Bismuth-Doped Nanohydroxyapatite Coatings on Titanium Implants for Improved Radiopacity and Antimicrobial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Solutions
2.3. Alkali-Thermal Treatment
2.4. Biomimetic Deposition
2.5. Sample Characterization
2.6. Antibacterial Tests
3. Results and Discussion
3.1. Mechanism of Coating Formation
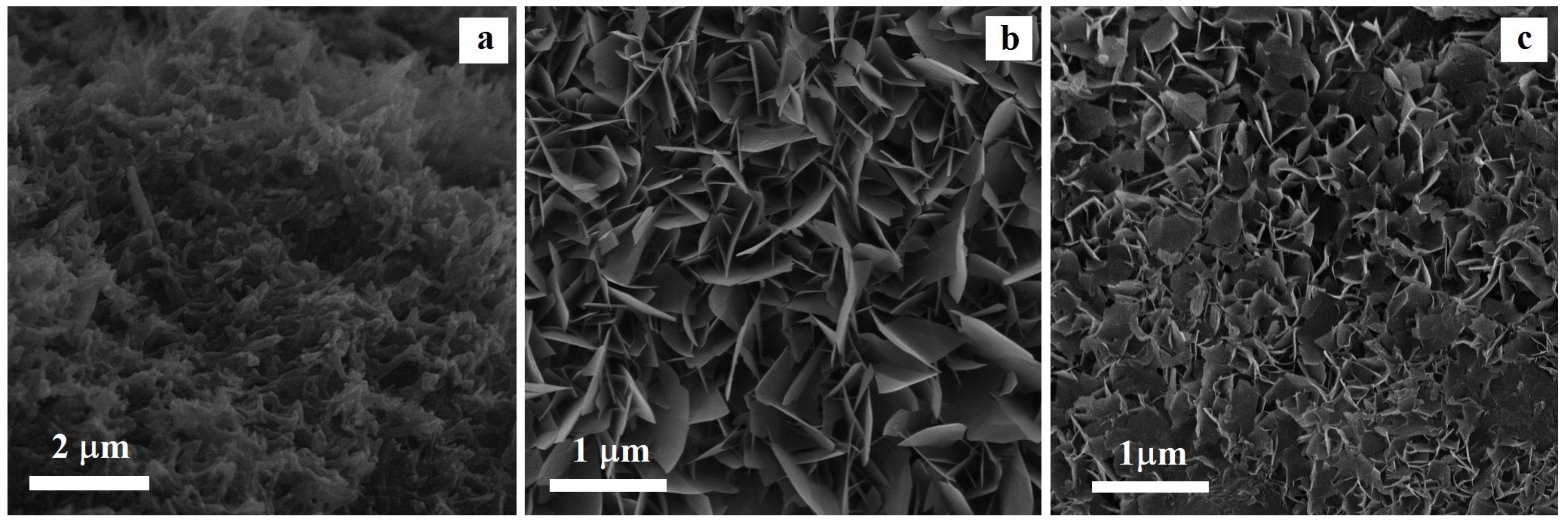
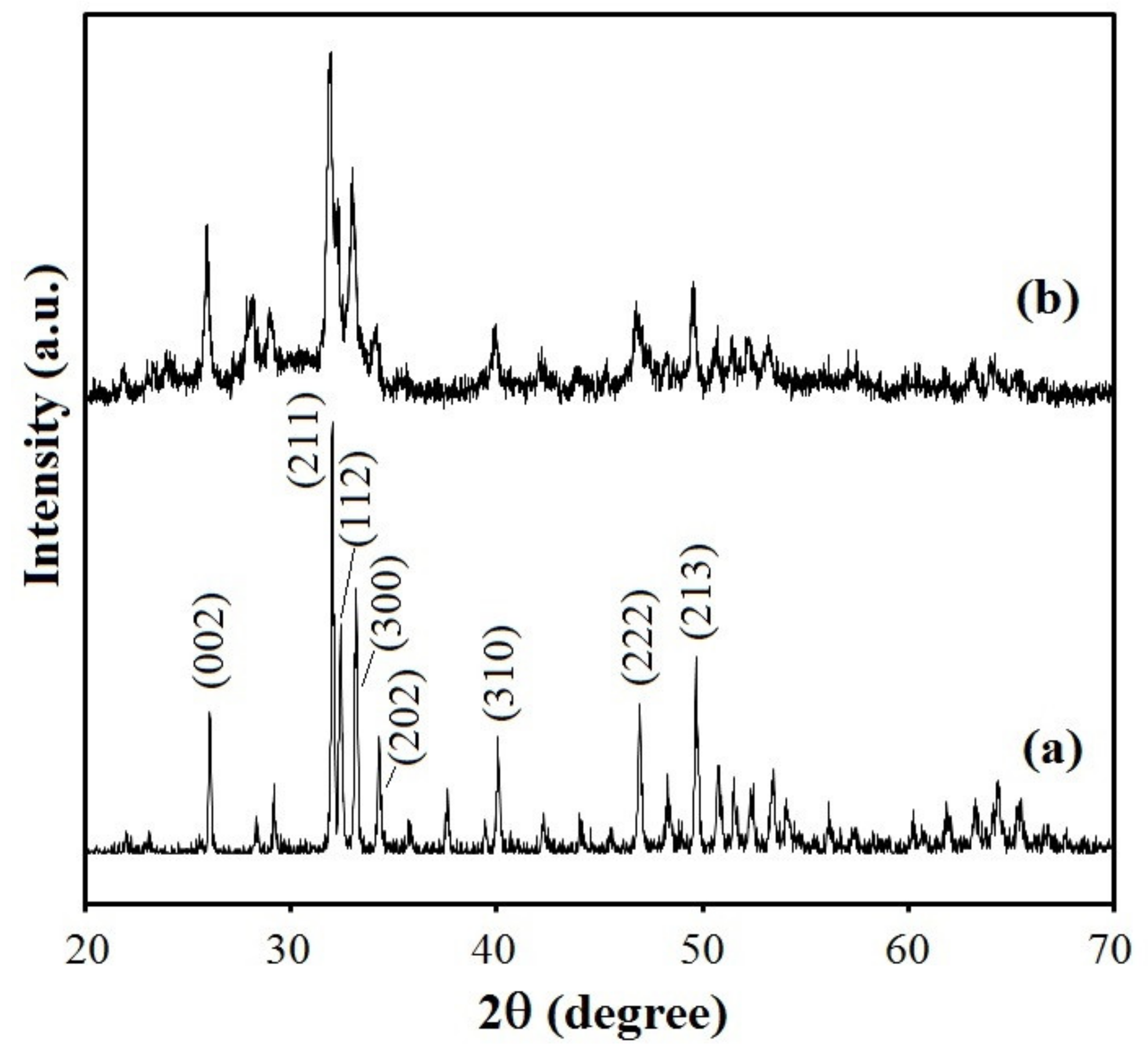
3.2. Coating Morphology and Structure
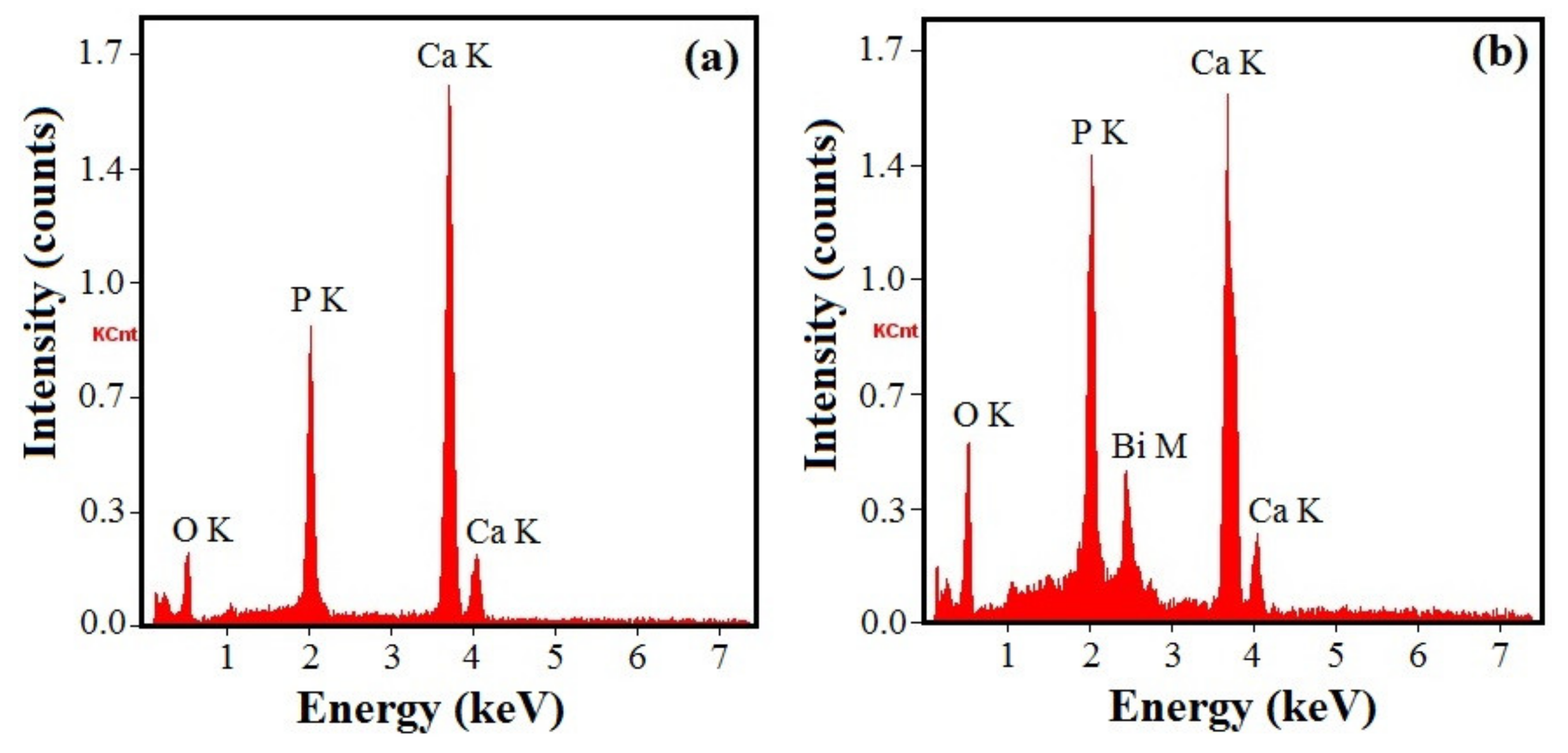
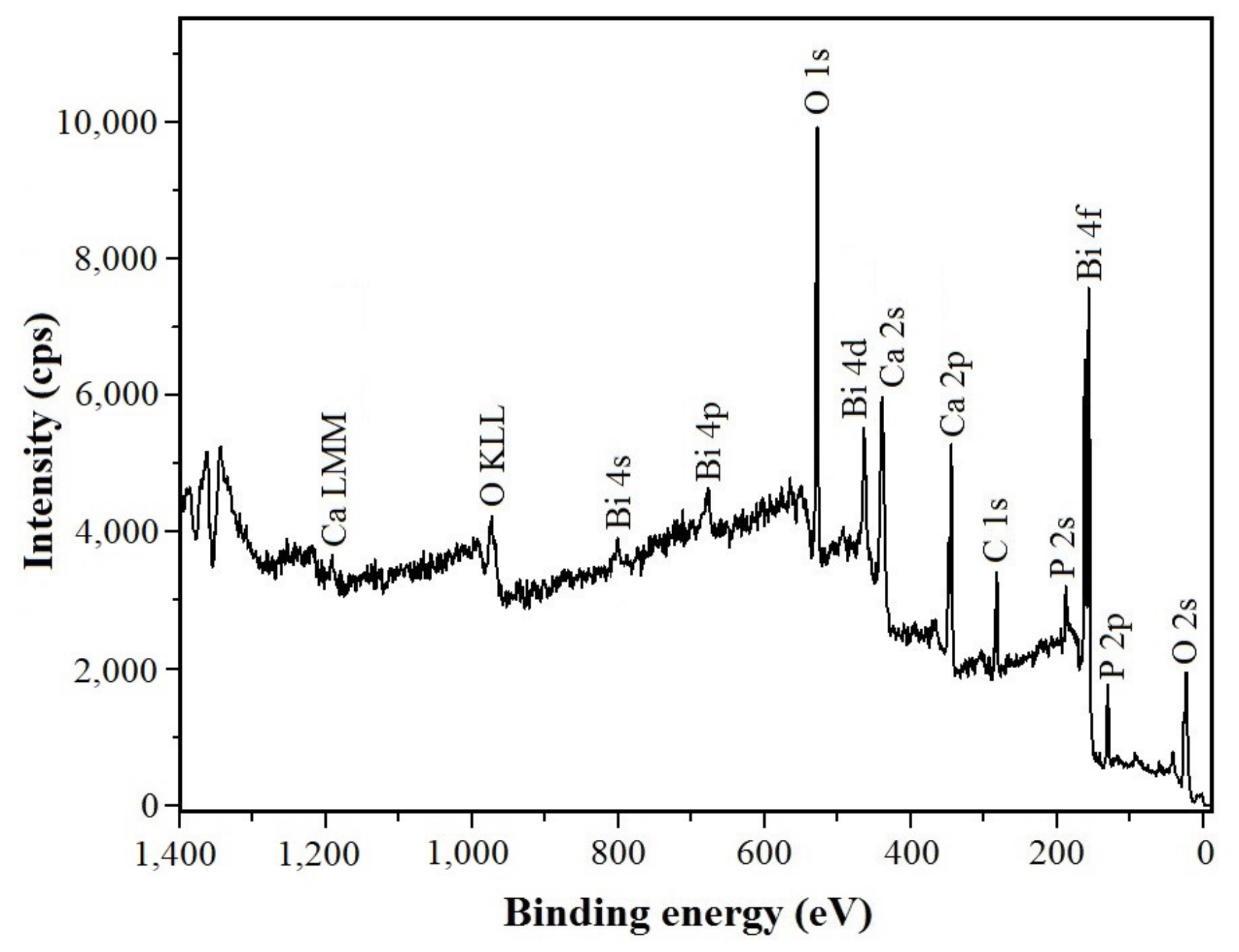
3.3. Coating Chemical Composition
3.4. Radiopacity
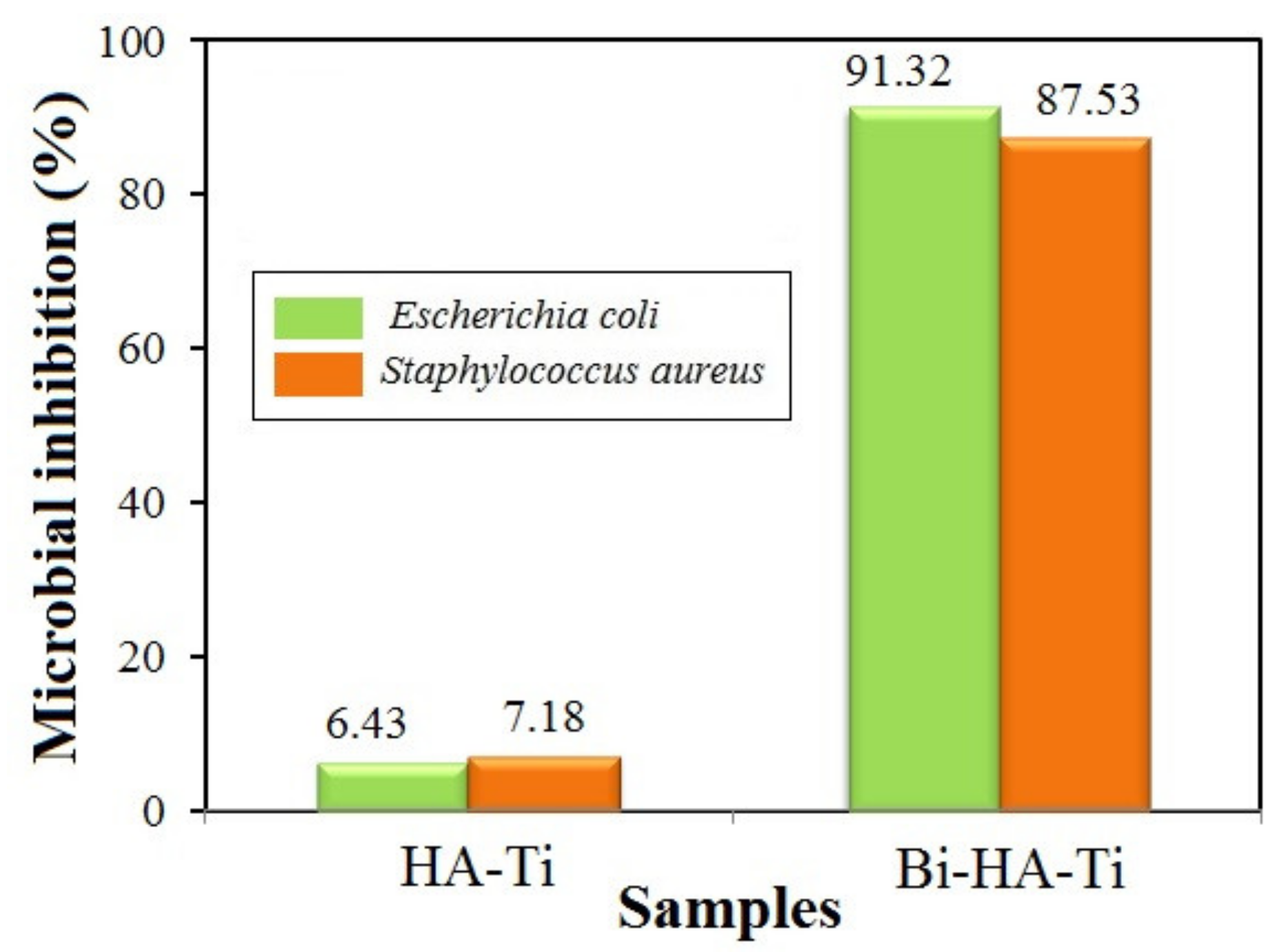
3.5. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, M.; Valanezhad, A.; Watanabe, I.; Yousefi, R. Surface and mechanical properties of modified porous titanium scaffold. Surf. Coat. Technol. 2017, 313, 61–66. [Google Scholar] [CrossRef]
- Pichugin, V.F.; Eshenko, E.V.; Surmenev, R.A.; Shesterikov, E.V.; Tverdokhlebov, S.I.; Ryabtseva, M.A.; Sokhoreva, V.V.; Khlusov, I.A. Application of high-frequency magnetron sputtering to deposit thin calcium-phosphate biocompatible coatings on a titanium surface. J. Surf. Ingestig. 2007, 1, 679–682. [Google Scholar] [CrossRef]
- Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Sadowska, B.; Więckowska-Szakiel, M.; Holopainen, J.; Ritala, M.; Leskelä, M.; Bartmański, M.; Szkodo, M.; et al. Titania nanotubes/hydroxyapatite nanocomposites produced with the use of the atomic layer deposition technique: Estimation of bioactivity and nanomechanical properties. Nanomaterials 2019, 9, 123. [Google Scholar] [CrossRef]
- Ciobanu, G.; Carja, G.; Ciobanu, O. Structural characterization of hydroxyapatite layer coatings on titanium supports. Surf. Coat. Technol. 2008, 202, 2467–2470. [Google Scholar] [CrossRef]
- Ciobanu, M.G.; Pop, G.; Ciobanu, O. Hydroxyapatite coatings on titanium implants. Rev. Chim. 2007, 58, 1313–1315. [Google Scholar]
- Domínguez-Trujillo, C.; Peón, E.; Chicardi, E.; Pérez, H.; Rodríguez-Ortiz, J.A.; Pavón, J.J.; García-Couce, J.; Galván, J.C.; García-Moreno, F.; Torresa, Y. Sol-gel deposition of hydroxyapatite coatings on porous titanium for biomedical applications. Surf. Coat. Technol. 2018, 333, 158–162. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate-containing biocomposites and hybrid biomaterials for biomedical applications. J. Funct. Biomater. 2015, 6, 708–832. [Google Scholar] [CrossRef]
- Lin, K.; Chang, J. Structure and properties of hydroxyapatite for biomedical applications. In Hydroxyapatite (Hap) for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–19. [Google Scholar]
- Ciobanu, G.; Ilisei, S.; Luca, C. The effect of vitamins to hydroxyapatite growth on porous polyurethane substrate. Prog. Org. Coat. 2012, 74, 648–653. [Google Scholar] [CrossRef]
- Ciobanu, G.; Ciobanu, O. Investigation on the effect of collagen and vitamins on biomimetic hydroxyapatite coating formation on titanium surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.H.; Shepherd, D.V.; Best, S.M. Substituted hydroxyapatites for bone repair. J. Mater. Sci. Mater. Med. 2012, 23, 2335–2347. [Google Scholar] [CrossRef] [PubMed]
- Zilm, M.E.; Chen, L.; Sharma, V.; McDannald, A.; Jain, M.; Ramprasada, R.; Wei, M. Hydroxyapatite substituted by transition metals: Experiment and theory. Phys. Chem. Chem. Phys. 2016, 18, 16457–16465. [Google Scholar] [CrossRef] [PubMed]
- Szyszka, K.; Targonska, S.; Gazinska, M.; Szustakiewicz, K.; Wiglusz, R.J. The comprehensive approach to preparation and investigation of the Eu3+ doped hydroxyapatite/poly(L-lactide) nanocomposites: Promising materials for theranostics application. Nanomaterials 2019, 9, 1146. [Google Scholar] [CrossRef]
- Ciobanu, G.; Bargan, A.M.; Luca, C. New cerium(IV)-substituted hydroxyapatite nanoparticles: Preparation and characterization. Ceram. Int. 2015, 41, 12192–12201. [Google Scholar] [CrossRef]
- Cox, S.C.; Jamshidi, P.; Grover, L.M.; Mallick, K.K. Preparation and characterisation of nanophase Sr, Mg, and Zn substituted hydroxyapatite by aqueous precipitation. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 35, 106–114. [Google Scholar] [CrossRef]
- Yang, N.; Sun, H. Biocoordination chemistry of bismuth: Recent advances. Coord. Chem. Rev. 2007, 251, 2354–2366. [Google Scholar] [CrossRef]
- Peng, M.; Dong, G.; Wondraczek, L.; Zhang, L.; Zhang, N.; Qiu, J. Discussion on the origin of NIR emission from Bi-doped materials. J. Non Cryst. Solids 2011, 357, 2241–2245. [Google Scholar] [CrossRef]
- Chen, F.; Liu, C.; Mao, Y. Bismuth-doped injectable calcium phosphate cement with improved radiopacity and potent antimicrobial activity for root canal filling. Acta Biomater. 2010, 6, 3199–3207. [Google Scholar] [CrossRef]
- Al-Hazmi, F.E. Synthesis and electrical properties of Bi doped hydroxyapatite ceramics. J. Alloy. Compd. 2016, 665, 119–123. [Google Scholar] [CrossRef]
- Ciobanu, G.; Bargan, A.M.; Luca, C. New bismuth-substituted hydroxyapatite nanoparticles for bone tissue engineering. JOM 2015, 67, 2534–2542. [Google Scholar] [CrossRef]
- Bargan, A.M.; Ciobanu, G.; Malutan, T.; Luca, C. Influence of cerium(IV) and bismuth(III) on the luminescence properties of the nanocrystalline hydroxyapatite. Rev. Chim. (Bucharest) 2015, 66, 1910–1913. [Google Scholar]
- Ciobanu, G.; Harja, M. Cerium-doped hydroxyapatite/collagen coatings on titanium for bone implants. Ceram. Int. 2019, 45, 2852–2857. [Google Scholar] [CrossRef]
- Fowler, B.A.; Sullivan, D.W., Jr.; Sexton, M.J. Chapter 31—Bismuth. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 655–666. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Kim, C.; Kendall, M.R.; Miller, M.A.; Long, C.L.; Larson, P.R.; Humphrey, M.B.; Madden, A.S.; Tas, A.C. Comparison of titanium soaked in 5 M NaOH or 5 M KOH solutions. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.B.; De Wijn, J.R.; Liu, Q.; De Groot, K.; Cui, F.Z. A simple method to prepare calcium phosphate coatings on Ti6Al4V. J. Mater. Sci. Mater. Med. 1997, 8, 765–770. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Lee, K.; Yoo, D. Large-area sodium titanate nanorods formed on titanium surface via NaOH alkali treatment. Arch. Metall. Mater. 2015, 60, 1371–1374. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Z.; Leng, Y. Biomimetic calcium phosphate coatings on nitric acid treated titanium surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 2007, 27, 700–708. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Surmenev, R.A.; Pichugin, V.F.; Chernousova, S.S.; Epple, M. In-vitro investigation of magnetron-sputtered coatings based on silicon-substituted hydroxyapatite. J. Surf. Ingestig. 2011, 5, 1202–1207. [Google Scholar] [CrossRef]
- Narasaraju, T.S.B.; Phebe, D.E. Some physico-chemical aspects of hydroxyapatite. J. Mater. Sci. 1996, 31, 1–21. [Google Scholar] [CrossRef]
- Salzedas, L.M.P.; Louzada, M.J.Q.; de Oliveira Filho, A.B. Radiopacity of restorative materials using digital images. J. Appl. Oral. Sci. 2006, 14, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Pekkan, G. Radiopacity of dental materials: An overview. Avicenna J. Dent. Res. 2016, 8, e36847. [Google Scholar] [CrossRef]
- Lim, P.N.; Teo, E.Y.; Ho, B.; Tay, B.Y.; Thian, E.S. Effect of silver content on the antibacterial and bioactive properties of silver-substituted hydroxyapatite. J. Biomed. Mater. Res. A 2013, 101, 2456–2464. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Róhycka, D. Substituted hydroxyapatites with antibacterial properties. Biomed. Res. Int. 2014, 2014, 178123. [Google Scholar] [CrossRef]
- Wright, J.A.; Nair, S.P. Interaction of staphylococci with bone. Int. J. Med. Microbiol. 2010, 300, 193–204. [Google Scholar] [CrossRef]
- Nair, S.; Meghji, S.; Wilson, M.; Reddi, K.; White, P.; Henderson, B. Bacterially induced bone destruction: Mechanisms and misconceptions. Infect. Immun. 1996, 64, 2371–2380. [Google Scholar]
- Marriott, I. Apoptosis-associated uncoupling of bone formation and resorption in osteomyelitis. Front. Cell. Infect. Microbiol. 2013, 3, 1–12. [Google Scholar] [CrossRef]
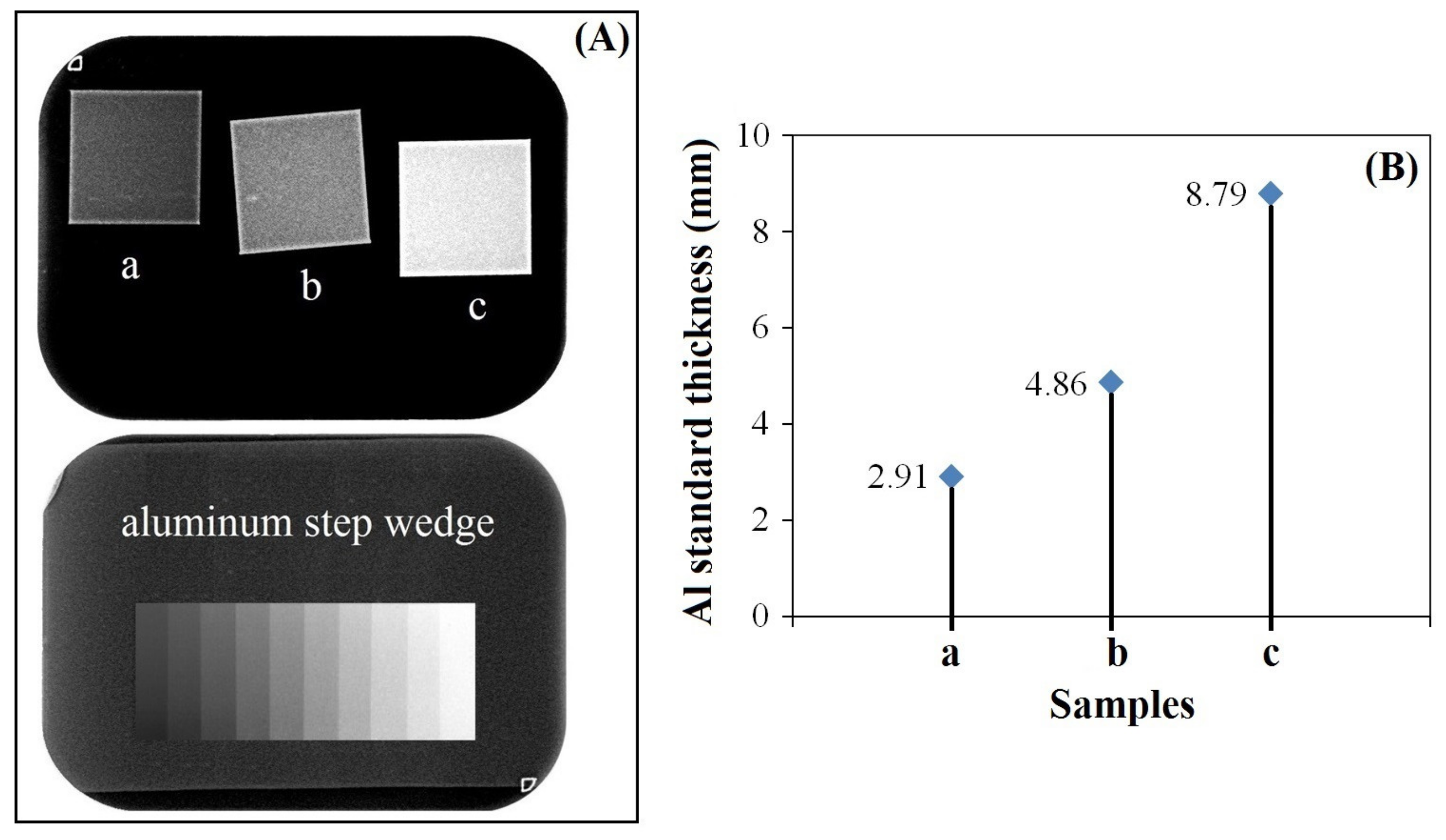
| Sample | SCS Solution | Final Coating | ||||
|---|---|---|---|---|---|---|
| Bi (%) | Bi (%) | |||||
| HA-Ti | 0 | 1.677 | 0 | 0 | 1.673 | 0 |
| Bi-HA-Ti | 0.01 | 1.677 | 1 | 0.0098 | 1.671 | 0.98 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciobanu, G.; Harja, M. Bismuth-Doped Nanohydroxyapatite Coatings on Titanium Implants for Improved Radiopacity and Antimicrobial Activity. Nanomaterials 2019, 9, 1696. https://doi.org/10.3390/nano9121696
Ciobanu G, Harja M. Bismuth-Doped Nanohydroxyapatite Coatings on Titanium Implants for Improved Radiopacity and Antimicrobial Activity. Nanomaterials. 2019; 9(12):1696. https://doi.org/10.3390/nano9121696
Chicago/Turabian StyleCiobanu, Gabriela, and Maria Harja. 2019. "Bismuth-Doped Nanohydroxyapatite Coatings on Titanium Implants for Improved Radiopacity and Antimicrobial Activity" Nanomaterials 9, no. 12: 1696. https://doi.org/10.3390/nano9121696
APA StyleCiobanu, G., & Harja, M. (2019). Bismuth-Doped Nanohydroxyapatite Coatings on Titanium Implants for Improved Radiopacity and Antimicrobial Activity. Nanomaterials, 9(12), 1696. https://doi.org/10.3390/nano9121696







