An Nd3+-Sensitized Upconversion Fluorescent Sensor for Epirubicin Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Characterization
2.3. Synthesis of Oleic Acid (OA)-Coated NaYF4:Yb/Er/Nd
2.4. Synthesis of OA-Coated Core@shell NaYF4:Yb/Er/Nd@NaYF4:Nd
2.5. Synthesis of Ligand-Free NaYF4:Yb/Er/Nd@NaYF4:Nd
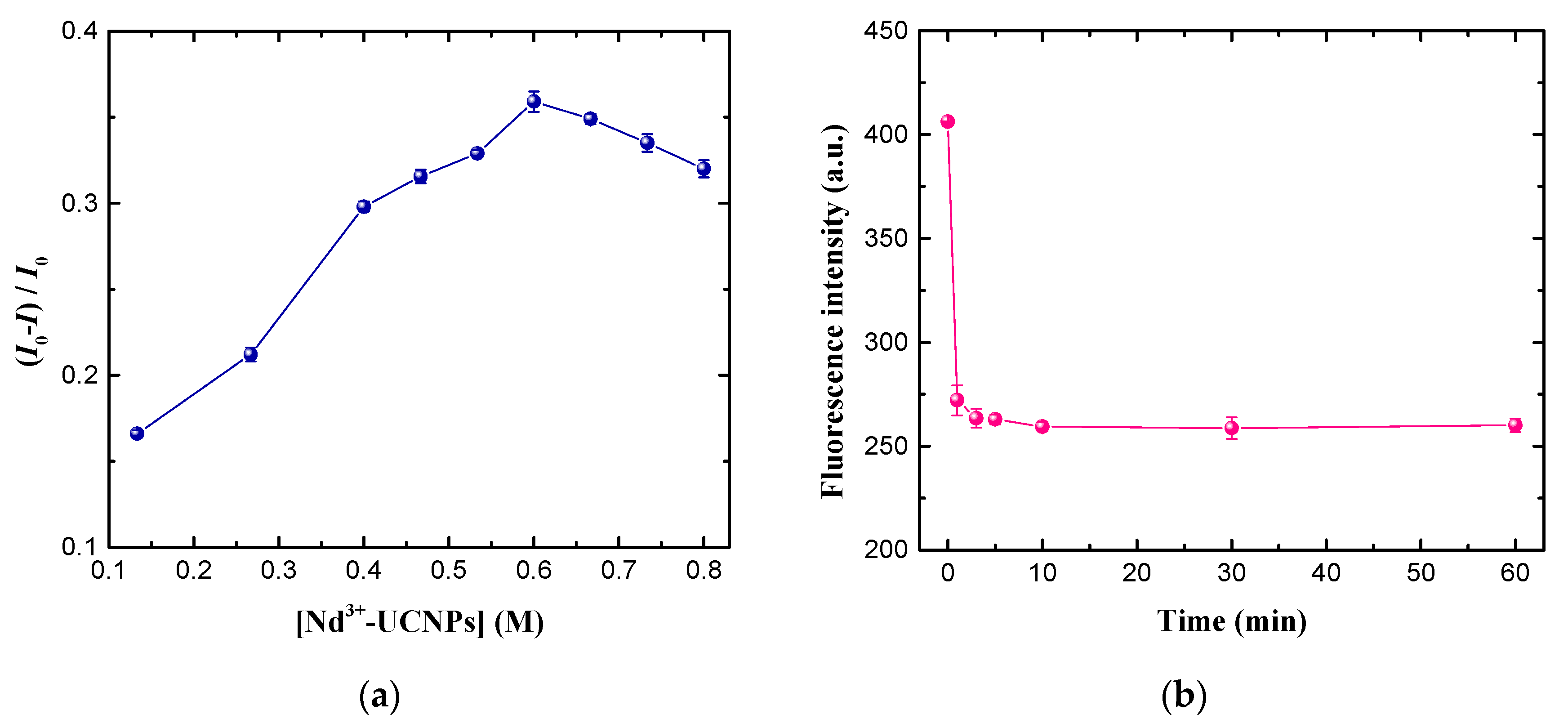
2.6. Optimization of Experimental Conditions
2.7. Assay Conditions for the detection of EPI
2.8. Real Sample Preparation
3. Results and Discussion
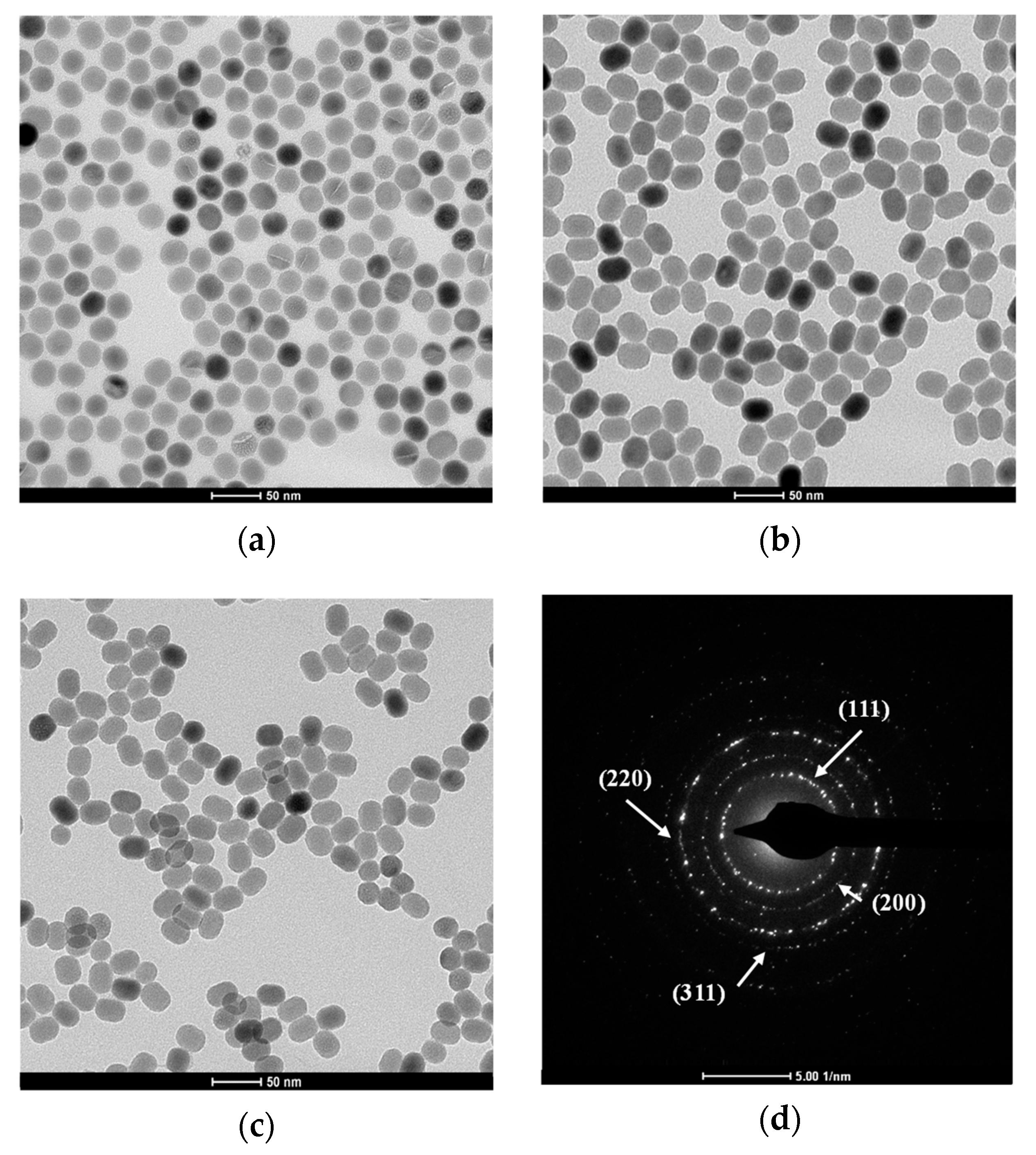
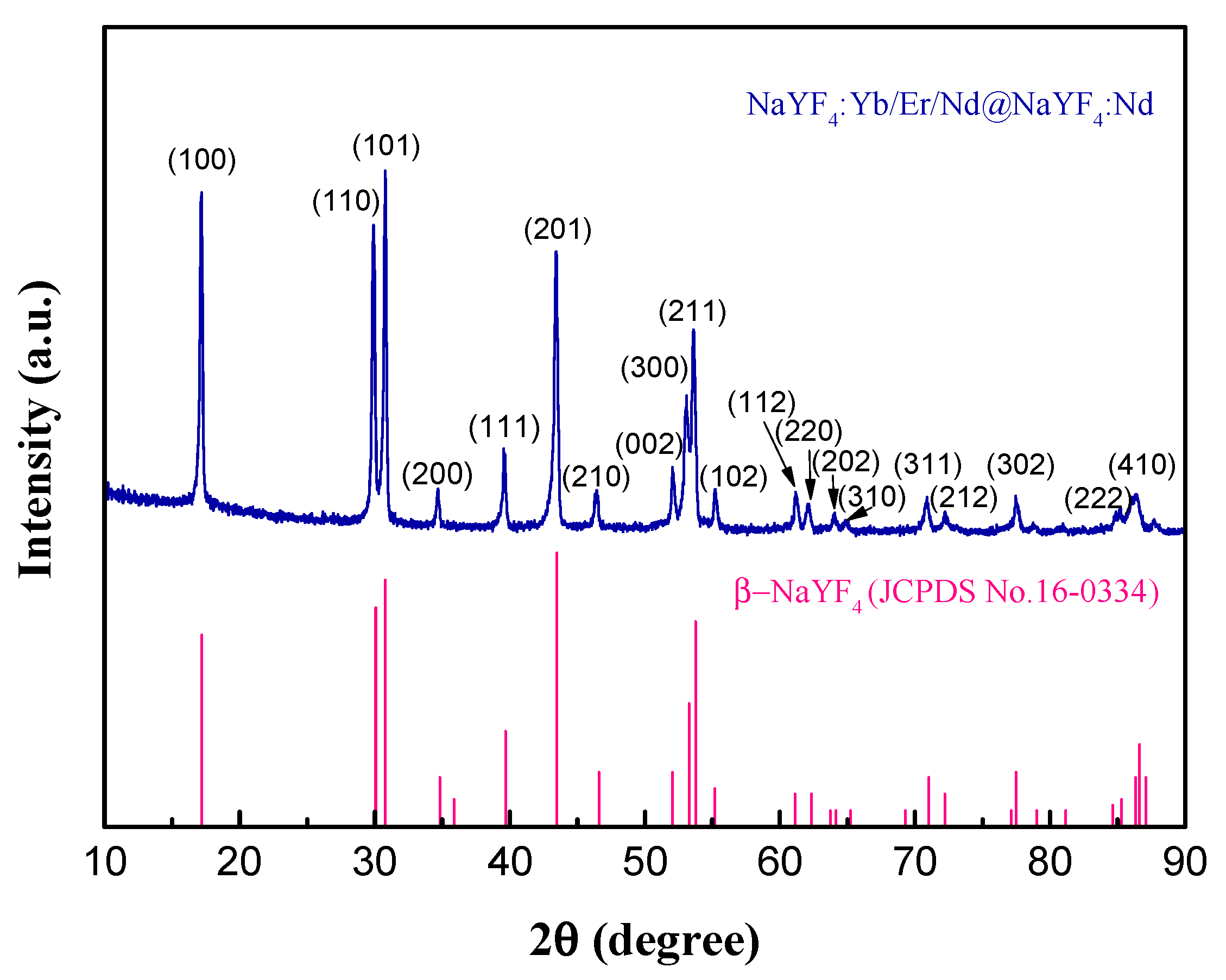
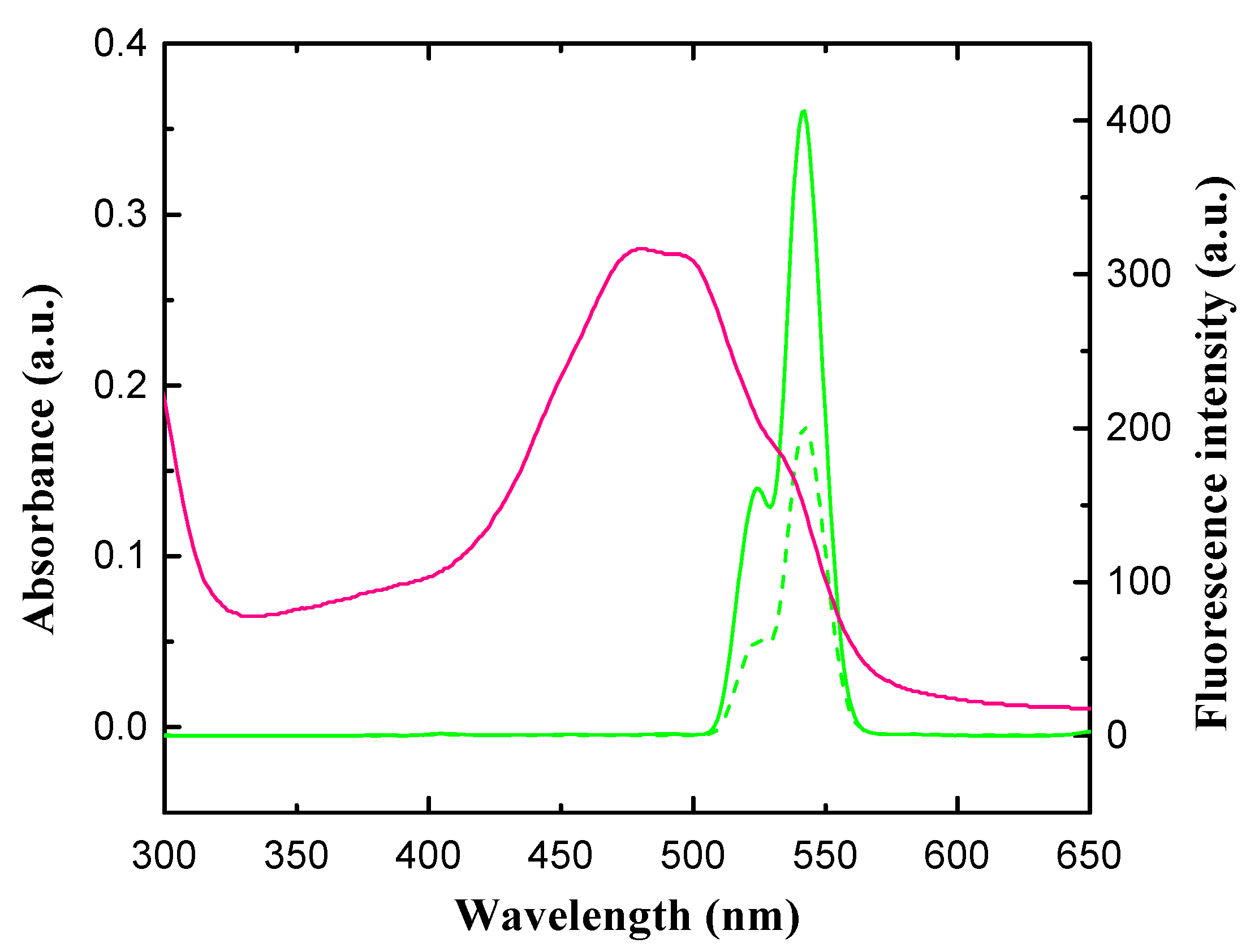
3.1. Characterization of UCNPs
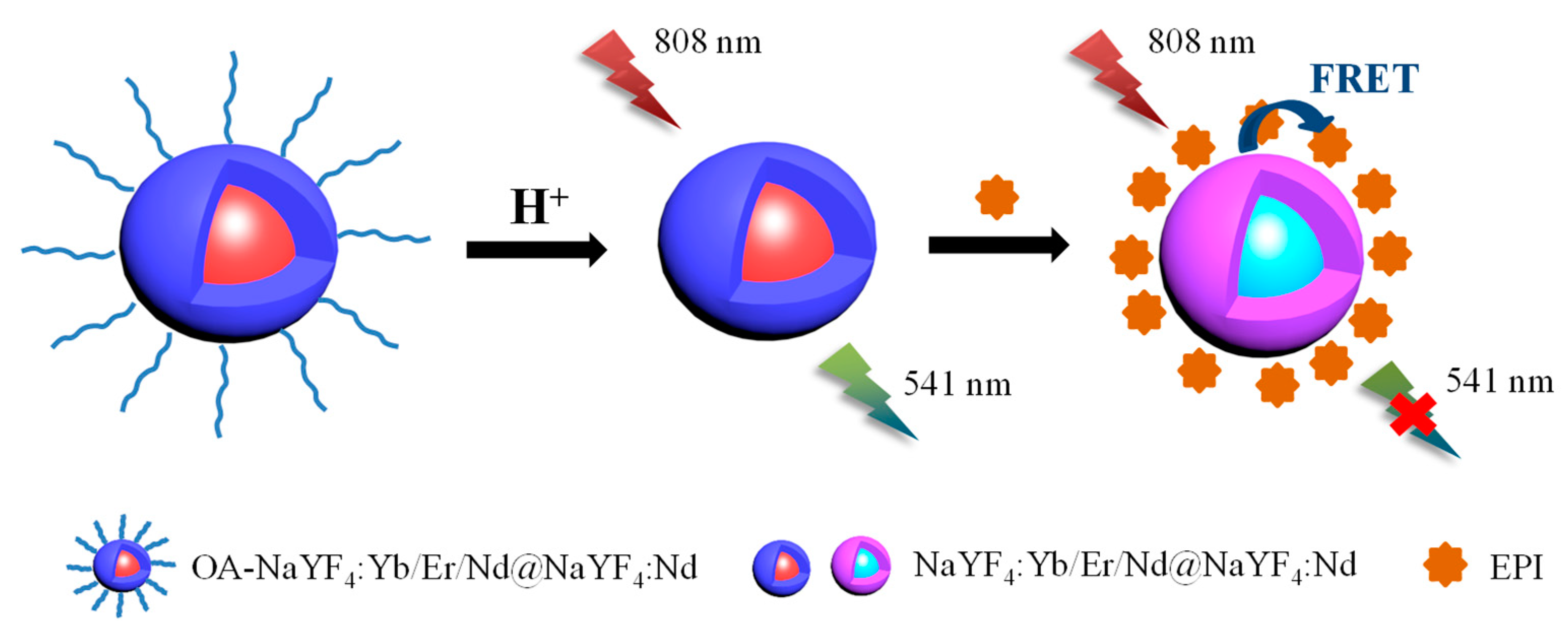
3.2. Principle of Detection
3.3. Optimization of Experimental Conditions
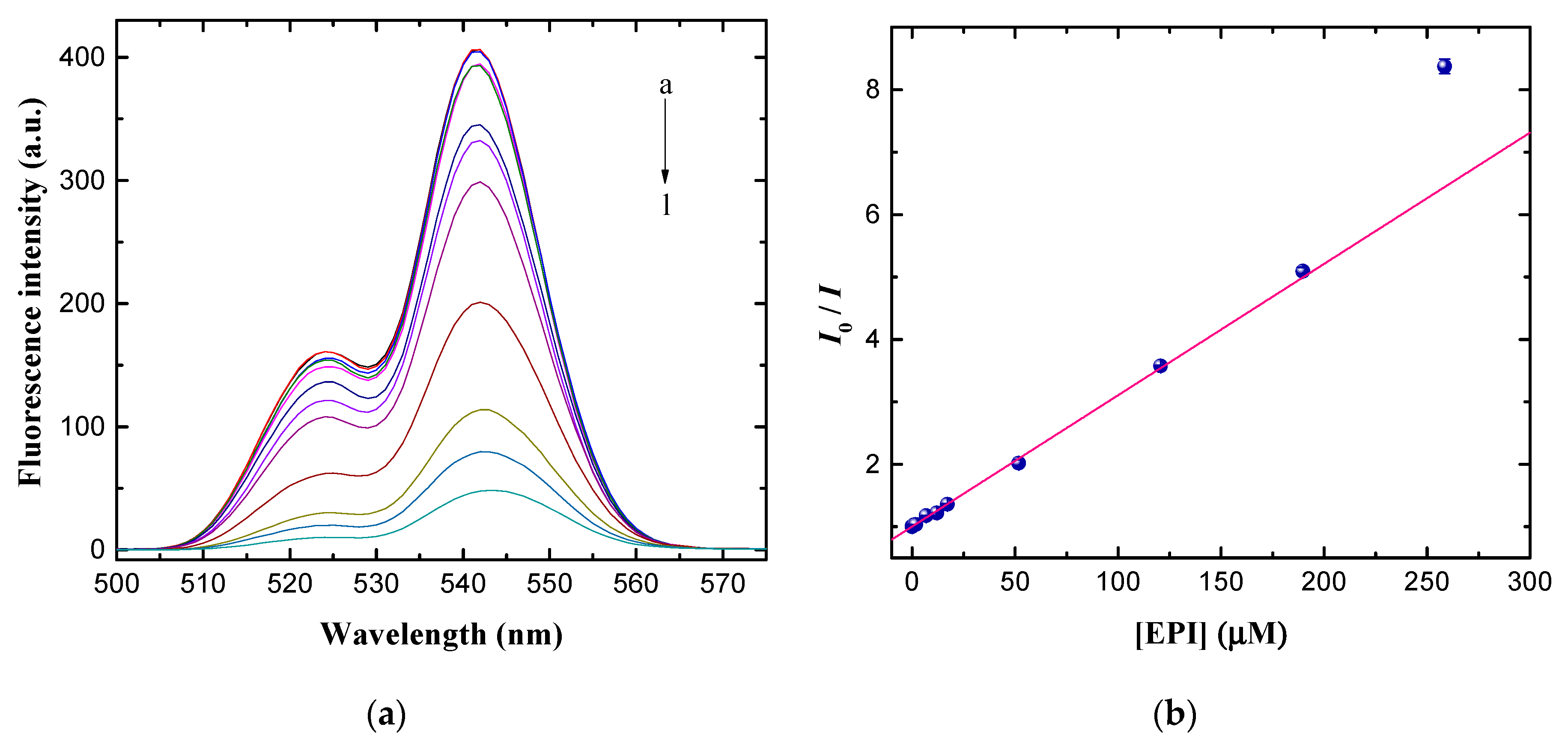
3.4. Fluorometric Detection of EPI
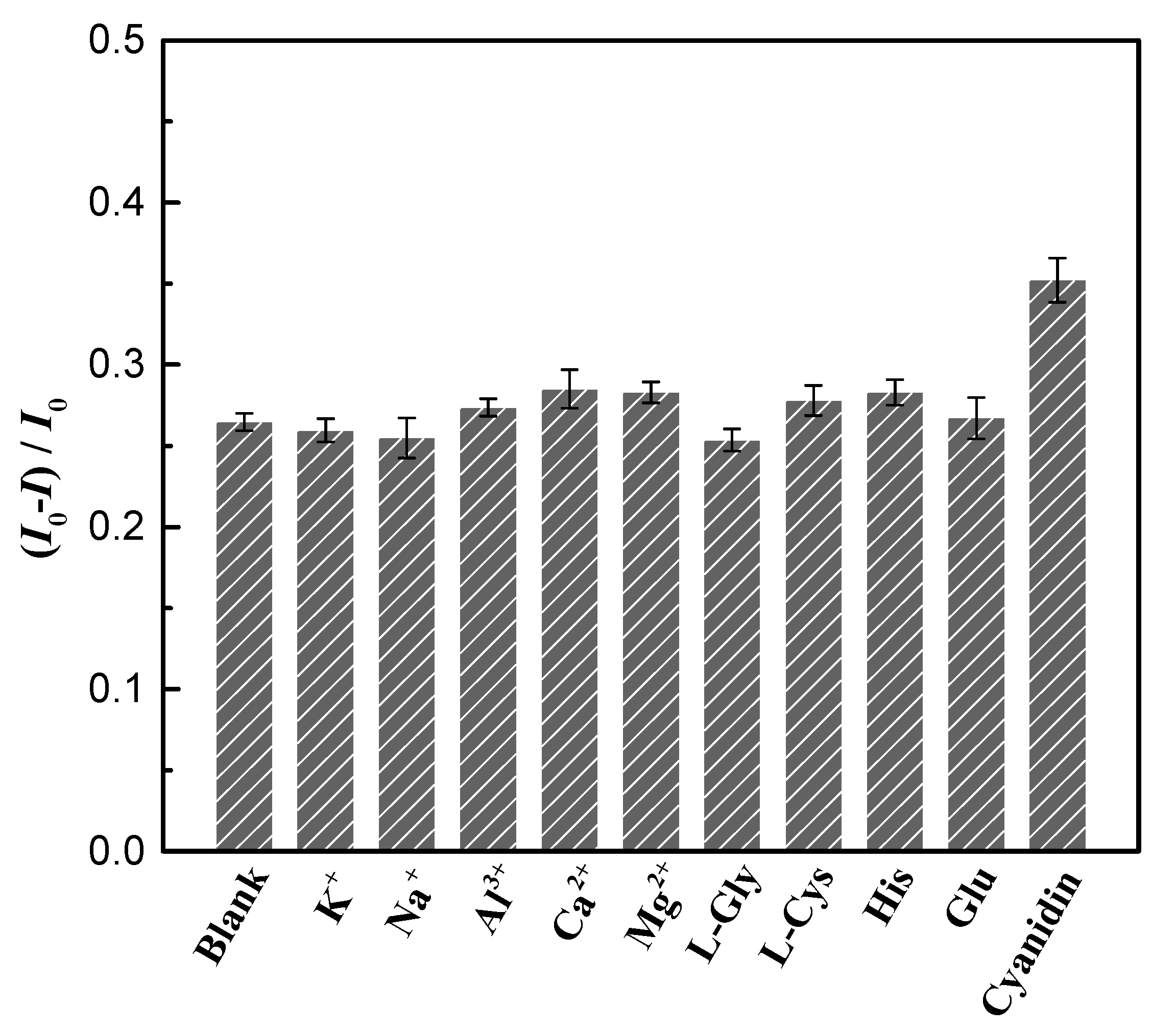
3.5. Selectivity for the Detection of EPI
3.6. Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cunningham, D.; Starling, N.; Rao, S.; Iveson, T.; Nicolson, M.; Coxon, F.; Middleton, G.; Daniel, F.; Oates, J.; Norman, A.R. Capecitabine and Oxaliplatin for Advanced Esophagogastric Cancer. N. Engl. J. Med. 2008, 358, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Bruix, J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003, 37, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Earl, H.; Iddawela, M. Epirubicin as adjuvant therapy in breast cancer. Expert Rev. Anticancer Ther. 2004, 4, 189–195. [Google Scholar] [CrossRef]
- Poole, C.J.; Earl, H.M.; Hiller, L.; Dunn, J.A.; Bathers, S.; Grieve, R.J.; Spooner, D.A.; Agrawal, R.K.; Fernando, I.N.; Brunt, A.M.; et al. Epirubicin and Cyclophosphamide, Methotrexate, and Fluorouracil as Adjuvant Therapy for Early Breast Cancer. N. Engl. J. Med. 2006, 355, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.; Rodríguez-Lescure, Á.; Ruiz, A.; Alba, E.; Calvo, L.; Ruiz-Borrego, M.; López García-Asenjo, J.A. Randomized Phase 3 Trial of Fluorouracil, Epirubicin, and Cyclophosphamide Alone or Followed by Paclitaxel for Early Breast Cancer. J. Natl. Cancer Inst. 2008, 100, 805–814. [Google Scholar] [CrossRef]
- Bhutani, M.; Kumar, L.; Vora, A.; Bhardwaj, N.; Pathak, A.K.; Singh, R.; Kochupillai, V. Randomized study comparing 4′-epi-doxorubicin (Epirubicin) versus doxorubicin as a part of induction treatment in adult acute lymphoblastic leukemia. Am. J. Hematol. 2002, 71, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Zinzani, P.; Tani, M.; Molinari, A.; Stefoni, V.; Zuffa, E.; Alinari, L.; Gabriele, A.; Bonifazi, F.; Salvucci, M.; Tura, S.; et al. Ifosfamide, epirubicin and etoposide regimen as salvage and mobilizing therapy for relapsed/refractory lymphoma patients. Haematologica 2002, 87, 816–821. [Google Scholar]
- Lehmann, J.; Retz, M.; Wiemers, C.; Beck, J.; Thüroff, J.; Weining, C.; Albers, P.; Frohneberg, D.; Becker, T.; Funke, P.-J.; et al. Adjuvant Cisplatin Plus Methotrexate Versus Methotrexate, Vinblastine, Epirubicin, and Cisplatin in Locally Advanced Bladder Cancer: Results of a Randomized, Multicenter, Phase III Trial (AUO-AB 05/95). J. Clin. Oncol. 2005, 23, 4963–4974. [Google Scholar] [CrossRef]
- Kuroda, M.; Kotake, T.; Akaza, H.; Hinotsu, S.; Kakizoe, T. Efficacy of Dose-intensified MEC (Methotrexate, Epirubicin and Cisplatin) Chemotherapy for Advanced Urothelial Carcinoma: A Prospective Randomized Trial Comparing MEC and M-VAC (Methotrexate, Vinblastine, Doxorubicin and Cisplatin). Jpn. J. Clin. Oncol. 1998, 28, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Florescu, M.; Magda, L.S.; Enescu, O.A.; Jinga, D.; Vinereanu, D. Early Detection of Epirubicin-Induced Cardiotoxicity in Patients with Breast Cancer. J. Am. Soc. Echocardiogr. 2014, 27, 83–92. [Google Scholar] [CrossRef]
- Guichard, N.; Guillarme, D.; Bonnabry, P.; Fleury-Souverain, S. Antineoplastic drugs and their analysis: A state of the art review. Analyst 2017, 142, 2273–2321. [Google Scholar] [CrossRef] [PubMed]
- Mahnik, S.N.; Rizovski, B.; Fuerhacker, M.; Mader, R.M. Development of an analytical method for the determination of anthracyclines in hospital effluents. Chemosphere 2006, 65, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Maudens, K.E.; Stove, C.P.; Cocquyt, V.F.J.; Denys, H.; Lambert, W.E. Development and validation of a liquid chromatographic method for the simultaneous determination of four anthracyclines and their respective 13-S-dihydro metabolites in plasma and saliva. J. Chromatogr. B 2009, 877, 3907–3915. [Google Scholar] [CrossRef] [PubMed]
- Dodde, W.I.; Maring, J.G.; Hendriks, G.; Wachters, F.M.; Groen, H.J.; de Vries, E.G.; Uges, D.R. Determination of epirubicin and its metabolite epirubicinol in saliva and plasma by HPLC. Ther. Drug Monit. 2003, 25, 433–440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tariq, M.; Thomas, S.; Singh, A.; Talegaonkar, S. Developed and validated stability indicating HPLC method for the determination of epirubicin in bulk drug, marketed injection and polymeric nanoparticles. Braz. J. Pharm. Sci. 2018, 54. [Google Scholar] [CrossRef]
- Gopinath, P.; Veluswami, S.; Thangarajan, R.; Gopisetty, G. RP-HPLC-UV Method for Estimation of Fluorouracil–Epirubicin–Cyclophosphamide and Their Metabolite Mixtures in Human Plasma (Matrix). J. Chromatogr. Sci. 2018, 56, 488–497. [Google Scholar] [CrossRef]
- Bermingham, S.; O’Connor, R.; Regan, F.; McMahon, G.P. Simultaneous determination of anthracyclines and taxanes in human serum using online sample extraction coupled to high performance liquid chromatography with UV detection. J. Sep. Sci. 2010, 33, 1571–1579. [Google Scholar] [CrossRef]
- Wall, R.; McMahon, G.; Crown, J.; Clynes, M.; O’Connor, R. Rapid and sensitive liquid chromatography–tandem mass spectrometry for the quantitation of epirubicin and identification of metabolites in biological samples. Talanta 2007, 72, 145–154. [Google Scholar] [CrossRef]
- Sottani, C.; Tranfo, G.; Bettinelli, M.; Faranda, P.; Spagnoli, M.; Minoia, C. Trace determination of anthracyclines in urine: A new high-performance liquid chromatography/tandem mass spectrometry method for assessing exposure of hospital personnel. Rapid Commun. Mass Spectrom. 2004, 18, 2426–2436. [Google Scholar] [CrossRef]
- Sottani, C.; Rinaldi, P.; Leoni, E.; Poggi, G.; Teragni, C.; Delmonte, A.; Minoia, C. Simultaneous determination of cyclophosphamide, ifosfamide, doxorubicin, epirubicin and daunorubicin in human urine using high-performance liquid chromatography/electrospray ionization tandem mass spectrometry: Bioanalytical method validation. Rapid Commun. Mass Spectrom. 2008, 22, 2645–2659. [Google Scholar] [CrossRef]
- Li, R.; Dong, L.; Huang, J. Ultra performance liquid chromatography–tandem mass spectrometry for the determination of epirubicin in human plasma. Anal. Chim. Acta 2005, 546, 167–173. [Google Scholar] [CrossRef]
- Whitaker, G.; Lillquist, A.; Pasas, S.A.; O’Connor, R.; Regan, F.; Lunte, C.E.; Smyth, M.R. CE-LIF method for the separation of anthracyclines: Application to protein binding analysis in plasma using ultrafiltration. J. Sep. Sci. 2008, 31, 1828–1833. [Google Scholar] [CrossRef] [PubMed]
- Mbuna, J.; Kaneta, T.; Imasaka, T. Micellar electrokinetic chromatographic analysis for in vitro accumulation of anthracyclines enhanced by inhibitors of cell membrane transporter-proteins in cancer cells. Biomed. Chromatogr. 2011, 25, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Mbuna, J.; Kaneta, T.; Imasaka, T. Measurement of intracellular accumulation of anthracyclines in cancerous cells by direct injection of cell lysate in MEKC/LIF detection. Electrophoresis 2010, 31, 1396–1404. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Pingarrón, J.M.; Yáñez-Sedeño, P.; González-Cortés, A. Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Komorsky-Lovrić, Š. Redox kinetics of adriamycin adsorbed on the surface of graphite and mercury electrodes. Bioelectrochemistry 2006, 69, 82–87. [Google Scholar] [CrossRef]
- Zhang, H. Fabrication of a single-walled carbon nanotube-modified glassy carbon electrode and its application in the electrochemical determination of epirubicin. J. Nanoparticle Res. 2004, 6, 665–669. [Google Scholar] [CrossRef]
- Bozal-Palabiyik, B.; Kurbanoglu, S.; Gumustas, M.; Uslu, B.; Ozkan, S. Electrochemical Approach for the Sensitive Determination of Anticancer Drug Epirubicin in Pharmaceuticals in the Presence of Anionic Surfactant. Rev. Roum. Chim. 2013, 58, 657–658. [Google Scholar]
- Hashkavayi, A.B.; Raoof, J.B. Design an aptasensor based on structure-switching aptamer on dendritic gold nanostructures/Fe3O4@SiO2/DABCO modified screen printed electrode for highly selective detection of epirubicin. Biosens. Bioelectron. 2017, 91, 650–657. [Google Scholar] [CrossRef]
- Karimi, F.; Shojaei, A.F.; Tabatabaeian, K.; Shakeri, S. CoFe2O4 nanoparticle/ionic liquid modified carbon paste electrode as an amplified sensor for epirubicin analysis as an anticancer drug. J. Mol. Liq. 2017, 242, 685–689. [Google Scholar] [CrossRef]
- Alavi-Tabari, S.A.R.; Khalilzadeh, M.A.; Karimi-Maleh, H.; Zareyee, D. An amplified platform nanostructure sensor for the analysis of epirubicin in the presence of topotecan as two important chemotherapy drugs for breast cancer therapy. New J. Chem. 2018, 42, 3828–3832. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, J.; Tao, L.; Tian, H.; Wang, S.; Ding, H. Simultaneous electrochemical determination of epirubicin and methotrexate in human blood using a disposable electrode modified with nano-Au/MWNTs-ZnO composites. Sens. Actuators B Chem. 2014, 204, 360–367. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Z.; Liu, M.; Liu, Y.; Ding, H. Application of Disposable Screen-printed Electrode as an Epirubicin Sensor and Relation among Whole Blood and Tissue Concentrations of Epirubicin. Int. J. Electrochem. Sci. 2012, 7, 1543–1555. [Google Scholar]
- Kokkinos, C.; Economou, A.; Prodromidis, M.I. Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. TrAC Trends Anal. Chem. 2016, 79, 88–105. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; La, H.; Zhu, R.; El-Banna, G.; Wei, Y.; Han, G. Upconverting NIR Photons for Bioimaging. Nanomaterials 2015, 5, 2148–2168. [Google Scholar] [CrossRef]
- Qiu, H.; Tan, M.; Ohulchanskyy, T.Y.; Lovell, J.F.; Chen, G. Recent Progress in Upconversion Photodynamic Therapy. Nanomaterials 2018, 8, 344. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, H.; Tang, J.; Deng, S.; Yan, F.; Li, W.; Qu, M. Carbon dots doped with heteroatoms for fluorescent bioimaging: A review. Microchim. Acta 2017, 184, 343–368. [Google Scholar] [CrossRef]
- Specht, E.A.; Braselmann, E.; Palmer, A.E. A Critical and Comparative Review of Fluorescent Tools for Live-Cell Imaging. Annu. Rev. Physiol. 2017, 79, 93–117. [Google Scholar] [CrossRef]
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Li, P.; Liu, S.; Yan, S.; Fan, X.; He, Y. A sensitive sensor for anthraquinone anticancer drugs and hsDNA based on CdTe/CdS quantum dots fluorescence reversible control. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 7–15. [Google Scholar] [CrossRef]
- Wei, J.-R.; Chen, H.-Y.; Zhang, W.; Pan, J.-X.; Dang, F.-Q.; Zhang, Z.-Q.; Zhang, J. Ratiometric fluorescence for sensitive and selective detection of mitoxantrone using a MIP@rQDs@SiO2 fluorescence probe. Sens. Actuators B Chem. 2017, 244, 31–37. [Google Scholar] [CrossRef]
- Ertas, N.; Satana Kara, H.E. l-Cysteine capped Mn-doped ZnS quantum dots as a room temperature phosphorescence sensor for in-vitro binding assay of idarubicin and DNA. Biosens. Bioelectron. 2015, 70, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Huang, X.; Fang, Y.; Zhang, Q. Determination of Idarubicin Using CdTe Quantum Dots as Fluorescence Probes. J. Nanosci. Nanotechnol. 2016, 16, 6992–6997. [Google Scholar] [CrossRef]
- Liu, C.-L.; Hou, C.-J.; Huo, D.-Q. Enhanced Room-Temperature Phosphorescence of Mn-Doped ZnS Quantum Dots Composited with PDDA for Detection of Adriamycin. J. Nanosci. Nanotechnol. 2018, 18, 2993–2999. [Google Scholar] [CrossRef]
- Yu, J.-C.; Chen, Y.-L.; Zhang, Y.-Q.; Yao, X.-K.; Qian, C.-G.; Huang, J.; Zhu, S.; Jiang, X.-Q.; Shen, Q.-D.; Gu, Z. pH-Responsive and near-infrared-emissive polymer nanoparticles for simultaneous delivery, release, and fluorescence tracking of doxorubicin in vivo. Chem. Commun. 2014, 50, 4699–4702. [Google Scholar] [CrossRef]
- Jiang, X.; Feng, D.-Q.; Liu, G.; Fan, D.; Wang, W. A fluorescent switch sensor for detection of anticancer drug and ctDNA based on the glutathione stabilized gold nanoclusters. Sens. Actuators B Chem. 2016, 232, 276–282. [Google Scholar] [CrossRef]
- Chen, C.; Li, C.; Shi, Z. Current Advances in Lanthanide-Doped Upconversion Nanostructures for Detection and Bioapplication. Adv. Sci. 2016, 3, 1600029. [Google Scholar] [CrossRef]
- Liu, Q.; Feng, W.; Li, F. Water-soluble lanthanide upconversion nanophosphors: Synthesis and bioimaging applications in vivo. Coord. Chem. Rev. 2014, 273–274, 100–110. [Google Scholar] [CrossRef]
- Ang, L.Y.; Lim, M.E.; Ong, L.C.; Zhang, Y. Applications of upconversion nanoparticles in imaging, detection and therapy. Nanomedicine 2011, 6, 1273–1288. [Google Scholar] [CrossRef]
- Wang, F.; Banerjee, D.; Liu, Y.; Chen, X.; Liu, X. Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst 2010, 135, 1839–1854. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Li, Z.; Zhang, Y.; Guo, S.; Pendharkar, A.I.; Lu, M.; Huang, L.; Huang, W.; Han, G. Emerging ≈ 800 nm Excited Lanthanide-Doped Upconversion Nanoparticles. Small 2017, 13, 1602843. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Gao, N.; Deng, R.; Sun, Q.; Xu, Q.-H.; Liu, X. Mechanistic Investigation of Photon Upconversion in Nd3+-Sensitized Core–Shell Nanoparticles. J. Am. Chem. Soc. 2013, 135, 12608–12611. [Google Scholar] [CrossRef]
- Xie, X.; Liu, X. Upconversion goes broadband. Nat. Mater. 2012, 11, 842. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Liu, G.-Y.; Sun, L.-D.; Xiao, J.-W.; Zhou, J.-C.; Yan, C.-H. Nd3+-Sensitized Upconversion Nanophosphors: Efficient In Vivo Bioimaging Probes with Minimized Heating Effect. ACS Nano 2013, 7, 7200–7206. [Google Scholar] [CrossRef]
- Wang, F.; Deng, R.; Liu, X. Preparation of core-shell NaGdF4 nanoparticles doped with luminescent lanthanide ions to be used as upconversion-based probes. Nat. Protoc. 2014, 9, 1634. [Google Scholar] [CrossRef]
- Cao, T.; Yang, T.; Gao, Y.; Yang, Y.; Hu, H.; Li, F. Water-soluble NaYF4:Yb/Er upconversion nanophosphors: Synthesis, characteristics and application in bioimaging. Inorg. Chem. Commun. 2010, 13, 392–394. [Google Scholar] [CrossRef]
- Tong, L.; Li, X.; Hua, R.; Li, X.; Zheng, H.; Sun, J.; Zhang, J.; Cheng, L.; Chen, B. Comparative study on upconversion luminescence and temperature sensing of α- and β-NaYF4:Yb3+/Er3+ nano-/micro-crystals derived from a microwave-assisted hydrothermal route. J. Lumin. 2015, 167, 386–390. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X. Upconversion Multicolor Fine-Tuning: Visible to Near-Infrared Emission from Lanthanide-Doped NaYF4 Nanoparticles. J. Am. Chem. Soc. 2008, 130, 5642–5643. [Google Scholar] [CrossRef]
- Ocak, I.; Kara, H.E.S. Phosphorescent detection of DNA- drug interaction based on emission quenching of ZnS quantum dots via photoinduced electron transfer. J. Lumin. 2018, 197, 112–118. [Google Scholar] [CrossRef]
- Bogdan, N.; Vetrone, F.; Ozin, G.A.; Capobianco, J.A. Synthesis of Ligand-Free Colloidally Stable Water Dispersible Brightly Luminescent Lanthanide-Doped Upconverting Nanoparticles. Nano Lett. 2011, 11, 835–840. [Google Scholar] [CrossRef]
- Yordanov, G.; Skrobanska, R.; Evangelatov, A. Entrapment of epirubicin in poly(butyl cyanoacrylate) colloidal nanospheres by nanoprecipitation: Formulation development and in vitro studies on cancer cell lines. Colloids Surf. B Biointerfaces 2012, 92, 98–105. [Google Scholar] [CrossRef]
- Kay, C.D.; Mazza, G.; Holub, B.J.; Wang, J. Anthocyanin metabolites in human urine and serum. Br. J. Nutr. 2007, 91, 933–942. [Google Scholar] [CrossRef]
- Chen, F.; Chen, H.; Duan, X.; Jia, J.; Kong, J. Molecularly imprinted polymers synthesized using reduction-cleavable hyperbranched polymers for doxorubicin hydrochloride with enhanced loading properties and controlled release. J. Mater. Sci. 2016, 51, 9367–9383. [Google Scholar] [CrossRef]
| Methods | LOD (μM) | Linear Range (μM) | Refs. |
|---|---|---|---|
| HPLC/Fluorometry | 0.00129 | 0.0043–4.311 | [13] |
| HPLC/UV | 0.05 | 0.172–86.2 | [16] |
| HPLC/MS/MS | 0.00017 | 0.00034–0.0069 | [20] |
| CE/LIF | 0.017 | 0.086–0.862 | [22] |
| Electrochemical/bare electrode | 0.126 | 0.86–70.0 | [29] |
| Electrochemical/nanoparticles | 0.01 | 0.04–450 | [31] |
| Electrochemical/carbon nanotube | 0.02 | 0.05–10 | [28] |
| Electrochemical/DNA | 0.04 | 0.07–21 | [30] |
| Fluorescence/Nd3+-UCNPs | 0.05 | 0.09–189.66 | This work |
| Sample | Added (μM) | Found (μM) | Recovery (%, n = 5) | RSD (%, n = 5) |
|---|---|---|---|---|
| Urine 1 | 5 | 4.9 | 98.2 | 4.7 |
| Urine 2 | 10 | 10.3 | 102.5 | 6.1 |
| Urine 3 | 20 | 23.2 | 97.5 | 5.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, J.; Shen, L.; Xu, Q.; Zeng, J.; Sha, J.; Hu, T.; Bi, K.; Chen, Y. An Nd3+-Sensitized Upconversion Fluorescent Sensor for Epirubicin Detection. Nanomaterials 2019, 9, 1700. https://doi.org/10.3390/nano9121700
Mo J, Shen L, Xu Q, Zeng J, Sha J, Hu T, Bi K, Chen Y. An Nd3+-Sensitized Upconversion Fluorescent Sensor for Epirubicin Detection. Nanomaterials. 2019; 9(12):1700. https://doi.org/10.3390/nano9121700
Chicago/Turabian StyleMo, Jingwen, Long Shen, Qian Xu, Jiaying Zeng, Jingjie Sha, Tao Hu, Kedong Bi, and Yunfei Chen. 2019. "An Nd3+-Sensitized Upconversion Fluorescent Sensor for Epirubicin Detection" Nanomaterials 9, no. 12: 1700. https://doi.org/10.3390/nano9121700
APA StyleMo, J., Shen, L., Xu, Q., Zeng, J., Sha, J., Hu, T., Bi, K., & Chen, Y. (2019). An Nd3+-Sensitized Upconversion Fluorescent Sensor for Epirubicin Detection. Nanomaterials, 9(12), 1700. https://doi.org/10.3390/nano9121700





