α- and β-Phase Ni-Mg Hydroxide for High Performance Hybrid Supercapacitors
Abstract
1. Introduction
2. Experimental Section
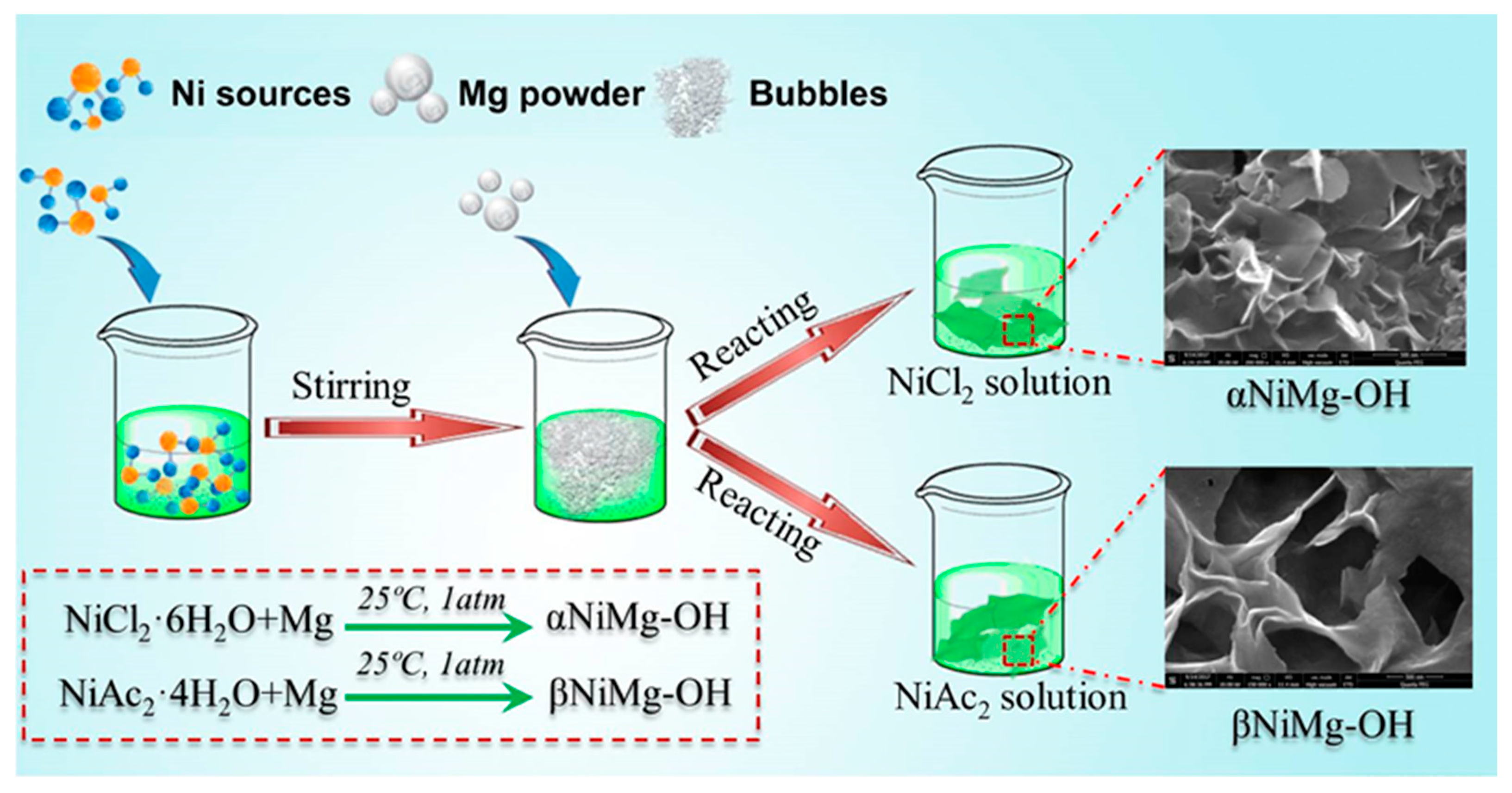
2.1. Preparation of Samples
2.2. Characterization of Samples
2.3. Electrochemical Measurements
3. Results and Discussion
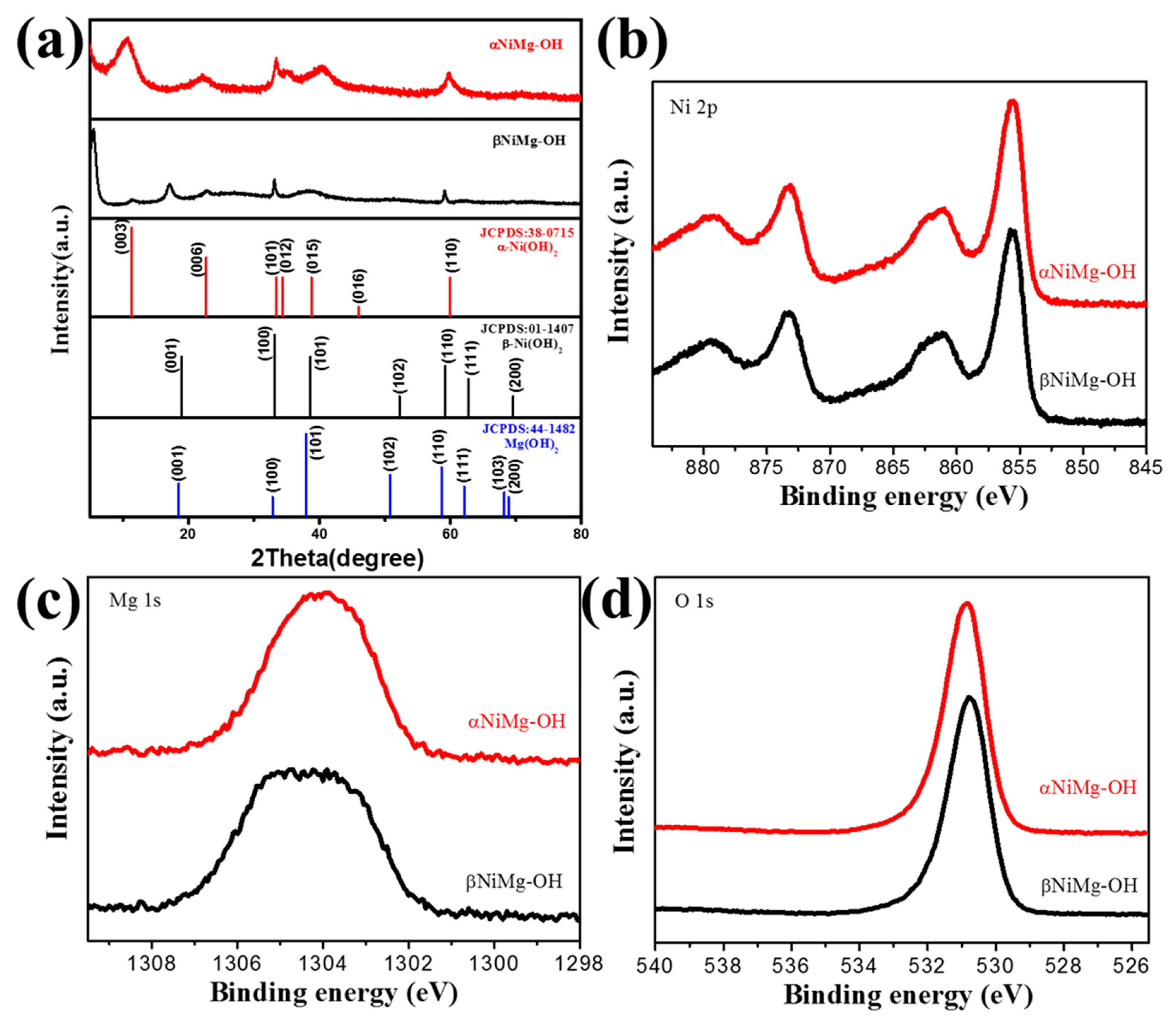
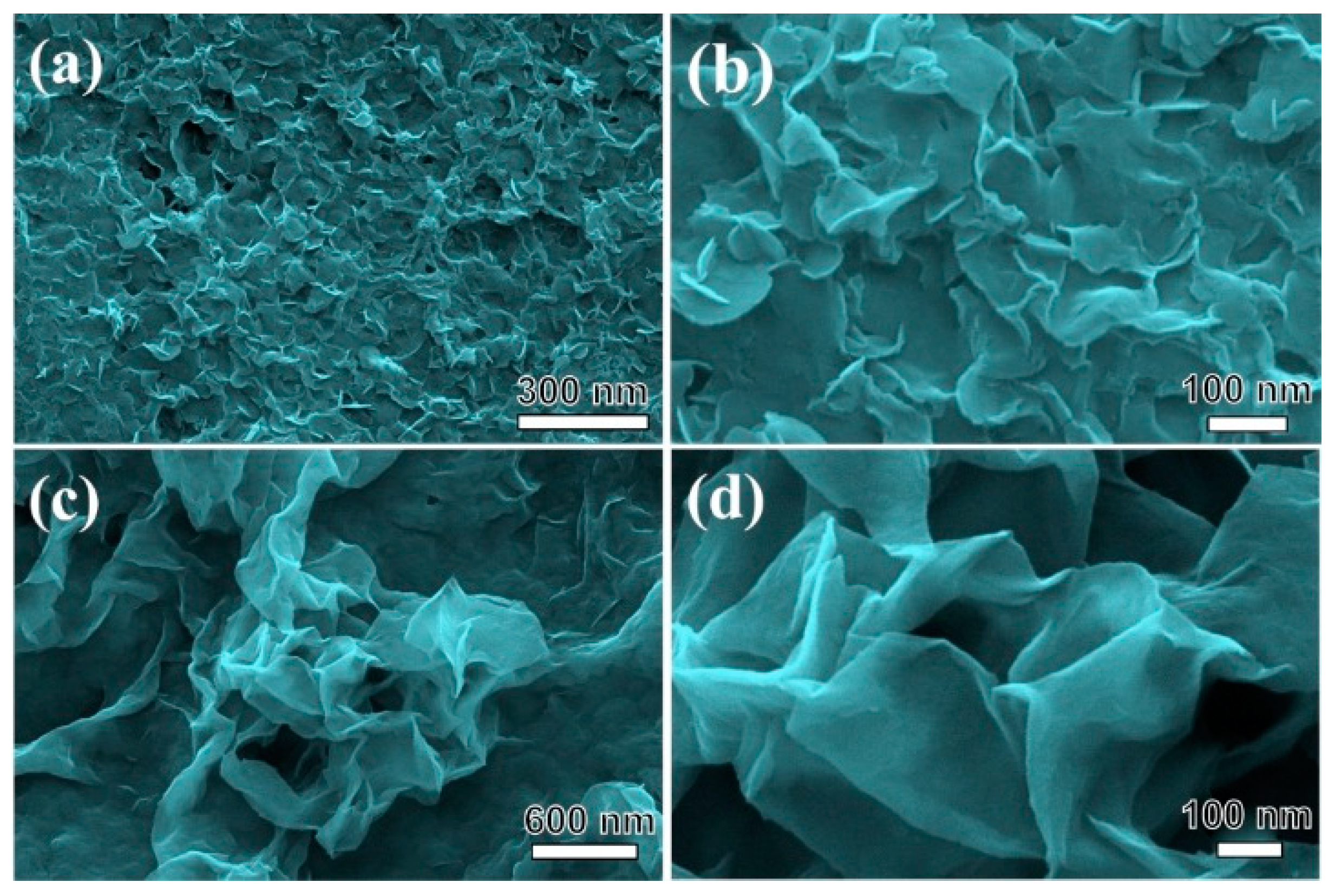
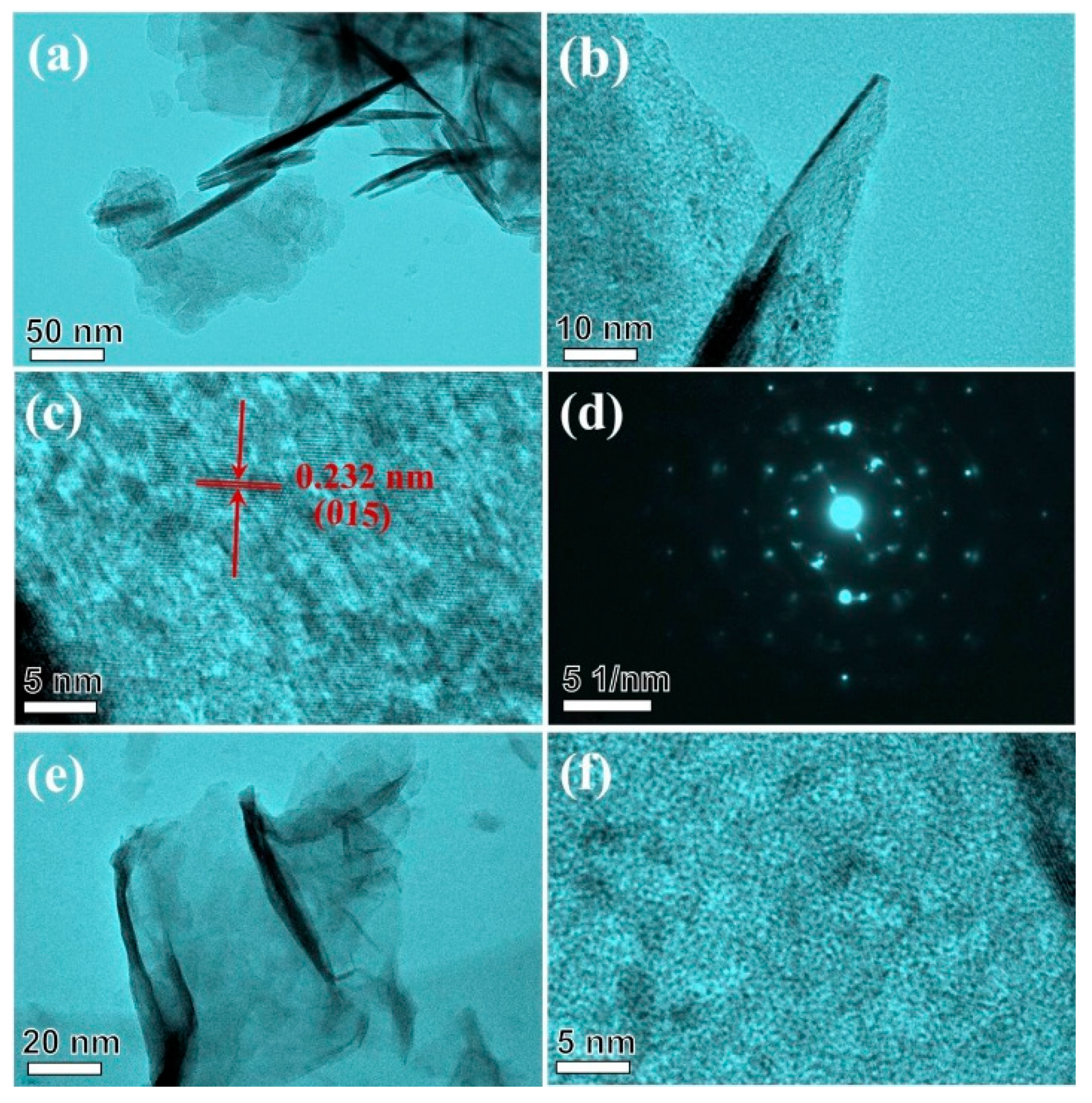
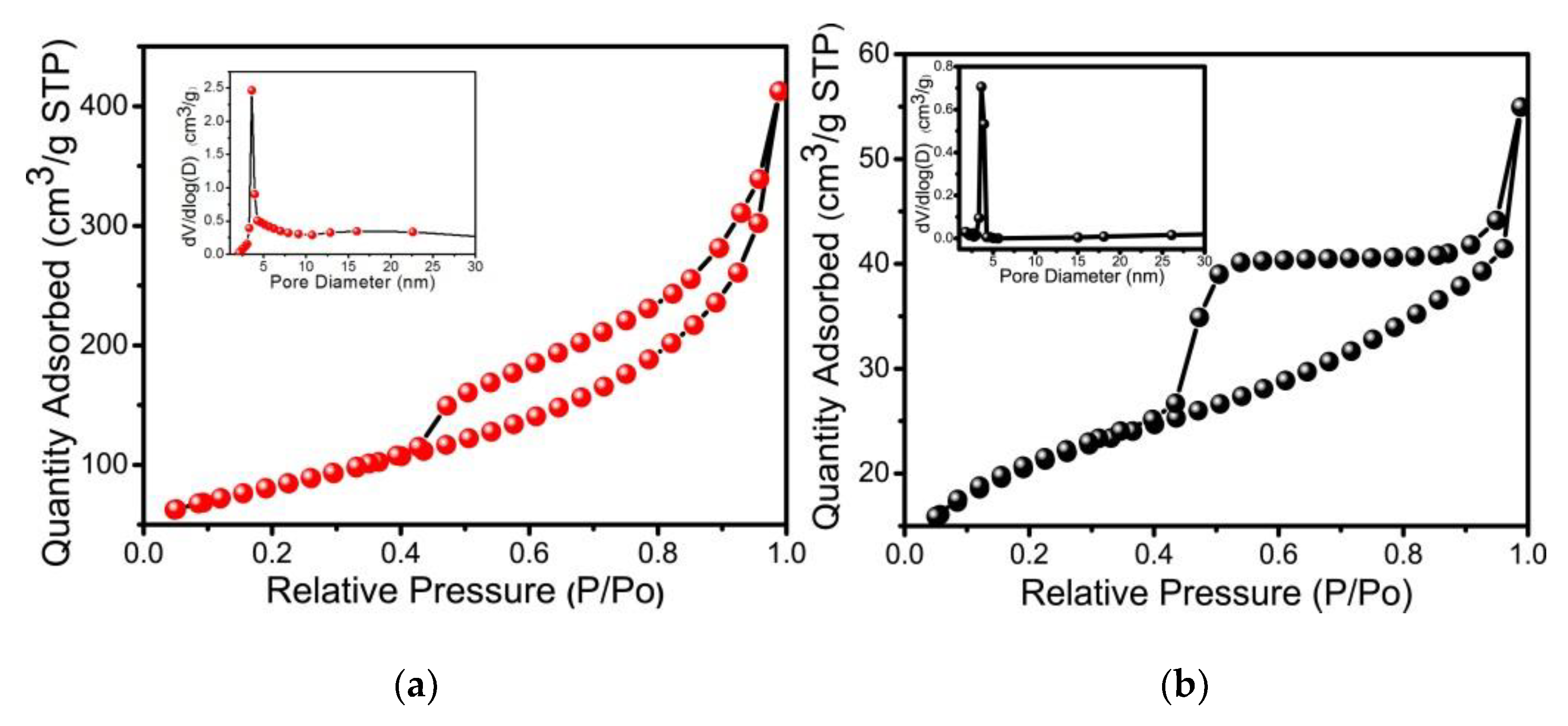
3.1. Characterization Results of NiMg-OH
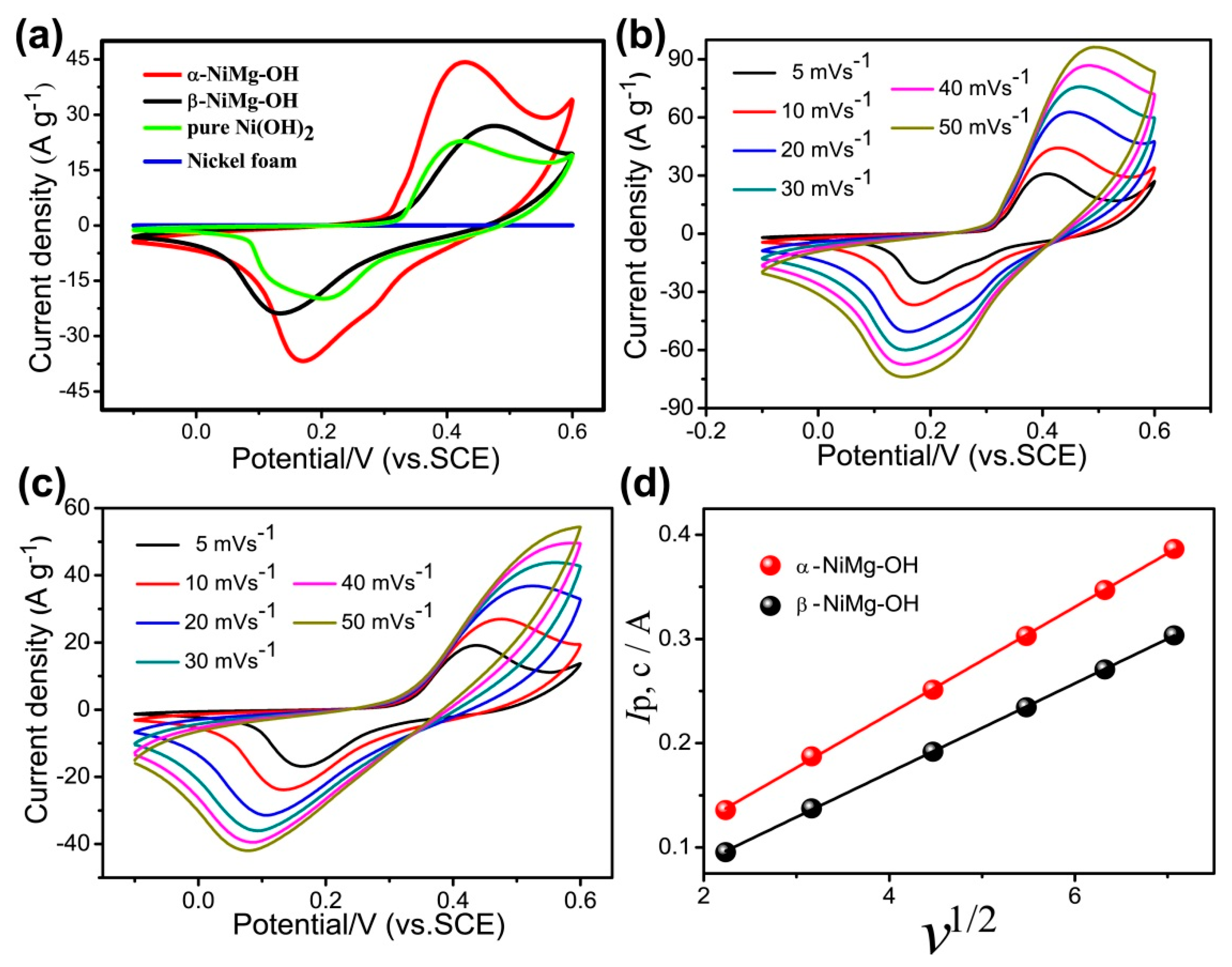
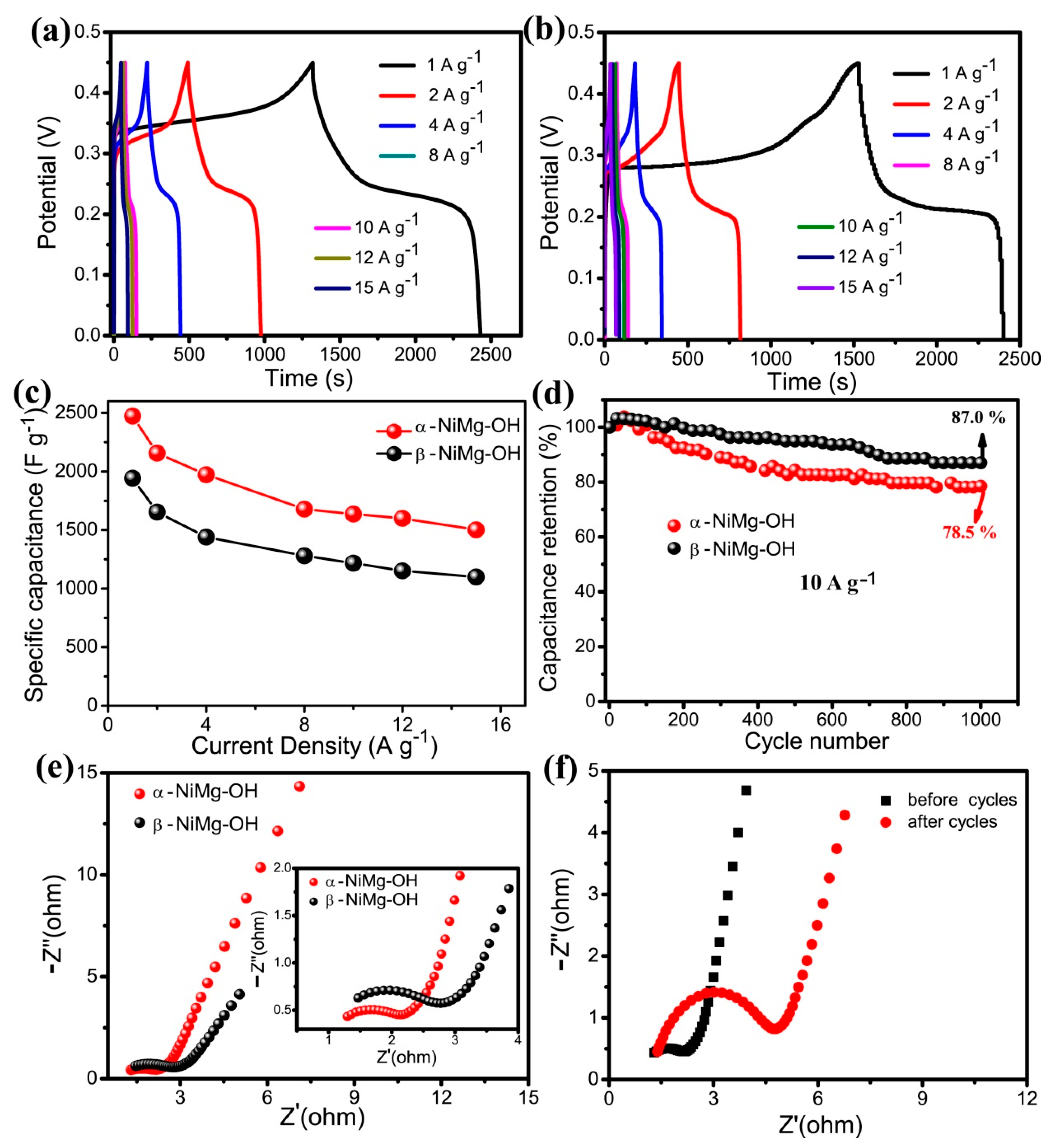
3.2. Electrochemical Performance of 2D NiMg-OH
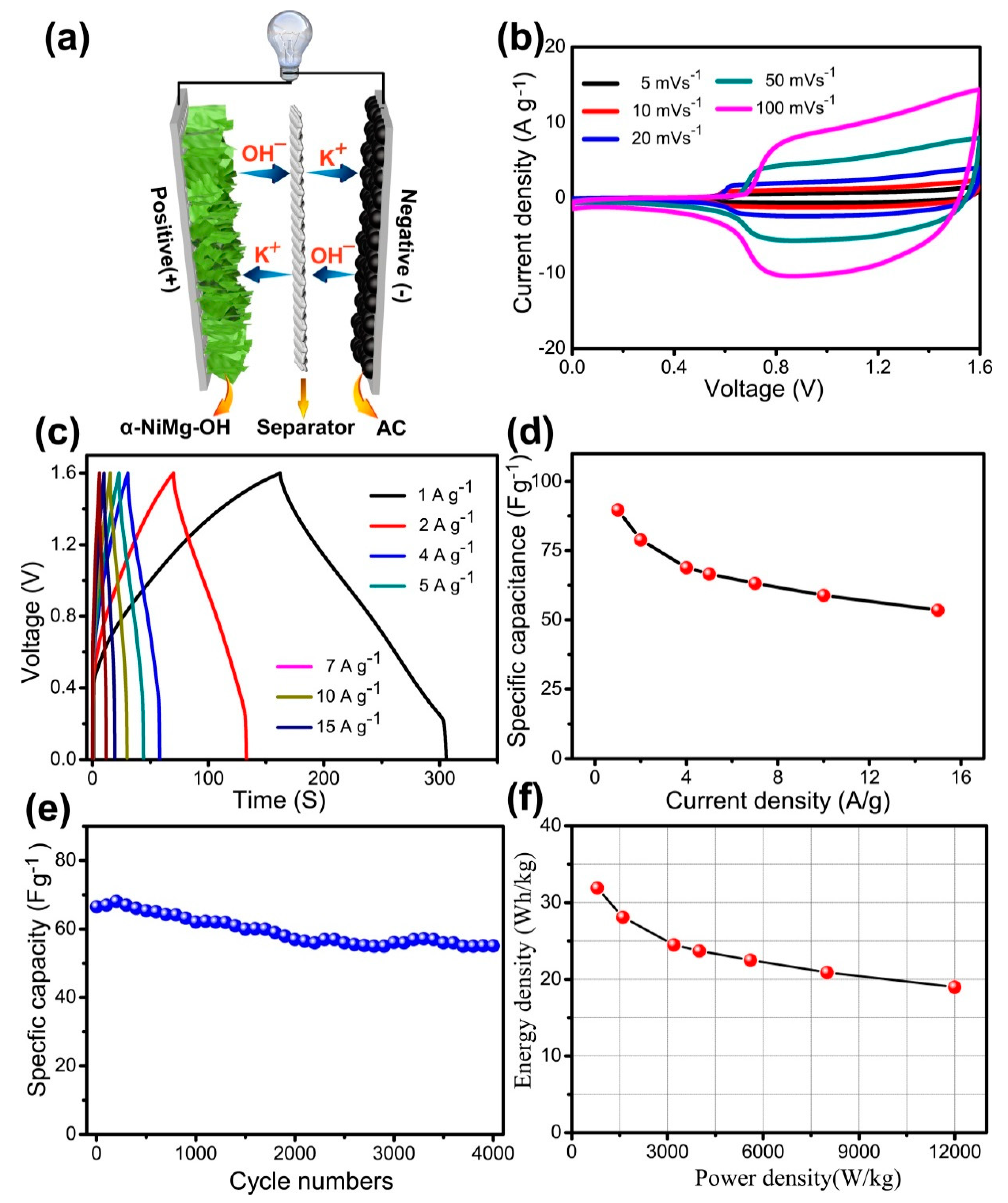
3.3. Asymmetric Supercapacitor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Miller, J.R.; Simon, P. Materials science-electrochemical capacitors for energy management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, W.; Huang, Y.; Yu, Y. Hierarchical mesoporous Co3O4@ZnCo2O4 hybrid nanowire arrays supported on Ni foam for high-performance asymmetric supercapacitors. Sci. China Mater. 2018, 61, 1167–1176. [Google Scholar] [CrossRef]
- Xu, J.; Sun, Y.; Lu, M.; Wang, L.; Zhang, J.; Tao, E.; Qian, J.; Liu, X. Fabrication of the porous MnCo2O4 nanorod arrays on Ni foam as an advanced electrode for asymmetric supercapacitors. Acta Mater. 2018, 152, 162–174. [Google Scholar] [CrossRef]
- Gregoire, B.; Ruby, C.; Carteret, C. Hydrolysis of mixed Ni2+-Fe3+ and Mg2+-Fe3+ solutions and mechanism of formation of layered double hydroxides. Dalton. Trans. 2013, 42, 15687–15698. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, H.; Xu, P.; Wang, R.; Tong, Y.; Lu, Q.; Gao, F. In situ construction of hierarchical Co/MnO@graphite carbon composites for highly supercapacitive and OER electrocatalytic performances. Nanoscale 2018, 10, 13702–13712. [Google Scholar] [CrossRef]
- Li, M.; Yang, W.; Li, J.; Feng, M.; Li, W.; Li, H.; Yu, Y. Porous layered stacked MnCo2O4 cubes with enhanced electrochemical capacitive performance. Nanoscale 2018, 10, 2218–2225. [Google Scholar] [CrossRef]
- Du, K.; Wei, G.; Zhao, F.; An, C.; Wang, H.; Li, J.; An, C. Urchin-like FeOOH hollow microspheres decorated with MnO2 for enhanced supercapacitor performance. Sci. China Mater. 2017, 61, 48–56. [Google Scholar] [CrossRef]
- Du, W.; Liu, R.; Jiang, Y.; Lu, Q.; Fan, Y.; Gao, F. Facile synthesis of hollow Co3O4 boxes for high capacity supercapacitor. J. Power Sources 2013, 227, 101–105. [Google Scholar] [CrossRef]
- Xie, M.J.; Xu, Z.C.; Duan, S.Y.; Tian, Z.F.; Zhang, Y.; Xiang, K.; Lin, M.; Guo, X.F.; Ding, W.P. Facile growth of homogeneous Ni(OH)2 coating on carbon nanosheets for high-performance asymmetric supercapacitor applications. Nano Res. 2018, 11, 216–224. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.J.; Sun, W.; Ning, G.Q.; Wei, T.; Zhang, Q.; Zhang, R.F.; Zhi, L.J.; Wei, F. Advanced asymmetric supercapacitors based on Ni(OH)2/graphene and porous graphene electrodes with high energy density. Adv. Funct. Mater. 2012, 22, 2632–2641. [Google Scholar] [CrossRef]
- Liu, F.; Chu, X.; Zhang, H.; Zhang, B.; Su, H.; Jin, L.; Wang, Z.; Huang, H.; Yang, W. Synthesis of self-assembly 3D porous Ni(OH)2 with high capacitance for hybrid supercapacitors. Electrochim. Acta 2018, 269, 102–110. [Google Scholar] [CrossRef]
- Wang, D.; Guan, B.; Li, Y.; Li, D.; Xu, Z.; Hu, Y.; Wang, Y.; Zhang, H. Morphology-controlled synthesis of hierarchical mesoporous α-Ni(OH)2 microspheres for high-performance asymmetric supercapacitors. J. Alloys Comp. 2018, 737, 238–247. [Google Scholar] [CrossRef]
- Li, M.; Lei, W.; Yu, Y.; Yang, W.; Li, J.; Chen, D.; Xu, S.; Feng, M.; Li, H. High-performance asymmetric supercapacitors based on monodisperse MnO nanocrystals with high energy densities. Nanoscale 2018, 10, 15926–15931. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, Y.; Lu, M.; Wang, L.; Zhang, J.; Liu, X. One-step electrodeposition fabrication of Ni3S2 nanosheet arrays on Ni foam as an advanced electrode for asymmetric supercapacitors. Sci. China Mater. 2018, 62, 699–710. [Google Scholar] [CrossRef]
- Cui, H.; Xue, J.; Wang, M. Synthesis of high electrochemical performance Ni(OH)2 nanosheets through a solvent-free reaction for application in supercapacitor. Adv. Powder Techol. 2015, 26, 434–438. [Google Scholar] [CrossRef]
- Jiang, L.; Qiu, Y.; Luo, P.; Yu, Y. Nickel hydroxide-impregnated and-coated carbon nanotubes using an easily manipulated solvothermal route for supercapacitors. Ceram. Int. 2016, 42, 11634–11639. [Google Scholar] [CrossRef]
- Ke, X.; Zhang, Z.; Cheng, Y.; Liang, Y.; Tan, Z.; Liu, J.; Liu, L.; Shi, Z.; Guo, Z. Ni(OH)2 nanoflakes supported on 3D hierarchically nanoporous gold/Ni foam as superior electrodes for supercapacitors. Sci. China Mater. 2017, 61, 353–362. [Google Scholar] [CrossRef]
- Lee, J.W.; Ko, J.M.; Kim, J.-D. Hierarchical microspheres based on α-Ni(OH)2 nanosheets intercalated with different anions: Synthesis, anion exchange, and effect of intercalated anions on electrochemical capacitance. J. Phys. Chem. C 2011, 115, 19445–19454. [Google Scholar] [CrossRef]
- Dai, J.; Li, S.F.Y.; Xiao, T.D.; Wang, D.M.; Reisner, D.E. Structural stability of aluminum stabilized alpha nickel hydroxide as a positive electrode material for alkaline secondary batteries. J. Power Sources 2000, 89, 40–45. [Google Scholar] [CrossRef]
- Nunes, C.V.; Danczuk, M.; Bortoti, A.A.; Gonçalves, J.M.; Araki, K.; Anaissi, F.J. Unexpected effect of drying method on the microstructure and electrocatalytic properties of bentonite/alpha-nickel hydroxide nanocomposite. J. Power Sources 2015, 297, 408–412. [Google Scholar] [CrossRef]
- Xie, M.; Duan, S.; Shen, Y.; Fang, K.; Wang, Y.; Lin, M.; Guo, X. In-situ-grown Mg(OH)2-derived hybrid α-Ni(OH)2 for highly stable supercapacitor. ACS Energy Lett. 2016, 1, 814–819. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, X.; Lu, C.; Chen, C. The fine control of porous pompon-like Mg-incorporated α-Ni(OH)2 for enhanced supercapacities. Funct. Mater. Lett. 2016, 9, 1650057. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Wang, J.M.; Chen, H.; Pan, T.; Zhang, J.Q.; Cao, C.N. Al-substituted α-nickel hydroxide prepared by homogeneous precipitation method with urea. Int. J. Hydrogen Energy 2004, 29, 889–896. [Google Scholar] [CrossRef]
- Huang, J.; Lei, T.; Wei, X.; Liu, X.; Liu, T.; Cao, D.; Yin, J.; Wang, G. Effect of Al-doped β-Ni(OH)2 nanosheets on electrochemical behaviors for high performance supercapacitor application. J. Power Sources 2013, 232, 370–375. [Google Scholar] [CrossRef]
- Shangguan, E.; Li, J.; Guo, D.; Guo, L.; Nie, M.; Chang, Z.; Yuan, X.-Z.; Wang, H. A comparative study of structural and electrochemical properties of high-density aluminum substituted α-nickel hydroxide containing different interlayer anions. J. Power Sources 2015, 282, 158–168. [Google Scholar] [CrossRef]
- Liu, H.; Yu, T.; Su, D.; Tang, Z.; Zhang, J.; Liu, Y.; Yuan, A.; Kong, Q. Ultrathin Ni-Al layered double hydroxide nanosheets with enhanced supercapacitor performance. Ceram. Int. 2017, 43, 14395–14400. [Google Scholar] [CrossRef]
- Xia, Q.X.; San Hui, K.; Hui, K.N.; Kim, S.D.; Lim, J.H.; Choi, S.Y.; Zhang, L.J.; Mane, R.S.; Yun, J.M.; Kim, K.H. Facile synthesis of manganese carbonate quantum dots/Ni(HCO3)2–MnCO3 composites as advanced cathode materials for high energy density asymmetric supercapacitors. J. Mater. Chem. A 2015, 3, 22102–22117. [Google Scholar] [CrossRef]
- Liang, D.; Wu, S.; Liu, J.; Tian, Z.; Liang, C. Co-doped Ni hydroxide and oxide nanosheet networks: Laser-assisted synthesis, effective doping, and ultrahigh pseudocapacitor performance. J. Mater. Chem. A 2016, 4, 10609–10617. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Z.; Wang, Z.; Li, X.; Guo, H.; Wang, J.; Zhang, D. Facile construction of Co(OH)2@Ni(OH)2 core-shell nanosheets on nickel foam as three dimensional free-standing electrode for supercapacitors. Electrochim. Acta 2019, 293, 40–46. [Google Scholar] [CrossRef]
- Jin, H.; Yuan, D.; Zhu, S.; Zhu, X.; Zhu, J. Ni-Co layered double hydroxide on carbon nanorods and graphene nanoribbons derived from MOFs for supercapacitors. Dalton. Trans. 2018, 47, 8706–8715. [Google Scholar] [CrossRef]
- Le, K.; Wang, Z.; Wang, F.; Wang, Q.; Shao, Q.; Murugadoss, V.; Wu, S.; Liu, W.; Liu, J.; Gao, Q.; et al. Sandwich-like NiCo layered double hydroxide/reduced graphene oxide nanocomposite cathodes for high energy density asymmetric supercapacitors. Dalton. Trans. 2019, 48, 5193–5202. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, C.; Shi, Y.; Dang, L.; Jin, Y.; Jiang, H.; Lu, Q.; Gao, F. Generalized low-temperature fabrication of scalable multi-type two-dimensional nanosheets with a green soft template. Chem. Eur. J 2016, 22, 5575–5582. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Shen, K.; Wu, Z.; Wang, X.; Kong, X. Electrodeposition of Zn-doped α-nickel hydroxide with flower-like nanostructure for supercapacitors. Appl. Surf. Sci. 2012, 258, 8117–8123. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, C.; Li, C.; Fan, M.; Shu, K.; Chen, H.C. Strong synergetic electrochemistry between transition metals of α phase Ni-Co-Mn hydroxide contributed superior performance for hybrid supercapacitors. J. Power Sources 2019, 412, 559–567. [Google Scholar] [CrossRef]
- Liang, T.; Xuan, H.; Xu, Y.; Gao, J.; Han, X.; Yang, J.; Han, P.; Wang, D.; Du, Y. Rational assembly of CoAl-layered double hydroxide on reduced graphene oxide with enhanced electrochemical performance for energy storage. ChemElectroChem 2018, 5, 2424–2434. [Google Scholar] [CrossRef]
- Xiao, Y.; Su, D.; Wang, X.; Wu, S.; Zhou, L.; Fang, S.; Li, F. Layered double hydroxides with larger interlayer distance for enhanced pseudocapacitance. Sci. China Mater. 2017, 61, 263–272. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Poirier, S.; Bock, C.; MacDougall, B.R. Raman and infrared spectroscopy of alpha and beta phases of thin nickel hydroxide films electrochemically formed on nickel. J. Phys. Chem. A 2012, 116, 6771–6784. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, S.; Li, R.; Sun, X. New insight into the conventional replacement reaction for the large-scale synthesis of various metal nanostructures and their formation mechanism. Chem. Eur. J 2010, 16, 10630–10634. [Google Scholar] [CrossRef]
- Huang, C.; Song, X.; Qin, Y.; Xu, B.; Chen, H.C. Cation exchange reaction derived amorphous bimetal hydroxides as advanced battery materials for hybrid supercapacitors. J. Mater. Chem. A 2018, 6, 21047–21055. [Google Scholar] [CrossRef]
- Miao, C.; Zhu, Y.; Zhao, T.; Jian, X.; Li, W. Synthesis and electrochemical performance of mixed phase α/β nickel hydroxide by codoping with ca2+ and PO4 3−. Ionics 2015, 21, 3201–3208. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Ghaemi, M.; Sabour, B.; Dalvand, S. Electrochemical preparation of α-Ni(OH)2 ultrafine nanoparticles for high-performance supercapacitors. J. Solid State Electr. 2014, 18, 1569–1584. [Google Scholar] [CrossRef]
- Gao, M.; Sheng, W.; Zhuang, Z.; Fang, Q.; Gu, S.; Jiang, J.; Yan, Y. Efficient water oxidation using nanostructured α-nickel-hydroxide as an electrocatalyst. J. Am. Chem. Soc. 2014, 136, 7077–7084. [Google Scholar] [CrossRef] [PubMed]
- Morishita, M.; Kakeya, T.; Ochiai, S.; Ozaki, T.; Kawabe, Y.; Watada, M.; Sakai, T. Structural analysis by synchrotron X-ray diffraction, X-ray absorption fine structure and transmission electron microscopy for aluminum-substituted α-type nickel hydroxide electrode. J. Power Sources 2009, 193, 871–877. [Google Scholar] [CrossRef]
- Cheng, M.Y.; Hwang, B.J. Control of uniform nanostructured α-Ni(OH)2 with self-assembly sodium dodecyl sulfate templates. J. Colloid Interf. Sci. 2009, 337, 265–271. [Google Scholar] [CrossRef]
- Aksoy, S.; Caglar, Y.; Ilican, S.; Caglar, M. Sol–gel derived Li–Mg Co-doped ZnO films: Preparation and characterization via XRD, XPS, FESEM. J. Alloys Comp. 2012, 512, 171–178. [Google Scholar] [CrossRef]
- Du, H.; Jiao, L.; Cao, K.; Wang, Y.; Yuan, H. Polyol-mediated synthesis of mesoporous α-Ni(OH)2 with enhanced supercapacitance. ACS Appl. Mater. Interfaces 2013, 5, 6643–6648. [Google Scholar] [CrossRef]
- Du, H.; Wang, Y.; Yuan, H.; Jiao, L. Facile synthesis and high capacitive performance of 3D hierarchical Ni(OH)2 microspheres. Electrochim. Acta 2016, 196, 84–91. [Google Scholar] [CrossRef]
- Yue, X.; Pan, J.; Sun, Y.; Wang, Z. Synthesis and electrochemical properties of nano–micro spherical β-Ni(OH)2 with super high charge–discharge speed. Ind. Eng. Chem. Res. 2012, 51, 8358–8365. [Google Scholar] [CrossRef]
- Dong, C.; He, G.; Zheng, W.; Bian, T.; Li, M.; Zhang, D. Study on antibacterial mechanism of Mg(OH)2 nanoparticles. Mater. Lett. 2014, 134, 286–289. [Google Scholar] [CrossRef]
- Bernard, M.C.; Bernard, P.; Keddam, M.; Senyarich, S.; Takenouti, H. Characterisation of new nickel hydroxides during the transformation of α-Ni(OH)2 to β-Ni(OH)2 by ageing. Electrochim. Acta 1996, 41, 91–93. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Li, C.; Huang, J.; Luv, J.; Xu, P.; Liu, J.; You, X. Facile and environmentally friendly synthesis of ultrathin nickel hydroxide nanosheets with excellent supercapacitor performances. Nanoscale 2016, 8, 11797–11802. [Google Scholar] [CrossRef] [PubMed]
- Szytula, A.; Murasik, A.; Balanda, M. Neutron diffraction study of Ni(OH)2. Phys. Status Solidi B 1971, 43, 125–128. [Google Scholar] [CrossRef]
- Bode, H.; Dehmelt, K.; Witte, J. Zur kenntnis der nickelhydroxidelektrode—I. Über das nickel (II)-hydroxidhydrat. Electrochim. Acta 1966, 11, 1079–1087. [Google Scholar] [CrossRef]
- Li, H.B.; Yu, M.H.; Wang, F.X.; Liu, P.; Liang, Y.; Xiao, J.; Wang, C.X.; Tong, Y.X.; Yang, G.W. Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials. Nat. Commun. 2013, 4, 1894. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, N.; Shi, Z.; Ye, Z.; Gao, Q.; Zhi, M.; Hong, Z. Preparation of Ni-Al layered double hydroxide hollow microspheres for supercapacitor electrode. Chem. Eng. J. 2018, 338, 55–61. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Y.; Yu, S.; Guo, W.; Mu, S.; Wang, H.; Zhao, Y.; Hou, L.; Fan, Y.; Gao, F. Template-free hydrothermal synthesis of nickel cobalt hydroxide nanoflowers with high performance for asymmetric supercapacitor. Electrochim. Acta 2015, 161, 279–289. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Zhou, G.; Gao, X.; Chen, J.; Zhang, L.; Xu, J.; Zhao, P.; Gao, F. α- and β-Phase Ni-Mg Hydroxide for High Performance Hybrid Supercapacitors. Nanomaterials 2019, 9, 1686. https://doi.org/10.3390/nano9121686
Yin J, Zhou G, Gao X, Chen J, Zhang L, Xu J, Zhao P, Gao F. α- and β-Phase Ni-Mg Hydroxide for High Performance Hybrid Supercapacitors. Nanomaterials. 2019; 9(12):1686. https://doi.org/10.3390/nano9121686
Chicago/Turabian StyleYin, Jingzhou, Guolang Zhou, Xiaoliang Gao, Jiaqi Chen, Lili Zhang, Jiaying Xu, Pusu Zhao, and Feng Gao. 2019. "α- and β-Phase Ni-Mg Hydroxide for High Performance Hybrid Supercapacitors" Nanomaterials 9, no. 12: 1686. https://doi.org/10.3390/nano9121686
APA StyleYin, J., Zhou, G., Gao, X., Chen, J., Zhang, L., Xu, J., Zhao, P., & Gao, F. (2019). α- and β-Phase Ni-Mg Hydroxide for High Performance Hybrid Supercapacitors. Nanomaterials, 9(12), 1686. https://doi.org/10.3390/nano9121686



