETRACTED: Reactive Mesoporous pH-Sensitive Amino-Functionalized Silica Nanoparticles for Efficient Removal of Coomassie Blue Dye
Abstract
1. Introduction
2. Experimental
2.1. Materials
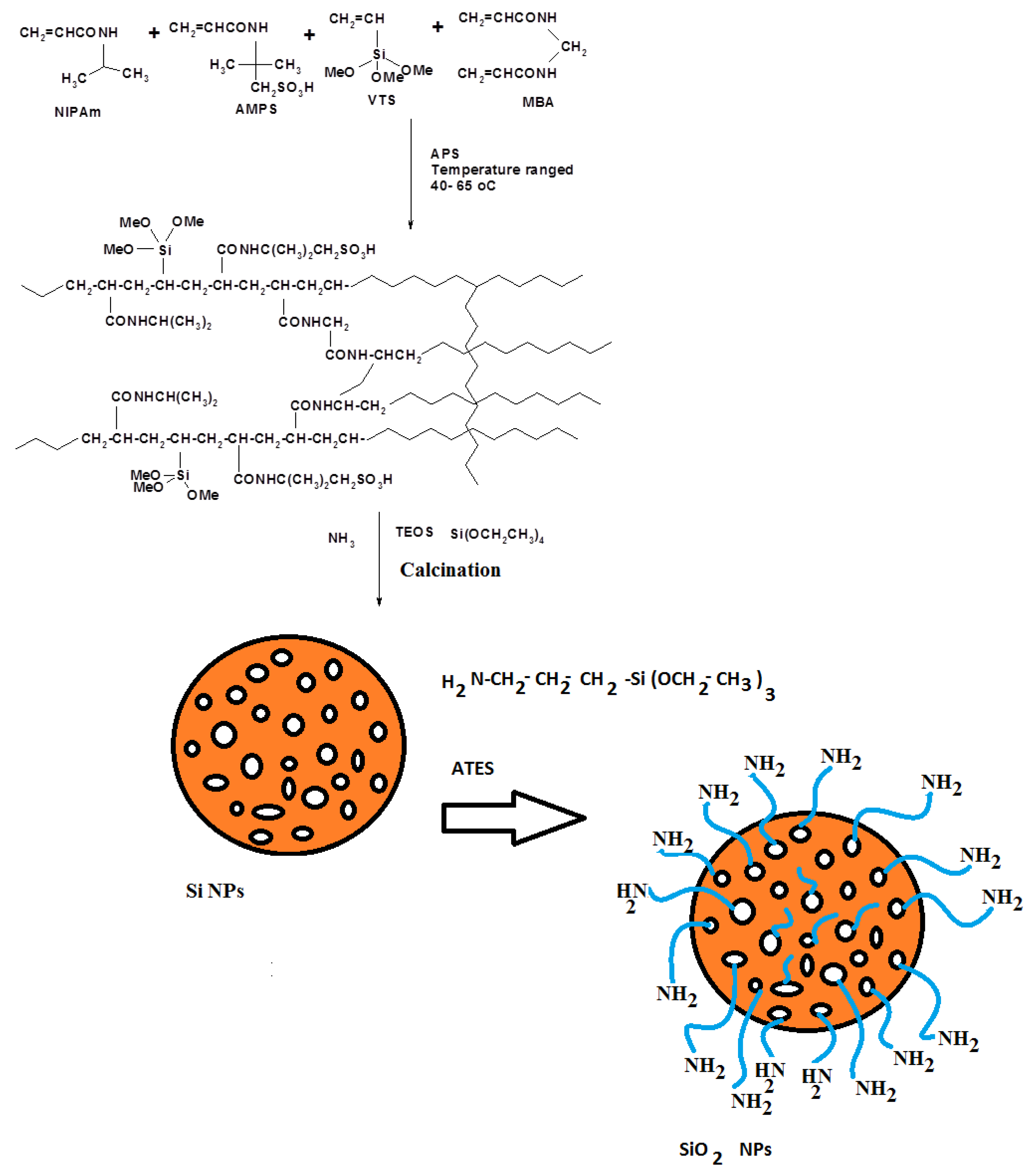
2.2. Preparation Techniques
2.2.1. Preparation of Silica Nanoparticles (Si NPs)
2.2.2. Preparation of SiO2 NPs
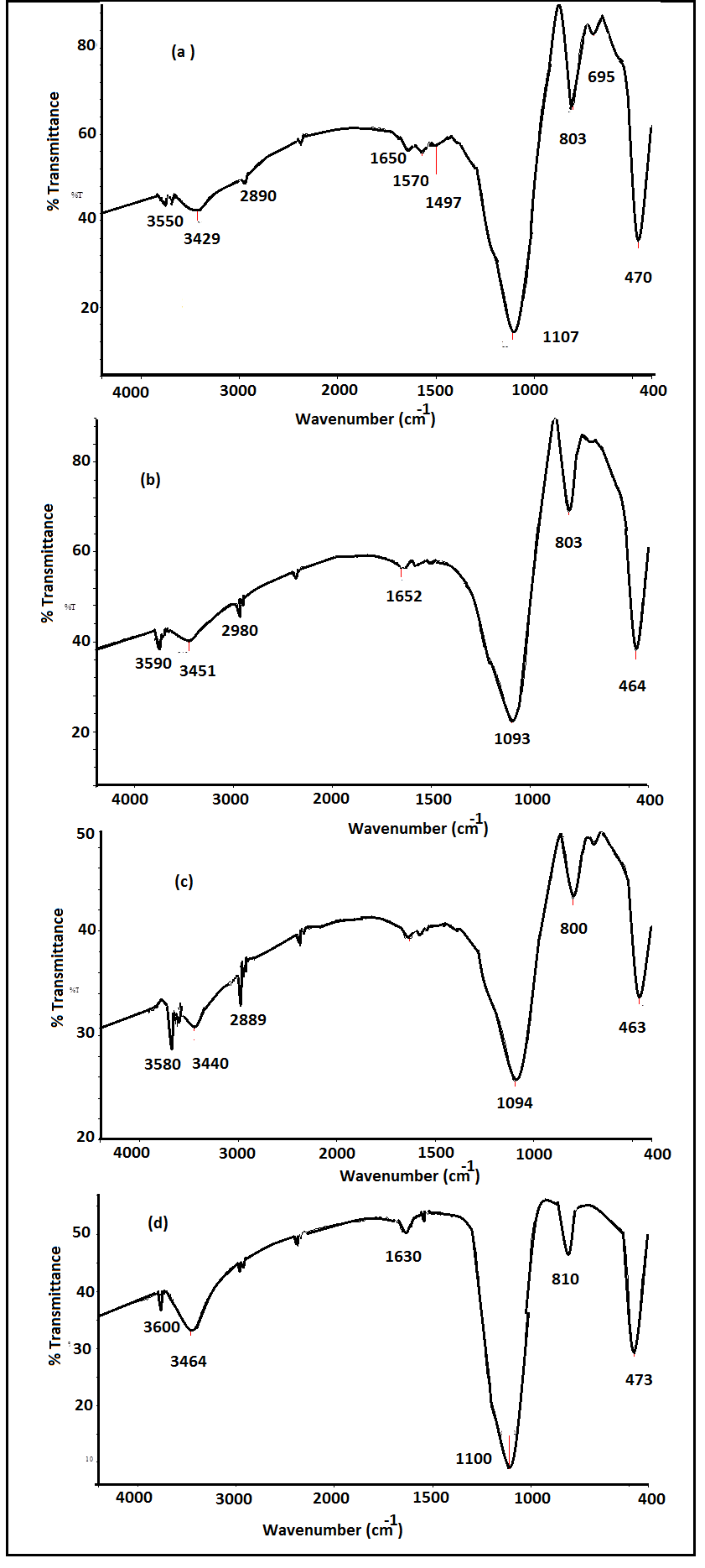
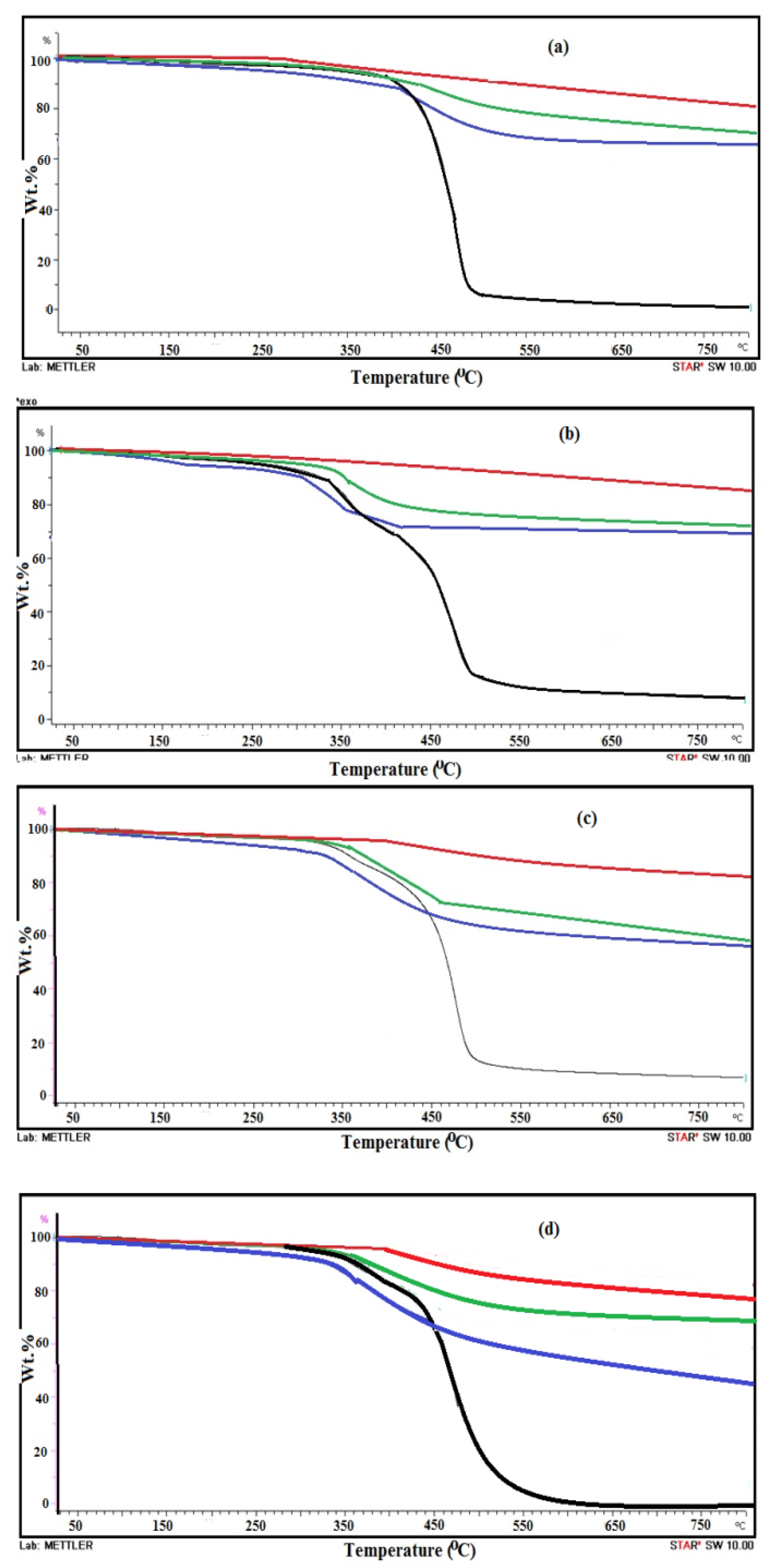
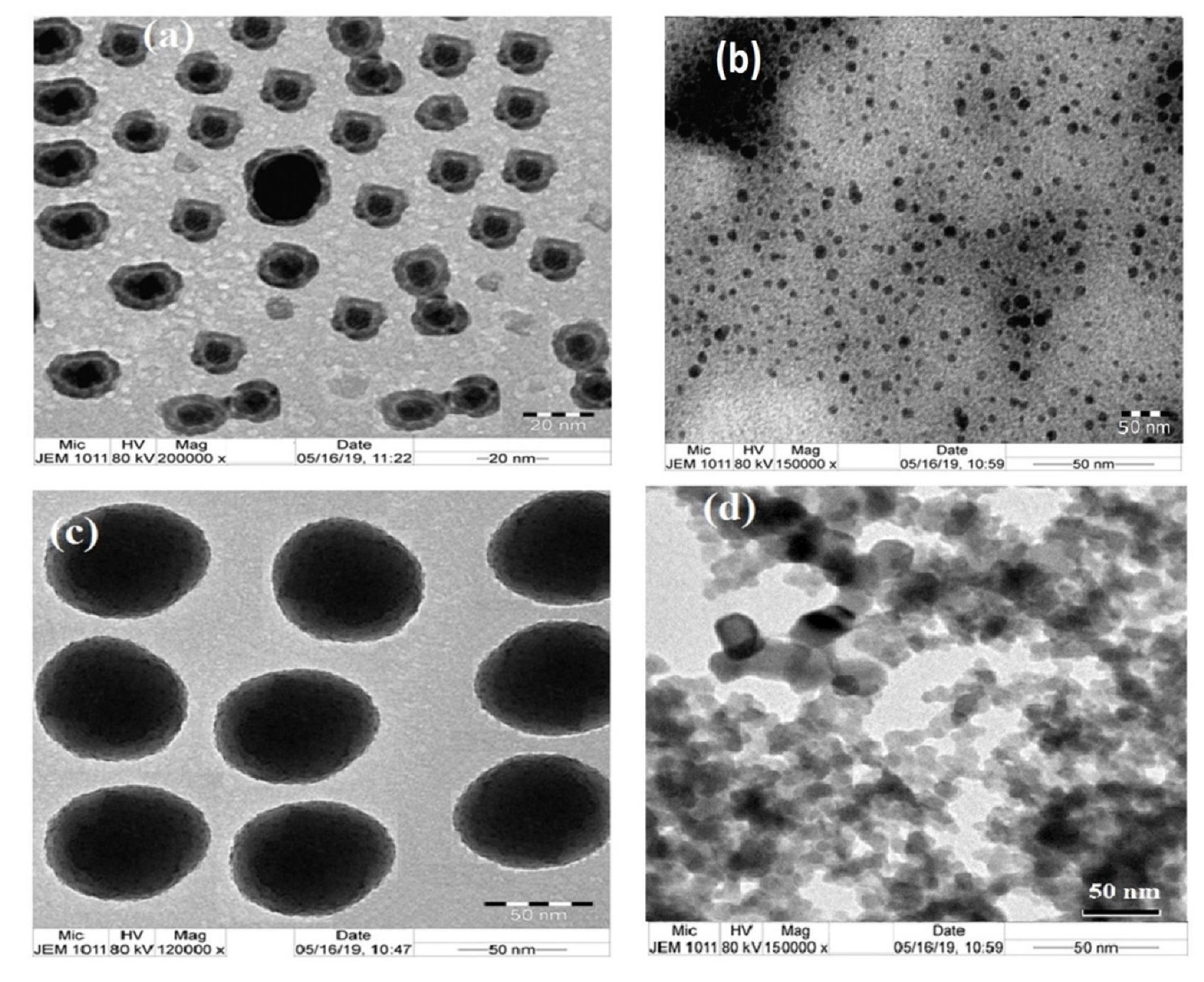
2.3. Characterization of the Prepared Composites
2.4. CB-R250 Adsorption Measurements
3. Results and Discussion
3.1. Characterization of Amine Modified SiO2 NPs
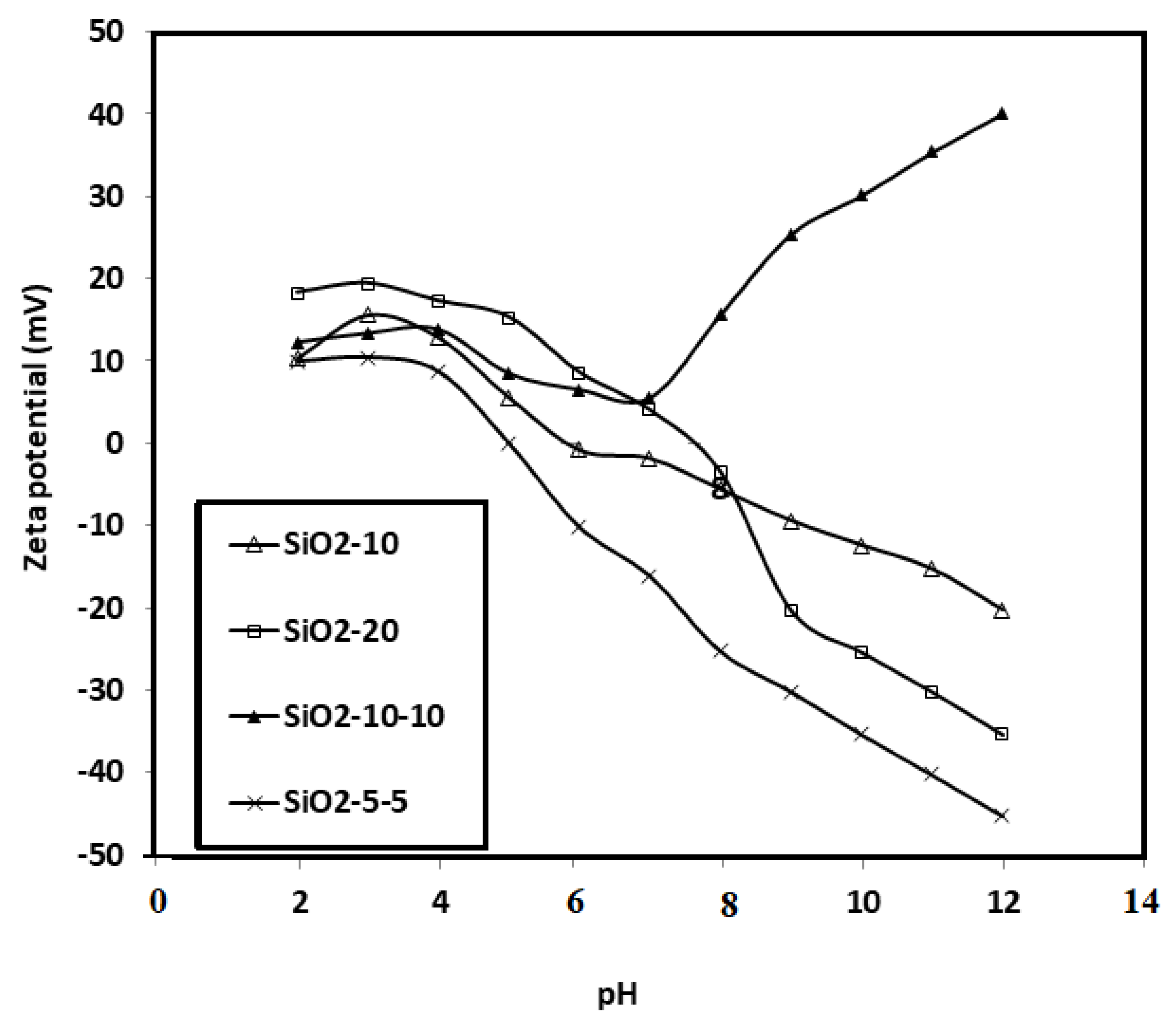
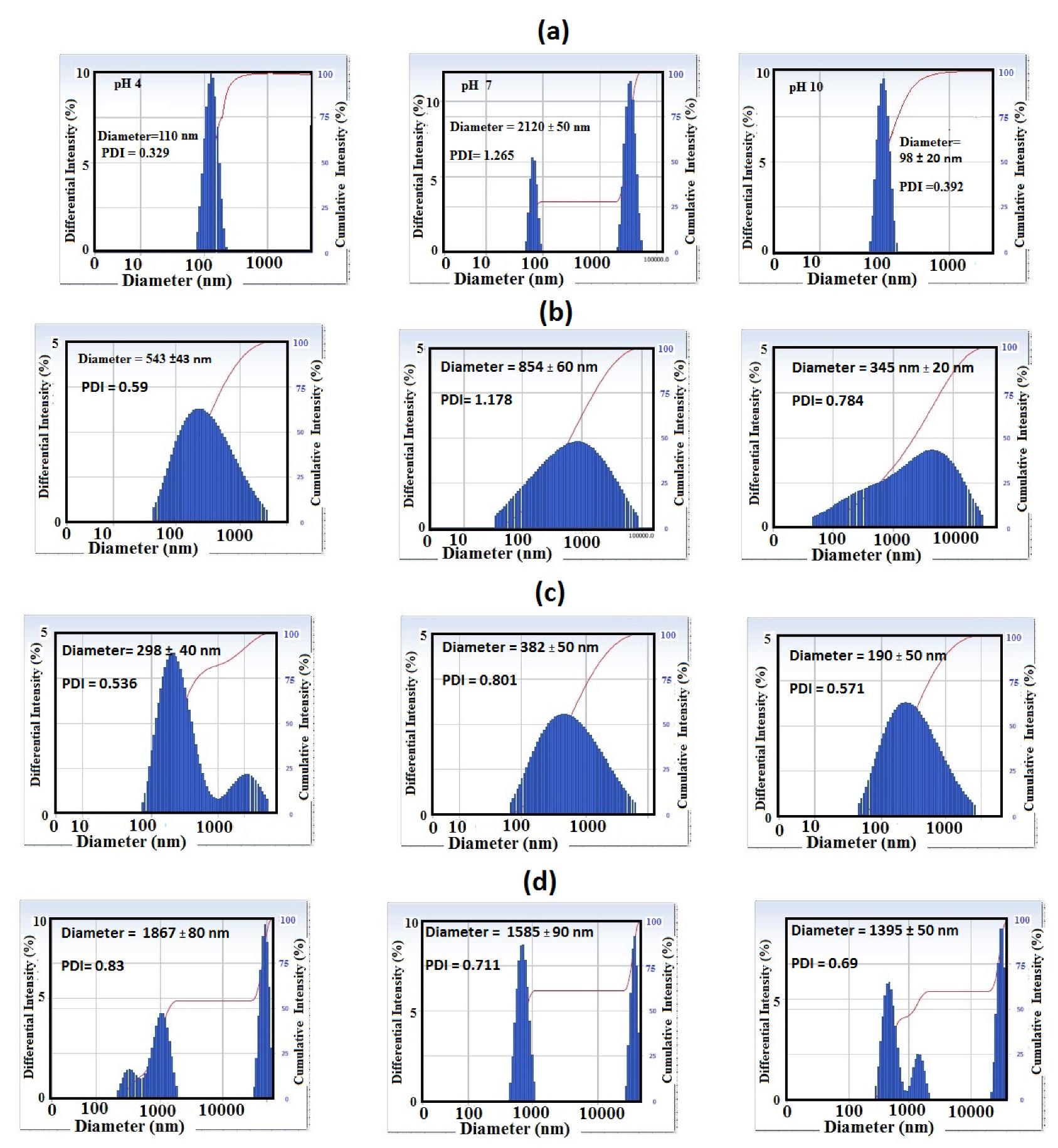
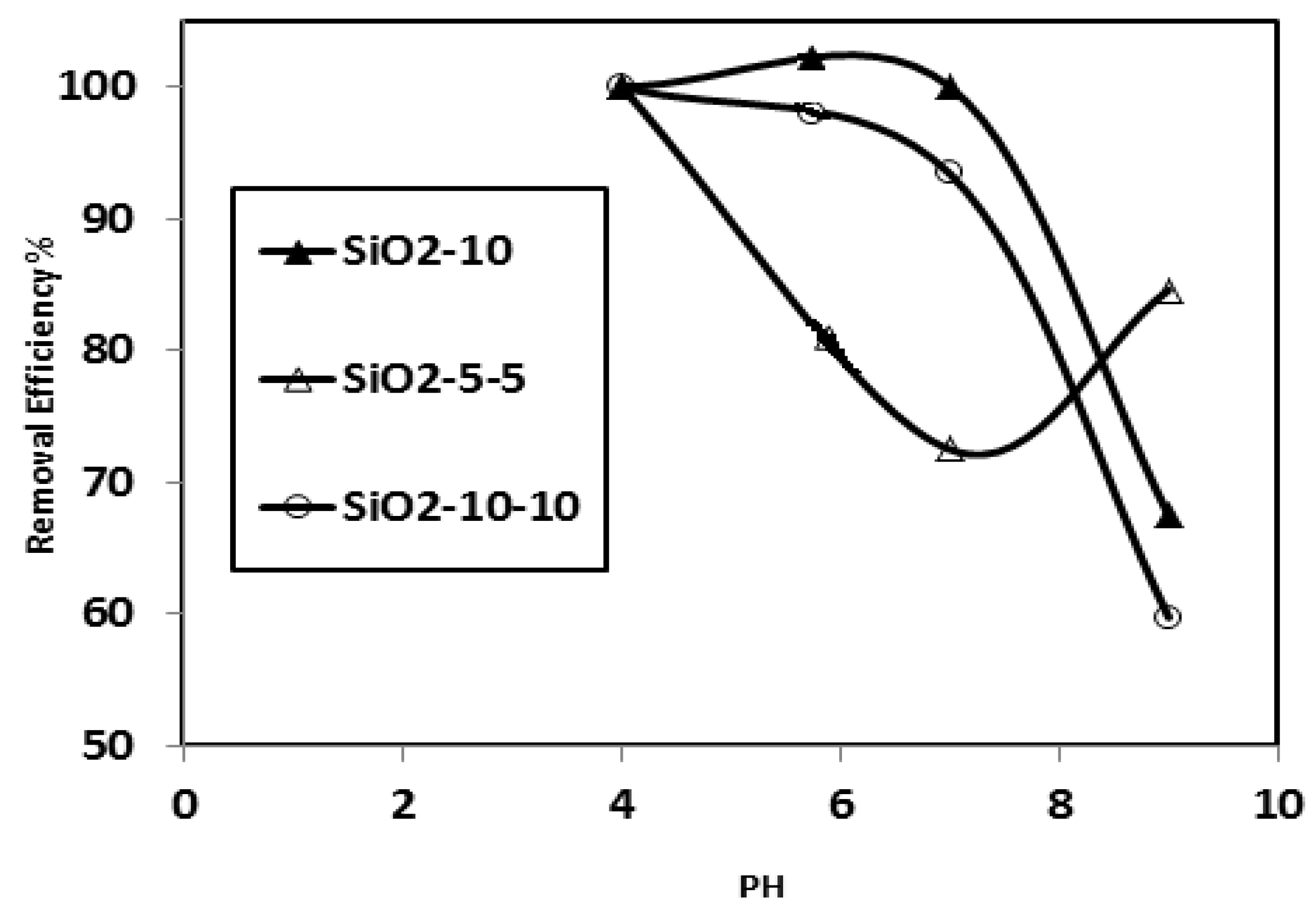
3.2. Effect of pH on the Surface and Particle Sizes of SiO2 NPs
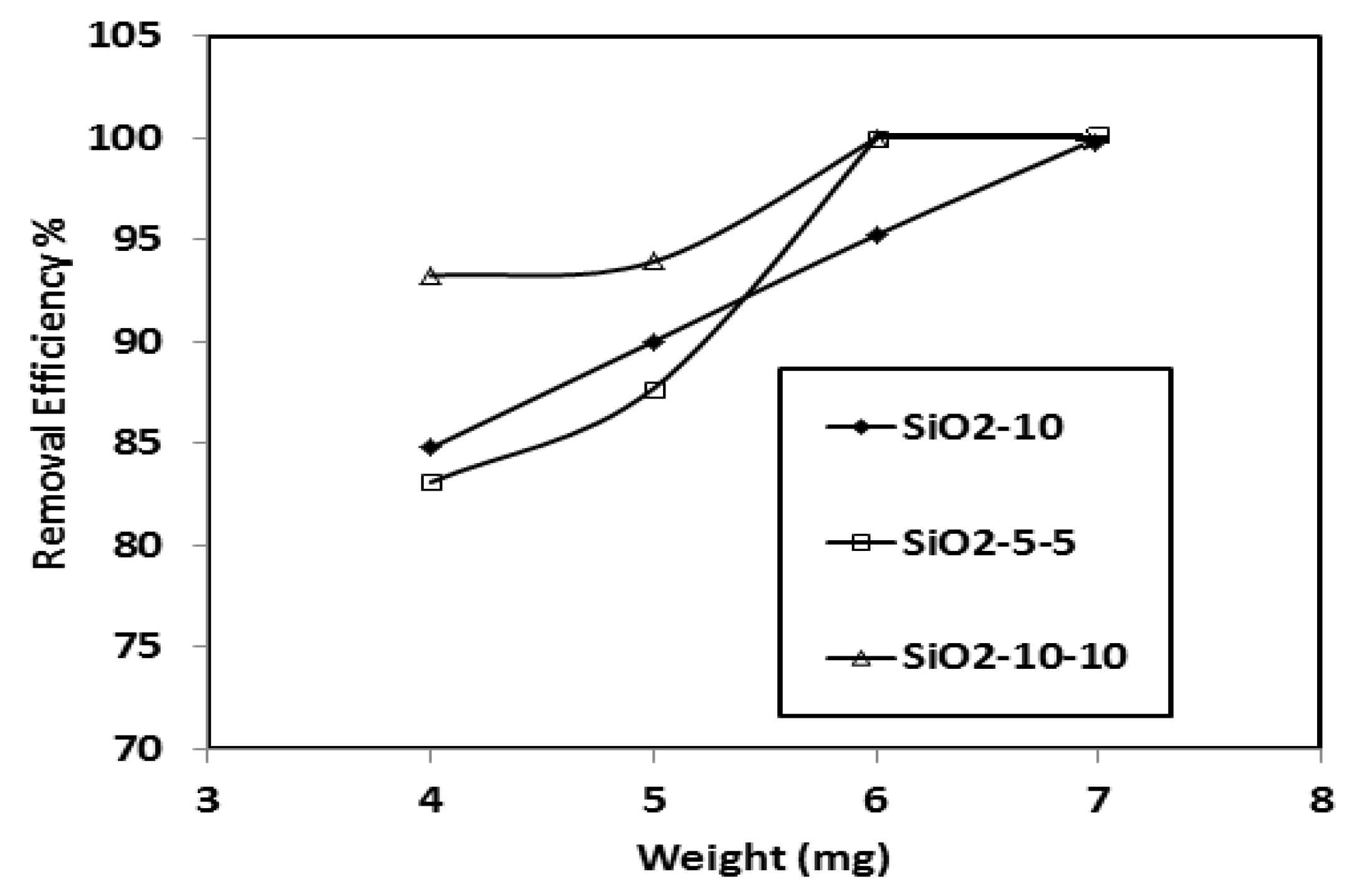
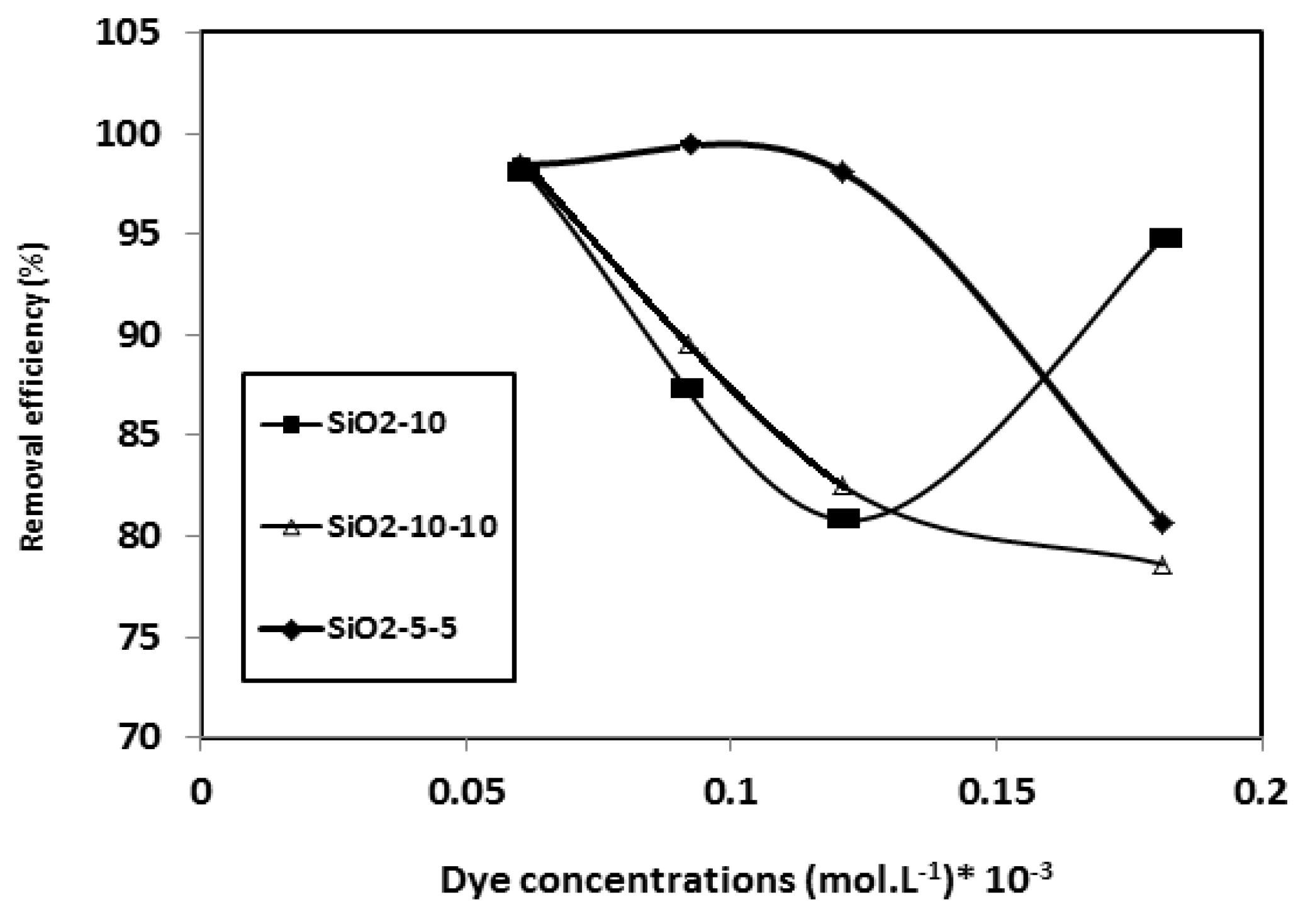
3.3. Sequestration and Optimization of CB-R250 Dye Adsorption
3.4. Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Zhao, X.; Lv, L.; Pan, B.; Zhang, W.; Zhang, S.; Zhang, Q. Polymer-supported nanocomposites for environmental application: A review. Chem. Eng. J. 2011, 170, 381–394. [Google Scholar] [CrossRef]
- Ren, X.; Chen, C.; Nagatsu, M.; Wang, X. Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem. Eng. J. 2011, 170, 395–410. [Google Scholar] [CrossRef]
- Galamboš, M.; Suchánek, P.; Rosskopfová, O. Sorption of anthropogenic radionuclides on natural and synthetic inorganic sorbents. J. Radioanal. Nucl. Chem. 2012, 293, 613–633. [Google Scholar] [CrossRef]
- Savage, N.; Diallo, M.S. Nanomaterials and water purification: Opportunities and challenges. JNR 2005, 7, 331–342. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X. Use of iron oxide nanomaterials in wastewater treatment: A review. ScTEn 2012, 424, 1–10. [Google Scholar] [CrossRef]
- Santra, S.; Tapec, R.; Theodoropoulou, N.; Dobson, J.; Hebard, A.; Tan, W. Synthesis and characterization of silica-coated iron oxide nanoparticles in microemulsion: The effect of nonionic surfactants. Langmuir 2001, 17, 2900–2906. [Google Scholar] [CrossRef]
- Ma, Z.; Guan, Y.; Liu, H. Superparamagnetic silica nanoparticles with immobilized metal affinity ligands for protein adsorption. JMMM 2006, 301, 469–477. [Google Scholar] [CrossRef]
- Hernandez, K.; Fernandez-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzym. Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Z.; Xing, J.; Liu, H. Preparation and characterization of amino-silane modified superparamagnetic silica nanospheres. JMMM 2004, 270, 1–6. [Google Scholar] [CrossRef]
- Diagboya, P.N.; Dikio, E.D. Silica-based mesoporous materials; emerging designer adsorbents for aqueous pollutants removal and water treatment. Microporous Mesoporous Mater. 2018, 266, 252–267. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Rafael, B.; Torres, R.B.; Vareda, J.P.; Lopes, D.; Ferreira, M.; Vanessa Valente, V.; Girão, A.V.; Valente, A.J.M.; Durães, L. Amine Modification of Silica Aerogels/Xerogels for Removal of Relevant Environmental Pollutants. Molecules 2019, 24, 3701. [Google Scholar] [CrossRef]
- Dou, B.; Hu, Q.; Li, J.; Qiao, S.; Hao, Z. Adsorption performance of VOCs in ordered mesoporous silicas with different pore structures and surface chemistry. J. Hazard. Mater. 2011, 186, 1615–1624. [Google Scholar] [CrossRef]
- Momčilović, M.Z.; Ranđelović, M.S.; Zarubica, A.R.; Onjia, A.E.; Kokunešoski, M.; Matović, B.Z. SBA-15 templated mesoporous carbons for 2, 4-dichlorophenoxyacetic acid removal. Chem. Eng. J. 2013, 220, 276–283. [Google Scholar] [CrossRef]
- Choi, J.W.; Lee, S.Y.; Lee, S.H.; Lee, K.B.; Kim, D.J.; Hong, S.W. Adsorption of phosphate by amino-functionalized and co-condensed SBA-15. Water Air Soil Pollut. 2012, 223, 2551–2562. [Google Scholar] [CrossRef]
- Diagboya, P.N.; Olu-Owolabi, B.I.; Adebowale, K.O. Microscale scavenging of pentachlorophenol in water using amine and tripolyphosphate-grafted SBA-15 silica: Batch and modeling studies. J. Environ. Manag. 2014, 146, 42–49. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Xu, Z.; Zheng, S.; Xue, M. Adsorption of tannic acid from aqueous solution by aminopropyl functionalized SBA-15. Desalin. Water Treat. 2015, 56, 475–484. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, Z.A.; Li, Y.; Liu, Y.; Huo, Q. Cooperative adsorbent based on mesoporous SiO2 for organic pollutants in water. JMCH 2011, 21, 17283–17289. [Google Scholar]
- Huang, Q.; Liu, M.; Chen, J.; Wang, K.; Xu, D.; Deng, F.; Huang, H.; Zhang, X.; Wei, Y. Mussel inspired preparation of functional silica nanocomposites for environmental adsorption applications. APSS 2016, 387, 285–293. [Google Scholar] [CrossRef]
- Liang, Z.; Zhao, Z.; Sun, T.; Shi, W.; Cui, F. Enhanced adsorption of the cationic dyes in the spherical CuO/meso-silica nano composite and impact of solution chemistry. JCIS 2017, 485, 192–200. [Google Scholar] [CrossRef]
- Atta, A.; Al-Lohedan, H.; Tawfik, A.; Ezzat, A. Application of Super-Amphiphilic Silica-Nanogel Composites for Fast Removal of Water Pollutants. Molecules 2016, 21, 1392. [Google Scholar] [CrossRef]
- Priya, B.S.; Kaith, U.; Shanker, B.; Bhatia Gupta, J.K. RSM-CCD optimized In-air synthesis of photocatalytic nanocomposite: Application in removal-degradation of toxic brilliant blue. React. Funct. Polym. 2018, 131, 107–122. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravind, U.K.; Aravindakumar, C.T. Sonochemical degradation of Coomassie Brilliant Blue: Effect of frequency, power density, pH and various additives. Chemosphere 2015, 119, 848–855. [Google Scholar] [CrossRef]
- El-Mahdy, G.A.; Atta, A.M.; Al-Lohedan, H.A. Synthesis and characterizations of Fe3O4 nanogel composite for enhancement of the corrosion resistance of steel in HCl solutions. J. Taiwan Inst. Chem. Eng. 2014, 45, 1947–1953. [Google Scholar] [CrossRef]
- Atta, A.M.; Dyab, A.K.; Allohedan, H.A. A novel route to prepare highly surface active nanogel particles based on nonaqueous emulsion polymerization. Polym. Adv. Technol. 2013, 24, 986–996. [Google Scholar] [CrossRef]
- Shoueir, K.R.; Sarhan, A.; Atta, A.M.; Akl, M.A. Adsorption studies of Cu2+ onto poly (vinyl alcohol)/poly (acrylamide-co-N-isopropylacrylamide) core–shell nanogels synthesized through surfactant-free emulsion polymerization. SsT 2016, 51, 1605–1617. [Google Scholar] [CrossRef]
- Atta, A.M.; Wahab, Z.A.E.; Shafey, Z.E.; Zidan, W.; Akl, Z. Uranyl Ions Uptake from Aqueous Solutions Using Crosslinked Ionic Copolymers Based on 2-Acrylamido-2-Methylpropane Sulfonic Acid Copolymers. JDST 2010, 31, 1601–1610. [Google Scholar] [CrossRef]
- Nanomaterials, S. For Imaging and Drug Delivery Applications. In Nanobiomaterials Handbook; CRC Press: Boca Rato, FL, USA, 2016. [Google Scholar]
- Whitby, C.P.; Krebsz, M.; Booty, S.J. Understanding the role of hydrogen bonding in the aggregation of fumed silica particles in triglyceride solvents. JCIS 2018, 527, 1–9. [Google Scholar] [CrossRef]
- Naderi, M. Surface Area: Brunauer-Emmett-Teller (BET), Progress in Filtration and Separation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 585–608. [Google Scholar]
- Leon, C.L.Y.; Solar, J.; Calemma, V.; Radovic, L.R. Evidence for the protonation of basal plane sites on carbon. Carbon 1992, 30, 797–811. [Google Scholar] [CrossRef]
- Montes-Moran, M.A.; Menéndez, J.A.; Fuente, E.; Suarez, D. Contribution of the basal planes to carbon basicity: An ab initio study of the H3O+−π interaction in cluster models. J. Phys. Chem. B 1998, 102, 5595–5601. [Google Scholar] [CrossRef]
- Ginés, L.; Mandal, S.; Cheng, C.L.; Sow, M.; Williams, O.A. Positive zeta potential of nanodiamonds. Nanoscale 2017, 9, 12549–12555. [Google Scholar] [CrossRef]
- Kim, K.-M.; Kim, H.M.; Lee, W.J.; Lee, C.W.; Kim, T.I.; Lee, J.K.; Jeong, J.; Paek, S.M.; Oh, J.M. Surface treatment of silica nanoparticles for stable and charge-controlled colloidal silica. Int. J. Nanomed. 2014, 9 (Suppl. S2), 29–40. [Google Scholar]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef]
- Csőgör, Z.; Nacken, M.; Sameti, M.; Lehr, C.M.; Schmidt, H. Modified silica particles for gene delivery. Mater. Sci. Eng. C 2003, 23, 93–97. [Google Scholar] [CrossRef]
- Morris, A.S.; Adamcakova-Dodd, A.; Lehman, S.E.; Wongrakpanich, A.; Thorne, P.S.; Larsen, S.C.; Salem, A.K. Amine modification of nonporous silica nanoparticles reduces inflammatory response following intratracheal instillation in murine lungs. Toxicol. Lett. 2016, 241, 207–215. [Google Scholar] [CrossRef]
- Shafqat, S.S.; Khan, A.A.; Zafar, M.N.; Alhaji, M.H.; Sanaullah, K.; Shafqat, S.R.; Murtaza, S.; Pang, S.C. Development of amino-functionalized silica nanoparticles for efficient and rapid removal of COD from pre-treated palm oil effluent. J. Mater. Res. Technol. 2019, 8, 385–395. [Google Scholar] [CrossRef]
- Park, J.K.; Choy, Y.B.; Oh, J.M.; Kim, J.Y.; Hwang, S.J.; Choy, J.H. Controlled release of donepezil intercalated in smectite clays. Int. J. Pharm. 2008, 359, 198–204. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kaur, Y.; Umar, A.; Chaudhary, G.R. Ionic liquid and surfactant functionalized ZnO nanoadsorbent for recyclable proficient adsorption of toxic dyes from waste water. J. Mol. Liq. 2016, 224, 1294–1304. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kaur, Y.; Umar, A.; Chaudhary, G.R. 1-butyl-3-methylimidazolium tetrafluoroborate functionalized ZnO nanoparticles for removal of toxic organic dyes. J. Mol. Liq. 2016, 220, 1013–1021. [Google Scholar] [CrossRef]
- Khan, M.A.; Alam, M.M.; Naushad, M.; Alothman, Z.A.; Kumar, M.; Ahamad, T. Sol-gel assisted synthesis of porous nano-crystalline CoFe2O4 composite and its application in the removal of brilliant blue-R from aqueous phase: An ecofriendly and economical approach. Chem. Eng. J. 2015, 279, 416–424. [Google Scholar] [CrossRef]
- Lai, B.H.; Yeh, C.C.; Chen, D.H. Surface modification of iron oxide nanoparticles with polyarginine as a highly positively charged magnetic nano-adsorbent for fast and effective recovery of acid proteins. Process Biochem. 2012, 47, 799–805. [Google Scholar] [CrossRef]
- Chaudhary, G.R.; Saharan, P.; Umar, A.; Mehta, S.; Mor, S. Well-crystalline ZnO nanostructures for the removal of acridine orange and coomassie brilliant blue R-250 hazardous dyes. Sci. Adv. Mater. 2013, 5, 1886–1894. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M.; Kumar, A.; Rana, S.; Sharma, S.; Bhatnagar, A.; Stadler, F.J.; Ghfar, A.A.; Khan, M.R. Efficient removal of coomassie brilliant blue R-250 dye using starch/poly (alginic acid-cl-acrylamide) nanohydrogel. Process Saf. Environ. Prot. 2017, 109, 301–310. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, J.; Zhang, H.; Yang, S.; Qi, J.; Wang, Z.; Xu, H. Use of rice husk-based porous carbon for adsorption of Rhodamine B from aqueous solutions. Dyes Pigment. 2005, 66, 123–128. [Google Scholar] [CrossRef]
- Mehta, D.; Mazumdar, S.; Singh, S. Magnetic adsorbents for the treatment of water/wastewater—A review. J. Water Process Eng. 2015, 7, 244–265. [Google Scholar] [CrossRef]
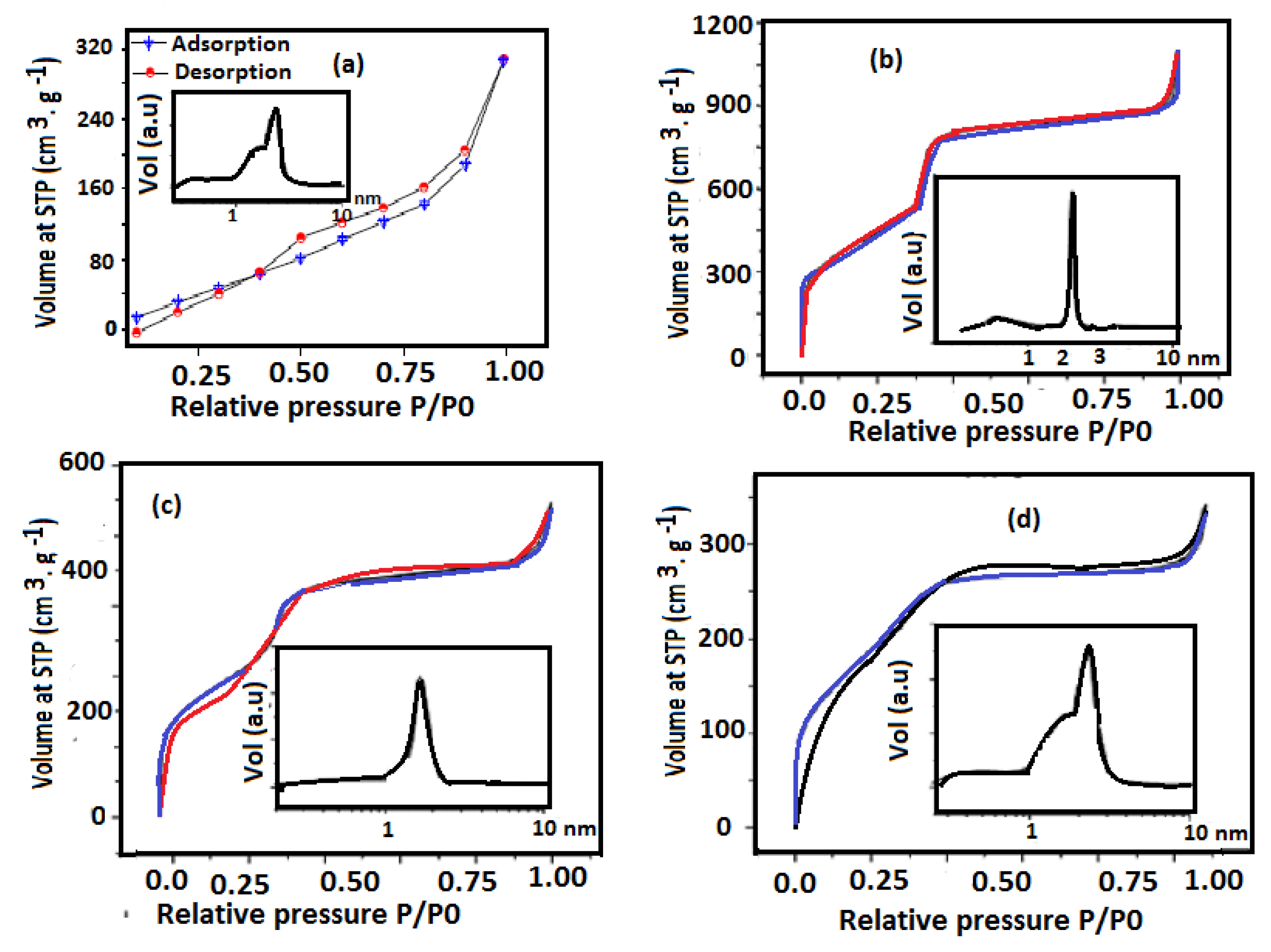

| Adsorbents | Surface Area m2·g−1 | Pore Volume cm3·g−1 | Pore Size nm |
|---|---|---|---|
| SiO2-10 | 738.8 | 4.91 | 1.12 |
| SiO2-20 | 220.3 | 14.097 | 8.36 |
| SiO2-10-10 | 1120.6 | 1.203 | 2.12 |
| SiO2-5-5 | 961.4 | 0.803 | 1.32 |
| Adsorbents | Langmuir Isotherm Parameters | Freundlich Isotherm Parameters | Exp. Adsorption Capacity | ||||
|---|---|---|---|---|---|---|---|
| Qmax mg·g−1 | Kl L·mg−1 | R² | n g·L−1 | Kf [(mg·g−1) (L·mg−1)(1/n))] | R² | qmax mg·g−1 | |
| SiO2-10 | 300.01 | 0.555 | 0.983 | 2.66 | 190.54 | 0.625 | 246.50 |
| SiO2-10-10 | 354.53 | 0.468 | 0.999 | 10.20 | 162.93 | 0.942 | 313.52 |
| SiO2-5-5 | 270.31 | 0.800 | 0.999 | 3.32 | 169.04 | 0.966 | 258.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabeela, N.I.; Almutairi, T.M.; Al-Lohedan, H.A.; Ezzat, A.O.; Atta, A.M. RETRACTED: Reactive Mesoporous pH-Sensitive Amino-Functionalized Silica Nanoparticles for Efficient Removal of Coomassie Blue Dye. Nanomaterials 2019, 9, 1721. https://doi.org/10.3390/nano9121721
Sabeela NI, Almutairi TM, Al-Lohedan HA, Ezzat AO, Atta AM. RETRACTED: Reactive Mesoporous pH-Sensitive Amino-Functionalized Silica Nanoparticles for Efficient Removal of Coomassie Blue Dye. Nanomaterials. 2019; 9(12):1721. https://doi.org/10.3390/nano9121721
Chicago/Turabian StyleSabeela, Nourah I., Tahani M. Almutairi, Hamad A. Al-Lohedan, Abdelrahman O. Ezzat, and Ayman M. Atta. 2019. "RETRACTED: Reactive Mesoporous pH-Sensitive Amino-Functionalized Silica Nanoparticles for Efficient Removal of Coomassie Blue Dye" Nanomaterials 9, no. 12: 1721. https://doi.org/10.3390/nano9121721
APA StyleSabeela, N. I., Almutairi, T. M., Al-Lohedan, H. A., Ezzat, A. O., & Atta, A. M. (2019). RETRACTED: Reactive Mesoporous pH-Sensitive Amino-Functionalized Silica Nanoparticles for Efficient Removal of Coomassie Blue Dye. Nanomaterials, 9(12), 1721. https://doi.org/10.3390/nano9121721






